Evaluation of Distillery Fractions in Direct Methanol Fuel Cells and Screening of Reaction Products
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant and Sampling
2.2. Chemicals
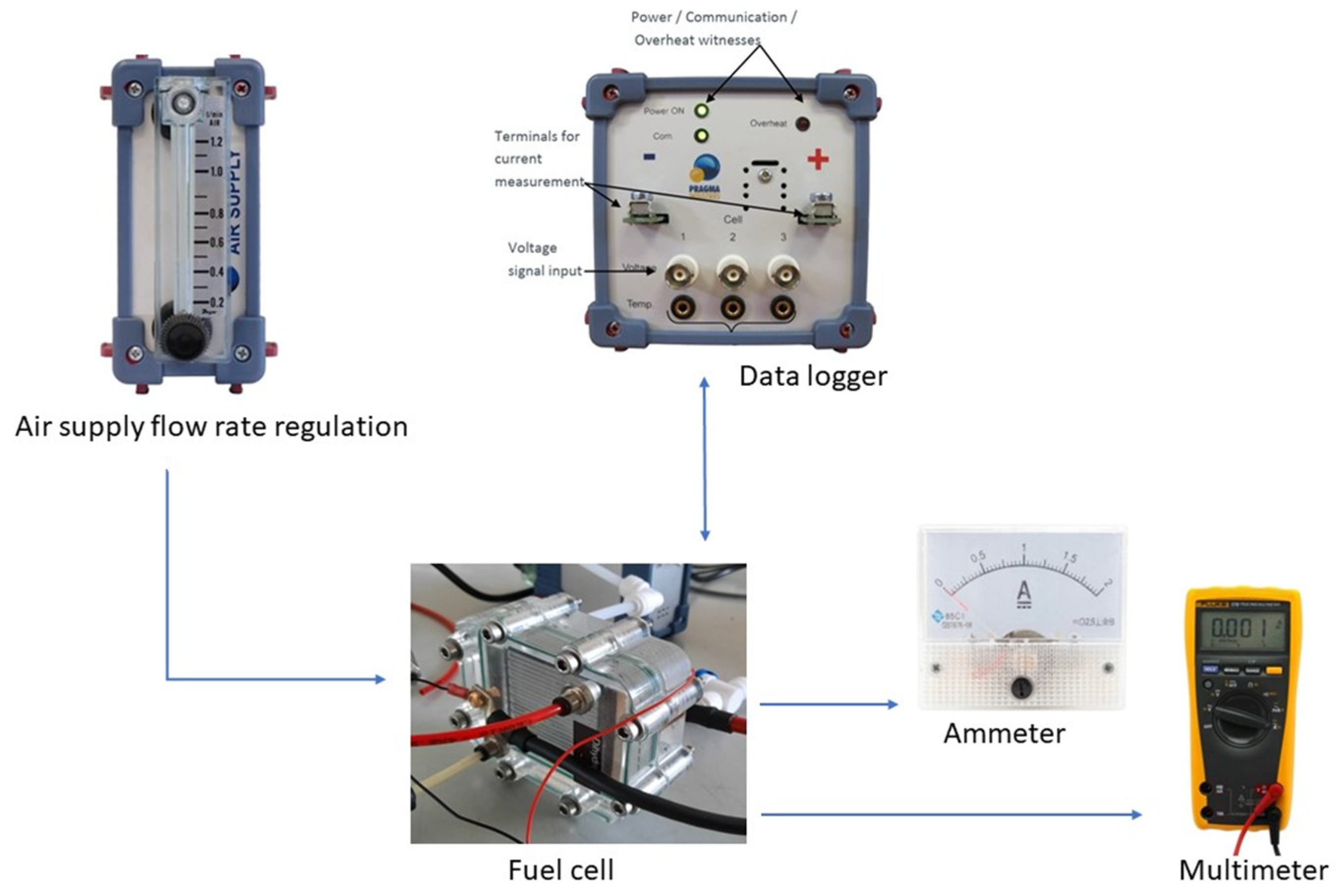
2.3. Direct Methanol Fuel Cell and Experiments’ Description
- Maximum gas pressure of 2 bars and a maximum internal temperature of 70 °C;
- Voltage control/voltage measurements: from 0.05 V to 5 V with a resolution of 0.01 V;
- Current control/measurement: from 0 A to 25 A with a resolution of 0.1 A;
- Temperature measurement: 0–110 °C with a resolution of 1%.
