Influence of Environment on Microbial Colonization of Historic Stone Buildings with Emphasis on Cyanobacteria
Abstract
1. Introduction
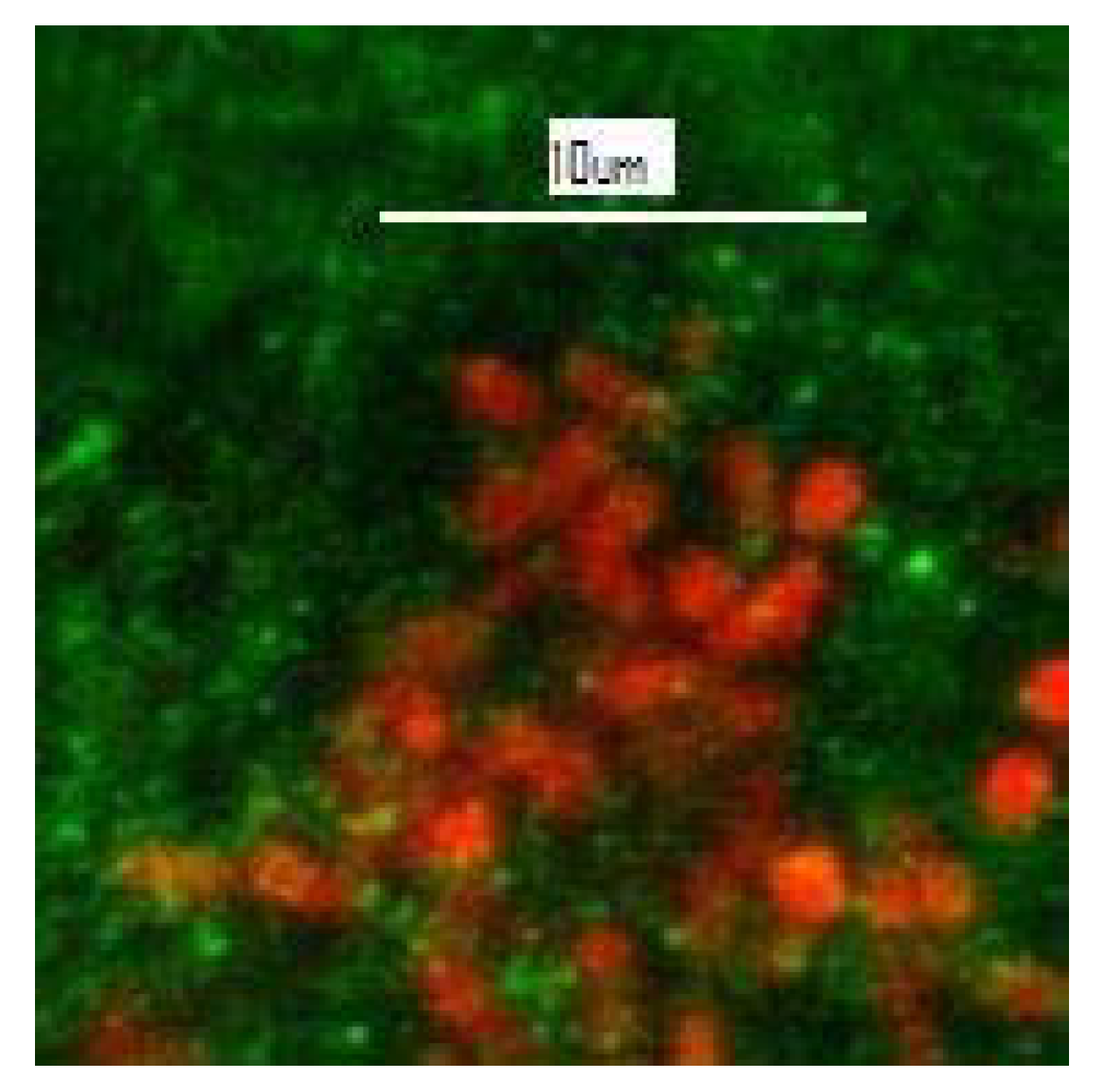
Colonization of Stone by Microorganisms
2. Deterioration of Stone Buildings by Microorganisms
3. Black Crusts: A Special Type of Biofilm?
4. Effects of Environment/Climate
5. Effect of Air Pollution
6. Potential Effects of Climate Change
7. Conclusions
Funding
Conflicts of Interest
References
- Mahdjoubi, L.; Hawas, S.; Fitton, R.; Dewidar, K.; Nagy, G.; Marshall, A.; Alzaatreh, A.; Abdelhady, E. A Guide for Monitoring the Effects of Climate Change on Heritage Building Materials and Elements. Report Prepared for the Funded Research Project: ‘Heritage Building Information Modelling and Smart Heritage Buildings Performance Measurements for Sustainability’. 2017. Available online: https://uwe-repository.worktribe.com/output/882571 (accessed on 16 October 2020).
