Structure and Properties of Supercritical Water: Experimental and Theoretical Characterizations
Abstract
1. Introduction
2. Experimental Studies on Density Fluctuation and Correlation Length of SCW
- (1)
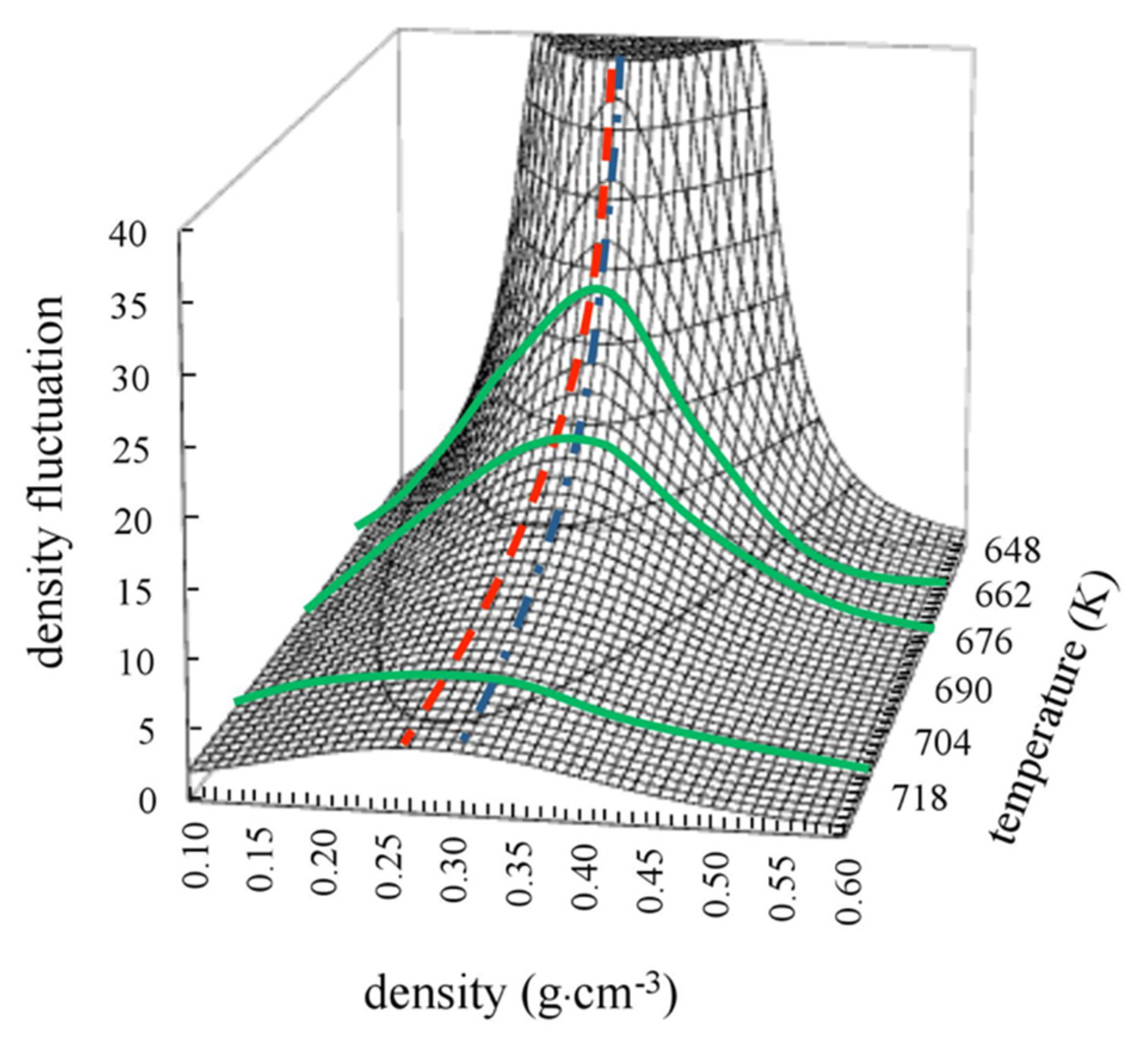
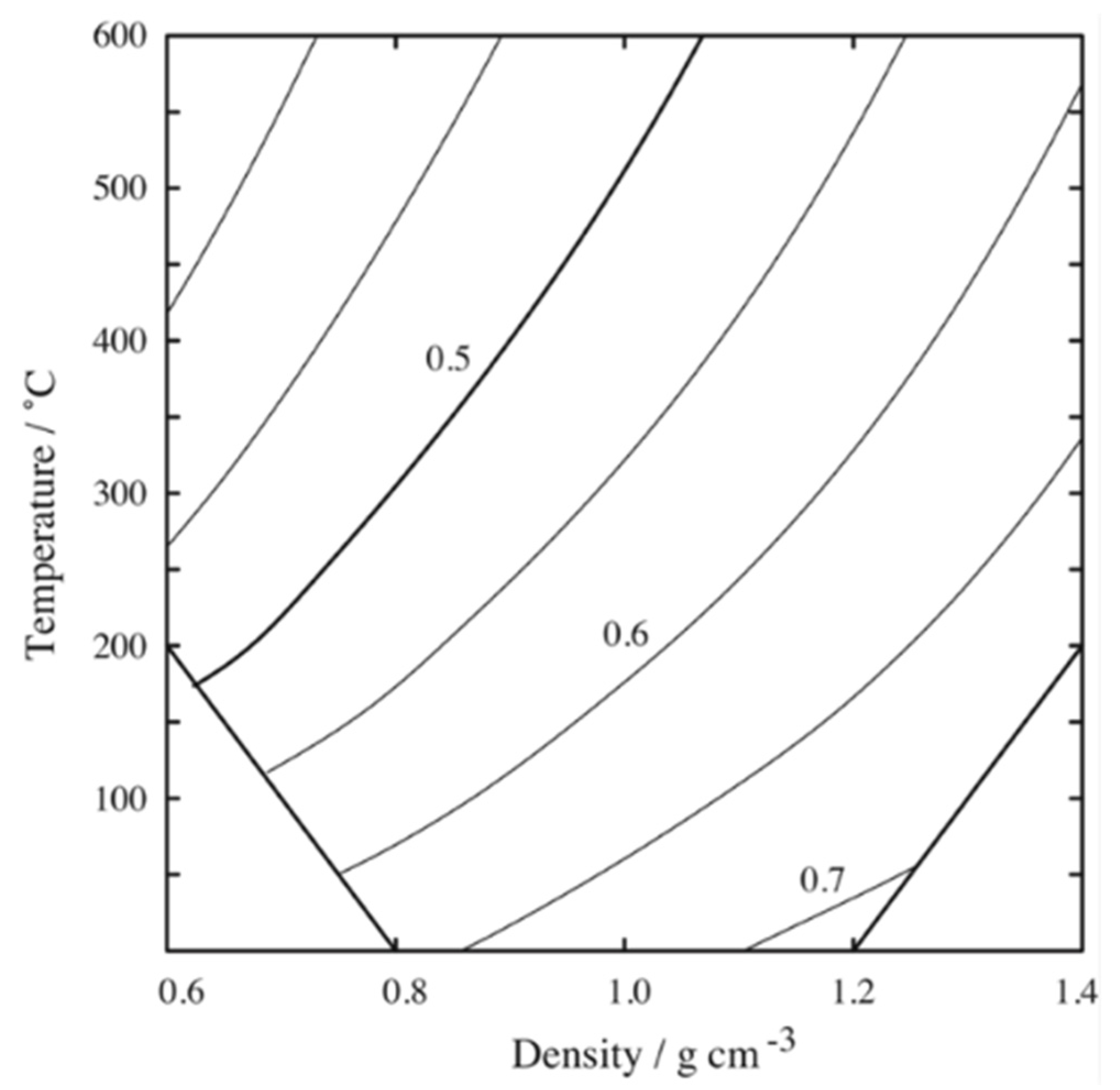
- The density fluctuation forms a ridge when the contour map of their values is drawn on the ρ–T phase diagram (ρ: density, T: absolute temperature). The ridge is the locus formed by the points at which the values of density fluctuation are their maximum in an isothermal change. The ridge does not coincide with but slightly deviates from the critical isodensity line.
- (2)
- The ridge corresponds to a trajectory of the maximum or minimum values of various physical quantities associated with the second derivative of Gibbs free energy, such as response functions of isothermal compressibility, heat capacity, and isobaric thermal expansion.
- (3)
- When the isothermal curves of density fluctuations and the ridges of supercritical CO2 and CF3H are drawn in the reduced variables such as , , and , they coincide with each other. This suggests the generality principle of the density fluctuation and ridge.