CH3CH2OH + 3 H2O → 2 CO2 + 12 H+ + 12 e−
3 O2 + 12 H+ + 12 e− → 6 H2O
CH3CH2OH + 3 O2 → 2 CO2 + 3 H2O
2.4. Determination of the Relationship of Voltage, Current Intensity, and Apparent Power during the DMFC Experiments
2.5. Determination of the Reaction Products in Steady-Voltage Experiments
2.6. GC-MS Determination of the Diluted Distillery Fractions
3. Results and Discussion
3.1. Chemical Characterization of the Distillery Fractions
3.2. Electrochemical Measurements of Diluted Distillation Fractions
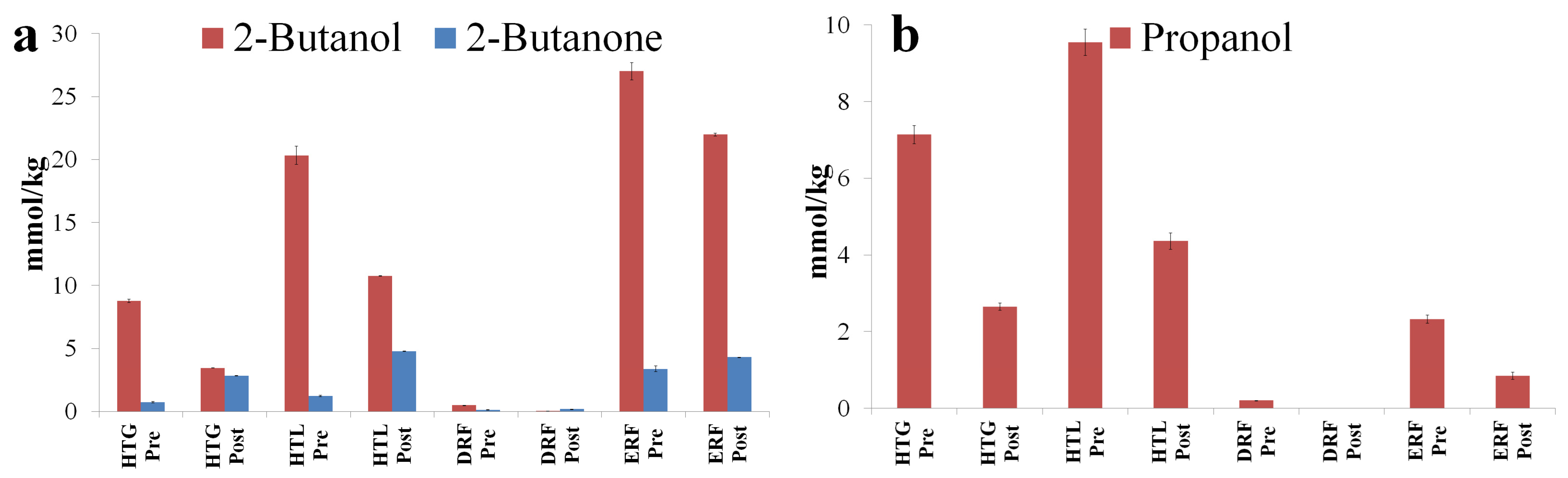
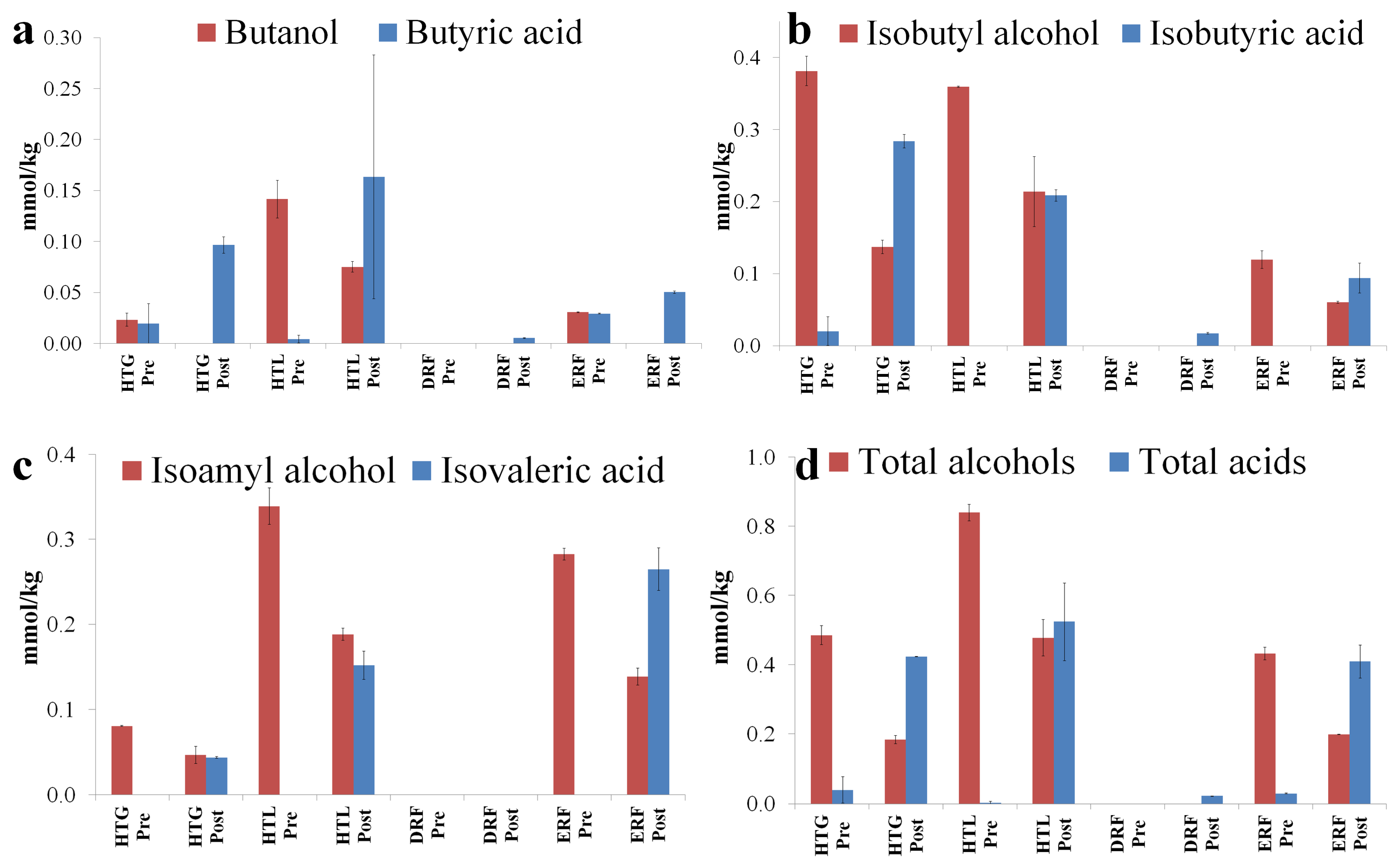
3.3. GC-MS Evaluation of the Diluted Distillery Fractions
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| DEFC | direct ethanol fuel cell |
| DMFC | direct methanol fuel cell |
| DRF | demethylation column reflux fraction |
| ERF | epuration column recycling fraction |
| FC | fuel cell |
| HTG | distillation heads and tails deriving from grape pomace |
| HTL | distillation heads and tails deriving from lees |
| m/z | Mass-to-charge ratio |
| PEM | polymer–electrolyte membrane |
| r | coefficient of correlation |
| V | voltage |
References
- Bahrami, H.; Faghri, A. Review and advances of direct methanol fuel cells: Part II: Modeling and numerical simulation. J. Power Sources 2013, 230, 303–320. [Google Scholar] [CrossRef]
- Ince, A.C.; Karaoglan, M.U.; Glüsen, A.; Colpan, C.O.; Müller, M.; Stolten, D. Semiempirical thermodynamic modeling of a direct methanol fuel cell system. Int. J. Energy Res. 2019, 43, 3601–3615. [Google Scholar] [CrossRef]
- Li, X.; Faghri, A. Review and advances of direct methanol fuel cells (DMFCs) part I: Design, fabrication, and testing with high concentration methanol solutions. J. Power Sources 2013, 226, 223–240. [Google Scholar] [CrossRef]