- Liu, X.; Koestler, R.J.; Warscheid, T.; Katayama, Y.; Gu, J.-D. Microbial deterioration and sustainable conservation of stone monuments and buildings. Nat. Sustain. 2020. [Google Scholar] [CrossRef]
- Mitchell, R.; Gu, J.-D. Changes in the biofilm microflora of limestone caused by atmospheric pollutants. Int Biodeterior. Biodegrad. 2000, 46, 299–303. [Google Scholar] [CrossRef]
- Guillitte, O. Bioreceptivity: A new concept for building ecology studies. Sci. Total Environ. 1995, 167, 215–220. [Google Scholar] [CrossRef]
- Sanmartín, P.; Grove, R.; Carballeira, R.; Viles, H. Impact of colour on the bioreceptivity of granite to the green alga Apatococcus lobatus: Laboratory and field testing. Sci. Total Environ. 2020, 745, 141179. [Google Scholar] [CrossRef]
- Vazquez-Nion, D.; Silva, B.; Prieto, B. Influence of the properties of granitic rocks on their bioreceptivity to subaerial phototrophic biofilms. Sci. Total Environ. 2018, 610–611, 44–54. [Google Scholar] [CrossRef]
- Caneva, G.; Di Stefano, D.; Giampaolo, C.; Ricci, S. Stone cavity and porosity as a limiting factor for biological colonisation: The travertine of Lungotevere (Rome). In Proceedings of the 10th International Congress on Deterioration and Conservation of Stone, Stockholm, Sweden, 27 June–2 July 2004; Kwiatkowski, D., Löfvendahl, R., Eds.; ICOMOS: Stockholm, Sweden, 2004; Volume 1, pp. 227–232. [Google Scholar]
- Del Mondo, A.; Pinto, G.; Carbone, D.A.; Pollio, A.; De Natale, A. Biofilm architecture on different substrates of an Oculatella subterranea (Cyanobacteria) strainisolated from Pompeii archaeological site (Italy). Environ. Sci. Pollut. Res. 2018. [Google Scholar] [CrossRef] [PubMed]
- Ortega-Morales, O.; Montero-Muñoz, J.L.; Baptista Neto, J.A.; Beech, I.B.; Sunner, J.; Gaylarde, C. Deterioration and cyanobacterial colonization of cultural heritage stone buildings in polluted and unpolluted tropical and subtropical climates: A meta-analysis. Int. Biodeterior. Biodegrad. 2019, 143, 104734. [Google Scholar] [CrossRef]
- Ortega-Morales, B.O.; Narvaéz-Zapata, J.A.; Schmalenberger, A.; Sosa-López, A.; Tebbe, C.C. Biofilms fouling ancient limestone Mayan monuments in Uxmal, Mexico: A cultivation-independent analysis. Biofilms 2004, 1, 79–90. [Google Scholar] [CrossRef]
- Li, Q.; Zhang, B.; Yang, X.; Ge, Q. Deterioration-associated microbiome of stone monuments: Structure, variation, and assembly. Appl. Environ. Microbiol. 2018, 84. [Google Scholar] [CrossRef] [PubMed]
- Morillas, H.; Maguregui, M.; Gallego-Cartagena, E.; Huallparimachi, G.; Marcaida, I.; Salcedo, I.; Silva, L.F.; Astete, F. Evaluation of the role of biocolonizations in the conservation state of Machu picchu (Peru): The sacred rock. Sci. Total Environ. 2019, 654, 1379–1388. [Google Scholar] [CrossRef] [PubMed]
- Gorbushina, A.A. Life on the rocks. Environ. Microbiol. 2007, 9, 1613–1631. [Google Scholar] [CrossRef] [PubMed]
- Crispim, C.A.; Gaylarde, C.C. Cyanobacteria and biodeterioration of cultural heritage: A review. Microb. Ecol. 2005, 49, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Zhang, B.; He, Z.; Yang, X. Distribution and diversity of bacteria and fungi colonization in stone monuments analyzed by high-throughput sequencing. PLoS ONE 2016, 11, e0163287. [Google Scholar] [CrossRef] [PubMed]
