Abstract
Locally sourced agricultural residues are a promising feedstock for the production of reinforcement fibers for wastepaper-based packaging papers. An eco-friendly high yield process to generate fibers from wheat straw using high pressure steam and sodium carbonate is presented. The wheat straw was impregnated with up to 16% of sodium carbonate and steam treated for 10 min at temperatures from 148 °C to 203 °C. The pulps were characterized concerning their chemical composition and test sheets with 100% straw fibers and with 15% and 30% straw fibers blended with recycled pulp were prepared. Fiber yields ranged from 70% to 45%, wherein more severe treatment conditions contributed to increased paper strength but lower yields. At comparable fiber yields, treatments featuring a higher chemical input, coupled with lower treatment temperatures, resulted in improved paper strength. By blending recycled pulp with up to 30% of straw fibers with a beating degree of roughly 45 °SR, the burst, compression and tensile strength was enhanced by up to 66%, 74% and 59%, respectively. As the enhancement effect decreases with a high steam treatment intensity and a high proportion of wheat straw, a moderate treatment and limited use of wheat straw may be the best choice.
1. Introduction
The demand for paper-based packaging has risen continuously in the past and is expected to continue to grow due to a significant increase in e-commerce in combination with a heightened interest in sustainability of the public [1]. While historically the demand for graphical paper and paper and board for packaging has risen together, in the last two decades the demand for graphical paper, has continuously dropped (Figure 1a). Concurrently, the growing environmental awareness, as well as constraints in available forest-based materials has led to an increasing utilization of recycled fibers and recycling rate (Figure 1b).
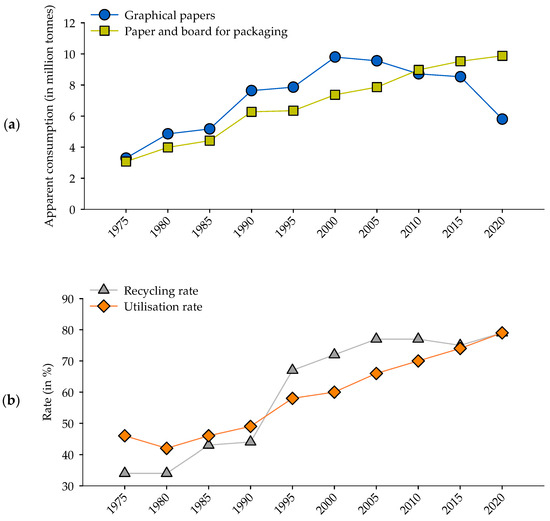
Figure 1.
(a) Apparent consumption (=production + import-export) of graphical and packaging paper and (b) recycling and recycled waste paper utilisation rate in Germany from 1975 to 2020 [2].
In Germany, paper-based packaging is made from 60% to 100% from recycled waste paper, whereas graphical paper has a waste paper utilization rate of around 50% [2]. Therefore, the declining production of graphical paper and further increase of packaging paper leads to a decreasing input of fresh fiber into the waste paper cycle. While the use of pulp from recycled waste paper has numerous environmental benefits in comparison to virgin pulp such as lower energy requirement and a lower carbon foot print [3], the repeated recycling process causes a reduction in the quality of the pulp [4,5]. Due to the lower amount of fresh fibers in the waste paper cycle and the rising recycling rate the overall quality of the recycled waste paper is declining. To counteract this trend, fresh virgin fibers can be blended directly to the recycled pulp [6,7]. However, increasing efforts in the preservation of forests due to climate change, biodiversity loss and changes in public perception, as well as rising procurement costs due to higher competition and increasing regulations are creating a progressively challenging market environment for the wood processing economic sectors. To face these changing market conditions, there is a growing interest in non-wood fibers in the pulp and paper industries.
Non-wood fibers can be obtained from many plants. These can be roughly divided into three groups: the naturally growing plants such as grass from meadows including wildflowers, herbs and weeds; the dedicated fiber feedstock plants such as flax, hemp or miscanthus; and agricultural residues. Of these, agricultural residues such as wheat and oat straws [8], bagasse [9] or corn stover [10,11] can serve as significant sources of non-wood fibers due to their abundant availability as by-products of food production without necessitating dedicated cultivation areas. Furthermore, recent research has indicated that paper-based packaging containing a visible share of agricultural residue is perceived as more environmentally sustainable than packaging devoid of visible waste materials [12]. This perception can be accompanied by a willingness to pay a higher premium [13], making agricultural residues a potentially economically attractive fiber source. Additionally, by valorizing agro-industrial waste, resilience of business operations can be improved through raw material diversification [14], while also strengthening the local economy.
Wheat straw is noted for its favorable capacity for fiber-to-fiber bonding when mixed with recycled softwood pulp and has shown a high recycling potential as the fibers did not suffer as much loss in tensile and burst strength as the softwood pulps did [15]. As of 2020, wheat is cultivated on 218 million ha of land [16], making it one of the most widely cultivated grown crops of the world [17]. Although a significant portion of the co-produced wheat straw is used to enhance soil quality by being incorporated into the field [18], approximately one-third of it can be responsibly harvested from the field without disrupting the humus balance [19]. This portion is therefore accessible for various uses. These properties position wheat straw as a suitable source for production of reinforcement fibers for packaging papers.
However, there are also significant challenges to using wheat straw as material for fiber production, such as high transportation and storage costs due to the low density and tendency toward rapid deterioration [20]. To handle these shortcomings, the wheat straw should be locally sourced to keep transportation distances short and reduce the need for extensive storage space. A favorable approach is to manufacture reinforcement fibers directly on site at the paper mill using small-scale processing units. This will require a process that involves few operational steps and can be realized with low economic expenditure while still offering satisfactory pulp yield and fiber strength.
Wheat straw has a lower lignin content in comparison to soft- and hardwood and is easier to impregnate, thereby enabling the utilization of less rigorous pulping procedures [21]. The Soda Process, which is based on sodium hydroxide-based alkaline treatments has been established as the principal pulping method for wheat straw [22,23]. However, the utilization of sodium hydroxide necessitates an elaborate chemical recovery, involving the combustion of the cooking liquor, causticization and subsequent burning in a lime kiln, which makes economic feasibility challenging on small scale.