- Shrivastava, N.K.; Thombre, S.B.; Chadge, R.B. Liquid feed passive direct methanol fuel cell: Challenges and recent advances. Ionics 2016, 22, 1–23. [Google Scholar] [CrossRef]
- Radenahmad, N.; Afif, A.; Petra, P.I.; Rahman, S.M.; Eriksson, S.-G.; Azad, A.K. Proton-conducting electrolytes for direct methanol and direct urea fuel cells—A state-of-the-art review. Renew. Sustain. Energy Rev. 2016, 57, 1347–1358. [Google Scholar] [CrossRef]
- Oumer, A.; Hasan, M.; Baheta, A.T.; Mamat, R.; Abdullah, A. Bio-based liquid fuels as a source of renewable energy: A review. Renew. Sustain. Energy Rev. 2018, 88, 82–98. [Google Scholar] [CrossRef]
- Sánchez Ballester, S.C. Synthesis and Characterization of New Polymer Electrolytes to Use in Fuel Cells Fed with Bio-Alcohols; Universitat Politècnica de València: Valencia, Spain, 2017. [Google Scholar]
- Revankar, S.T.; Majumdar, P. Fuel Cells: Principles, Design, and Analysis; CRC Press: Boca Raton, FL, USA, 2016. [Google Scholar]
- Araya, S.S.; Liso, V.; Cui, X.; Li, N.; Zhu, J.; Sahlin, S.L.; Jensen, S.H.; Nielsen, M.P.; Kær, S.K. A Review of The Methanol Economy: The Fuel Cell Route. Energies 2020, 13, 596. [Google Scholar] [CrossRef]
- Hodson, G.; Wilkes, E.; Azevedo, S.; Battaglene, T. Methanol in wine. BIO Web Conf. 2017, 9, 02028. [Google Scholar] [CrossRef]
- Shamsul, N.S.; Kamarudin, S.K.; Rahman, N.A.; Kofli, N.T. An overview on the production of bio-methanol as potential renewable energy. Renew. Sustain. Energy Rev. 2014, 33, 578–588. [Google Scholar] [CrossRef]
- Roode-Gutzmer, Q.I.; Kaiser, D.; Bertau, M. Renewable Methanol Synthesis. ChemBioEng Rev. 2019, 6, 209–236. [Google Scholar] [CrossRef]