- (4)
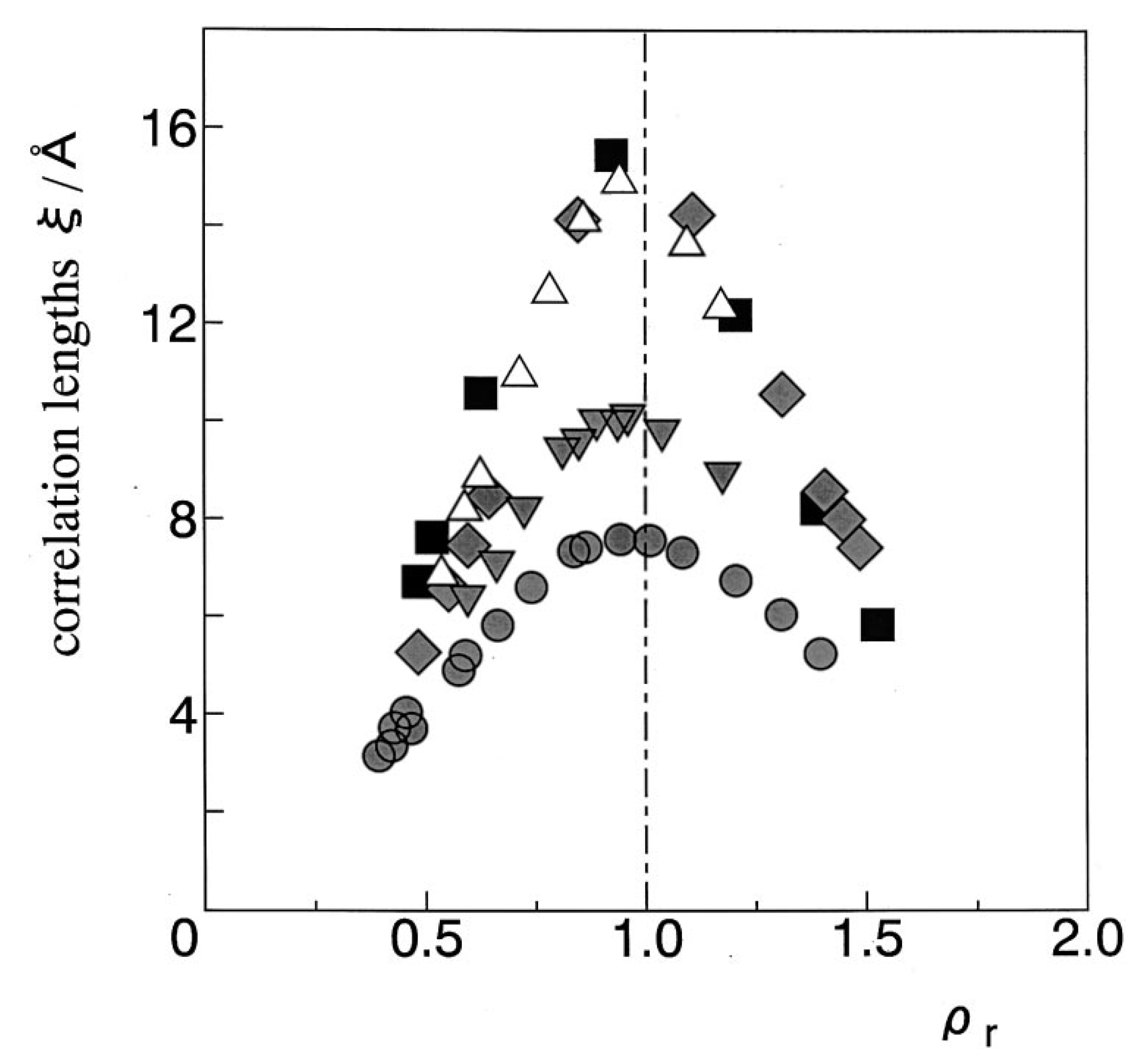
- The same statements (1)–(3) apply to the correlation length.
2.1. Experiment
2.2. Results and Discussion
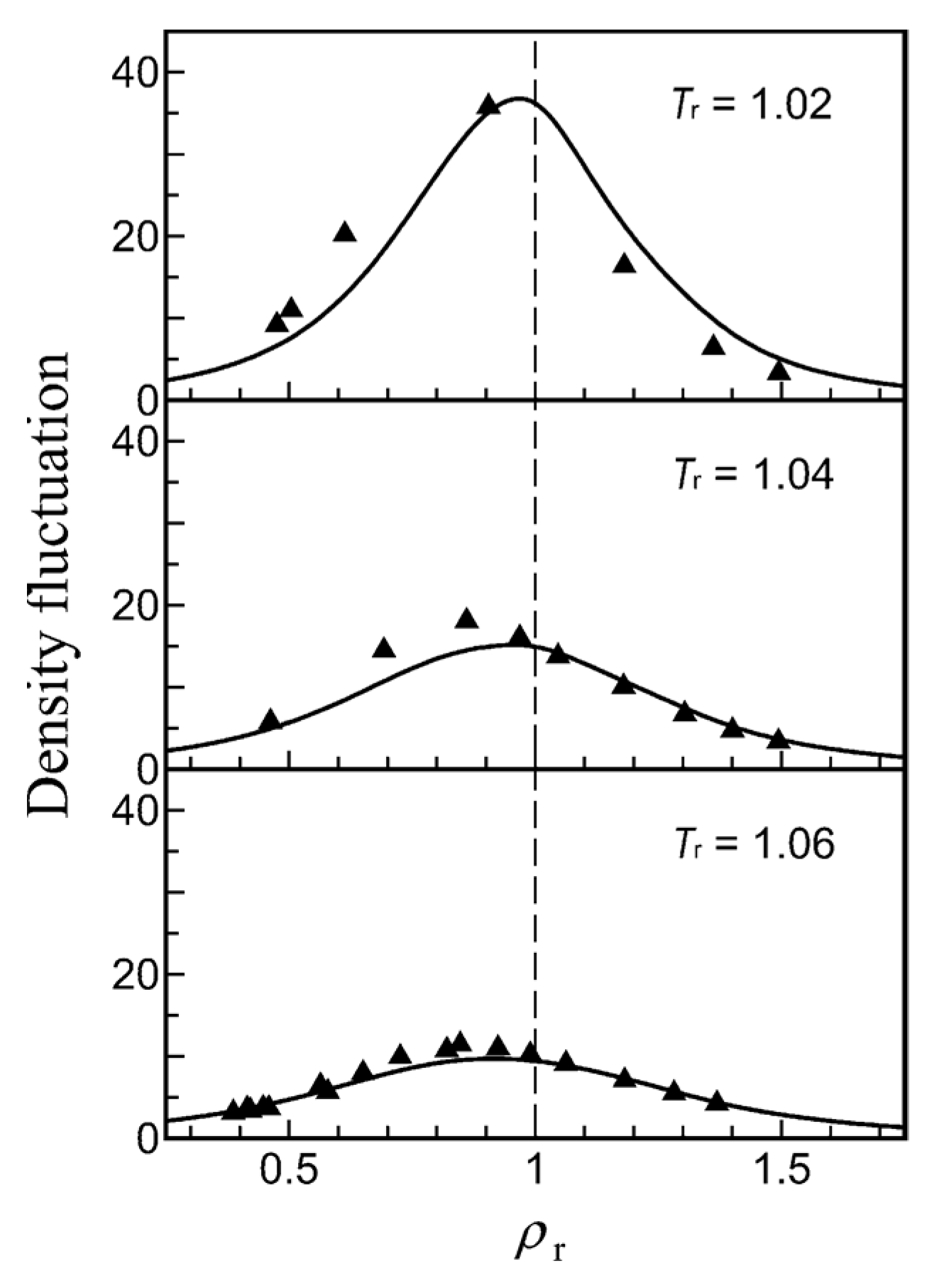
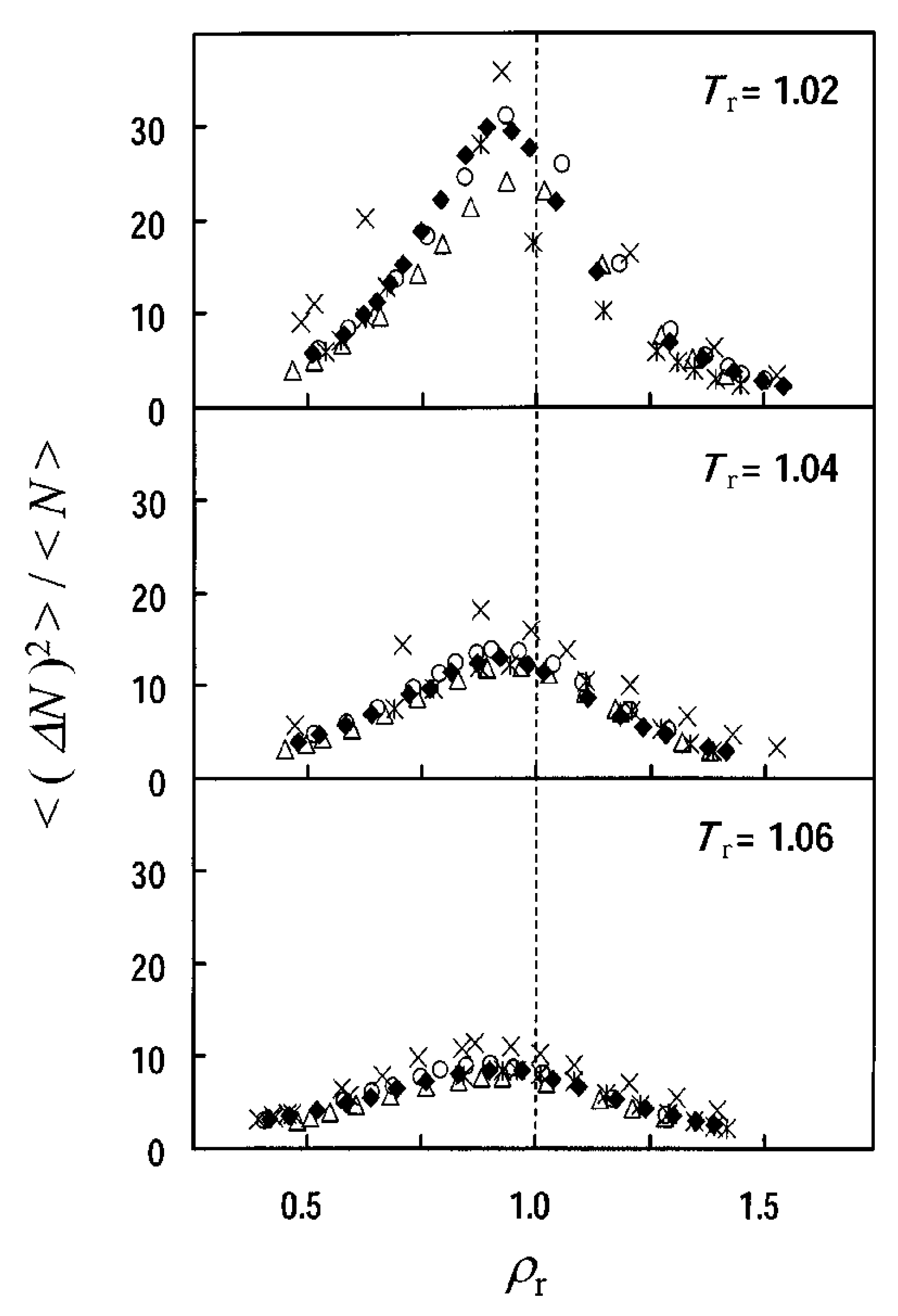
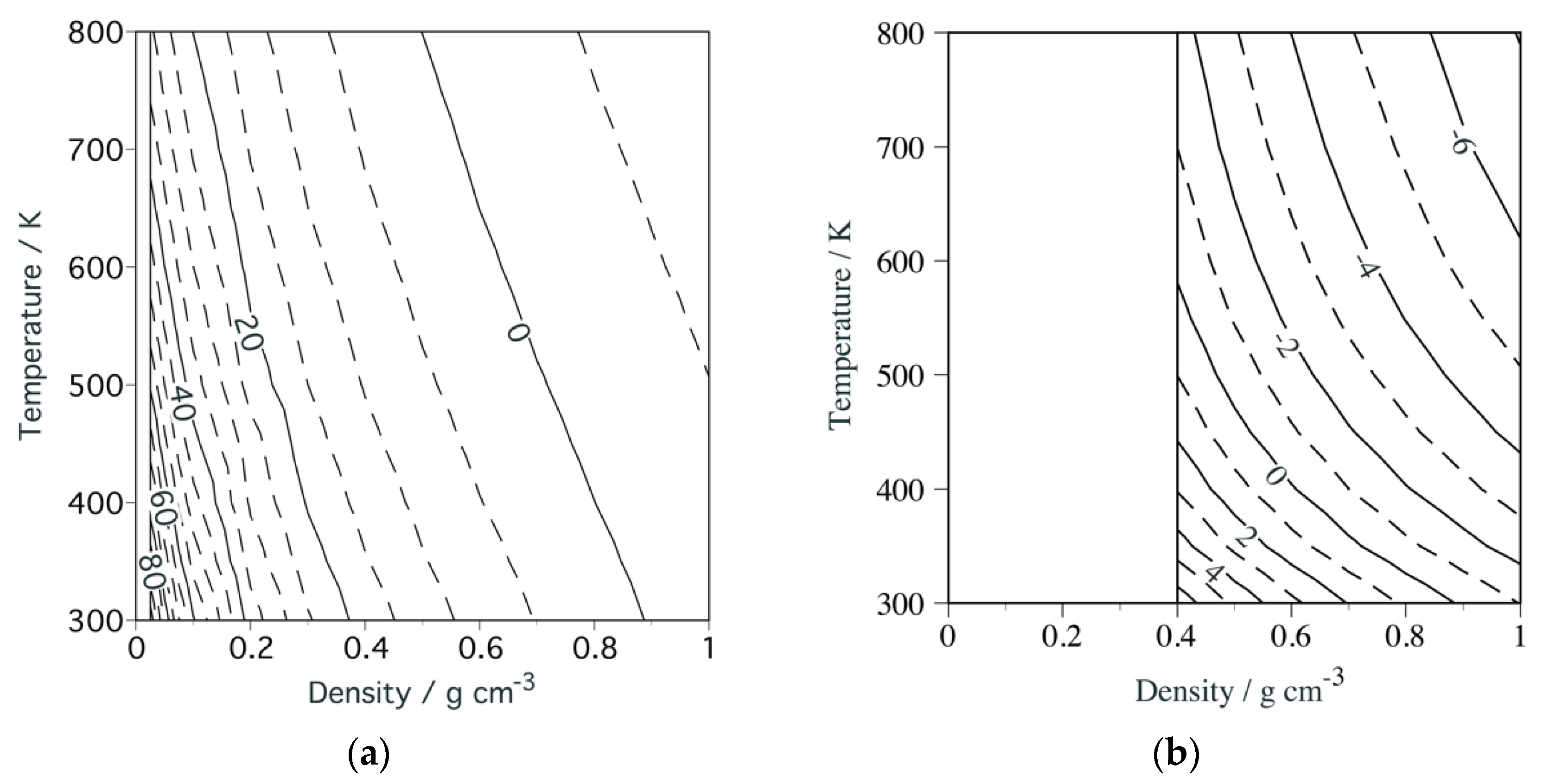
2.2.1. Density Fluctuations
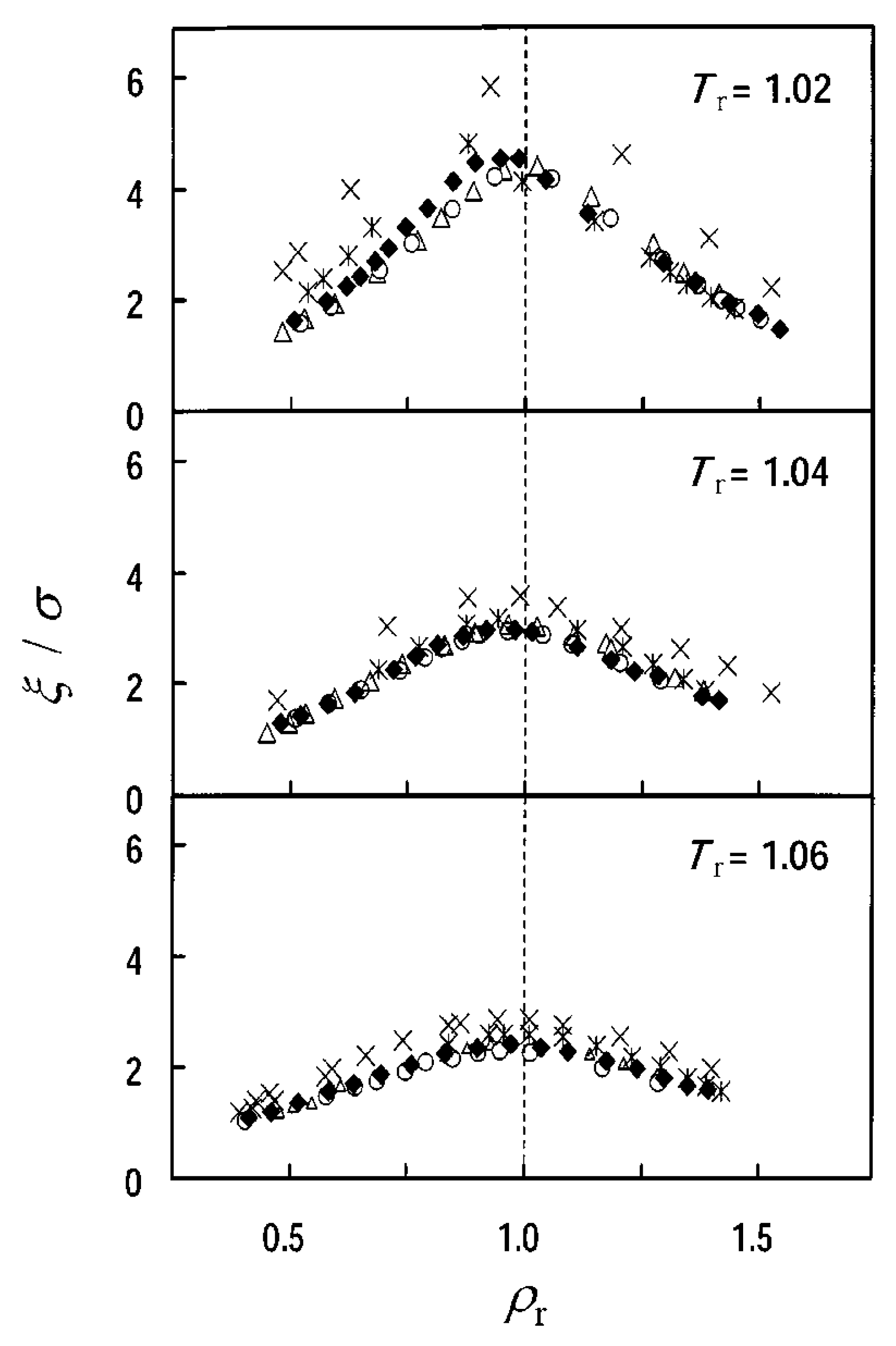
2.2.2. Correlation Lengths
2.2.3. Substance Dependences of Density Fluctuation and Correlation Length
2.3. Summary
3. Theoretical Characterization of the SCW Region in Terms of the Concept of “Ridge” in the Phase Diagram
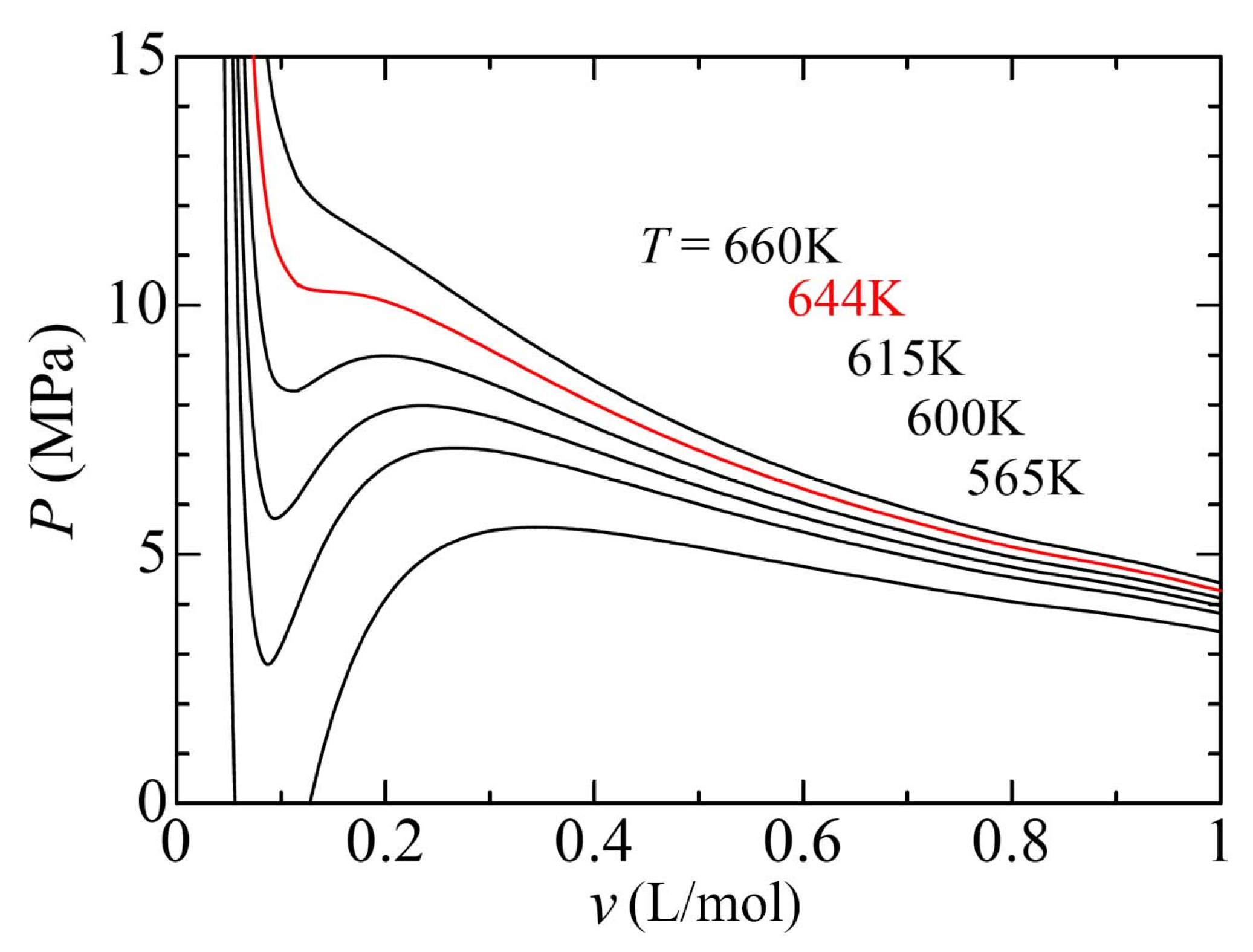
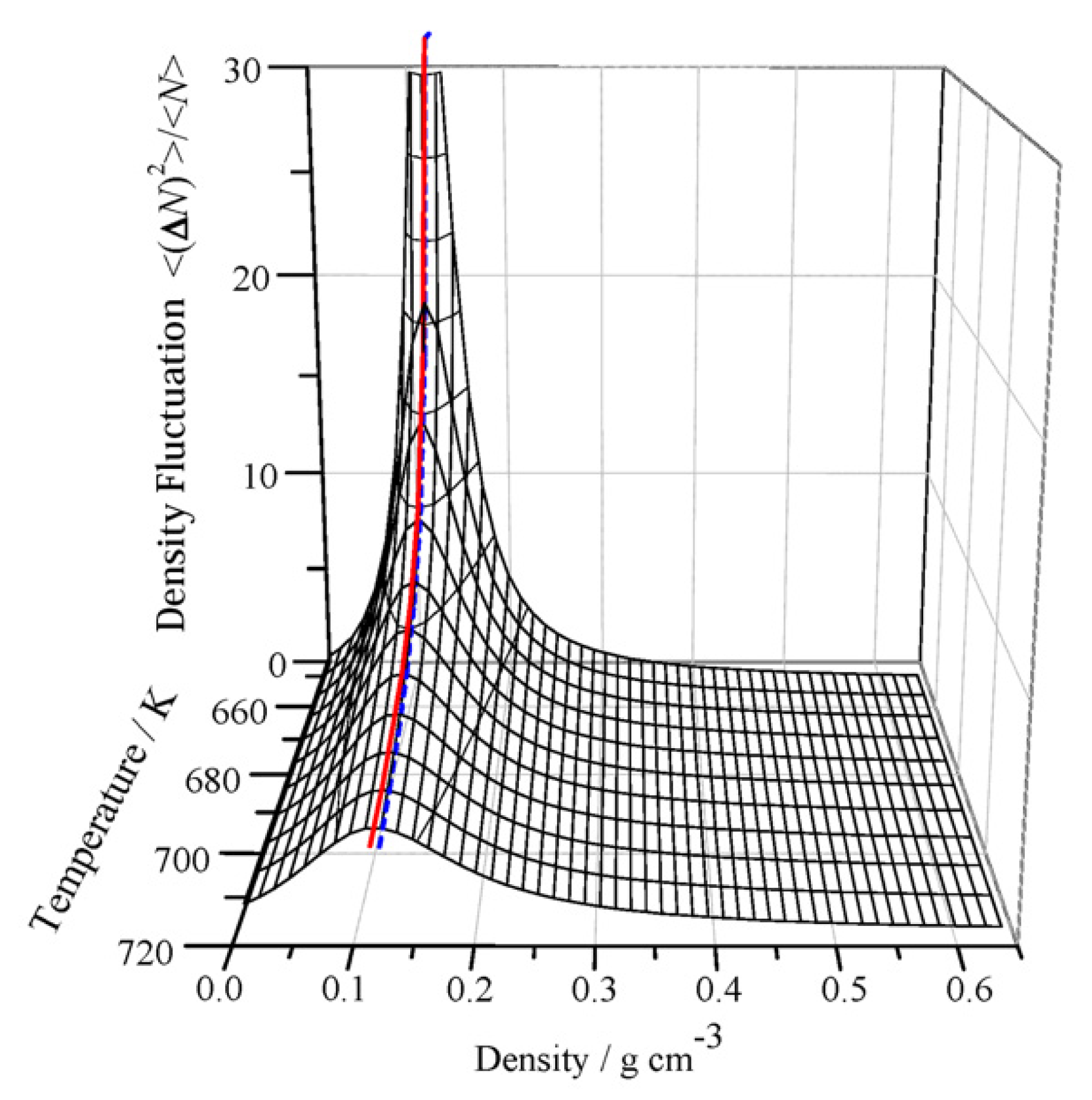
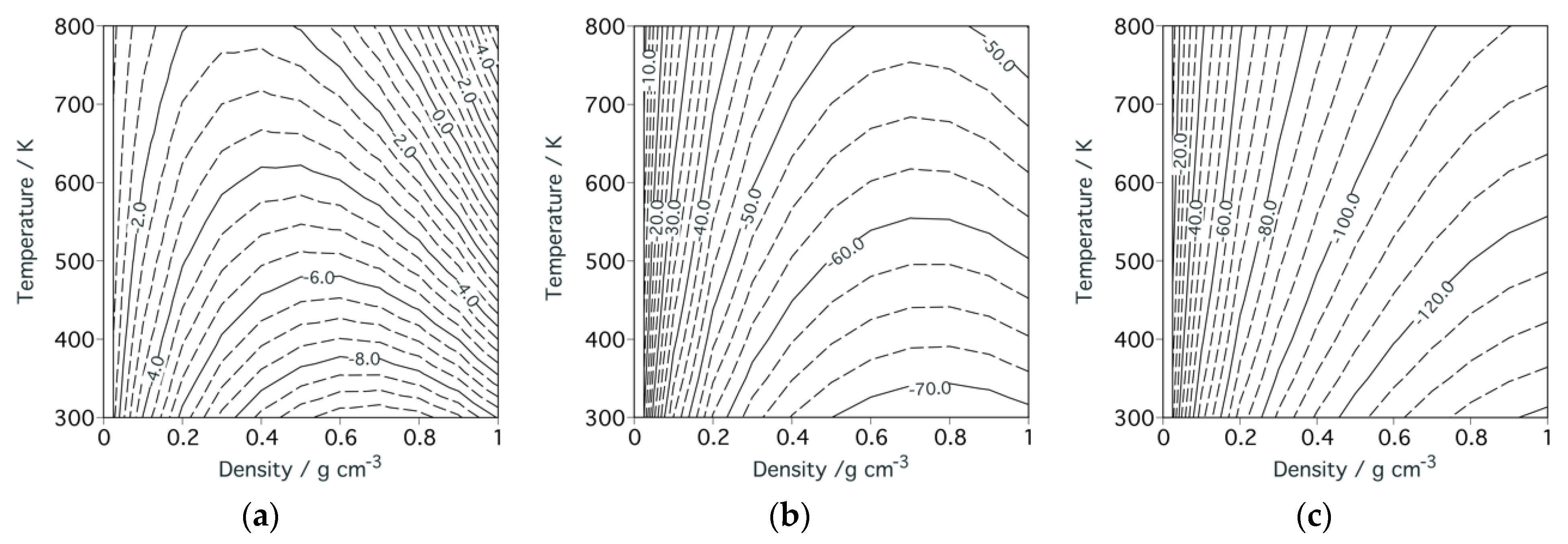
3.1. Theoretical Framework of Density Fluctuation
3.2. Temperature and Density Dependence of Density Fluctuation
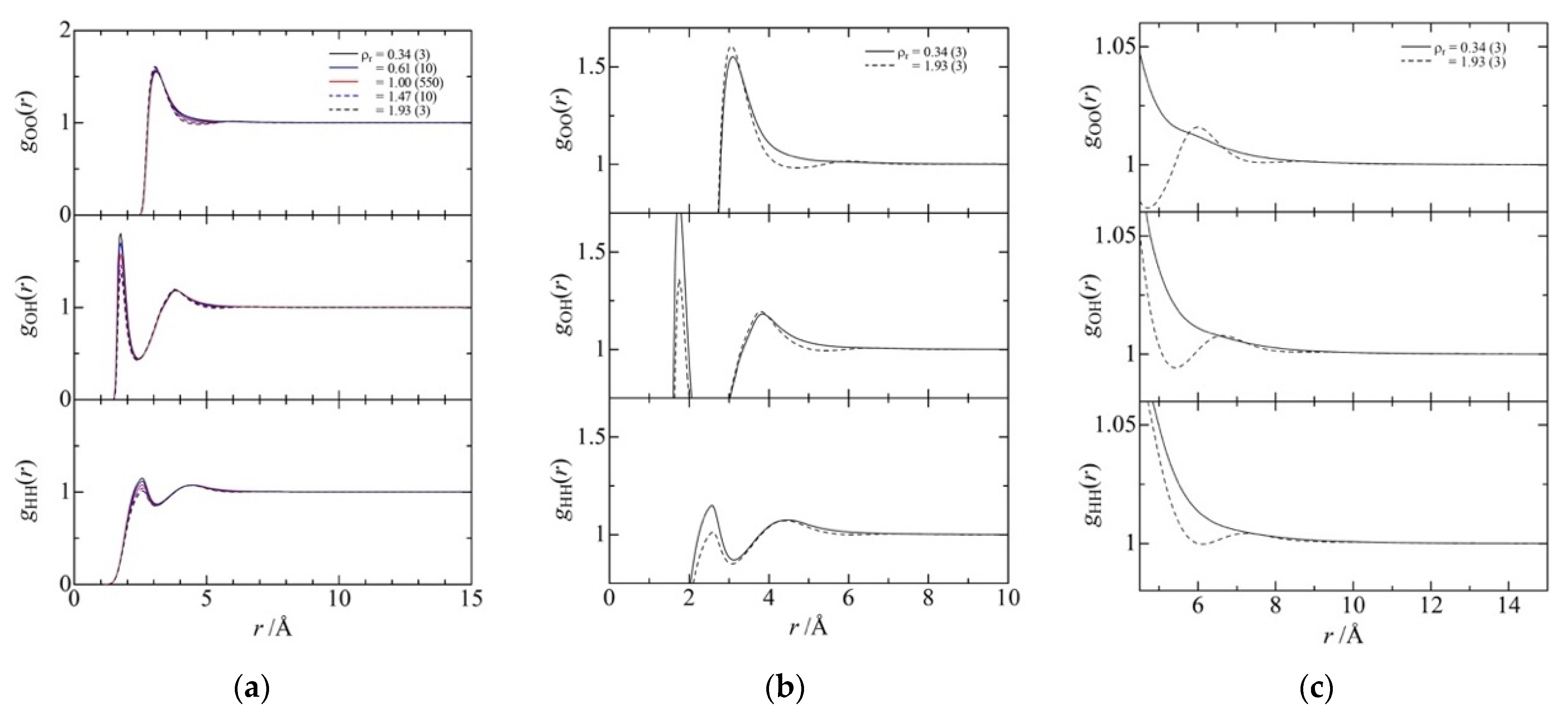
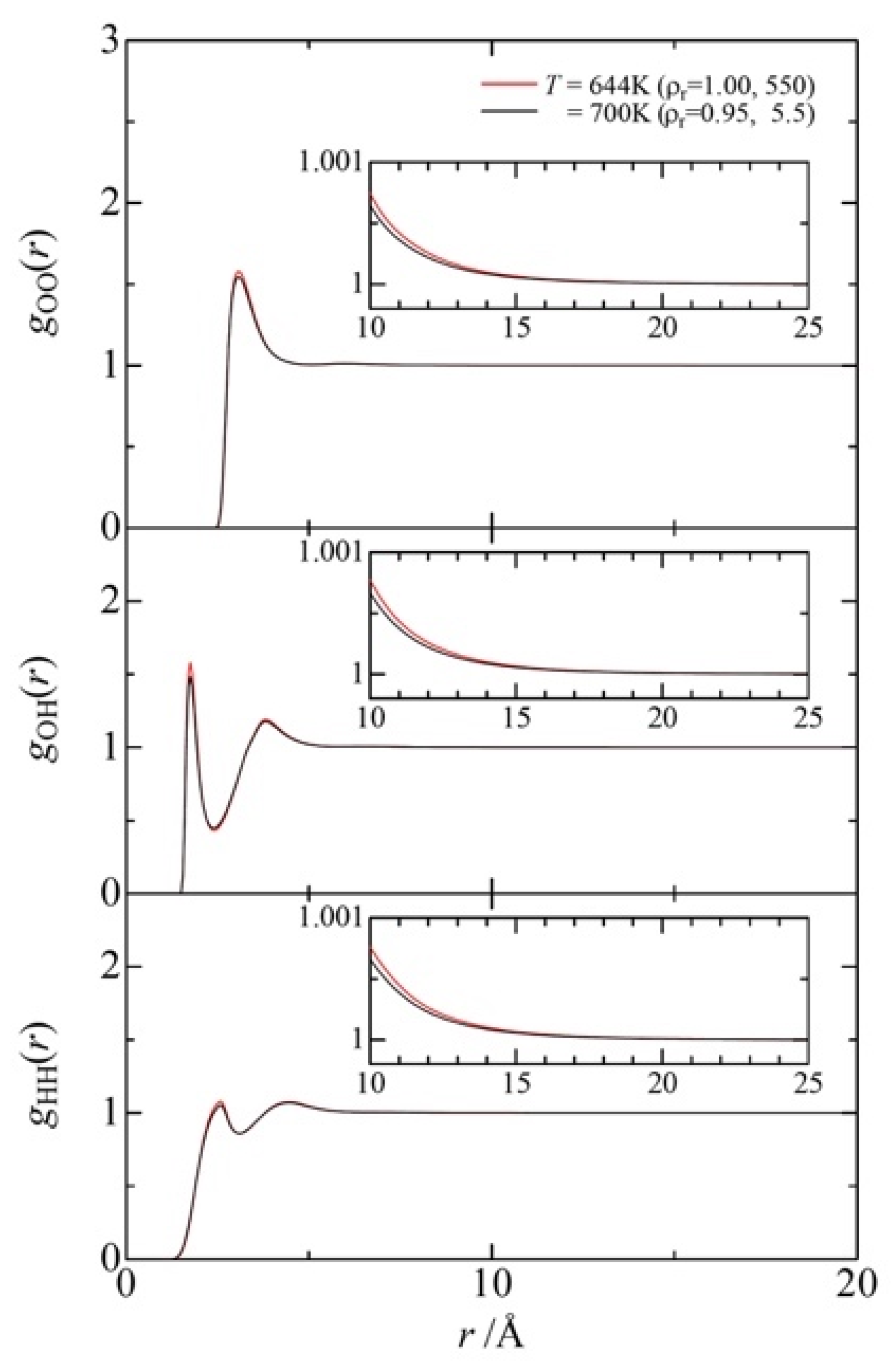
3.3. Fluid Structure around the Ridge
3.4. Summary
4. Structure of Water Using Ab Initio Modeling
4.1. Theoretical Framework Considering Polarization
- (i)
- First, the electronic structure of an isolated molecule is calculated. (In this study, the Hartree–Fock level approximation with a double zeta with polarization functions (DZP)-type basis set has been employed.)
- (ii)
- The effective charges of atoms in the molecule are determined by the electrostatic potential method based on the electronic structure calculations.
- (iii)
- The RISM equation for the neat liquid system is solved using the effective charges, and the pair correlation function is determined.
- (iv)
- From the pair correlation function, the microscopic reaction field is calculated using the same definition as the Fock operator in the original RISM–SCF.
- (v)
- The electronic structure of a molecule is calculated with the modified Fock operator including the microscopic reaction field.
4.2. Electronic Polarization of Water
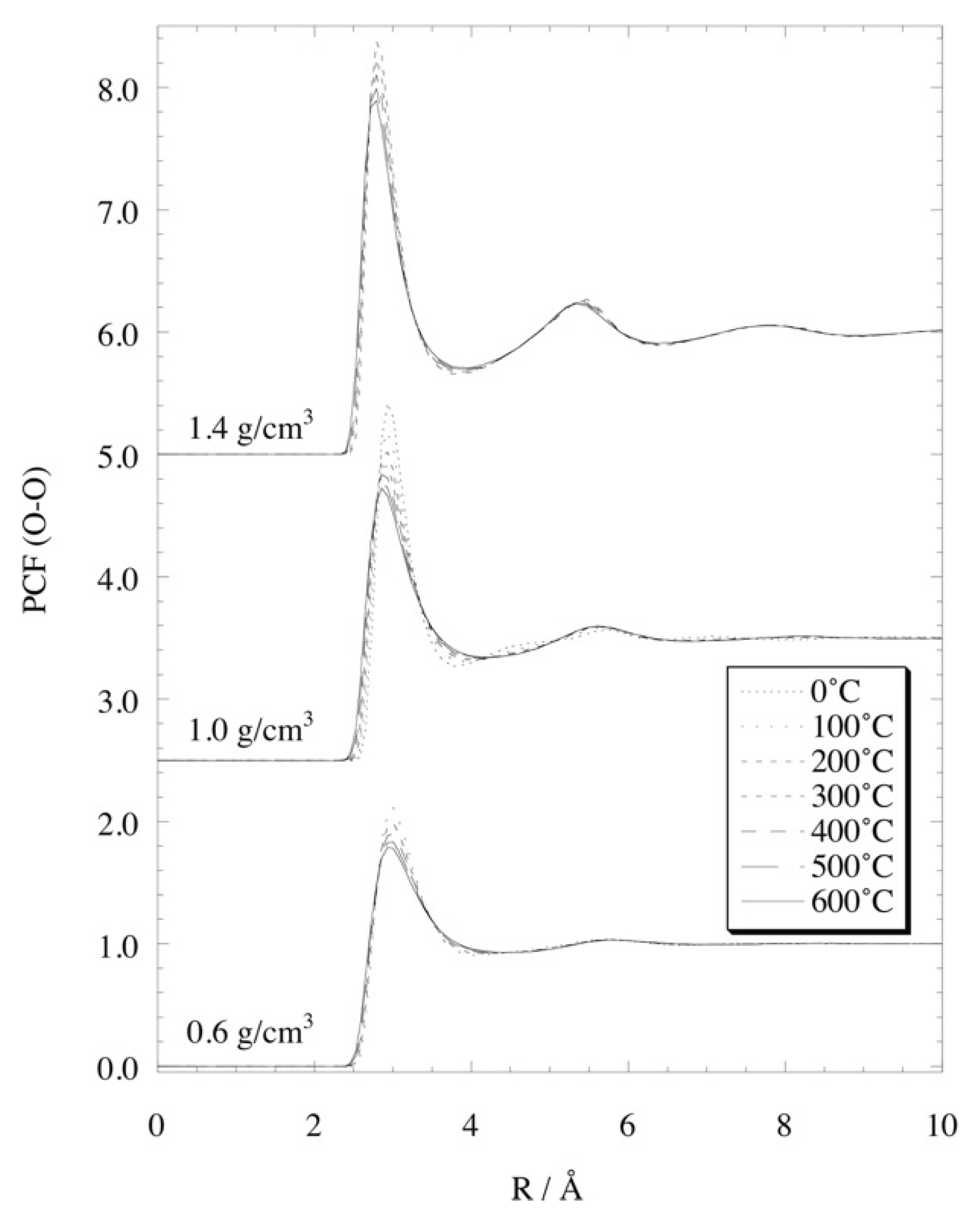
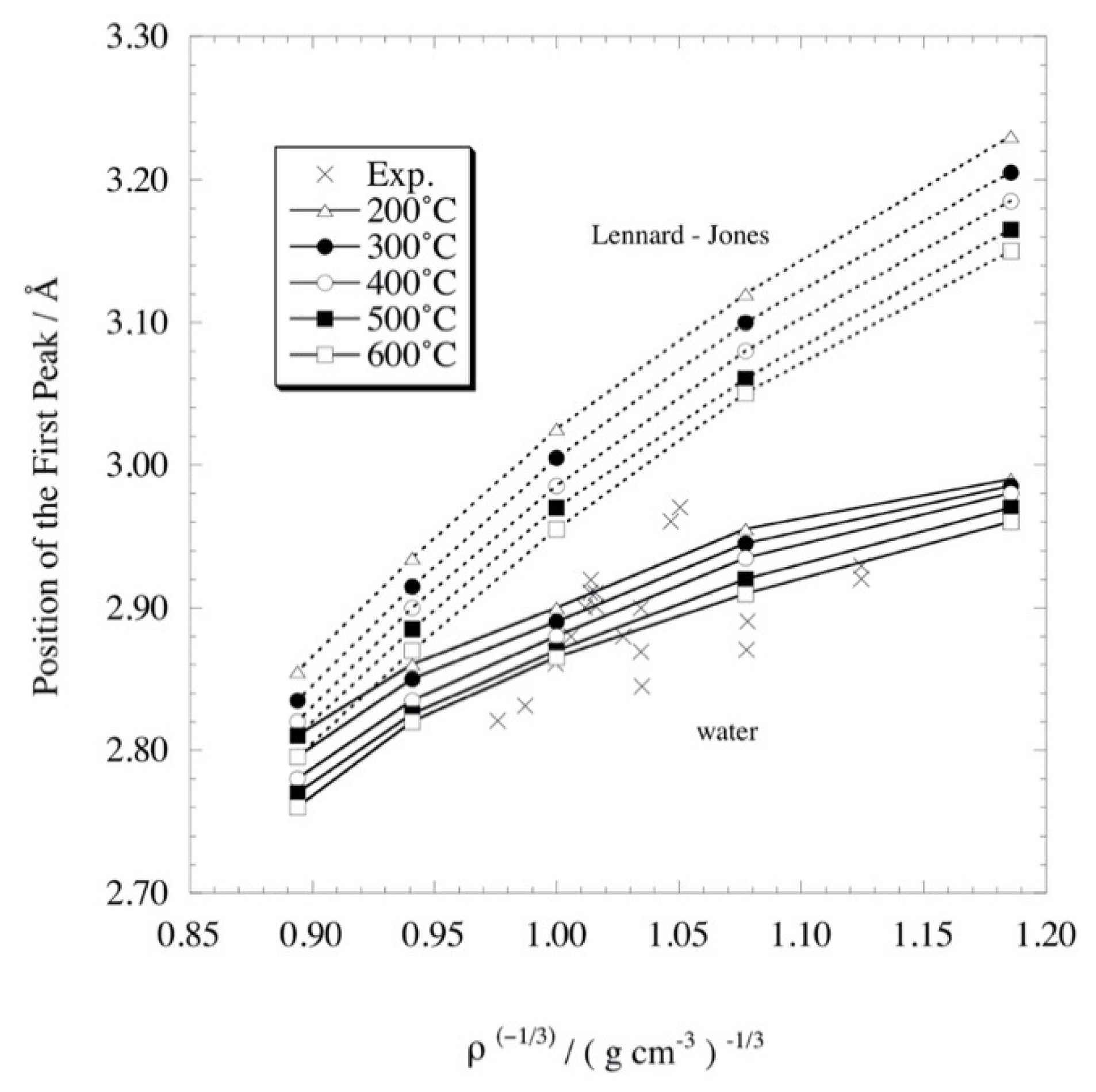
4.3. Liquid Structure
4.4. Summary
5. Autoionization in Supercritical Water
5.1. Theoretical Framework of pKw
5.2. Free Energy and pKw
5.3. Summary
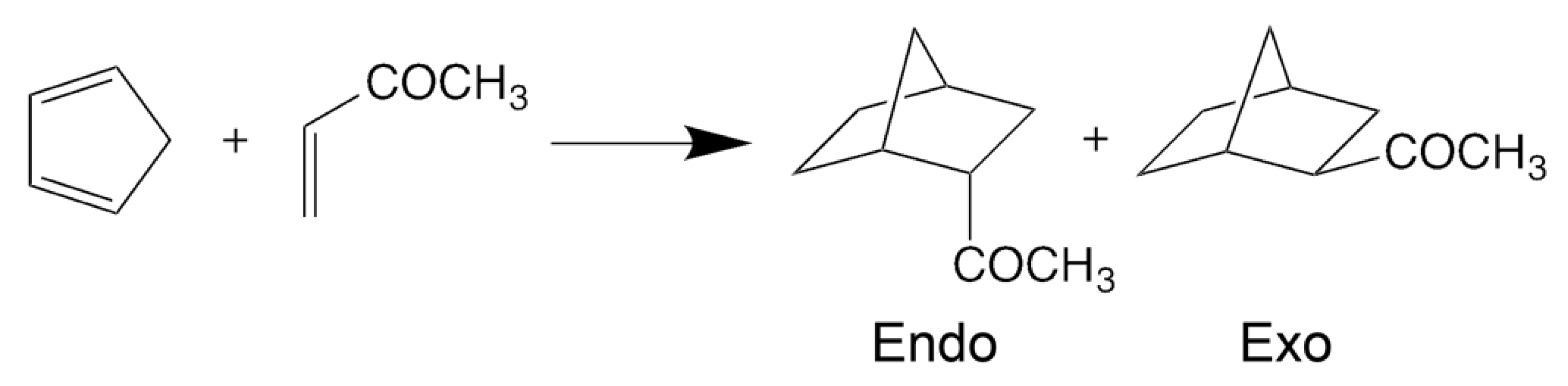
6. The Diels–Alder Reaction in Supercritical Water
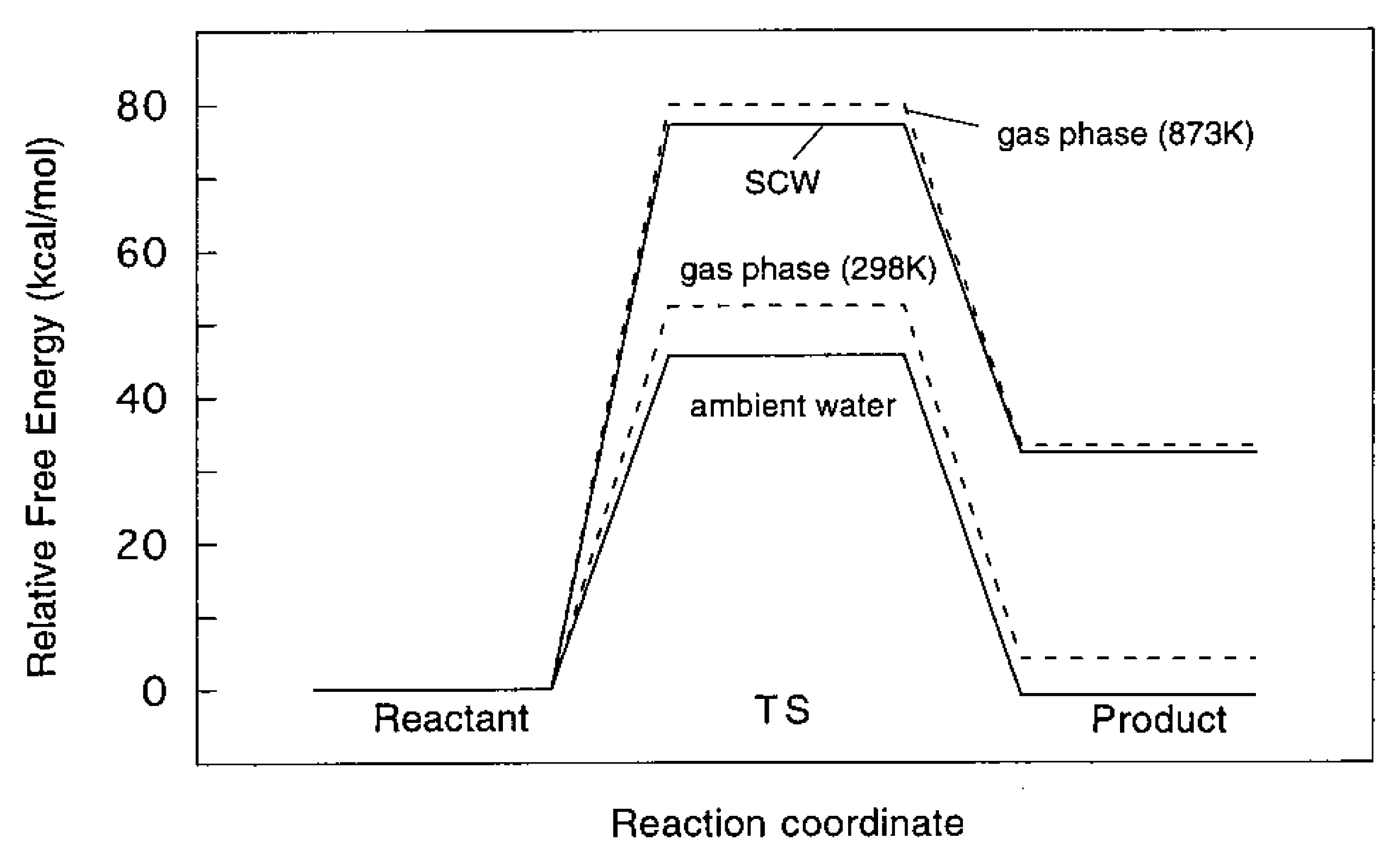
6.1. Stabilization of the Transition State in Ambient Water
6.2. Physical Origin of the High Yield in Supercritical Water
6.3. Summary
7. Concluding Remarks
Author Contributions
Funding
Conflicts of Interest
References
- Nakahara, M.; Yamaguchi, T.; Ohtaki, H. The structure of water and aqueous electrolyte solutions under extreme conditions. Recent Res. Devel. Phys. Chem. 1997, 1, 17. [Google Scholar]
- Fischer, K.; Schulenberg, T.; Laurien, E. Design of a supercritical water-cooled reactor with a three-pass core arrangement. Nucl. Eng. Des. 2009, 239, 800–812. [Google Scholar] [CrossRef]