- Becerra, J.; Zaderenko, A.P.; Gómez-Morón, M.A.; Ortiz, P. Nanoparticles applied to stone buildings. Int. J. Architect. Herit. 2019. [Google Scholar] [CrossRef]
- Ogawa, A.; Celikkol-Aydin, S.; Gaylarde, C.; Baptista-Neto, J.A.; Beech, I. Microbial communities on painted wet and dry external surfaces of a historic fortress in Niteroi, Brazil. Int. Biodeterior. Biodegrad. 2017, 123, 164–173. [Google Scholar] [CrossRef]
- Häubner, N.; Schumann, R.; Karsten, U. Aeroterrestrial algae growing in biofilms on facades e response to temperature and water stress. Microb. Ecol. 2006, 51, 285–293. [Google Scholar] [CrossRef]
- Ortega-Morales, B.O.; Gaylarde, C.; Anaya-Hernandez, A.; Chan-Bacab, M.J.; De la Rosa-García, S.C.; Arano-Recio, D.; Montero-M, J. Orientation affects Trentepohlia-dominated biofilms on Mayan monuments of the Rio Bec style. Int. Biodeterior. Biodegrad. 2013, 84, 351–356. [Google Scholar] [CrossRef]
- Warscheid, T.; Braams, J. Biodeterioration of stone: A review. Int. Biodeterior. Biodegrad. 2000, 46, 343–368. [Google Scholar] [CrossRef]
- Golubic, S.; Perkins, R.D.; Lukas, K.J. Boring microorganisms and microborings in carbonate substrates. In The Study of Trace Fossils; Frey, R.W., Ed.; Springer: Berlin/Heidelberg, Germany, 1975. [Google Scholar] [CrossRef]
- Wierzchos, J.; Casero, M.C.; Artieda, O.; Ascaso, C. Endolithic microbial habitats as refuges for life in polyextreme environment of the Atacama Desert. Curr. Opin. Microbiol. 2018, 43, 124–131. [Google Scholar] [CrossRef]
- Ascaso, C.; Wierzchos, J.; Castello, R. Study of the biogenic weathering of calcareous litharenite stones caused by lichen and endolithic microorganisms. Int. Biodeterior. Biodegrad. 1998, 42, 29–38. [Google Scholar] [CrossRef]
- Qu, E.B.; Omelon, C.R.; Oren, A.; Meslier, V.; Cowan, D.A.; Maggs-Kölling, G.; DiRuggiero, J. Trophic selective pressures organize the composition of endolithic microbial communities from global deserts. Front. Microbiol. 2020, 10, 2952. [Google Scholar] [CrossRef] [PubMed]
- Gaylarde, P.; Englert, G.; Ortega-Morales, O.; Gaylarde, C. Lichen-like colonies of pure Trentepohlia on limestone monuments. Int. Biodeterior. Biodegrad. 2006, 58, 119–123. [Google Scholar] [CrossRef]
- Gaylarde, P.; Gaylarde, C. Deterioration of siliceous stone monuments in Latin America: Microorganisms and mechanisms. Corros. Revs. 2004, 22, 395–415. [Google Scholar] [CrossRef]
- Gaylarde, C.C.; Gaylarde, P.M.; Neilan, B.A. Endolithic phototrophs in built and natural stone. Curr. Microbiol. 2012, 65, 183–188. [Google Scholar] [CrossRef]
- Zhang, G.; Gong, C.; Gu, J.; Katayama, Y.; Someya, T.; Gu, J.D. Biochemical reactions and mechanisms involved in the biodeterioration of stone world cultural heritage under the tropical climate conditions. Int. Biodeterior. Biodegrad. 2019, 143, 104723. [Google Scholar] [CrossRef]
- Esposito, A.; Borruso, L.; Rattray, J.E.; Brusetti, L.; Ahmed, E. Taxonomic and functional insights into rock varnish microbiome using shotgun metagenomics. FEMS Microbiol. Ecol. 2019, 95, fiz180. [Google Scholar] [CrossRef] [PubMed]