- Dias, M.O.; Modesto, M.; Ensinas, A.V.; Nebra, S.A.; Filho, R.M.; Rossell, C.E. Improving bioethanol production from sugarcane: Evaluation of distillation, thermal integration and cogeneration systems. Energy 2011, 36, 3691–3703. [Google Scholar] [CrossRef]
- Gupta, C.; Prakash, D.; Gupta, S. A Biotechnological Approach to Microbial Based Perfumes and Flavours. J. Microbiol. Exp. 2015, 2, 11–18. [Google Scholar] [CrossRef]
- Ottone, C.; Romero, O.; Aburto, C.; Illanes, A.; Wilson, L. Biocatalysis in the winemaking industry: Challenges and opportunities for immobilized enzymes. Compr. Rev. Food Sci. Food Saf. 2020, 19, 595–621. [Google Scholar] [CrossRef] [PubMed]
- Pu, H. Polymers for PEM Fuel Cells; Wiley: Hoboken, NJ, USA, 2014. [Google Scholar] [CrossRef]
- Carvallo, J.; Labbe, M.; Pérez-Correa, J.; Zaror, C.; Wisniak, J. Modelling methanol recovery in wine distillation stills with packing columns. Food Control 2011, 22, 1322–1332. [Google Scholar] [CrossRef]
- Junqueira, T.L.; Dias, M.O.S.; Filho, R.M.; Wolf-Maciel, M.R.; Rossell, C.E.V.; Atala, D.I.P. Proposition of alternative configurations of the distillation columns for bioethanol production using vacuum extractive fermentation process. Chem. Eng. Trans. 2009, 17, 1627–1632. [Google Scholar] [CrossRef]
- Batista, F.R.; Follegatti-Romero, L.A.; Meirelles, A.J. A new distillation plant for neutral alcohol production. Sep. Purif. Technol. 2013, 118, 784–793. [Google Scholar] [CrossRef]
- Bastidas, P.; Parra, J.; Gil, I.; Rodríguez, G. Alcohol Distillation Plant Simulation: Thermal and Hydraulic Studies. Procedia Eng. 2012, 42, 80–89. [Google Scholar] [CrossRef]
- Aylott, R.I. Flavoured spirits. In Fermented Beverage Production; Springer: New York, NY, USA, 1995. [Google Scholar] [CrossRef]
- Wiley, H.W. Industrial Alcohol: Uses and Statistics; US Department of Agriculture: Washington, DC, USA, 1906.