- Imre, A.R.; Groniewsky, A.; Györke, G.; Katona, A.; Velmovszki, D. Anomalous Properties of Some Fluids−with High Relevance in Energy Engineering− in Their Pseudo-critical (Widom) Region. Period. Polytech. Chem. Eng. 2019, 63, 276–285. [Google Scholar] [CrossRef]
- Padilla, R.V.; Too, Y.C.S.; Benito, R.; Stein, W. Exergetic analysis of supercritical CO2 Brayton cycles integrated with solar central receivers. Appl. Energy 2015, 148, 348–365. [Google Scholar] [CrossRef]
- Nakahara, M. The Structure and Properties of Supercritical Water. Netsu Sokutei 2004, 31, 14–22. [Google Scholar]
- Hirata, F. Exploring Life Phenomena with Statistical Mechanics of Molecular Liquids; CRC Press: Boca Raton, FL, USA, 2020. [Google Scholar]
- Stanley, H.E. Introduction to Phase Transitions and Critical Phenomena; Oxford University Press: New York, NY, USA, 1987. [Google Scholar]
- Banuti, D. Crossing the Widom-line–supercritical pseudo-boiling. J. Supercrit. Fluids 2015, 98, 12–16. [Google Scholar] [CrossRef]
- Kajimoto, O. Solvation in Supercritical Fluids: Its Effects on Energy Transfer and Chemical Reactions. Chem. Rev. 1999, 99, 355–390. [Google Scholar] [CrossRef]
- Tucker, S.C.; Maddox, M.W. The effect of solvent density inhomogeneities on solute dynamics in supercritical fluids: A theoretical perspective. J. Phys. Chem. B 1998, 102, 2437–2453. [Google Scholar] [CrossRef]
- Tucker, S.C. Solvent density inhomogeneities in supercritical fluids. Chem. Rev. 1999, 99, 391–418. [Google Scholar] [CrossRef] [PubMed]
- Nishikawa, K.; Morita, T. Fluid Behavior at Supercritical States Studied by Small-Angle X-Ray Scattering. J. Supercrit. Fluids 1998, 13, 143–148. [Google Scholar] [CrossRef]
- Nishikawa, K.; Morita, T. Inhomogeneity of molecular distribution in supercritical fluids. Chem. Phys. Lett. 2000, 316, 238–242. [Google Scholar] [CrossRef]
- Nishikawa, K.; Kusano, K.; Arai, A.A.; Morita, T. Density fluctuation of a van der Waals fluid in supercritical state. J. Chem. Phys. 2003, 118, 1341–1346. [Google Scholar] [CrossRef]
- Saitow, K.; Kajiya, D.; Nishikawa, K. Dynamics of density fluctuation of supercritical fluid mapped on phase diagram. J. Am. Chem. Soc. 2004, 126, 422–423. [Google Scholar] [CrossRef] [PubMed]
- Nishikawa, K.; Takematsu, M. X-Ray-Scattering Study of Carbon-Dioxide at Supercritical States. Chem. Phys. Lett. 1994, 226, 359–363. [Google Scholar] [CrossRef]
- Nishikawa, K.; Tanaka, I. Correlation Lengths and Density-Fluctuations in Supercritical States of Carbon-Dioxide. Chem. Phys. Lett. 1995, 244, 149–152. [Google Scholar] [CrossRef]
- Nishikawa, K.; Tanaka, I.; Amemiya, Y. Small-angle X-ray scattering study of supercritical carbon dioxide. J. Phys. Chem. 1996, 100, 418–421. [Google Scholar] [CrossRef]
- Nishikawa, K.; Morita, T. Small-Angle X-ray-Scattering Study of Supercritical Trifluoromethane. J. Phys. Chem. B 1997, 101, 1413–1418. [Google Scholar] [CrossRef]