- Barrionuevo, M.R.E.; Hidalgo, G.E.; Gaylarde, C.C. Physical and microbiological analysis of sandstone deterioration in the Argentine Jesuit missions. Geomicrobiol. J. 2016. [Google Scholar] [CrossRef]
- Gaylarde, C.; Baptista-Neto, J.A.; Ogawa, A.; Kowalski, M.; Celikkol-Aydin, S.; Beech, I. Epilithic and endolithic microorganisms and deterioration on stone church facades subject to urban pollution in a sub-tropical climate. Biofouling 2017, 33, 113–127. [Google Scholar] [CrossRef]
- Duffy, A.P.; Cooper, T.P.; Perry, S.H. Repointing mortars for conservation of a historic stone building in Trinity College, Dublin. Mater. Struct. 1993, 26, 302–306. [Google Scholar] [CrossRef][Green Version]
- Grube, M.; Cernava, T.; Soh, J.; Fuchs, S.; Aschenbrenner, I.; Lassek, C.; Wegner, U.; Becher, D.; Riedel, K.; Sensen, C.W.; et al. Exploring functional contexts of symbiotic sustain within lichen-associated bacteria by comparative omics. ISME J. 2015, 9, 412–424. [Google Scholar] [CrossRef]
- Scheerer, S.; Ortega-Morales, O.; Gaylarde, C. Microbial deterioration of stone monuments—An updated overview. Adv. Appl. Microbiol. 2009, 66, 97–139. [Google Scholar]
- Garcia-Vallès, M.; Topal, T.; Vendrell-Saz, M. Lichenic growth as a factor in the physical deterioration or protection of Cappadocian monuments. Environ. Geol. 2003, 43, 776–781. [Google Scholar] [CrossRef]
- Ausset, P.; Del Monte, M.; Lefèvre, R.A. Embryonic sulphated black crusts on carbonate rocks in atmospheric simulation chamber and in the field: Role of carbonaceous fly-ash. Atmos. Environ. 1999, 33, 1525–1534. [Google Scholar] [CrossRef]
- Castenholz, R.W.; Wilmotte, A.; Herdman, M.; Rippka, R.; Waterbury, J.B.; Iteman, I.; Hoffmann, L. Phylum BX. Cyanobacteria. In Bergey’s Manual® of Systematic Bacteriology; Boone, D.R., Castenholz, R.W., Garrity, G.M., Eds.; Springer: New York, NY, USA, 2001. [Google Scholar] [CrossRef]
- Gaylarde, C.C.; Ortega-Morales, B.O.; Bartolo-Perez, P. Biogenic black crusts on buildings in unpolluted environments. Curr. Microbiol. 2007, 54, 162–166. [Google Scholar] [CrossRef] [PubMed]
- García de Miguel, J.M.; Sánchez-Castillo, L.; Ortega-Calvo, J.J.; Gil, J.A.; Saiz-Jimenez, C. Deterioration of building materials from the Great Jaguar Pyramid at Tikal, Guatemala. Build. Environ. 1995, 30, 591–598. [Google Scholar] [CrossRef]
- Louati, M.; Ennis, N.J.; Ghodhbane-Gtari, F.; Hezbri, K.; Sevigny, J.L.; Fahnestock, M.F.; Cherif-Silini, H.; Bryce, J.G.; Tisa, L.S.; Gtari, M. Elucidating the ecological networks in stone-dwelling microbiomes. Environ. Microbiol. 2019. [Google Scholar] [CrossRef] [PubMed]
- Keshari, N.; Adhikary, S.P. Characterization of cyanobacteria isolated from biofilms on stone monuments at Santiniketan, India. Biofouling 2013, 29. [Google Scholar] [CrossRef] [PubMed]
- Gaylarde, C.C.; Gaylarde, P.M. A comparative study of the major microbial biomass of biofilms on exteriors of buildings in Europe and Latin America. Int. Biodeterior. Biodegrad. 2005, 55, 131–139. [Google Scholar] [CrossRef]