As an alternative, the use of sodium carbonate as main pulping agent for agricultural residues has been suggested previously [24,25,26]. Advantages of sodium carbonate over sodium hydroxide include a more affordable procurement price, easier handling due to lower toxicity, and simpler recovery since causticization is not required. Kulkarni et al. [27] have pulped rice straw using a mixture of sodium carbonate and sodium hydroxide (in a 60:40 ratio). They discovered that employing milder pulping conditions can yield satisfactory pulps, along with reduced pollution levels and decreased silica content in the spent liquors. The decrease of the silica content is noteworthy, as elevated silica content in the black liquor can pose issues for chemical recovery systems, impacting the operations in evaporators, recovery furnaces, causticization, and limekilns [28]. Aravamuthan and Yayin [29] investigated the impact of the sodium hydroxide fraction in the pulping of wheat straw on the concora crush resistance using combinations of sodium carbonate and sodium hydroxide as pulping agents. It was found that soda-carbonate pulping using negligible amounts of sodium hydroxide (less than 0.01 fraction) gives comparable results to soda pulping concerning the concora crush resistance. Puitel et al. [8] have shown that it is possible to produce pulps with satisfactory strength for blending in corrugated paperboard from wheat straw using sodium carbonate as sole pulping agent with liquid phase cooking. In direct comparison with pulp generated with sodium hydroxide, the sodium carbonate pulps showed a higher pulp yield and a slightly lower paper strength. The authors attributed the higher yield to the larger quantity of lignin retained in the fibers after pulping, resulting from the limited cleavage of alpha-aryl ether bonds when sodium carbonate is employed in contrast to sodium hydroxide. Another recent study by Marin et al. [30] investigated the mutual influence of the sodium carbonate to sodium hydroxide mass ratio (1:1–9:1), alkali charge (16–20%) and treatment temperature (150 °C–170 °C) on the wheat straw pulp yield, kappa number and resulting paper strength. It was observed that, for the production of pulps with high yield and strength, employing a moderate to low treatment temperature, a low alkali charge (16%), and a higher sodium carbonate to sodium hydroxide mass ratio proved advantageous. Conversely, when the objective is to generate pulps with lower kappa numbers, selecting a lower sodium carbonate to sodium hydroxide mass ratio and a higher alkali charge is recommended. However, in the latter scenario, the augmented presence of sodium hydroxide results in a notable reduction in yield. This literature review shows that several investigations on the use of sodium carbonate have been carried out. However, most of these studies are missing trials to evaluate the final goal of enhancing paper strength in a mixture with recovered waste paper.
Building upon these findings, the objective of this study is to further develop and optimize a pulping process for wheat straw utilizing sodium carbonate as the pulping agent, with the intended application being the incorporation into packaging paper blends. The aim is to generate a high yield pulp with satisfactory blending properties that can be generated in small pulping facilities. Since no bleaching is necessary for the intended application and a straightforward chemical recovery process is desired, sodium carbonate serves as the exclusive pulping agent. The study investigated how the treatment temperature and sodium carbonate concentration in a steam-based process influence the chemical composition of wheat straw fibers. Moreover, their influence on the resultant paper properties, both independently and in conjunction with recycled waste paper, was investigated.
2. Materials and Methods
2.1. Raw Materials
The wheat straw used in the initial pulping trials for optimization of the steaming process was collected in Hungary (WS1) and the wheat straw used for the blending trials was collected in Germany in the area of lower Saxony (WS2). Both samples were cut into pieces of a length of 3 to 10 cm by a shredder and stored at room temperature until use (dry matter content of around 90%). The recycled waste paper (RWP) used in the blending trials consisted of recovered paper grade 1.02 and 1.04 (as described in EN 643) and represents a typical raw material mixture for the production of test liners. The paper was manufactured on a commercial scale paper machine with disabled size press to eliminate the influence of starch content.
2.2. Impregnation and Steam Treatment
Material input per trial was 200 g of dry material. Prior to the steam treatment, the wheat straw was impregnated with 8% and 16% (based on dry raw material) of sodium carbonate (Carl Roth GmbH, Karlsruhe, Germany) using a ratio of 1:1 (liquor:straw) by soaking in a rotary stirrer (Hobart A20, Offenburg, Germany) for 30 min. The wheat straw fully absorbed the sodium carbonate solution and was then inserted into the cylindrical 10 L reactor (Martin Busch & Sohn GmbH, Schermbeck, Germany). The reactor was equipped with a blade system with four blades reaching over the whole length of the reactor, which could be rotated at 1455 rpm by an electric motor with a rated power of 6.8 kW. The wall of the reactor contained five sets of bars. At the end of each steam treatment, the blade system was rotated for 30 s. Thus, defibration of the softened material occurred between the blades and the bars at the wall of the reactor. Experimental runs were done at temperatures of 148 °C, 165 °C, 175 °C, 184 °C, 193 °C and 203 °C for 10 min each. For comparison, steaming experiments without impregnation were conducted using otherwise identical treatment conditions. The overarching process flow-chart is depicted in Figure 2.
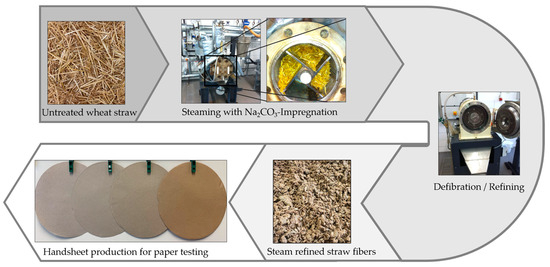
Figure 2.
Process flowchart for sodium carbonate steam refining of wheat straw and subsequent processing of fibers.
2.3. Refining and Beating
After the steam treatment, the pressure was released through a valve. The material was flushed out of the reactor with water and the pH of the spent liquor determined with a pH-Meter ph 330i (WTW, Weilheim, Germany). For further defibration the pulp was passed through a 12″ Sprout-Bauer laboratory refiner (Andritz, Graz, Austria) twice. In the first pass, the gap distance was adjusted to 0.5 mm and in the second pass the gap distance was reduced to 0.2 mm. After refining, the pulp was thoroughly washed and dewatered in a spin dryer to a consistency of about 25–30%.
The pulp was beaten in a Jokro-mill (Frank-PTI, Birkenau, Germany) to the targeted beating degree range and subsequently disintegrated according to German Zellcheming Standard (V/5/60 and V/4/61). The beating degree was determined using a Schopper-Riegler tester Type SR1 (Karl Schröder KG, Weinhem, Germany) as described in ISO 5267-1:1999.
2.4. Pulp Evaluation
Laboratory sheets with a basis weight of 75 g/m2 were created from the produced pulps for evaluation according to ISO 5269-2:2004 with a Rapid-Köthen sheet forming machine (FRANK-PTI, Birkenau, Germany). The laboratory sheets were conditioned for at least 24 h at 23 (±1) °C and 55 (±2)% relative humidity before testing. From the laboratory sheets the burst strength (ISO 2758:2014), short span compressive strength (DIN 54518:2022), tensile strength (ISO 1924-2:2008) and tear strength (ISO 1974:2012-09) were determined using instruments by FRANK-PTI GmbH (Birkenau, Germany). From the determined strength values and weights of the laboratory sheets the strength indices were calculated according to TAPPI T 220. The length weighted fiber length (l w) of the pulp samples was measured using a kajaaniFiberLab (Metso, Helsinki, Finland) after shive removal.