- Morita, T.; Kusano, K.; Ochiai, H.; Saitow, K.; Nishikawa, K. Study of inhomogeneity of supercritical water by small-angle X-ray scattering. J. Chem. Phys. 2000, 112, 4203–4211. [Google Scholar] [CrossRef]
- Arai, A.A.; Morita, T.; Nishikawa, K. Analysis to obtain precise density fluctuation of supercritical fluids by small-angle X-ray scattering. Chem. Phys. 2005, 310, 123–128. [Google Scholar] [CrossRef]
- Amemiya, Y.; Wakabayashi, K.; Hamanaka, T.; Wakabayashi, T.; Matsushita, T.; Hashizume, H. Design of a Small-Angle X-Ray Diffractometer Using Synchrotron Radiation at the Photon-Factory. Nucl. Instrum. Methods 1983, 208, 471–477. [Google Scholar] [CrossRef]
- Morita, T.; Miyagi, H.; Shimokawa, Y.; Maisuo, H.; Nishikawa, K. Construction of the sample holder and small-angle X-ray scattering measurement for supercritical water. Jpn. J. Appl. Phys. Part 2 Lett. 1998, 37, L768–L770. [Google Scholar] [CrossRef]
- Nishikawa, K.; Arai, A.A.; Morita, T. Density fluctuation of supercritical fluids obtained from small-angle X-ray scattering experiment and thermodynamic calculation. J. Supercrit. Fluids 2004, 30, 249–257. [Google Scholar] [CrossRef]
- Morita, T.; Kusano, K.; Nishikawa, K.; Miyagi, H.; Shimokawa, Y.; Matsuo, H. Titanium sample holder for small-angle x-ray scattering measurements of supercritical aqueous solutions. Rev. Sci. Instrum. 2001, 72, 3013–3018. [Google Scholar] [CrossRef]
- Wagner, W.; Pruss, A. The IAPWS formulation 1995 for the thermodynamic properties of ordinary water substance for general and scientific use. J. Phys. Chem. Ref. Data 2002, 31, 387–535. [Google Scholar] [CrossRef]
- Yoshida, N.; Ishizuka, R.; Sato, H.; Hirata, F. Ab initio theoretical study of temperature and density dependence of molecular and thermodynamic properties of water in the entire fluid region: Autoionization processes. J. Phys. Chem. B 2006, 110, 8451–8458. [Google Scholar] [CrossRef] [PubMed]
- Shibuta, S.; Imamura, H.; Nishikawa, K.; Morita, T. Fluctuational parameters based on the Bhatia-Thornton theory for supercritical solutions: Application to a supercritical aqueous solution of n-pentane. Chem. Phys. 2017, 487, 30–36. [Google Scholar] [CrossRef]
- Ploetz, E.A.; Smith, P.E. Gas or Liquid? The Supercritical Behavior of Pure Fluids. J. Phys. Chem. B 2019, 123, 6554–6563. [Google Scholar]
- Carome, E.F.; Cykowski, C.B.; Havlice, J.F.; Swyt, D.A. Temperature and Pressure Dependence of Velocity of Ultrasound in Argon. Physica 1968, 38, 307. [Google Scholar] [CrossRef]
- Nishikawa, K.; Ochiai, H.; Saitow, K.; Morita, T. Static inhomogeneity of supercritical ethylene studied by small-angle X-ray scattering. Chem. Phys. 2003, 286, 421–430. [Google Scholar] [CrossRef]