- Khandelwal, M.; van Dril, T. Decarbonisation Options for the Dutch Biofuels Industry; PBL Planbureau voor de Leefomgeving: Hague, The Netherlands, 2020; Available online: https://www.pbl.nl/sites/default/files/downloads/pbl-2020-decarbonisation-options-for-the-dutch-biofuels-industry_3887.pdf (accessed on 17 March 2024).
- Montevecchi, G.; Cancelli, U.; Masino, F.; Antonelli, A. Composition and applications of fractions discharged from a distillation plant for neutral ethanol production. Chem. Eng. Res. Des. 2021, 171, 80–85. [Google Scholar] [CrossRef]
- Wongyao, N.; Therdthianwong, A.; Therdthianwong, S. The fading behavior of direct methanol fuel cells under a start-run-stop operation. Fuel 2010, 89, 971–977. [Google Scholar] [CrossRef]
- Mazzapioda, L.; Lo Vecchio, C.; Aricò, A.S.; Navarra, M.A.; Baglio, V. Performance Improvement in Direct Methanol Fuel Cells by Using CaTiO3-δ Additive at the Cathode. Catalysts 2019, 9, 1017. [Google Scholar] [CrossRef]
- Bhuvanendran, N.; Ravichandran, S.; Zhang, W.; Ma, Q.; Xu, Q.; Khotseng, L.; Su, H. Highly efficient methanol oxidation on durable PtxIr/MWCNT catalysts for direct methanol fuel cell applications. Int. J. Hydrogen Energy 2020, 45, 6447–6460. [Google Scholar] [CrossRef]
- Leo, T.J.; Raso, M.A.; Navarro, E.; Mora, E. Long Term Performance Study of a Direct Methanol Fuel Cell Fed with Alcohol Blends. Energies 2013, 6, 282–293. [Google Scholar] [CrossRef]
- Kim, J.H.; Choi, S.M.; Nam, S.H.; Seo, M.H.; Choi, S.H.; Kim, W.B. Influence of Sn content on PtSn/C catalysts for electrooxidation of C1–C3 alcohols: Synthesis, characterization, and electrocatalytic activity. Appl. Catal. B Environ. 2008, 82, 89–102. [Google Scholar] [CrossRef]
- Puthiyapura, V.K.; Lin, W.-F.; Russell, A.E.; Brett, D.J.L.; Hardacre, C. Effect of Mass Transport on the Electrochemical Oxidation of Alcohols Over Electrodeposited Film and Carbon-Supported Pt Electrodes. Top. Catal. 2018, 61, 240–253. [Google Scholar] [CrossRef]
- Geng, W.; Zheng, M.; Chen, D.; Wang, X.; Zhang, Z.; Lin, Y.; Cai, D.; Li, H.; Huang, G.; Yu, Y.J.; et al. The Performance of a Novel PdFe/B-N-G Catalys for Ethanol Electro-oxidation in Alkaline Medium. Int. J. Electrochem. Sci. 2020, 15, 2528–2538. [Google Scholar] [CrossRef]
- Wnuk, P.; Jurczakowski, R.; Lewera, A. Electrochemical Characterization of Low-Temperature Direct Ethanol Fuel Cells using Direct and Alternate Current Methods. Electrocatalysis 2020, 11, 121–132. [Google Scholar] [CrossRef]
- Sánchez-Monreal, J.; García-Salaberri, P.A.; Vera, M. A mathematical model for direct ethanol fuel cells based on detailed ethanol electro-oxidation kinetics. Appl. Energy 2019, 251, 113264. [Google Scholar] [CrossRef]