2.5. Analytical Procedures
To determine the extractives content, the wheat straw was ground to a particle size of ≤1 mm using a cutting mill (SM 2000, Retsch, Haan, Germany). Sequential extraction was carried out using an ASE 350 system (Dionex, Sunnyvale, CA, USA). The extraction solvents employed were petroleum ether (70 °C), acetone/water with a ratio of 9:1 (70 °C), and finally water (90 °C), applying a pressure of 10 MPa for 10 min each.
To determine the carbohydrate composition, the extracted material and the pulped wheat straw fibers were air-dried and milled into a fine powder with a T-1000 vibrating disc mill (Siebtechnik GmbH, Mülheim an der Ruhr, Germany). Subsequently, two-step acid hydrolyses were performed in triplicate on 200 mg samples. The fine powder was prehydrolyzed with 2 mL of sulfuric acid (72%, Fisher Scientific, Hampton, NH, USA) at 30 °C for 60 min to break up crystalline cellulose. The reaction was stopped by dilution of the sulfuric acid to a concentration of 4%. Post-hydrolysis was conducted in an autoclave (120 °C and 0.12 MPa) for 40 min to obtain monomeric sugars. Once cooled, the samples were filtered using a G4 sintered glass crucible and the weight of the acid-insoluble residue was determined. The carbohydrate content in the filtrates was determined with Borate-HPAEC using a Dionex Ultimate 3000 (Dionex, Sunnyvale, CA, USA) with anion exchange resin MCL Gel CA80F (Mitsubishi Chemicals, Tokyo, Japan) and a 5 × 120 mm column packed at 65 °C as described comprehensively in the work of Lorenz et al. [31]. Additionally, the acid soluble lignin content was measured in the filtrate with a UV-Spectrophotometer LAMBDA 650 (PerkinElmer, Waltham, MA, USA) at a wavelength of 205 nm. Ash and silica contents were determined according to TAPPI T211 and specifications by Merck KGaA [32], respectively.
3. Results
3.1. Raw Material Characterization
The results of the raw material characterization of the two wheat straw samples used in this study are presented in Table 1. The wheat straw samples show high extractives contents of 10.5% and 11.4% in comparison to poplar and spruce wood chips, with 2.9% and 1.8%, respectively. The highest fraction of extractives was gained with water as the solvent, pointing to a substantial presence of water-soluble, low-molecular-weight polysaccharides, proteins and starch [33].

Table 1.
Chemical composition of the wheat straw samples (in % based on o.d. raw material).
The carbohydrate content of the wheat straw samples ranges from 59.4% to 63.3%, with glucose representing the highest proportion at 35.0% and 39.3% of the raw material, respectively. Xylose accounts for 20.8% and 20.6% of the raw material, whereas arabinose makes up around 2.5% of the content. Galactose, arabinose, and rhamnose were only detected in minor amounts. The acid-insoluble residue, which is approximately equivalent to the lignin content after considering the ash present in the residue, was found to be around 15% of the material. The ash content comprised 6.3% in one sample and 10.5% in the other. The silicate content also varied, with one sample having 4.8% and the other 7.0%, accounting for 76% and 66% of the total ash content, respectively.
Generally, when compared to wood [34], the cellulose and lignin content in the wheat straw samples is lower, while the hemicellulose, ash, and extractives contents are correspondingly higher. Overall the results align with previously published findings. Chemical analysis of wheat straw grown in Egypt, conducted by El-Sayed et al. [21], revealed 47.7% cellulose, 17.1% hemicellulose, 18.2% lignin, 8.3% ash, and 3.4% silica. Another study on wheat straw collected in Romania showed 43.1% cellulose, 31.3% hemicellulose, 17.5% lignin, 5.5% extractives, and 5.3% ash [8]. Mayank et al. [15] reported approximately 41.6% cellulose, 19.7% hemicellulose, 21.6% lignin, and 8.2% ash for wheat straw collected in Uttar Pradesh, India. These disparities in chemical composition between different wheat straw samples can be partly explained by variations in species, harvesting season, and share of morphological parts, such as nodes, internodes, leaf sheaths and blades in the samples, as these parts contain differing amounts of chemical components [21]. Tutus and Eroglu [35] reported a high concentration of ash (14.0%) and silica (9.9%) in the leaves, while the internodes contain only a small amount of ash (3.1%) and silica (1.9%) in a wheat straw sample from Turkey. Thus, it can be advantageous to remove a large part of the leaves before pulping to keep the amount of silica in the process water low [36]. This, however, reduces the overall yield based on raw material input, as the leaves account for around 20% of the mass of the wheat straw [35]. Ideally, the leaves should be removed on the field to save transportation costs and support the humus balance.
3.2. Influence of the Treatment Conditions on the Wheat Straw Fibers
The steam treatments were carried out on the wheat straw sample from Hungary (WS1) using a laboratory-scale plant, with treatment temperatures ranging from 148 °C to 203 °C. Preliminary trials indicated that the steaming duration has a relatively low impact on the pulp characteristics in comparison to temperature and chemical charge. Thus, in order to ensure better comparability, the steaming duration was kept constant at 10 min. Prior to steaming, the wheat straw underwent impregnation with either 8% or 16% sodium carbonate for 30 min. Additionally, steam treatments without chemical additives were conducted, resulting in a total of 18 experimental runs for comparison. It was observed that across all evaluated treatment temperatures, the higher level of sodium carbonate impregnation of 16% leads to a higher pH of the cooking liquor and a reduced fiber yield in comparison to the lower level of sodium carbonate addition (Figure 3). In the absence of sodium carbonate, the pH of the pulping liquor decreases from 7 to approximately 4.5 as the treatment temperature rises. This reduction can be attributed to the liberation of various organic acids from the wheat straw during steaming [37,38]. In contrast to this the cooking liquor of wheat straw impregnated with 8% to 16% of sodium carbonate (based on raw material weight) maintains a slightly alkaline pH level. At 8% addition, pH values are lowering slightly from 9 to 8 at higher treatment temperature, while 16% addition are sufficient to keep the pH stable at approximately 9 to 10, independent of the treatment temperature.
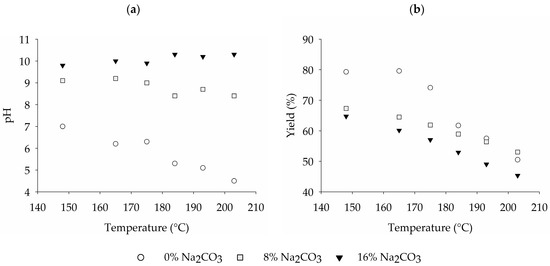
Figure 3.
Influence of the treatment temperature and sodium carbonate impregnation on the pH of (a) the cooking liquor and (b) the fiber yield.