- Arai, A.A.; Morita, T.; Nishikawa, K. Investigation of structural fluctuation of supercritical benzene by small-angle x-ray scattering. J. Chem. Phys. 2003, 119, 1502–1509. [Google Scholar] [CrossRef]
- Reid, R.C.; Prausnitz, J.M.; Poling, J.E. The Properties of Gases and Liquids, 4 ed.; McGraw-Hill: New York, NY, USA, 1987. [Google Scholar]
- Chialvo, A.A.; Cummings, P.T. Solute-induced Effects on the Structure and the Thermodynamics of Infinitely Dilute Mixtures. AICHE J. 1994, 40, 1558–1573. [Google Scholar] [CrossRef]
- Gray, C.; Goldman, S.; Tomberli, B.; Li, W. Correlation lengths and density fluctuations in supercritical states of carbon dioxide—Comment. Chem. Phys. Lett. 1997, 271, 185–187. [Google Scholar] [CrossRef]
- Nishikawa, K. Reply to Comment on "Correlation lengths and density fluctuations in supercritical states of carbon dioxide". Chem. Phys. Lett. 1997, 271, 188. [Google Scholar] [CrossRef]
- Narikiyo, O. Comment on "correlation length and density fluctuations in supercritical states of carbon dioxide". Chem. Phys. Lett. 1998, 290, 549–550. [Google Scholar] [CrossRef]
- Matsugami, M.; Yoshida, N.; Hirata, F. Theoretical characterization of the "ridge" in the supercritical region in the fluid phase diagram of water. J. Chem. Phys. 2014, 140, 104511. [Google Scholar] [CrossRef]
- Hansen, J.P.; McDonald, I.R. Theory of Simple Liquids, 3rd ed.; Academic Press: Amsterdam, The Netherlands, 2006. [Google Scholar]
- Kovalenko, A.; Hirata, F. Molecular Theory of Solvation; Hirata, F., Ed.; Kluwer: Dordrecht, The Netherlands, 2003. [Google Scholar]
- Kovalenko, A.; Hirata, F. First-principles realization of a van der Waals-Maxwell theory for water. Chem. Phys. Lett. 2001, 349, 496–502. [Google Scholar] [CrossRef]
- Matubayasi, N.; Nakahara, M. Super- and subcritical hydration of nonpolar solutes. I. Thermodynamics of hydration. J. Chem. Phys. 2000, 112, 8089–8109. [Google Scholar] [CrossRef][Green Version]
- Ohmine, I.; Tanaka, H. Fluctuation, Relaxations, and Hydration in Liquid Water—Hydrogen-Bond Rearrangement Dynamics. Chem. Rev. 1993, 93, 2545–2566. [Google Scholar] [CrossRef]
- Hirata, F. Chemical processes in solution studied by an integral equation theory of molecular liquids. Bull. Chem. Soc. Jpn. 1998, 71, 1483–1499. [Google Scholar] [CrossRef]
- Maw, S.; Sato, H.; Ten-No, S.; Hirata, F. Ab initio study of water: Self-consistent determination of electronic structure and liquid state properties. Chem. Phys. Lett. 1997, 276, 20–25. [Google Scholar] [CrossRef]
- Dorsey, N. Properties of Ordinary Water-Substance; Hafner Publishing Co.: New York, NY, USA, 1968. [Google Scholar]
- Ten-No, S.; Hirata, F.; Kato, S. A Hybrid Approach for the Solvent Effect on the Electronic-Structure of a Solute Based on the RISM and Hartree-Fock Equations. Chem. Phys. Lett. 1993, 214, 391–396. [Google Scholar] [CrossRef]