- Choudhary, A.K.; Pramanik, H. Enhancement of ethanol electrooxidation in half cell and single direct ethanol fuel cell (DEFC) using post-treated polyol synthesized Pt-Ru nano electrocatalysts supported on HNO3-functionalized acetylene black carbon. Int. J. Hydrogen Energy 2020, 45, 574–594. [Google Scholar] [CrossRef]
- Ong, B.; Kamarudin, S.; Basri, S. Direct liquid fuel cells: A review. Int. J. Hydrogen Energy 2017, 42, 10142–10157. [Google Scholar] [CrossRef]
- Li, N.-H.; Sun, S.-G.; Chen, S.-P. Studies on the role of oxidation states of the platinum surface in electrocatalytic oxidation of small primary alcohols. J. Electroanal. Chem. 1997, 430, 57–67. [Google Scholar] [CrossRef]
- Puthiyapura, V.K.; Brett, D.J.L.; Russell, A.E.; Lin, W.-F.; Hardacre, C. Biobutanol as Fuel for Direct Alcohol Fuel Cells—Investigation of Sn-Modified Pt Catalyst for Butanol Electro-oxidation. ACS Appl. Mater. Interfaces 2016, 8, 12859–12870. [Google Scholar] [CrossRef] [PubMed]
- Masino, F.; Montevecchi, G.; Riponi, C.; Antonelli, A. Composition of some commercial grappas (grape marc spirit): The anomalous presence of 1,1-diethoxy-3-methylbutane: A case study. Eur. Food Res. Technol. 2009, 228, 565–569. [Google Scholar] [CrossRef]
- Liu, J.; Solem, C.; Jensen, P.R. Harnessing biocompatible chemistry for developing improved and novel microbial cell factories. Microb. Biotechnol. 2020, 13, 54–66. [Google Scholar] [CrossRef] [PubMed]
- Ochoa-Gómez, J.R.; Fernández-Carretero, F.; Río-Pérez, F.; García-Luis, A.; Roncal, T.; García-Suárez, E.J. Electrosynthesis of 2,3-butanediol and methyl ethyl ketone from acetoin in flow cells. Green Chem. 2019, 21, 164–177. [Google Scholar] [CrossRef]
- Vaze, A.S.; Sawant, S.B.; Pangarkar, V.G. Electrochemical oxidation of isobutanol to isobutyric acid at nickel oxide electrode: Improvement of the anode stability. J. Appl. Electrochem. 1997, 27, 584–588. [Google Scholar] [CrossRef]



| IUPAC Nomenclature | Common Names | Monitored Ions | HTG | HTL | DRF | ERF |
|---|---|---|---|---|---|---|
| m/z | mg/kg | mg/kg | mg/kg | mg/kg | ||
| Ethanal | Acetaldehyde | 44, 43 | 531 | 41 | 7 | 38 |
| Ethyl acetate | 43, 45, 61 | 14,000 | 9333 | 38 | 4333 | |
| Methanol | 31, 32 | 105,333 | 6000 | 17,000 | 4000 | |
| Butan-2-one | 2-Butanone | 43, 72, 57 | 1007 | 1783 | 261 | 4220 |
| 2-Methylpropyl ethanoate | Isobutyl acetate | 42, 56, 73 | 67 | n.d. | n.d. | 41 |
| Butan-2-ol | 2-Butanol | 43, 41, 74 | 17,503 | 20,872 | 106 | 14,088 |
| Propan-1-ol | Propanol | 31, 42, 59 | 8383 | 8121 | 8 | 2611 |
| Ethyl 2-methylpropanoate | Ethyl isobutyrate | 43, 71, 116 | 67 | 20 | 10 | 10 |
| Ethyl pentanoate | Ethyl valerate | 57, 88, 101 | 298 | 112 | n.d. | 52 |
| 2-Methylpropan-1-ol | Isobutyl alcohol | 43, 74, 55 | 2325 | n.d. | 4 | n.d. |
