Exploration of In Vitro Voltage Production by a Consortium of Chemolithotrophic Microorganisms Using Galena (PbS) as a Sulphur Source
Abstract
1. Introduction
2. Materials and Methods
2.1. Growing Medium
2.2. Isolation
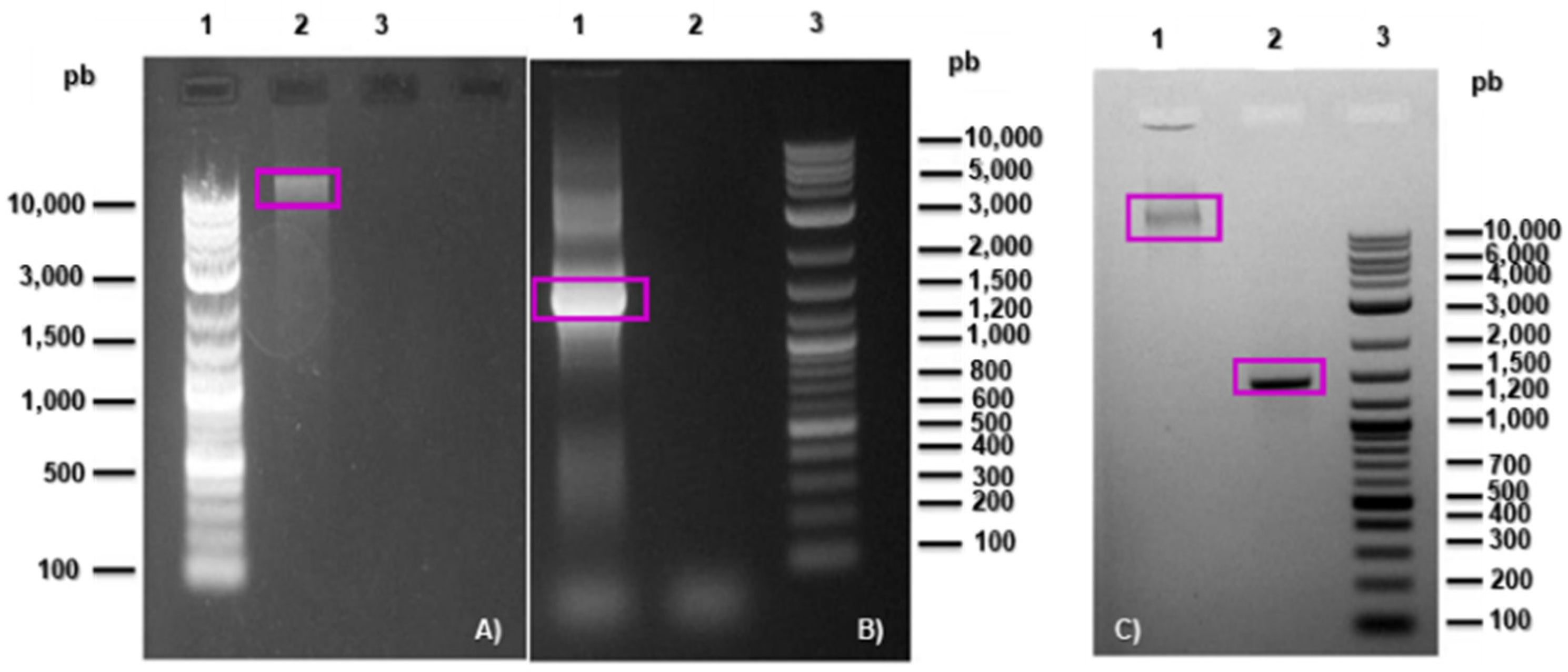
2.3. Microorganism Identification
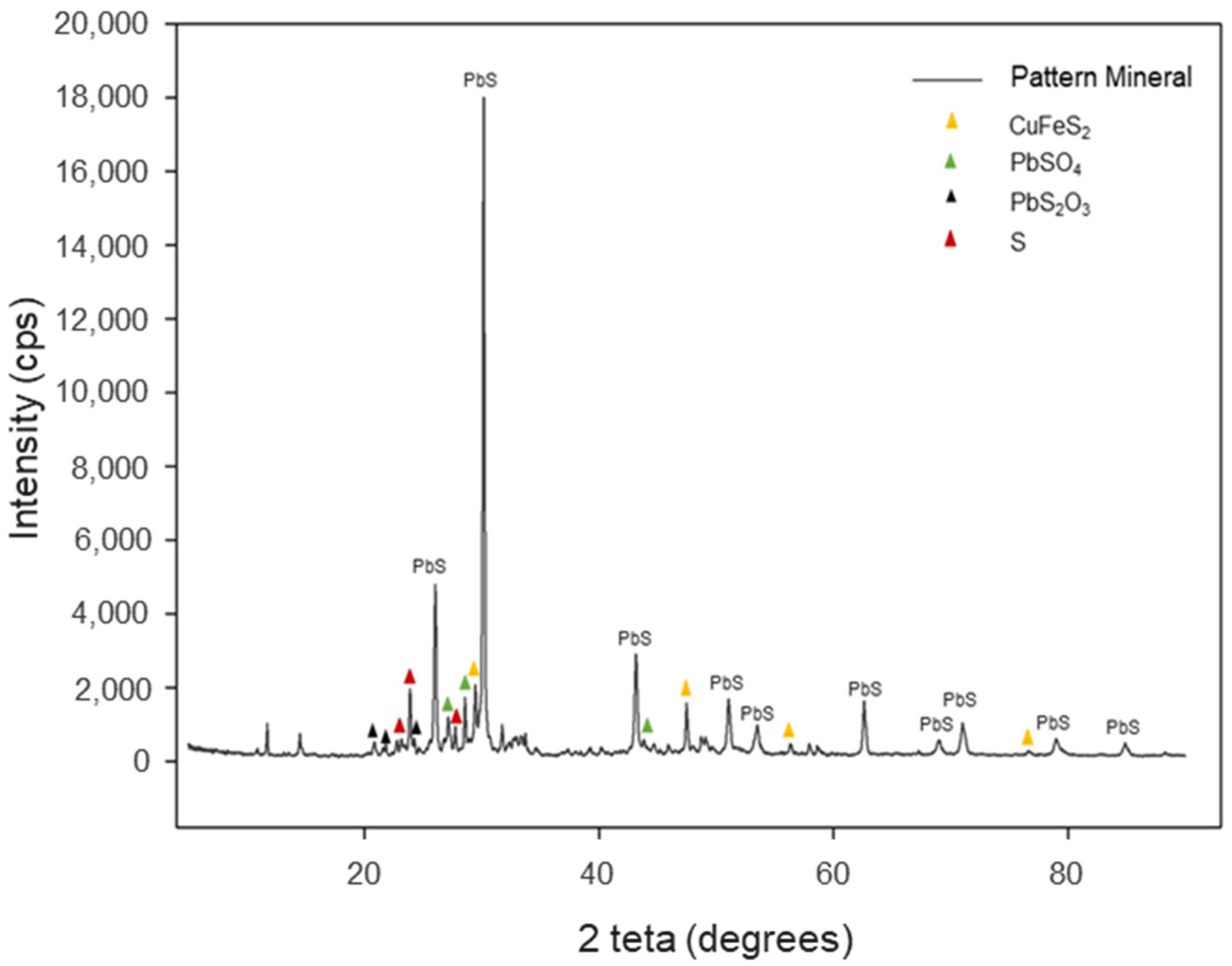
