Crystallographic Studies in Cultural Heritage: Solid State Behaviour of Inorganic Pigments
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Instrumentation
2.3. Experimental
3. Results and Discussion
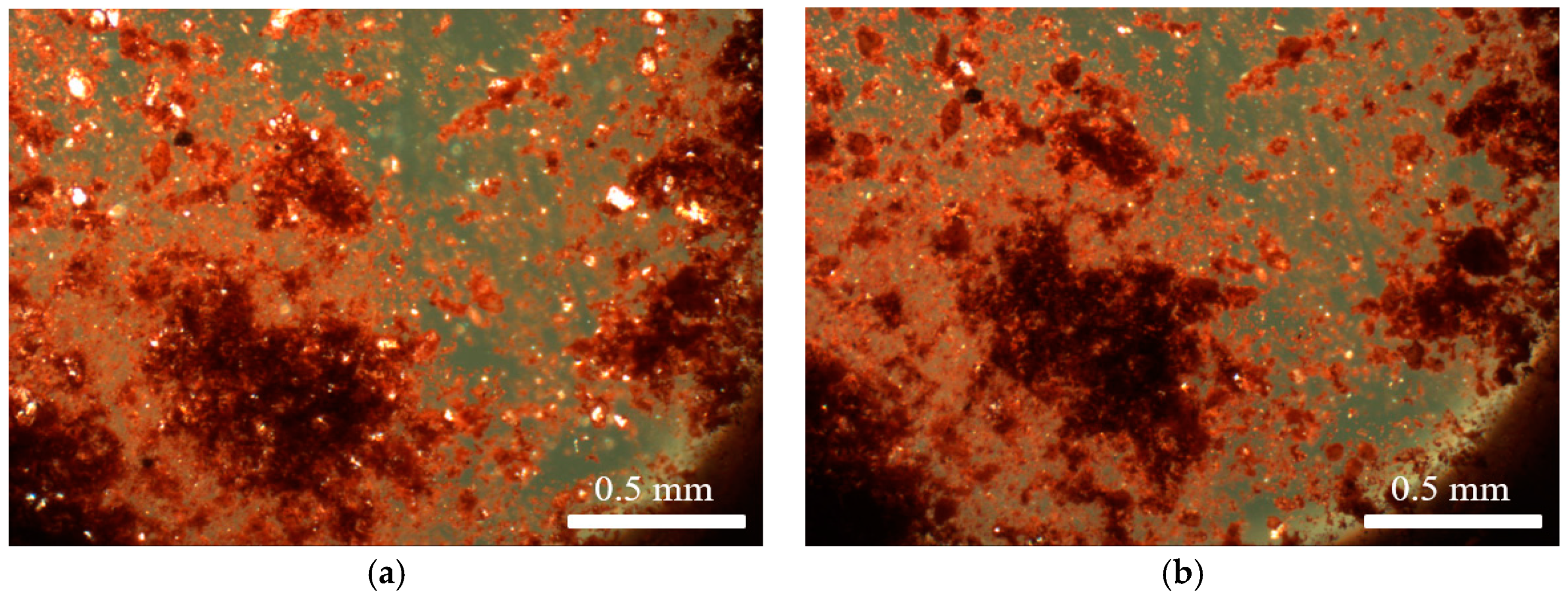
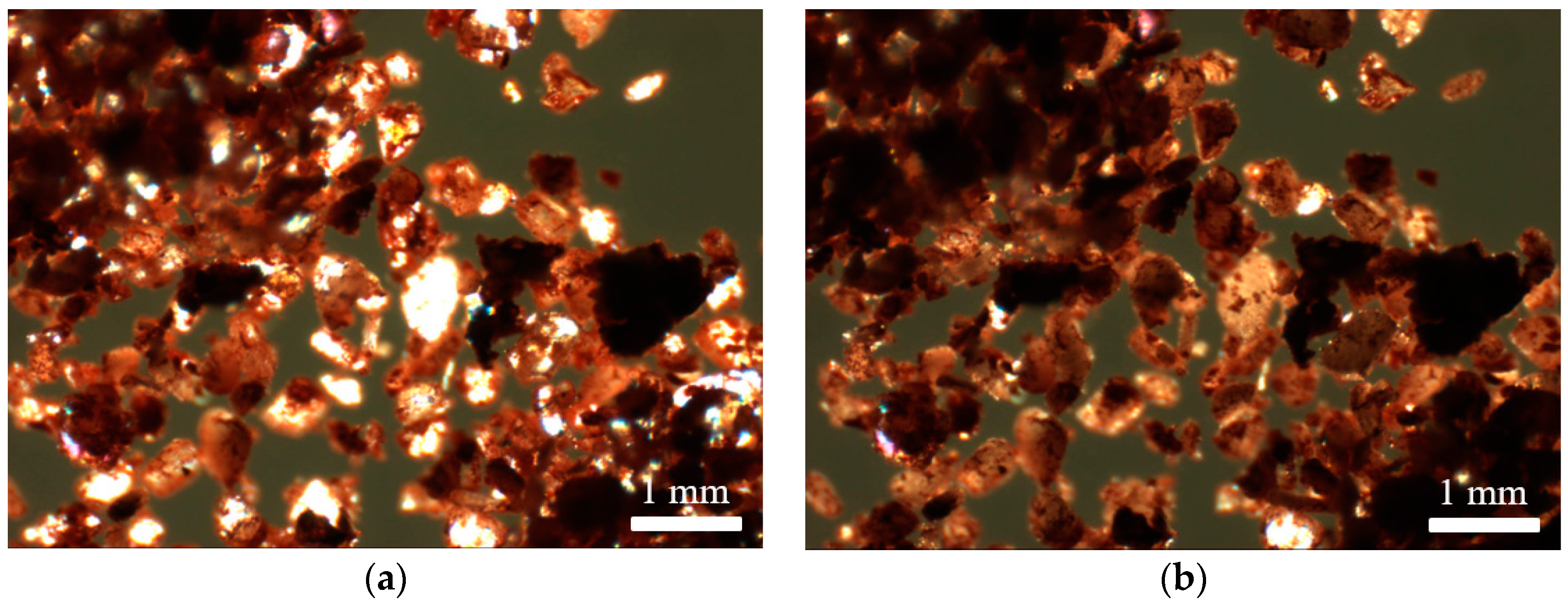
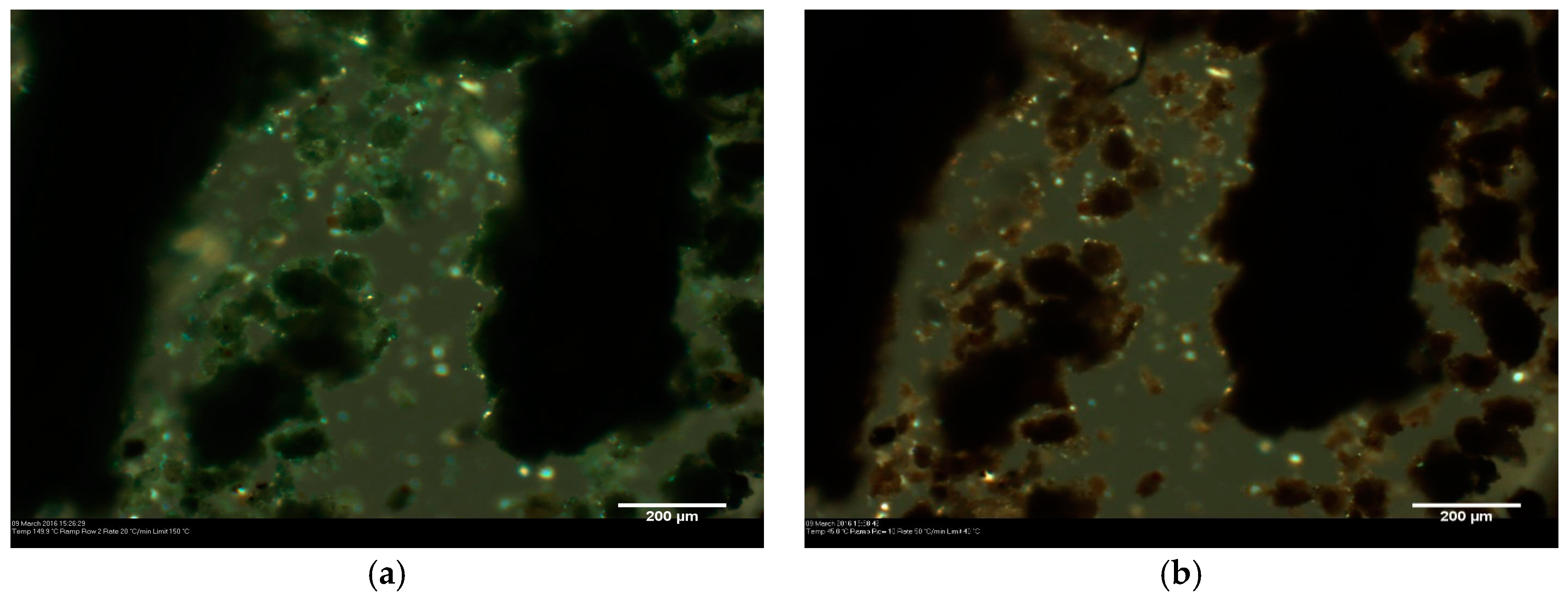
3.1. Solid State Behaviour of Venetian Red Ochre at High Temperatures
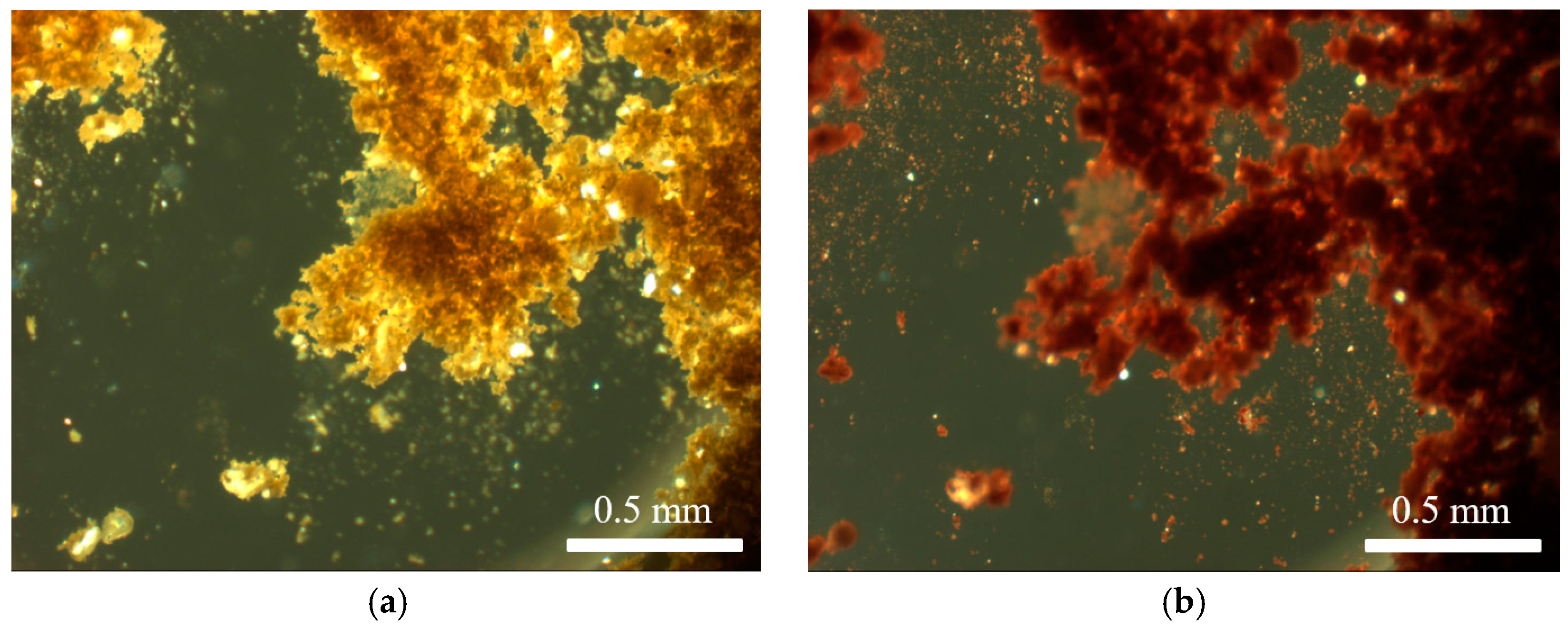
3.2. Characterisation of Venetian Red Ochre and Comparison with Verona Terra Verde
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Cornell, R.M.; Schwertmann, U. The Iron Oxides: Structure, Properties, Reactions, Occurrences and Uses; WILEY-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2006. [Google Scholar]