- Ten-No, S.; Hirata, F.; Kato, S. Reference Interaction Site Model Self-Consistent-Field Study For Solvation Effect On Carbonyl-Compounds In Aqueous-Solution. J. Chem. Phys. 1994, 100, 7443–7453. [Google Scholar] [CrossRef]
- Sato, H.; Hirata, F.; Kato, S. Analytical energy gradient for the reference interaction site model multiconfigurational self-consistent-field method: Application to 1,2-difluoroethylene in aqueous solution. J. Chem. Phys. 1996, 105, 1546–1551. [Google Scholar] [CrossRef]
- Gregory, J.K.; Clary, D.C.; Liu, K.; Brown, M.G.; Saykally, R.J. The water dipole moment in water clusters. Science 1997, 275, 814–817. [Google Scholar] [CrossRef] [PubMed]
- Matubayasi, N.; Wakai, C.; Nakahara, M. Structural study of supercritical water. II. Computer simulations. J. Chem. Phys. 1999, 110, 8000–8011. [Google Scholar] [CrossRef]
- Eisenberg, D.; Kauzmann, W. The Structure and Properties of Water; Oxford at the Clarendon Press: London, UK, 1969. [Google Scholar]
- Ohmine, I. Liquid Water Dynamics—Collective Motions, Fluctuation, and Relaxation. J. Phys. Chem. 1995, 99, 6767–6776. [Google Scholar] [CrossRef]
- Trout, B.; Parrinello, M. Analysis of the dissociation of H2O in water using first-principles molecular dynamics. J. Phys. Chem. B 1999, 103, 7340–7345. [Google Scholar] [CrossRef]
- Trout, B.; Parrinello, M. The dissociation mechanism of H2O in water studied by first-principles molecular dynamics. Chem. Phys. Lett. 1998, 288, 343–347. [Google Scholar] [CrossRef]
- Sato, H.; Hirata, F. Theoretical study for autoionization of liquid water: Temperature dependence of the ionic product (pKw). J. Phys. Chem. A 1998, 102, 2603–2608. [Google Scholar] [CrossRef]
- Sato, H.; Hirata, F. Ab initio study of water. II. Liquid structure, electronic and thermodynamic properties over a wide range of temperature and density. J. Chem. Phys. 1999, 111, 8545–8555. [Google Scholar] [CrossRef]
- Sato, H.; Hirata, F. Ab initio study on molecular and thermodynamic properties of water: A theoretical prediction of pK(w) over a wide range of temperature and density. J. Phys. Chem. B 1999, 103, 6596–6604. [Google Scholar] [CrossRef]
- Kido, K.; Sato, H.; Sakaki, S. Systematic Assessment on Aqueous pK(a) and pK(b) of an Amino Acid Base on RISM-SCF-SEDD Method: Toward First Principles Calculations. Int. J. Quantum Chem. 2012, 112, 103–112. [Google Scholar] [CrossRef]
- Seno, Y.; Yoshida, N.; Nakano, H. Theoretical analysis of complex formation of p-carboxybenzeneboronic acid with a monosaccharide. J. Mol. Liq. 2016, 217, 93–98. [Google Scholar] [CrossRef]
- Fujiki, R.; Kasai, Y.; Seno, Y.; Matsui, T.; Shigeta, Y.; Yoshida, N.; Nakano, H. A computational scheme of pKa values based on the three-dimensional reference interaction site model self-consistent field theory coupled with the linear fitting correction scheme. Phys. Chem. Chem. Phys. 2018, 20, 27272–27279. [Google Scholar] [CrossRef]