The fiber yield ranges from 80% without sodium carbonate at a treatment temperature of 148 °C to 45% yield at a treatment temperature of 203 °C using 16% of sodium carbonate. While the pulping trials involving sodium carbonate impregnation exhibit a gradual reduction in fiber yield with ascending temperature within the evaluated range, pulping trials solely utilizing steam demonstrate a higher fiber yield at lower temperatures, followed by a notable decline between 175 °C and 184 °C. This culminates in a fiber yield slightly below that of the pulping trials with 8% sodium carbonate but still exceeding the results of pulping with 16% sodium carbonate.
Examining the chemical composition of the fibers after pulping (Figure 4a–f), it becomes evident that the gradual reduction in fiber yield with increasing treatment temperature observed in trials involving sodium carbonate impregnation is mainly attributed to the successive solubilization of the hemicellulose and lignin fractions (Figure 4b–d). During alkaline treatments alkali-labile linkages between lignin compounds and between lignin and polysaccharides are broken [39]. The fragmented lignin becomes soluble under alkaline conditions due to the acidic moieties, such as carboxylic and phenolic groups generated by the hydrolysis of ether and ester bonds [40]. While alkaline treatments cause swelling in the cellulose matrix due to the weakening of the inter-molecular hydrogen bonds [41], a dissolution of the cellulose cannot be observed [42]. Thus, the solubilization of the hemicellulose and lignin aligns with a simultaneous relative increase in the glucose content of the fibers as seen in Figure 4a. Hence, while the yields of pulps generated with and without sodium carbonate may converge at high treatment temperatures, there is a marked distinction in the chemical fiber composition.
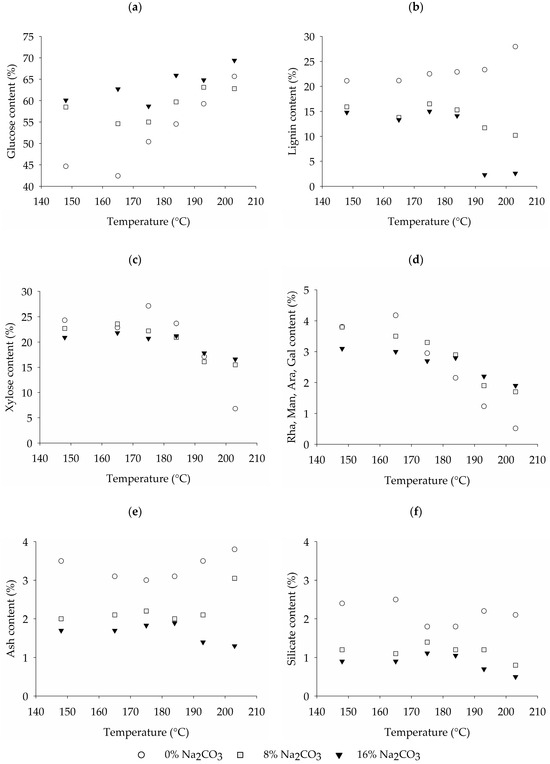
Figure 4.
Influence of the treatment temperature and sodium carbonate impregnation on the (a) glucose, (b) lignin, (c) xylose, (d) and rhamnose, mannose, arabinose, galactose, (e) ash, and (f) silicate content.
In the absence of sodium carbonate, it is primarily the hemicellulose fraction that is solubilized (Figure 4c,d). The high temperature of the steam treatment first leads to dissociation of the condensed water [43]. Subsequently, acetic acid is released from acetylated hemicellulose, which leads to further hydrolysis of the hemicellulose [44]. The ester and ether bonds are cleaved, progressively depolymerizing and solubilizing the polysaccharides. Conversely, the glucose content (Figure 4a) and lignin content (Figure 4b) of the fibers increase, as lignin remains insoluble in neutral or acidic treatment environments, and cellulose does not undergo significant hydrolysis under hydrothermal treatments at low severities such as the ones used in this study.
Looking at the ash and silica content of the fibers (Figure 4e,f), it becomes obvious that increasing the amount of sodium carbonate during pulping reduces the amount of ash and silica in the fibers as more silicate is dissolved due to the higher alkalinity (Figure 3a). Specifically, the silicate content decreases from a range of 1.8% to 2.5% when pulped without sodium carbonate to a range of 0.5% to 1.1% when pulped with 16% sodium carbonate. This indicates a silica removal rate of 48% to 63% for the pulping trials without sodium carbonate and 77% to 90% with 16% sodium carbonate. The treatment temperature on the other hand does not seem to significantly influence the ash and silica content, as no clear trend can be observed.
The removal of ash and silicate from the fibers can be beneficial as their presence can reduce the service life of machinery in subsequent converting processes of the paper. On the other hand, the silica in the black liquor can cause problems during chemical recovery. Different solutions have been suggested in the past, such as a alkaline pre-extraction of the wheat straw to remove silica [45,46] or the precipitation of silica in the spent liquor [27]. However, all of these methods necessitate additional process steps. Thus, a lower silicate removal rate from the fibers should be considered advantageous and as much silicate as possible should be removed from the process with the paper.
Moreover, during the sodium carbonate steam refining process, a black liquor is generated, comprising solubilized and partially degraded lignin and hemicellulose, along with silicate. As the treatment intensity increases, the quantity of solubilized hemicellulose and lignin in the liquor increases. Notably, acid-catalyzed degradation products, such as furfural or 5-(hydroxymethyl)furfural, were not detected in the pulping liquor or steam condensate, attributed to the elevated alkalinity of the process. The black liquor, enriched in hemicellulose, holds potential for valorization in biogas production. Consequently, the diminished fiber yield at higher treatment intensities may be be partially compensated by the heightened biogas potential. Another approach to a more holistic utilization of the raw material involves an integrated processing strategy, wherein hemicelluloses are extracted before pulping-either through hot water or alkaline extraction methods [47,48].
3.3. Paper Properties
The main goal of the study was, to optimize the pulping in such a way that good fiber strength can be reached at the highest possible fiber yield. Therefore, the paper strength values in Figure 5 are presented depending on the fiber yield to give the best overview about the relation of these values. All pulps are compared at a beating degree of about 45 °SR and the pulping conditions can be found in the legend.
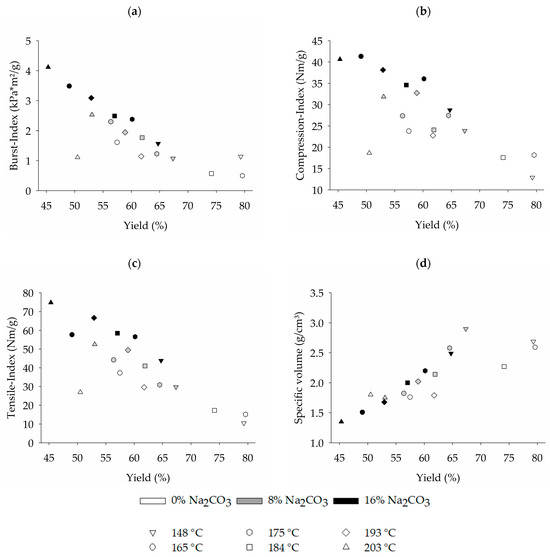
Figure 5.