2.4. Determination of Inorganic Mineral Sulphur Species
2.5. Salt Bridge
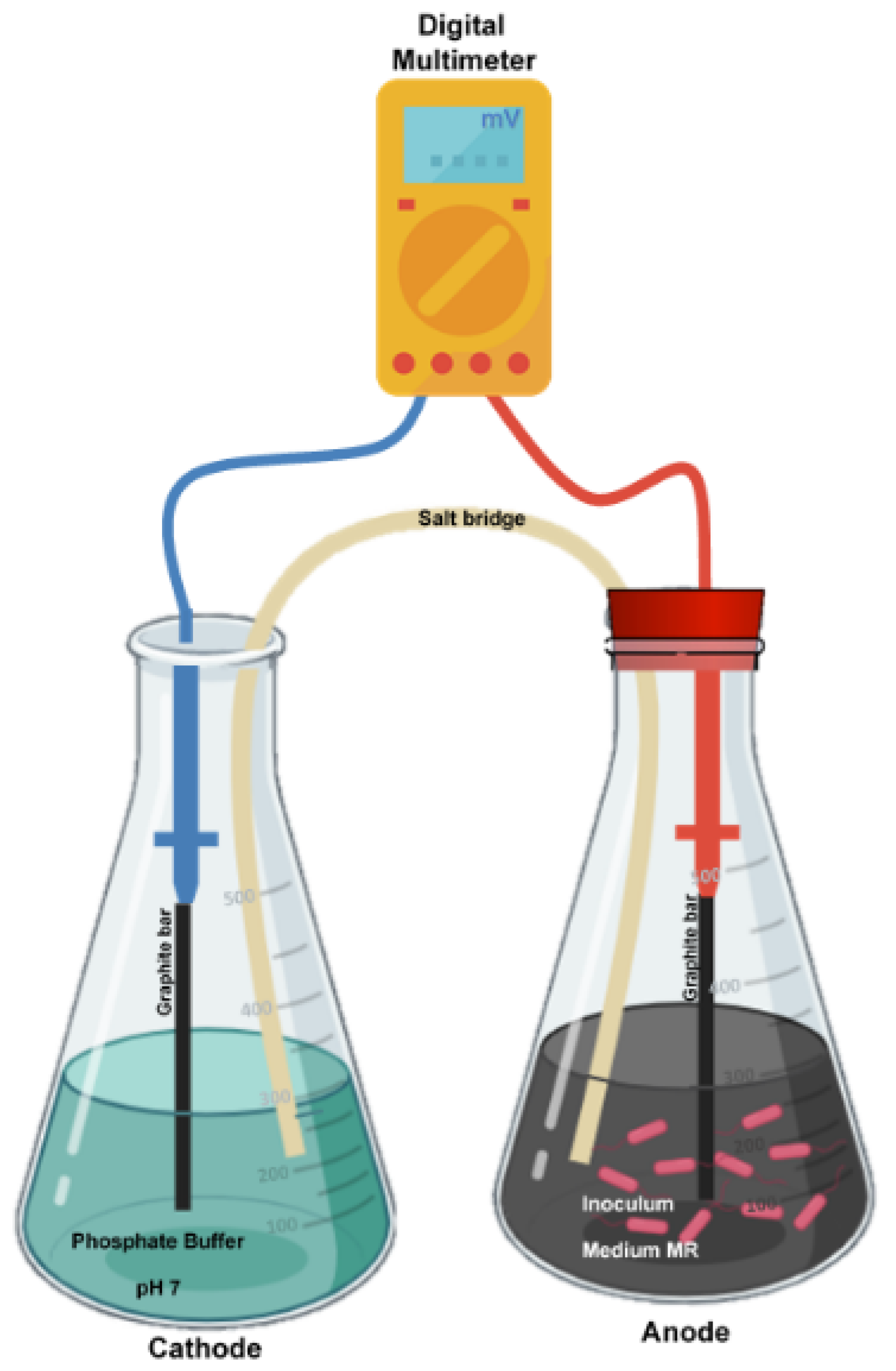
2.6. Cathode
2.7. Anode
2.8. Experimental Design
2.9. Voltage Measurements