- Kido, K.; Sato, H.; Sakaki, S. First Principle Theory for pK(a) Prediction at Molecular Level: pH Effects Based on Explicit Solvent Model. J. Phys. Chem. B 2009, 113, 10509–10514. [Google Scholar] [CrossRef] [PubMed]
- Kovalenko, A.; Hirata, F. Self-Consistent Description Of A Metal-Water Interface By The Kohn-Sham Density Functional Theory And The Three-Dimensional Reference Interaction Site Model. J. Chem. Phys. 1999, 110, 10095–10112. [Google Scholar] [CrossRef]
- Mesmer, R.E.; Marshall, W.L.; Palmer, D.A.; Simonson, J.M.; Holmes, H.F. Thermodynamics of Aqueous Association and Ionization Reactions at High-Temperatures and Pressures. J. Solut. Chem. 1988, 17, 699–718. [Google Scholar] [CrossRef]
- Yagasaki, T.; Iwahashi, K.; Saito, S.; Ohmine, I. A theoretical study on anomalous temperature dependence of pK(w) of water. J. Chem. Phys. 2005, 122, 144504. [Google Scholar] [CrossRef] [PubMed]
- Rideout, D.C.; Breslow, R. Hydrophobic acceleration of Diels-Alder reactions. J. Am. Chem. Soc. 1980, 102, 7816–7817. [Google Scholar] [CrossRef]
- Shaw, R.W.; Brill, T.B.; Clifford, A.A.; Eckert, C.A.; Franck, E.U. Supercritical Water—A Medium for Chemistry. Chem. Eng. News 1991, 69, 26–39. [Google Scholar]
- Savage, P.E. Organic chemical reactions in supercritical water. Chem. Rev. 1999, 99, 603–621. [Google Scholar] [CrossRef] [PubMed]
- Korzenski, M.B.; Kolis, J.W. Diels-Alder reactions using supercritical water as an aqueous solvent medium. Tetrahedron Lett. 1997, 38, 5611–5614. [Google Scholar] [CrossRef]
- Blake, J.F.; Jorgensen, W.L. Solvent Effects on a Diels-Alder Reaction from Computer-Simulations. J. Am. Chem. Soc. 1991, 113, 7430–7432. [Google Scholar] [CrossRef]
- Blake, J.F.; Lim, D.; Jorgensen, W.L. Enhanced Hydrogen-Bonding of Water to Diels-Alder Transition-States—Ab-Initio Evidence. J. Org. Chem. 1994, 59, 803–805. [Google Scholar] [CrossRef]
- Otto, S.; Blokzijl, W.; Engberts, J.B.F.N. Diels-Alder Reactions in Water—Effects of Hydrophobicity and Hydrogen-Bonding. J. Org. Chem. 1994, 59, 5372–5376. [Google Scholar] [CrossRef]
- Furlani, T.R.; Gao, J.L. Hydrophobic and hydrogen-bonding effects on the rate of Diels-Alder reactions in aqueous solution. J. Org. Chem. 1996, 61, 5492–5497. [Google Scholar] [CrossRef]
- Jorgensen, W.L.; Lim, D.C.; Blake, J.F. Abinitio Study of Diels-Alder Reactions of Cyclopentadiene with Ethylene, Isoprene, Cyclopentadiene, Acrylonitrile, and Methyl Vinyl Ketone. J. Am. Chem. Soc. 1993, 115, 2936–2942. [Google Scholar] [CrossRef]
- Assfeld, X.; Ruiz-López, M.F.; García, J.I.; Mayoral, J.A.; Salvatella, L. Importance of Electronic and Nuclear-Polarization Energy on Diastereofacial Selectivity of Diels-Alder Reactions in Aqueous-Solution. J. Chem. Soc. Chem. Comm. 1995, 1371–1372. [Google Scholar] [CrossRef]