Influence of treatment temperature and sodium carbonate input on 100% wheat straw paper properties at a beating degree of around 45 °SR: (a) Burst-Index; (b) Compression-Index; (c) Tensile-Index and (d) specific volume.
Examining the achievable burst (Figure 5a), compression (Figure 5b), and tensile strength (Figure 5c), it becomes evident that, with increasing treatment temperature, the strength properties of the pulp generally improve. The highest paper strength was achieved at the maximum treatment temperature of 203 °C and with the addition of 16% of sodium carbonate, while the lowest paper strength was observed at the lowest temperature and without the use of sodium carbonate. This is attributed to two different reasons. On the one hand, it was found that an increase in treatment intensity, i.e., treatment temperature and chemical input, led to a reduction in specific volume of the paper sheets (Figure 5d). This suggests a rise in fiber flexibility, resulting in more easily bent fibers that come into closer contact with each other, reducing the overall thickness of the test sheet. With the increased fiber flexibility, there is a larger surface area available for fiber-to-fiber bonding, consequently enhancing the paper strength. This explains why at the lower range the paper strength increases with higher treatment severity even without the addition of chemicals (see Figure 5a–c), even though no lignin was dissolved from the fibers (Figure 4b). However, with further rising treatment temperatures, no further enhancement of paper strength can be achieved, resulting even in a distinct drop-off at a high treatment temperature of 203 °C. This is probably due to an advancing depolymerization of cellulose and the loss of hemicelluloses at high treatment temperatures, caused by the low pH values that lead to acid hydrolyses of the carbohydrates (Figure 4c). The second reason for increasing paper strength is the reduction of the lignin content in the fibers at high-intensity treatments including the addition of sodium carbonate (see Figure 4b), as lignin impedes the hydrogen bonding between fibers.
The length-weighted fiber length of the pulped wheat straw samples ranges from 0.72 mm to 0.79 mm, demonstrating a shorter fiber length compared to the reported fiber lengths for recycled pulp samples [49,50], as well as untreated wheat straw, hardwood, and softwood [39]. Due to the absence of a discernible trend concerning different treatment temperatures and sodium carbonate input levels on the measured fiber length, it is unlikely that changes in the average fiber length of the wheat straw pulp are responsible for the observed variations in paper strength.
The strength values determined in this study after impregnation with the highest chemical dose fall within a comparable range to the results reported by Puitel et al. [8], who employed sodium carbonate with an alkali charge from 16% to 20% NaOH units in a liquid cooking process at 160 °C for 60 min. At a fiber yield of 49.5% to 51.3%, the authors determined a burst index of 2.9 to 3.7 kPa*m2/g, a short-span compressive strength index of 28.9 to 27.2 Nm/g and a tensile index of 50.8 to 57.7 Nm/g.
Overall it was found that employing a 16% sodium carbonate impregnation resulted in the highest paper strength and the lowest fiber yields over the whole temperature range. It is however noteworthy that when comparing different combinations of steam treatment process conditions that result in comparable fiber yields, treatments with a higher amount of sodium carbonate input (coupled with lower treatment temperatures) always result in increased paper strength. Therefore, when solely assessing the fiber yield and attainable paper strength of test sheets produced from pure wheat straw, it was determined that a higher sodium carbonate input proved to be advantageous within the experimental parameters of this study. However, in practice, the higher costs for sodium carbonate input and the necessity of a chemical recovery process have to be considered when setting up a process.
Since the goal for paper mills producing packaging papers will not be, to make paper from pure wheat straw fibers, but use them as a make up for diminishing waste paper qualities, the possible reinforcement effect was evaluated in blending trials of sodium carbonate pulped wheat straw (WS2) with recycled waste paper (RWP) at two different ratio levels (70% RWP/30% WS2 and 85% RWP/15% WS2). Due to the low paper strength achievable at low treatment temperatures (Figure 5a–c) and low fiber yield at high treatment temperatures (Figure 3a), the evaluated treatment temperature region was reduced to a range of 165 °C to 193 °C for the blending trials. The recycled waste paper, supplied by a packaging paper producer, was disintegrated and subsequently blended with pulped wheat straw. Given the suboptimal quality of recycled waste paper pulp, the majority of packaging paper producers in Germany refrain from subjecting the recycled pulp to beating. Therefore, blending trials were carried out with the unbeaten recycled pulp. The burst-, compression- and tensile strength of the paper test sheets made from wheat straw and recycled waste paper blends are given in Figure 6.
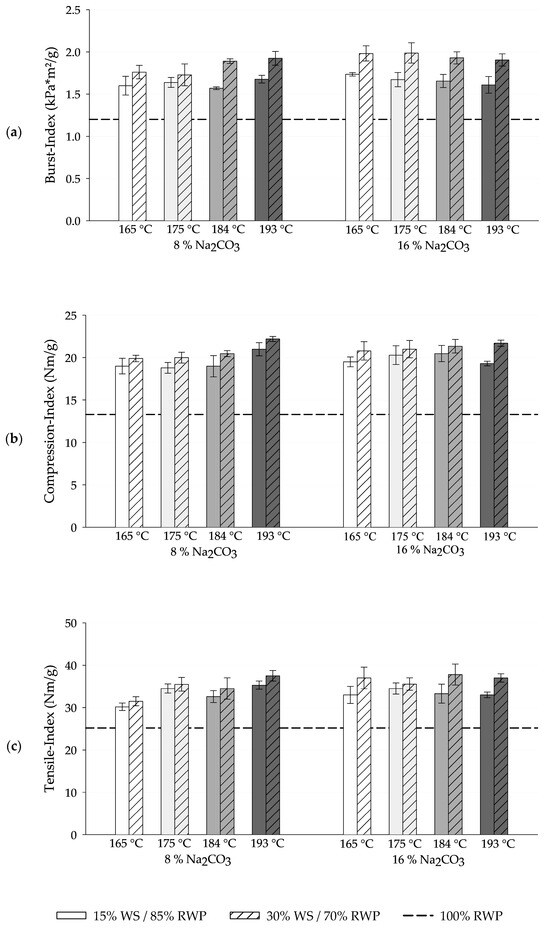
Figure 6.
Influence of treatment temperature and sodium carbonate input on (a) burst-, (b) compression-, and (c) tensile strength of paper test sheets made from blends of wheat straw (WS) and recycled waste paper (RWP) in comparison to pure recycled waste paper samples. Values reported as mean values (n = 4) ± sd.