| 3-Methylbutyl acetate | Isoamyl acetate | 43, 70, 55 | 80 | 91 | n.d. | 191 |
| Butan-1-ol | Butanol | 56, 41, 72 | 56 | 237 | n.d. | 36 |
| Pentan-1-ol | Pentanol | 42, 55, 70 | n.d. | n.d. | n.d. | 4 |
| 3-Methylbutan-1-ol | Isoamyl alcohol | 42, 55, 70 | 321 | 959 | 16 | 730 |
| Ethyl hexanoate | Ethyl caproate | 88, 99, 115 | 28 | 83 | n.d. | 79 |
| 3-Hydroxybutan-2-one | Acetoin | 45, 43, 88 | 340 | n.d. | n.d. | n.d. |
| Hexyl acetate | Capryl acetate | 43, 56, 84 | n.d. | n.d. | n.d. | 2 |
| Ethyl 2-hydroxypropanoate | Ethyl lactate | 45, 75 | n.d. | n.d. | n.d. | 15 |
| Hexan-1-ol | Hexanol | 56, 69, 84 | n.d. | 1 | 2 | 30 |
| Ethyl octanoate | Ethyl caprylate | 88, 101, 127 | n.d. | 4 | 2 | 181 |
| Ethanoic acid | Acetic acid | 43, 45, 60 | 2001 | 48 | 1 | 59 |
| Ethanol (g/100 g) | 45, 46 | 54 | 67 | 75 | 70 |
| %EtOH %MeOH | 0% 3.101% | 0.894% 2.261% | 1.635% 1.564% | 2.464% 0.786% | 2.784% 0.485% | 3.291% 0% |
|---|---|---|---|---|---|---|
| V | mA (A) | mA (B) | mA (C) | mA (D) | mA (E) | mA (F) |
| 0.00 | 134.08 | 71.55 | 47.73 | 51.00 | 61.98 | 49.68 |
| 0.05 | 120.53 | 61.90 | 41.60 | 44.28 | 57.33 | 43.48 |
| 0.10 | 107.93 | 53.83 | 36.03 | 38.20 | 50.98 | 39.73 |
| 0.15 | 95.63 | 46.03 | 30.75 | 32.35 | 45.55 | 34.95 |
| 0.20 | 85.85 | 37.63 | 25.70 | 27.05 | 38.78 | 30.15 |
| 0.25 | 72.38 | 30.20 | 21.15 | 21.93 | 32.85 | 25.10 |
| 0.30 | 59.93 | 22.93 | 16.73 | 17.05 | 27.20 | 20.63 |
| 0.35 | 44.55 | 16.80 | 12.65 | 12.73 | 21.65 | 16.20 |
| 0.40 | 34.08 | 11.73 | 9.10 | 8.03 | 17.30 | 12.23 |
| 0.45 | 25.03 | 6.55 | 5.63 | 4.95 | 13.68 | 8.70 |
| 0.50 | 19.28 | 2.83 | 2.70 | 1.95 | 10.28 | 5.68 |
| 0.55 | 14.65 | 0.00 | 0.00 | 0.00 | 6.68 | 3.18 |
| 0.60 | 9.78 | / | / | / | 3.55 | 0.00 |
| 0.65 | 2.88 | / | / | / | 0.00 | / |
| 0.70 | 0.00 | / | / | / | / | / |
| %EtOH %MeOH | 0% 3.101% | 0.894% 2.261% | 1.635% 1.564% | 2.464% 0.786% | 2.784% 0.485% | 3.291% 0% |
|---|---|---|---|---|---|---|
| V | mW (A) | mW (B) | mW (C) | mW (D) | mW (E) | mW (F) |
| 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| 0.05 | 6.03 | 3.10 | 2.08 | 2.21 | 2.87 | 2.17 |
| 0.10 | 10.79 | 5.38 | 3.60 | 3.82 | 5.10 | 3.97 |
| 0.15 | 14.34 | 6.90 | 4.61 | 4.85 | 6.83 | 5.24 |
| 0.20 | 17.17 | 7.53 | 5.14 | 5.41 | 7.76 | 6.03 |
| 0.25 | 18.09 | 7.55 | 5.29 | 5.48 | 8.21 | 6.28 |
| 0.30 | 17.98 | 6.88 | 5.02 | 5.12 | 8.16 | 6.19 |
| 0.35 | 15.59 | 5.88 | 4.43 | 4.45 | 7.58 | 5.67 |
| 0.40 | 13.63 | 4.69 | 3.64 | 3.21 | 6.92 | 4.89 |
| 0.45 | 11.26 | 2.95 | 2.53 | 2.23 | 6.15 | 3.92 |
| 0.50 | 9.64 | 1.41 | 1.35 | 0.97 | 5.14 | 2.84 |
| 0.55 | 8.06 | 0.00 | 0.00 | 0.00 | 3.67 | 1.75 |
| 0.60 | 5.87 | / | / | / | 2.13 | 0.00 |
| 0.65 | 1.87 | / | / | / | 0.00 | / |