- Breslow, R.; Maitra, U. Unusual Structural Effects on the Chemical Degradation of Steroid Sidechains. Tetrahedron Lett. 1984, 25, 5843–5846. [Google Scholar] [CrossRef]
- Harano, Y.; Sato, H.; Hirata, F. Solvent effects on a Diels-Alder reaction in supercritical water: RISM-SCF study. J. Am. Chem. Soc. 2000, 122, 2289–2293. [Google Scholar] [CrossRef]
- Harano, Y.; Sato, H.; Hirata, F. A theoretical study on a Diels-Alder reaction in ambient and supercritical water: Viewing solvent effects through frontier orbitals. Chem. Phys. 2000, 258, 151–161. [Google Scholar] [CrossRef]
- Jorgensen, W.L.; Briggs, J.M.; Contreras, M.L. Relative Partition-coefficients for Organic Solutes from Fluid Simulations. J. Phys. Chem. 1990, 94, 1683–1686. [Google Scholar] [CrossRef]
- Jorgensen, W.L.; Madura, J.D.; Swenson, C.J. Optimized Intermolecular Potential Functions for Liquid Hydrocarbons. J. Am. Chem. Soc. 1984, 106, 6638–6646. [Google Scholar] [CrossRef]
- Peach, J.; Eastoe, J. Supercritical carbon dioxide: A solvent like no other. Beilstein J. Org. Chem. 2014, 10, 1878–1895. [Google Scholar] [CrossRef] [PubMed]
- Saitow, K.; Kajiya, D.; Nishikawa, K. Time evolution of density fluctuation in supercritical region. I. Non-hydrogen-bonded fluids studied by dynamic light scattering. J. Phys. Chem. A 2005, 109, 83–91. [Google Scholar] [CrossRef] [PubMed]
- Nakayama, H.; Saitow, K.; Sakashita, M.; Ishii, K.; Nishikawa, K. Raman spectral changes of neat CO2 across the ridge of density fluctuation in supercritical region. Chem. Phys. Lett. 2000, 320, 323–327. [Google Scholar] [CrossRef]

(kcal/mol) | (kcal/mol) | (kcal/mol) | |
|---|---|---|---|
| AW | |||
| trans | |||
| cis | |||
| SCW | |||
| trans | |||
| cis | |||
(kcal/mol) | (kcal/mol) | (kcal/mol) | |
|---|---|---|---|
| AW | |||
| trans | |||
| cis | |||
| SCW | |||
| trans | |||
| cis | |||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yoshida, N.; Matsugami, M.; Harano, Y.; Nishikawa, K.; Hirata, F. Structure and Properties of Supercritical Water: Experimental and Theoretical Characterizations. J 2021, 4, 698-726. https://doi.org/10.3390/j4040049
Yoshida N, Matsugami M, Harano Y, Nishikawa K, Hirata F. Structure and Properties of Supercritical Water: Experimental and Theoretical Characterizations. J. 2021; 4(4):698-726. https://doi.org/10.3390/j4040049
Chicago/Turabian StyleYoshida, Norio, Masaru Matsugami, Yuichi Harano, Keiko Nishikawa, and Fumio Hirata. 2021. "Structure and Properties of Supercritical Water: Experimental and Theoretical Characterizations" J 4, no. 4: 698-726. https://doi.org/10.3390/j4040049
APA StyleYoshida, N., Matsugami, M., Harano, Y., Nishikawa, K., & Hirata, F. (2021). Structure and Properties of Supercritical Water: Experimental and Theoretical Characterizations. J, 4(4), 698-726. https://doi.org/10.3390/j4040049