- Auras, M.; Bundschuh, P.; Eichhorn, J.; Kirchner, D.; Mach, M.; Seewald, B.; Scheuvens, D.; Snethlage, R. Traffic-induced emissions on stone buildings. In Future for Stone, Proceedings of the 13th International Congress on the Deterioration and Conservation of Stone, Paisley, Scotland, 6–10 September 2016; Hughes, J., Howind, T., Eds.; University of the West of Scotland: Paisley, Scotland, 2016; Volume 1, pp. 3–12. ISBN 978-1-903978-57-3. [Google Scholar]
- Cutler, N.; Viles, H. Eukaryotic microorganisms and stone biodeterioration. Geomicrobiol. J. 2010, 27, 630–646. [Google Scholar] [CrossRef]
- Mihajlovski, A.; Seyer, D.; Benamara, H.; Bousta, F.; Di Martino, P. An overview of techniques for the characterization and quantification of microbial colonization on stone monuments. Ann. Microbiol. 2015, 65, 1243–1255. [Google Scholar] [CrossRef]
- Ibarrondo, I.; Prieto-Taboada, N.; Martinez-Arkarazo, I.; Madariaga, J.M. Resonance Raman imaging as a tool to assess the atmospheric pollution level: Carotenoids in Lecanoraceae lichens as bioindicators. Environ. Sci. Pollut. Res. 2016, 23, 6390–6399. [Google Scholar] [CrossRef] [PubMed]
- Basu, S.; Orr, S.A.; Aktas, Y.D. A geological perspective on climate change and building stone deterioration in London: Implications for urban stone-built heritage research and management. Atmosphere 2020, 11, 788. [Google Scholar] [CrossRef]
- Pinheiro, A.C.; Mesquita, N.; Trovão, J.; Soares, F.; Tiago, I.; Coelho, C.; de Carvalho, H.P.; Gil, F.; Catarino, L.; Piñar, G.; et al. Limestone biodeterioration: A review on the Portuguese cultural heritage scenario. J. Cult. Herit. 2019, 36, 275–285. [Google Scholar] [CrossRef]
- Matos, P.; Vieira, J.; Rocha, B.; Branquinho, C.; Pinho, P. Modeling the provision of air-quality regulation ecosystem service provided by urban green spaces using lichens as ecological indicators. Sci. Total Environ. 2019, 665, 521–530. [Google Scholar] [CrossRef] [PubMed]
- De Wit, T. Lichens as indicators for air quality. In Ecological Indicators for the Assessment of the Quality of Air, Water, Soil, and Ecosystems; Best, E.P.H., Haeck, J., Eds.; Springer: Dordrecht, The Netherlands, 1983. [Google Scholar] [CrossRef]
- Nuhoglu, Y.; Oguz, E.; Uslu, H.; Ozbek, A.; Ipekoglu, B.; Ocak, I.; Hasenekoglu, I. The accelerating effects of the microorganisms on biodeterioration of stone monuments under air pollution and continental-cold climatic conditions in Erzurum, Turkey. Sci. Total Environ. 2006, 364, 272–283. [Google Scholar] [CrossRef]
- Villa, F.; Stewart, P.S.; Klapper, I.; Jacob, J.M.; Cappitelli, F. Subaerial biofilms on outdoor stone monuments: Changing the perspective toward an ecological framework. BioScience 2016, 66, 285–294. [Google Scholar] [CrossRef]
- Viles, H.A.; Cutler, N.A. Global environmental change and the biology of heritage structures. Glob. Chang. Biol. 2012, 18, 2406–2418. [Google Scholar] [CrossRef]
- Miller, A.Z.; Dionísio, A.; Macedo, M.F.; Sáiz-Jiménez, C. Primary bioreceptivity of limestones to phototrophic microorganisms: A laboratory-based stone colonization experiment. Technoheritage 2017, 97. Available online: http://hdl.handle.net/10261/155200 (accessed on 30 October 2020).