- Bonneau, A.; Pearce, D.G.; Pollard, A.M. A multi-technique characterization and provenance study of the pigments used in San rock art, South Africa. J. Archaeol. Sci. 2012, 39, 287–294. [Google Scholar] [CrossRef]
- Román, R.S.; Bañón, C.B.; Landete Ruiz, M.D. Analysis of the red ochre of the El Mirón burial (Ramales de la Victoria, Cantabria, Spain). J. Archaeol. Sci. 2015, 60, 84–98. [Google Scholar] [CrossRef]
- Toschi, F.; Paladini, A.; Colosi, F.; Cafarelli, P.; Valentini, V.; Falconieri, M.; Gagliardi, S.; Santoro, P. A multi-technique approach for the characterization of Roman mural paintings. Appl. Surf. Sci. 2013, 284, 291–296. [Google Scholar] [CrossRef]
- Grissom, C.A. Green Earth. In Artists’ Pigments: A Handbook of their History and Characteristics; Feller, R.L., Ed.; Archetype Publications: London, UK, 2012; Volume 1, pp. 141–168. [Google Scholar]
- Granger, F. Vitruvius on Architecture Book VII; Harvard University Press: Cambridge, UK, 1970. [Google Scholar]
- Piovesan, R.; Siddall, R.; Mazzoli, C.; Nodari, L. The Temple of Venus (Pompeii): A study of the pigments and painting techniques. J. Archaeol. Sci. 2011, 38, 2633–2643. [Google Scholar] [CrossRef]
- Thompson, D.V. The Practice of Tempera Paintings; Dover Publications Inc.: New York, NY, USA, 1962. [Google Scholar]
- Laurie, A.P. The Painters’ Methods and Materials; Dover Publications Inc.: New York, NY, USA, 1967. [Google Scholar]
- Elias, M.; Chartier, C.; Prévot, G.; Garay, H.; Vignaud, C. The Colour of Ochres Explained by Their Composition. Mater. Sci. Eng. B 2006, 127, 70–80. [Google Scholar] [CrossRef]