Paper test sheets made for reference purposes from the pure disintegrated recycled waste paper feature a burst index of 1.2 kPa*m2/g, a compression index of 13.3 Nm/g and a tensile index of 25.2 Nm/g at a beating degree of 18 °SR. Incorporating wheat straw with a beating degree of approximately 45 °SR into the disintegrated recycled waste paper with a beating degree of 18 °SR results in an overall increase in the beating degree of the blend to approximately 25 °SR at 15% share of wheat straw and 30 °SR at 30% share of wheat straw. The incorporation of 15% wheat straw resulted in notable improvements of 30% to 45% in the burst index, 40% to 60% in the compression index, and 20% to 40% in the tensile index when compared to test sheets composed solely of disintegrated recycled waste paper. Depending on the treatment conditions, increasing the wheat straw incorporation of 30% led to even more substantial strength enhancements, with a 45% to 65% increase in the burst index, a 50% to 74% increase in the compression index, and a 25% to 59% increase in the tensile index. Consequently, in all scenarios, the addition of wheat straw resulted in a significant boost in paper strength, with the higher mixing level of 30% wheat straw demonstrating an enhanced reinforcement effect. However, the increase in paper strength from a 15% to a 30% share of wheat straw is considerably smaller compared to the step from pure recycled waste paper to a 15% share of wheat straw, indicating diminishing returns of strength enhancement at higher straw content.
When utilizing straw fibers from treatments with 8% sodium carbonate, the blends exhibit a modest overall increase in paper strength with rising treatment temperatures. Conversely, with 16% sodium carbonate, the tensile and compression indices stagnate at elevated treatment temperatures, and the burst index even experiences a slight decrease. Consequently, the process conditions yielding the highest paper strength in pure wheat straw samples do not necessarily translate to the highest paper strength in the blends.
The achieved strength properties demonstrate that wheat straw can be effectively utilized within the specified ranges with at a tolerable level of raising drainage resistance. Especially the substantial increase in compression strength is promising for the intended use, since an essential aspect of packaging material is its capability to safeguard its contents during transportation, making resistance to compressive forces crucial. Stacking strength, a critical factor for corrugated board boxes, is substantially impacted by the edgewise compression strength. Moreover, there exists a direct correlation between the edgewise compression strength of a corrugated board and the compression strength of its individual components [51]. Therefore, the short span compressive strength of the test sheets serves as a reliable indicator for the compressive strength of corrugated paperboard and the final box products.
In Figure 7a, paper test sheets under varying pulping conditions are presented. With increasing treatment intensity, the paper transitions from a light brown, almost yellowish color to a progressively darker brown shade. Furthermore, increasing the sodium carbonate addition from 8% to 16% results in a reduction of visible fibers on the outer surface, perceptible to the naked eye. As treatment intensity rises, the paper surface becomes smoother, accompanied by a decrease in paper thickness, aligning with the decline in specific volume illustrated in Figure 5d. As depicted in Figure 7b, the incorporation of wheat straw imparts a subtle brown hue to the otherwise grayish recycled waste paper. The slightly diminished brightness and brown hue introduced by the sodium carbonate pulped wheat straw should not be detrimental to the intended use in packaging material. In fact, currently packaging paper producers occasionally add brown coloring to the grayish waste pulp to provide the paper a more natural appearance. Thus, the addition of wheat straw pulp may offer the potential to reduce the required amount of additional coloring.
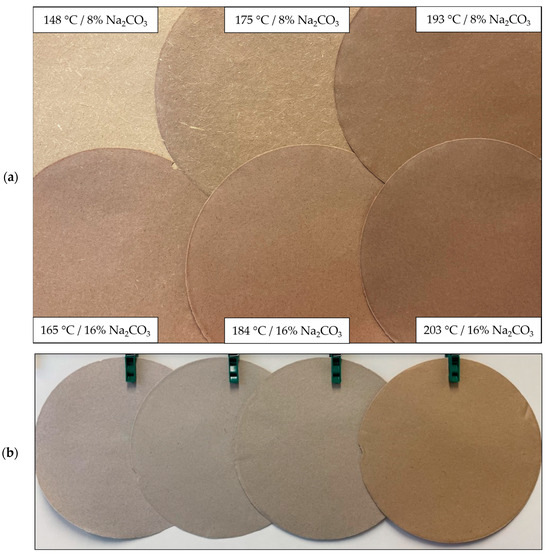
Figure 7.
Visual representation of paper test sheets made out of (a) 100% wheat straw pulped at differing pulping conditions and (b) 100% RWP, 15% WS/85% RWP, 30% WS/70% RWP and 100% of WS (from left to right) using wheat straw pulped at 184 °C and 8% of Na2CO3.
4. Conclusions
With sodium carbonate pulping, it is possible to produce fibers from wheat straw that can be used to reinforce recycled waste paper pulp for packaging applications. By replacing up to 30% recycled waste paper pulp with sodium carbonate pulped wheat straw, an increase of up to 66% in the burst, 74% in the compression strength and 59% of the tensile strength can be achieved, depending on the baseline paper strength of the recycled waste paper.
It was observed that when producing paper from pure wheat straw, a higher sodium carbonate input level resulted in increased paper strength at comparable fiber yields. However, in the blending trials, a higher sodium carbonate level did not significantly enhance paper strength, and in some instances, it even led to reduced paper strength compared to lower sodium carbonate levels. Moreover, diminishing returns were noted when increasing the wheat straw content in the blends. Going from 0% to 15% showed a much more substantial improvement than increasing the share of wheat straw from 15% to 30%. These findings suggest that low-intensity process conditions may be the preferred choice, as they result in minimal polysaccharide degradation and lignin removal, which helps maintain yield at high levels for economic feasibility, while still allowing a significant reinforcing effect when blended in limited quantities into recycled waste paper pulp. However, for a more comprehensive assessment, it is necessary to consider the chemical, raw material, and energy costs. This is because factors such as fiber yield, paper strength, silicate removal rate, energy, and chemical input move in part diametrically opposed to each other and the optimal process conditions will be contingent on the particular production site circumstances and paper requirements.
In future studies, process variants such as using mixtures of sodium hydroxide and sodium carbonate pulping could be examined as former research has shown that this can be beneficial in the sodium carbonate pulping of wheat straw. Additionally, the production of reinforcement fibers from other agricultural residues through sodium carbonate pulping should be explored. This could create alternative valorization pathways, aiding in the preservation of forest resources and promoting a circular economy approach, all while supplying packaging producers with much-needed fresh fibers.
Author Contributions
Conceptualization, S.H. and F.S.; methodology, S.H. and F.S.; validation, S.H.; investigation, S.H.; data curation, S.H.; writing—original draft preparation, S.H. and F.S.; writing—review and editing, S.H. and F.S.; visualization, S.H.; supervision, F.S.; project administration, F.S.; funding acquisition, F.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Fachagentur Nachwachsende Rohstoffe, grant number 22024518.
Data Availability Statement
All data analyzed during this study is included in this published article.