- Adhikary, S.P.; Keshari, N.; Urzi, C.; De Phillipis, R. Cyanobacteria in biofilms on stone temples of Bhubaneswar, Eastern India. Algol. Stud. 2015, 147, 67–93. [Google Scholar] [CrossRef]
- Aptroot, A.; van Herk, C.M. Further evidence of the effects of global warming on lichens, particularly those with Trentepohlia phycobionts. Environ. Poll. 2007, 146, 293–298. [Google Scholar] [CrossRef]
- Prieto, B.; Vázquez-Nion, D.; Fuentes, E.; Durán-Román, A.G. Response of subaerial biofilms growing on stone-built cultural heritage to changing water regime and CO2 conditions. Int. Biodeterior. Biodegrad. 2020, 148, 104882. [Google Scholar] [CrossRef]
- Orr, S.A.; Young, M.; Stelfox, D.; Curran, J.; Viles, H. Wind-driven rain and future risk to built heritage in the United Kingdom: Novel metrics for characterising rain spells. Sci. Total Environ. 2018, 640–641, 1098–1111. [Google Scholar] [CrossRef]
- Orr, S.A.; Cassar, M. Exposure indices of extreme wind-driven rain events for Built Heritage. Atmosphere 2020, 11, 163. [Google Scholar] [CrossRef]
- Pérez-Bella, J.M.; Domínguez-Hernández, J.; Cano-Suñén, E.; Alonso-Martínez, M.; del Coz-Díaz, J.J. Equivalence between the methods established by ISO 15927-3 to determine wind-driven rain exposure: Reanalysis and improvement proposal. Build. Environ. 2020, 174, 106777. [Google Scholar] [CrossRef]
- Traversetti, L.; Bartoli, F.; Caneva, G. Wind-driven rain as a bioclimatic factor affecting the biological colonization at the archaeological site of Pompeii. Italy. Int. Biodeterior. Biodegrad. 2018, 134, 31–38. [Google Scholar] [CrossRef]
- Lee, C.G.; Watanabe, T.; Fujita, Y.; Asakawa, S.; Kimura, M. Heterotrophic growth of cyanobacteria and phage-mediated microbial loop in soil: Examination by stable isotope probing (SIP) method. Soil Sci. Plant Nutrit. 2012, 58, 161–168. [Google Scholar] [CrossRef]
- Meireles dos Santos, A.; Vieira, K.R.; Basso, S.R.; Meireles dos Santos, A.; Queiroz, M.I.; Queiroz, Z.L.; Jacob-Lopes, E. Heterotrophic cultivation of cyanobacteria: Study of effect of exogenous sources of organic carbon, absolute amount of nutrients, and stirring speed on biomass and lipid productivity. Front. Bioeng. Biotechnol. 2017, 5, 12. [Google Scholar] [CrossRef]
- Miller, A.Z.; Sanmartín, P.; Pereira-Pardo, L.; Dionísio, A.; Saiz-Jimenez, C.; Macedo, M.F.; Prieto, B. Bioreceptivity of building stones: A review. Sci. Total Environ. 2012, 426, 1–12. [Google Scholar] [CrossRef]
- André, M.-F.; Vautier, F.; Voldoire, O.; Roussel, E. Accelerated stone deterioration induced by forest clearance around the Angkor temples. Sci. Total Environ. 2014, 493, 98–108. [Google Scholar] [CrossRef]
- Gómez-Bolea, A.; Llop, E.; Ariño, X.; Saiz-Jiménez, C.; Bonazza, A.; Messina, P.; Sabbioni, C. Mapping the impact of climate change on biomass accumulation on stone. J. Cult. Herit. 2012, 13, 254–258. [Google Scholar] [CrossRef]








| Pigment | Biological Function | Color(s) | Comments |
|---|---|---|---|
| Chlorophylls | Light absorption for energy production−photosynthesis | Green | Cyanobacteria contain only chlorophyll a |
| Carotenoids | Accessory pigments, light absorption, photoprotection | Orange, red, yellow, brown | Mainly membrane-bound |
| Phycobilins | Accessory pigments, light harvesting | Blue, red | Water-soluble, absorb green/red wavelengths |
| Mycosporine-like amino acids (MAAs) | Protective “sunscreen” | Dark brown | Found in cyanobacteria and some algae |
| Scytonemin | UV (perhaps general) protection | Dark brown/red | Only found in sheathed cyanobacteria |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
C. Gaylarde, C. Influence of Environment on Microbial Colonization of Historic Stone Buildings with Emphasis on Cyanobacteria. Heritage 2020, 3, 1469-1482. https://doi.org/10.3390/heritage3040081
C. Gaylarde C. Influence of Environment on Microbial Colonization of Historic Stone Buildings with Emphasis on Cyanobacteria. Heritage. 2020; 3(4):1469-1482. https://doi.org/10.3390/heritage3040081
Chicago/Turabian StyleC. Gaylarde, Christine. 2020. "Influence of Environment on Microbial Colonization of Historic Stone Buildings with Emphasis on Cyanobacteria" Heritage 3, no. 4: 1469-1482. https://doi.org/10.3390/heritage3040081
APA StyleC. Gaylarde, C. (2020). Influence of Environment on Microbial Colonization of Historic Stone Buildings with Emphasis on Cyanobacteria. Heritage, 3(4), 1469-1482. https://doi.org/10.3390/heritage3040081