- Jercher, M.; Pring, A.; Jones, P.G.; Raven, M.D. Rietveld X-Ray Diffraction and X-Ray Fluorescence Analysis of Australian Aboriginal Ochres. Archaeometry 1998, 40, 383–401. [Google Scholar] [CrossRef]
- Coelho, A. TOPAS and TOPAS-Academic: An optimization program integrating computer algebra and crystallographic objects written in C++. J. Appl. Crystallogr. 2018, 51, 210–218. [Google Scholar] [CrossRef]
- Micallef, D.; Spiteri, R.; Baisch, U.; Vella-Zarb, L. Terra Verde: Overcoming the Problem of Transparency by Crystal Engineering. J. Cult. Herit. 2019, in press. [Google Scholar] [CrossRef]
- Welton, J.E. SEM Petrology Atlas; The American Association of Petroleum Geologists: Tulsa, OK, USA, 2003; Volume 4. [Google Scholar]
- Dooley, J.H. Glauconite. In Industrial Minerals and Rocks: Commodities, Markets, and Uses; Kogel, J.E., Trivedi, N.C., Barker, J.M., Eds.; Society for Mining, Metallurgy, and Exploration, Inc.: Littleton, CO, USA, 2006; pp. 495–506. [Google Scholar]
- Ospitali, F.; Bersani, D.; Di Lonardo, G.; Lottici, P.P. ‘Green earths’: Vibrational and elemental characterization of glauconites, celadonites and historical pigments. J. Raman Spectrosc. 2008, 39, 1066–1073. [Google Scholar] [CrossRef]
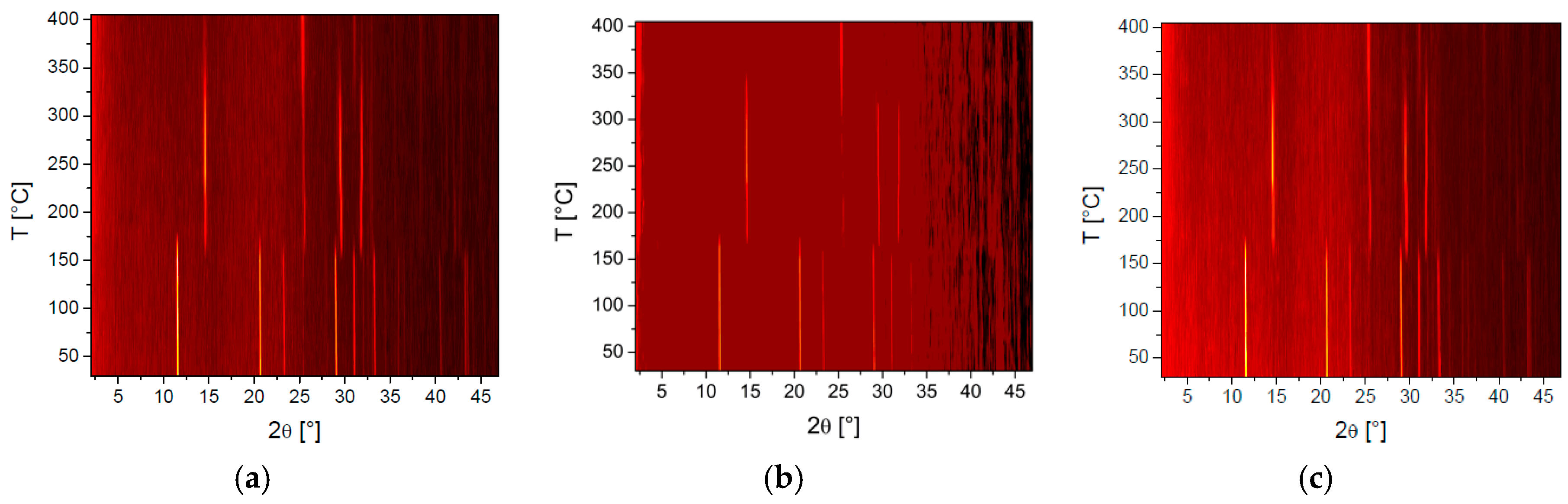
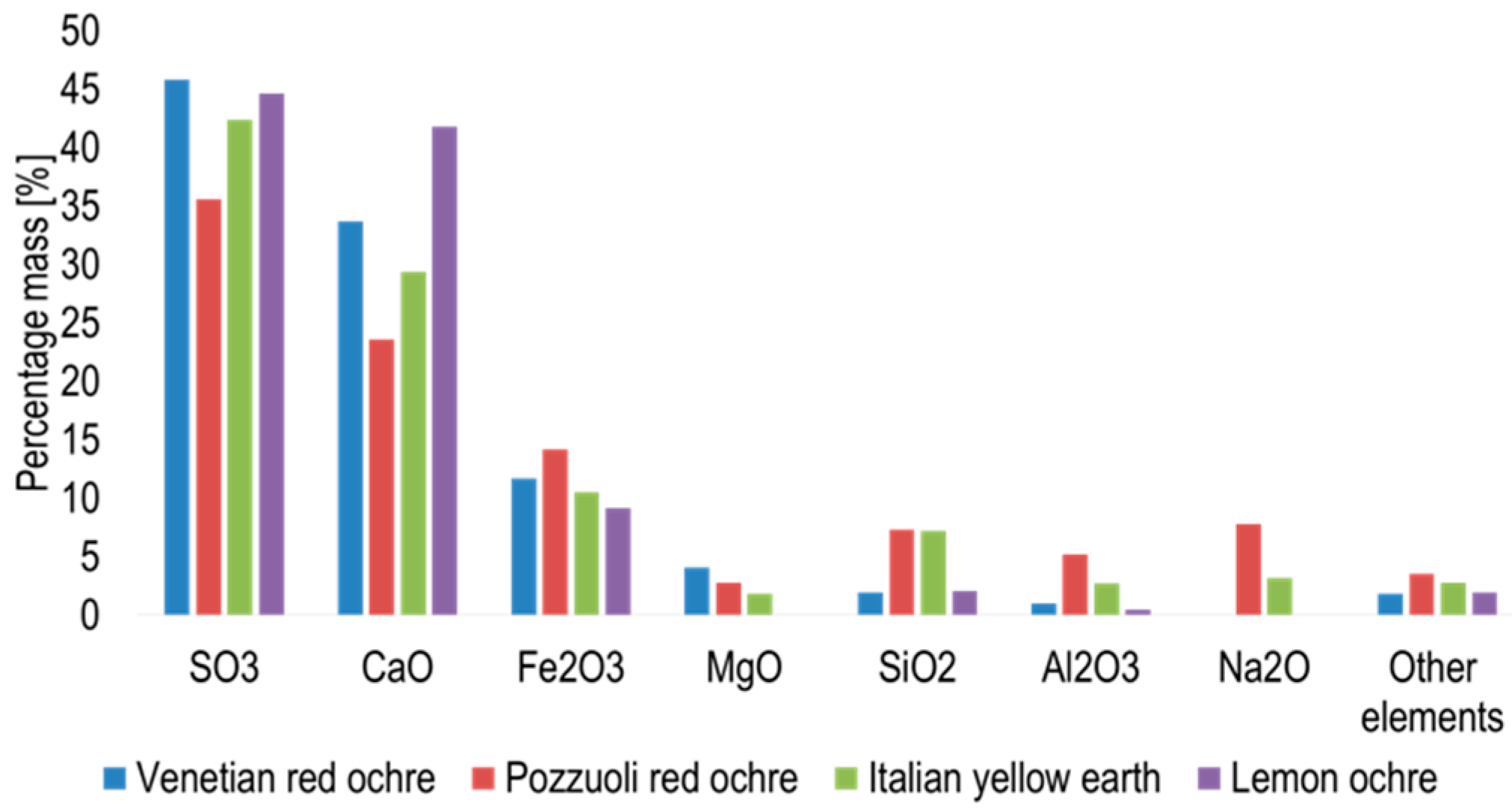
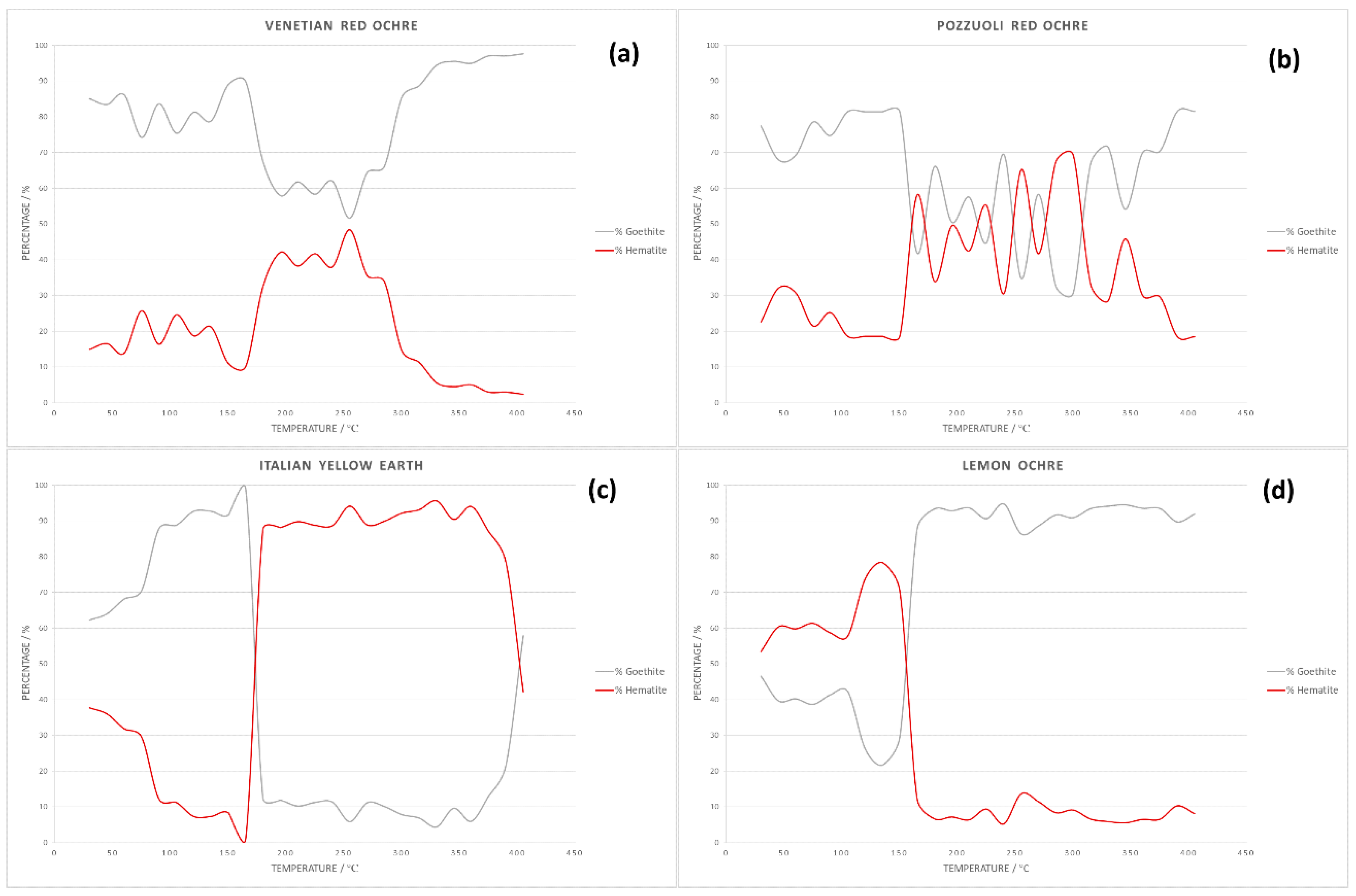
| Solvent | Temperature Range/°C |
|---|---|
| Water | 130–145 |
| Ethanol | 125–135 |
| Methanol | 125–140 |
| Acetone | 115–145 |
| Dimethyl sulfoxide | 120–130 |
| 1:1 ratio of dimethyl sulfoxide and water | 125–145 |