Acknowledgments
Special thanks go to Thorben Bendler and Nicole Erasmy for their technical support in the laboratory.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Berg, P.; Lingqvist, O. Pulp, Paper, and Packaging in the Next Decade: Transformational Change. 2019. Available online: https://www.mckinsey.com/industries/paper-forest-products-and-packaging/our-insights/pulp-paper-and-packaging-in-the-next-decade-transformational-change (accessed on 14 December 2023).
- Moldenhauer, T.; Burkard, A.; Geiger, G. VDP-Leistungsbericht PAPIER 2021. 2021. Available online: https://www.papierindustrie.de/fileadmin/0002-PAPIERINDUSTRIE/07_Dateien/XX-LB/PAPIER2021-digital.pdf (accessed on 14 December 2023).
- Grimes, S.; Donaldson, J.; Grimes, J. Report on the Environmental Benefits of Recycling—2016 Edition; Brussels. 2015. Available online: https://www.bir.org/publications/facts-figures/download/172/174/36?method=view (accessed on 14 December 2023).
- Hubbe, M. Prospects for Maintaining Strength of Paper and Paperboard Products While Using Less Forest Resources: A Review. BioResources 2014, 9, 1634–1763. [Google Scholar] [CrossRef]
- Li, M.; Hu, K.; Shao, S. Tensile strength estimation of paper sheets made from recycled wood and non-wood fibers using machine learning. Cogent Eng. 2023, 10, 2116828. [Google Scholar] [CrossRef]
- Hubbe, M.A.; Venditti, R.A.; Rojas, O.J. What happens to cellulosic fibers during papermaking and recycling? A Review. BioResources 2007, 2, 739–788. [Google Scholar]
- Salehi, K.; Kordsachia, O.; Saake, B. The Potential of Wheat Straw High Yield MEA Pulp for Enhancing Strength Properties of Recycled Paper. BioResources 2017, 12, 8255–8271. [Google Scholar] [CrossRef]
- Puitel, A.C.; Marin, N.; Puiu, P.; Gavrilescu, D. Lignocellulosic Agrcultural Residues—A Virgin Fibre Supply Solution For Paper-Based Packaging. Cellul. Chem. Technol. 2015, 49, 633–639. [Google Scholar]
- O’Hara, I.M. The sugarcane industry, biofuel, and bioproduct perspectives. In Sugarcane-Based Biofuels and Bioproducts; O’Hara, I.M., Mundree, S.G., Eds.; John Wiley & Sons, Inc: Hoboken, NJ, USA, 2016; pp. 1–22. [Google Scholar]
- Chesca, A.; Nicu, R.; Tofanica, B.; Puițel, A.; Vlase, R.; Gavrilescu, D. Pulping of Corn Stalks—Assessment for Bio-based Packaging Materials. Cellul. Chem. Technol. 2018, 52, 645–653. [Google Scholar]
- Krafft, M.J.; Bendler, M.; Schreiber, A.; Saake, B. Steam Refining with Subsequent Alkaline Lignin Extraction as an Alternative Pretreatment Method to Enhance the Enzymatic Digestibility of Corn Stover. Agronomy 2020, 10, 811. [Google Scholar] [CrossRef]
- Van Schoubroeck, S.; Chacon, L.; Reynolds, A.M.; Lavoine, N.; Hakovirta, M.; Gonzalez, R.; Van Passel, S.; Venditti, R.A. Environmental sustainability perception toward obvious recovered waste content in paper-based packaging: An online and in-person survey best-worst scaling experiment. Resour. Conserv. Recycl. 2023, 188, 13. [Google Scholar] [CrossRef]
- Arce Salazar, H.; Oerlemans, L. Do We Follow the Leader or the Masses? Antecedents of the Willingness to Pay Extra for Eco-Products. J. Consum. Aff. 2016, 50, 286–314. [Google Scholar] [CrossRef]
- Kennedy, S.; Linnenluecke, M.K. Circular economy and resilience: A research agenda. Bus. Strategy Environ. 2022, 31, 2754–2765. [Google Scholar] [CrossRef]
- Mayank, G.; Amit, K.G.; Surendra, P.S. Wheat Straw Pulp as Reinforcing Aid for Recycled Softwood Pulp. IPPTA J. 2008, 20, 113–117. [Google Scholar]
- FAO. FAOSTAT Statistical Database; FAO: Rome, Italy, 2023. [Google Scholar]
- Erenstein, O.; Jaleta, M.; Mottaleb, K.A.; Sonder, K.; Donovan, J.; Braun, H.-J. Global trends in Wheat Production, Consumption and Trade. In Wheat Improvement; Reynolds, M.P., Braun, H.-J., Eds.; Springer: Cham, Switzerland, 2022. [Google Scholar]
- Liu, C.; Lu, M.; Cui, J.; Li, B.; Fang, C.M. Effects of straw carbon input on carbon dynamics in agricultural soils: A meta-analysis. Glob. Chang. Biol. 2014, 20, 1366–1381. [Google Scholar] [CrossRef]
- Weiser, C.; Zeller, V.; Reinicke, F.; Wagner, B.; Majer, S.; Vetter, A.; Thraen, D. Integrated assessment of sustainable cereal straw potential and different straw-based energy applications in Germany. Appl. Energy 2014, 114, 749–762. [Google Scholar] [CrossRef]
- Puițel, A.C.; Tofanica, B.M.; Gavrilescu, D.A. Fibrous Raw Materials from Agricultural Residues. In Pulp Production and Processing: High-Tech Applications; Popa, V.I., Ed.; Walter de Gruyter GmbH & Co KG.: Berlin, Germany, 2020; pp. 49–72. [Google Scholar]
- El-Sayed, E.S.A.; El-Sakhaway, M.; El-Sakhawy, M.A.-M. Non-wood fibers as raw material for pulp and paper industry. Nord. Pulp Pap. Res. J. 2020, 35, 215–230. [Google Scholar] [CrossRef]
- Feng, G.; Shen, K. Wheat Straw Pulping for Paper and Paperboard Production. In Global Wheat Production; Fahad, S., Basir, A., Adnan, M., Eds.; IntechOpen: London, UK, 2018; pp. 223–239. [Google Scholar]
- Hart, P. Wheat straw as an alternative pulp fiber. TAPPI J. 2020, 19, 41–52. [Google Scholar] [CrossRef]
- Steffen, F.; Kordsachia, T.; Heizmann, T.; Eckardt, M.P.; Chen, Y.; Saake, B. Sodium Carbonate Pulping of Wheat Straw—An Alternative Fiber Source for Various Paper Applications. Agronomy 2024, 14, 162. [Google Scholar] [CrossRef]
- Salem, K.; Naithani, V.; Jameel, H.; Lucia, L.; Pal, L. Lignocellulosic Fibers from Renewable Resources Using Green Chemistry for a Circular Economy. Glob. Chall. 2020, 5, 2000065. [Google Scholar] [CrossRef]