| 0.70 | 0.00 | / | / | / | / | / |
| mA | mW | mA | mW | mA | mW | mA | mW | |
|---|---|---|---|---|---|---|---|---|
| HT G | HT L | DW | EW | |||||
| 0 Volts | 63.2 ± 1.8 | 60.9 ± 2.0 | 75.9 ± 3.0 | 68.8 ± 2.1 | ||||
| 0.05 Volts | 56.6 ± 1.3 | 2.8 ± 0.1 | 54.3 ± 2.3 | 2.7 ± 0.1 | 67.7 ± 3.8 | 3.4 ± 0.2 | 60.5 ± 4.0 | 3.0 ± 0.2 |
| 0.10 Volts | 49.2 ± 0.9 | 4.9 ± 0.1 | 48.5 ± 2.7 | 4.9 ± 0.3 | 59.2 ± 3.0 | 5.9 ± 0.3 | 54.3 ± 2.9 | 5.4 ± 0.3 |
| 0.15 Volts | 42.8 ± 1.0 | 6.4 ± 0.2 | 41.5 ± 2.2 | 6.2 ± 0.3 | 50.6 ± 2.1 | 7.6 ± 0.3 | 47.7 ± 2.0 | 7.1 ± 0.3 |
| 0.20 Volts | 36.1 ± 0.8 | 7.2 ± 0.2 | 35.7 ± 1.6 | 7.1 ± 0.3 | 42.2 ± 1.3 | 8.4 ± 0.3 | 41.4 ± 1.7 | 8.3 ± 0.3 |
| 0.25 Volts | 30.7 ± 0.7 | 7.7 ± 0.2 | 30.4 ± 1.4 | 7.6 ± 0.3 | 34.1 ± 1.4 | 8.5 ± 0.3 | 35.3 ± 1.7 | 8.8 ± 0.4 |
| 0.30 Volts | 25.7 ± 0.9 | 7.7 ± 0.3 | 25.2 ± 0.9 | 7.6 ± 0.3 | 28.0 ± 0.4 | 8.4 ± 0.1 | 29.5 ± 1.5 | 8.9 ± 0.5 |
| 0.35 Volts | 20.8 ± 0.7 | 7.3 ± 0.3 | 20.6 ± 0.9 | 7.2 ± 0.3 | 22.2 ± 0.8 | 7.8 ± 0.3 | 24.0 ± 1.7 | 8.4 ± 0.6 |
| 0.40 Volts | 16.7 ± 0.3 | 6.7 ± 0.1 | 16.3 ± 1.0 | 6.5 ± 0.4 | 17.2 ± 1.0 | 6.9 ± 0.4 | 19.2 ± 1.5 | 7.7 ± 0.6 |
| 0.45 Volts | 14.1 ± 0.5 | 6.4 ± 0.3 | 12.4 ± 1.0 | 5.6 ± 0.5 | 13.3 ± 0.6 | 6.0 ± 0.3 | 15.0 ± 1.4 | 6.7 ± 0.6 |
| 0.50 Volts | 9.7 ± 1.0 | 4.8 ± 0.6 | 9.1 ± 1.1 | 4.5 ± 0.6 | 9.8 ± 0.5 | 4.9 ± 0.2 | 11.2 ± 1.1 | 5.6 ± 0.6 |
| 0.55 Volts | 6.4 ± 0.6 | 3.5 ± 0.4 | 6.0 ± 1.0 | 3.3 ± 0.5 | 6.6 ± 0.6 | 3.6 ± 0.3 | 6.9 ± 0.5 | 3.8 ± 0.3 |
| 0.60 Volts | 3.3 ± 0.4 | 2.0 ± 0.3 | 2.9 ± 0.5 | 1.7 ± 0.3 | 3.2 ± 0.5 | 1.9 ± 0.3 | 3.0 ± 0.5 | 1.8 ± 0.3 |
| 0.65 Volts | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Montevecchi, G.; Cannio, M.; Cancelli, U.; Antonelli, A.; Romagnoli, M. Evaluation of Distillery Fractions in Direct Methanol Fuel Cells and Screening of Reaction Products. Clean Technol. 2024, 6, 513-527. https://doi.org/10.3390/cleantechnol6020027
Montevecchi G, Cannio M, Cancelli U, Antonelli A, Romagnoli M. Evaluation of Distillery Fractions in Direct Methanol Fuel Cells and Screening of Reaction Products. Clean Technologies. 2024; 6(2):513-527. https://doi.org/10.3390/cleantechnol6020027
Chicago/Turabian StyleMontevecchi, Giuseppe, Maria Cannio, Umberto Cancelli, Andrea Antonelli, and Marcello Romagnoli. 2024. "Evaluation of Distillery Fractions in Direct Methanol Fuel Cells and Screening of Reaction Products" Clean Technologies 6, no. 2: 513-527. https://doi.org/10.3390/cleantechnol6020027
APA StyleMontevecchi, G., Cannio, M., Cancelli, U., Antonelli, A., & Romagnoli, M. (2024). Evaluation of Distillery Fractions in Direct Methanol Fuel Cells and Screening of Reaction Products. Clean Technologies, 6(2), 513-527. https://doi.org/10.3390/cleantechnol6020027