| 1:1 ratio of ethanol and water | 120–140 |
| 1:1 ratio of acetone and water | 125–140 |
| 1:1 ratio of methanol and water | 135–145 |
| Detected Compound | Concentration, % |
|---|---|
| Potassium Oxide | 0.875 |
| Sodium Oxide | 5.02 |
| Calcium Oxide | 24.6 |
| Iron (III) Oxide | 5.54 |
| Aluminium Oxide | 8.13 |
| Magnesium Oxide | 2.3 |
| Titanium Oxide | 0.658 |
| Silicon Oxide | 25.8 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Baisch, U.; Camilleri, M.; Micallef, D.; Rhauderwiek, T.; Stock, N.; Spiteri, R.; Vella-Zarb, L. Crystallographic Studies in Cultural Heritage: Solid State Behaviour of Inorganic Pigments. Heritage 2019, 2, 967-975. https://doi.org/10.3390/heritage2010063
Baisch U, Camilleri M, Micallef D, Rhauderwiek T, Stock N, Spiteri R, Vella-Zarb L. Crystallographic Studies in Cultural Heritage: Solid State Behaviour of Inorganic Pigments. Heritage. 2019; 2(1):967-975. https://doi.org/10.3390/heritage2010063
Chicago/Turabian StyleBaisch, Ulrich, Marie Camilleri, Duncan Micallef, Timo Rhauderwiek, Norbert Stock, Rebecca Spiteri, and Liana Vella-Zarb. 2019. "Crystallographic Studies in Cultural Heritage: Solid State Behaviour of Inorganic Pigments" Heritage 2, no. 1: 967-975. https://doi.org/10.3390/heritage2010063
APA StyleBaisch, U., Camilleri, M., Micallef, D., Rhauderwiek, T., Stock, N., Spiteri, R., & Vella-Zarb, L. (2019). Crystallographic Studies in Cultural Heritage: Solid State Behaviour of Inorganic Pigments. Heritage, 2(1), 967-975. https://doi.org/10.3390/heritage2010063