- Naithani, V.; Tyagi, P.; Jameel, H.; Lucia, L.; Pal, L. Ecofriendly and innovative processing of hemp hurds fibers for tissue and towel paper. BioResources 2020, 15, 706–720. [Google Scholar] [CrossRef]
- Kulkarni, A.G.; Rao, N.R.M.; Mathur, R.M. Pulping of agricultural residues problems and suggestions. IPPTA J. 1983, 20, 55–64. [Google Scholar]
- Kulkarni, A.G.; Mathur, R.M.; Dixit, A.K. Desilication of wheat straw black liquor. In Proceedings of the International Symposium on Wood, Fibre and Pulping Chemistry, Auckland, New Zealand, 16–19 May 2005; Appita: Carlton, Australia, 2005; pp. 615–621. [Google Scholar]
- Aravamuthan, R.; Yayin, I. Application of Response Surface Methodology for the Mixaimization of Concora Crush Resistance of Paperboard. Qual. Eng. 1993, 6, 1–20. [Google Scholar] [CrossRef]
- Marin, N.; Puitel, A.C.; Chesca, A.-M.; Gavrilescu, D. Response Surface Modeling Of Wheat Straw Pulping Using Sodium Carbonate And Sodium Hydroxide Mixtures. Cellul. Chem. Technol. 2017, 51, 745–753. [Google Scholar]
- Lorenz, D.; Erasmy, N.; Akil, Y.; Saake, B. A new method for the quantification of monosaccharides, uronic acids and oligosaccharides in partially hydrolyzed xylans by HPAEC-UV/VIS. Carbohydr. Polym. 2016, 140, 181–187. [Google Scholar] [CrossRef]
- Merck, E. Chemisch-Technische Untersuchungsmethoden—Zellstoff und Papier; Verlag Chemie GmbH: Weinheim, Germany, 1957. [Google Scholar]
- Malik, S.; Rana, V.; Joshi, G.; Gupta, P.K.; Sharma, A. Valorization of Wheat Straw for the Paper Industry: Pre-extraction of Reducing Sugars and Its Effect on Pulping and Papermaking Properties. ACS Omega 2020, 5, 30704–30715. [Google Scholar] [CrossRef]
- Hagel, S.; Saake, B. Fractionation of Waste MDF by Steam Refining. Molecules 2020, 25, 2165. [Google Scholar] [CrossRef]
- Tutus, A.; Eroglu, H. An alternative solution to the silica problem in wheat straw pulping. Appita Technol. Innov. Manuf. Environ. 2004, 57, 214–217. [Google Scholar]
- Fahmy, Y.; Fahmy, T.; Mobarak, F.; El-Sakhawy, M.; Fadl, M.H. Agricultural Residues (Wastes) for Manufacture of Paper, Board, and Miscellaneous Products: Background Overview and Future Prospects. Int. J. ChemTech Res. 2017, 10, 424–448. [Google Scholar] [CrossRef]
- Chen, X.; Li, H.; Sun, S.; Cao, X.; Sun, R. Co-production of oligosaccharides and fermentable sugar from wheat straw by hydrothermal pretreatment combined with alkaline ethanol extraction. Ind. Crops Prod. 2018, 111, 78–85. [Google Scholar] [CrossRef]
- Puiţel, A.C.; Suditu, G.D.; Nechita, M.T.; Gavrilescu, D. Fractionation of Agricultural Waste Biomass by Means of Integradted Biorefinery Concept. Environ. Eng. Manag. J. 2021, 20, 389–396. [Google Scholar] [CrossRef]
- Zhang, L.; Larsson, A.; Moldin, A.; Edlund, U. Comparison of lignin distribution, structure, and morphology in wheat straw and wood. Ind. Crops Prod. 2022, 187, 115432. [Google Scholar] [CrossRef]
- Scalbert, A.; Monties, B. Comparison of Wheat Straw Lignin Preparations II. Straw Lignin Solubilisation in Alkali. Holzforschung 1986, 40, 249–254. [Google Scholar] [CrossRef]
- Budtova, T.; Navard, P. Cellulose in NaOH–water based solvents: A review. Cellulose 2016, 23, 5–55. [Google Scholar] [CrossRef]
- Zikeli, F.; Ters, T.; Feckler, K.; Srebotnik, E.; Li, J. Wheat straw lignin fractionation and characterization as lignin-carbohydrate complexes. Ind. Crops Prod. 2016, 85, 309–317. [Google Scholar] [CrossRef]
- Sato, H.; Hirata, F. Theoretical Study for Autoionization of Liquid Water: Temperature Dependence of the Ionic Product (pKw). J. Phys. Chem. A 1998, 102, 2603–2608. [Google Scholar] [CrossRef]
- Bassani, A.; Fiorentini, C.; Vadivel, V.; Moncalvo, A.; Spigno, G. Implementation of Auto-Hydrolysis Process for the Recovery of Antioxidants and Cellulose from Wheat Straw. Appl. Sci. 2020, 10, 6112. [Google Scholar] [CrossRef]
- Pekarovic, J.; Pekarovicova, A.; Joyce, T. Desilication of agricultural residues—The first step prior to pulping. Appita J. 2005, 58, 130–134. [Google Scholar]
- Pekarovic, J.; Pekarovica, A.; Fleming, P.D., III. Two-step straw processing—A new concept of silica problem solution. In Proceedings of the 2006 Engineering, Pulping and Environmental Conference, Atlanta, GA, USA, 5–8 November 2006. [Google Scholar]
- Puițel, A.C.; Balan, C.D.; Ailiesei, G.-L.; Drăgoi, E.N.; Nechita, M.T. Integrated Hemicellulose Extraction and Papermaking Fiber Production from Agro-Waste Biomass. Polymers 2023, 15, 4597. [Google Scholar] [CrossRef]
- Puitel, A.C.; Suditu, G.D.; Danu, M.; Ailiesei, G.-L.; Nechita, M.T. An Experimental Study on the Hot Alkali Extraction of Xylan-Based Hemicelluloses from Wheat Straw and Corn Stalks and Optimization Methods. Polymers 2022, 14, 1662. [Google Scholar] [CrossRef]
- Hagel, S.; Joy, J.; Cicala, G.; Saake, B. Recycling of Waste MDF by Steam Refining: Evaluation of Fiber and Paper Strength Properties. Waste Biomass Valorization 2021, 12, 5701–5713. [Google Scholar] [CrossRef]
- Zambrano, F.; Marquez, R.; Jameel, H.; Vendititti, R.; Gonzales, R. Upcycling strategies for old corrugated containerboard to attain high-performance tissue paper: A viable answer to the packaging waste generation dilemma. Resour. Conserv. Recycl. 2021, 175, 105854. [Google Scholar] [CrossRef]
- Dimitrov, K.; Heydenrych, M. Relationship between the edgewise compression strength of corrugated board and the compression strength of liner and fluting medium papers. South. For. 2009, 71, 227–233. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).