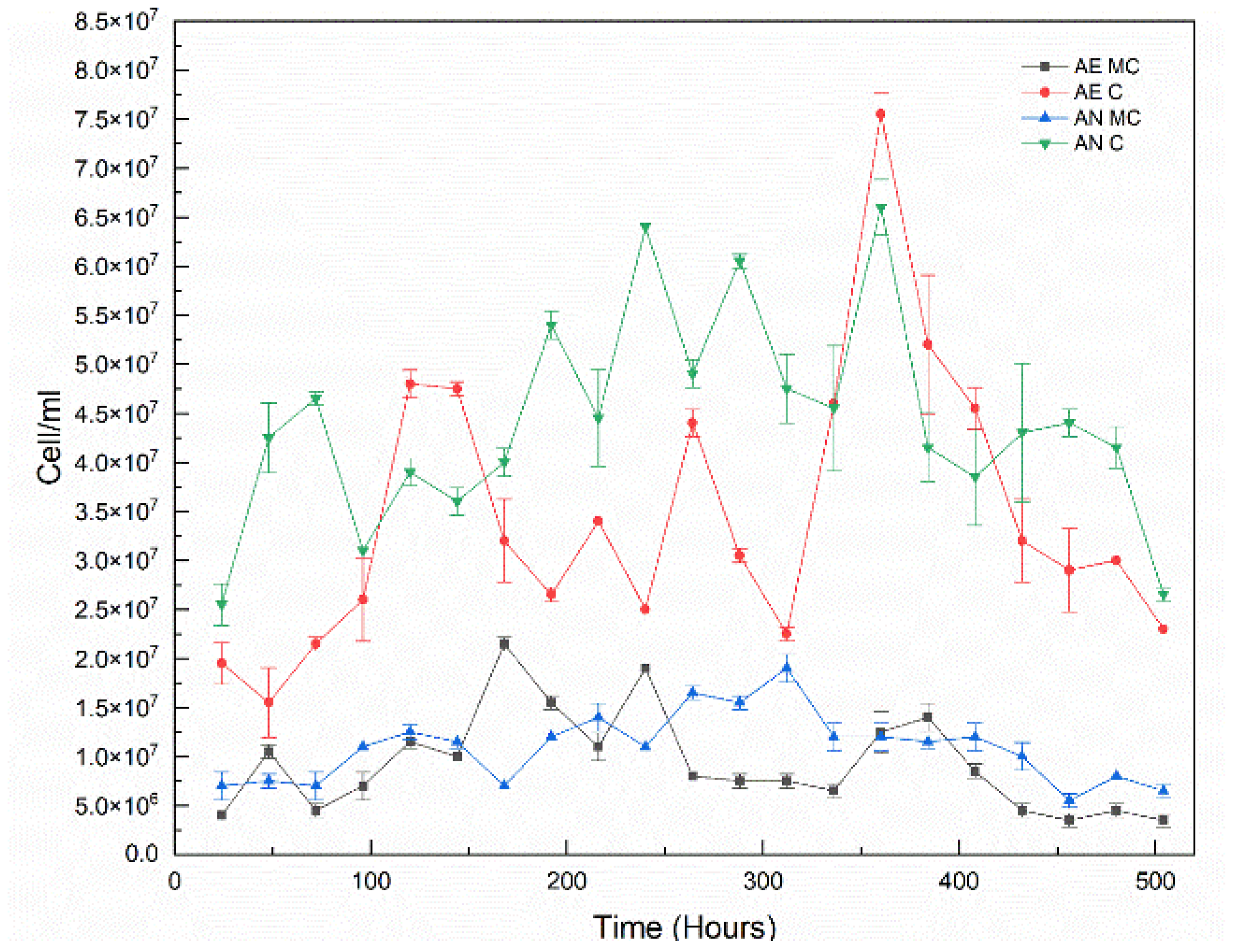
2.10. Anode Cell Density
3. Results and Discussion
3.1. Isolation and Identification
3.2. Characterization of Inorganic Mineral Substrate
3.3. Cell Growth
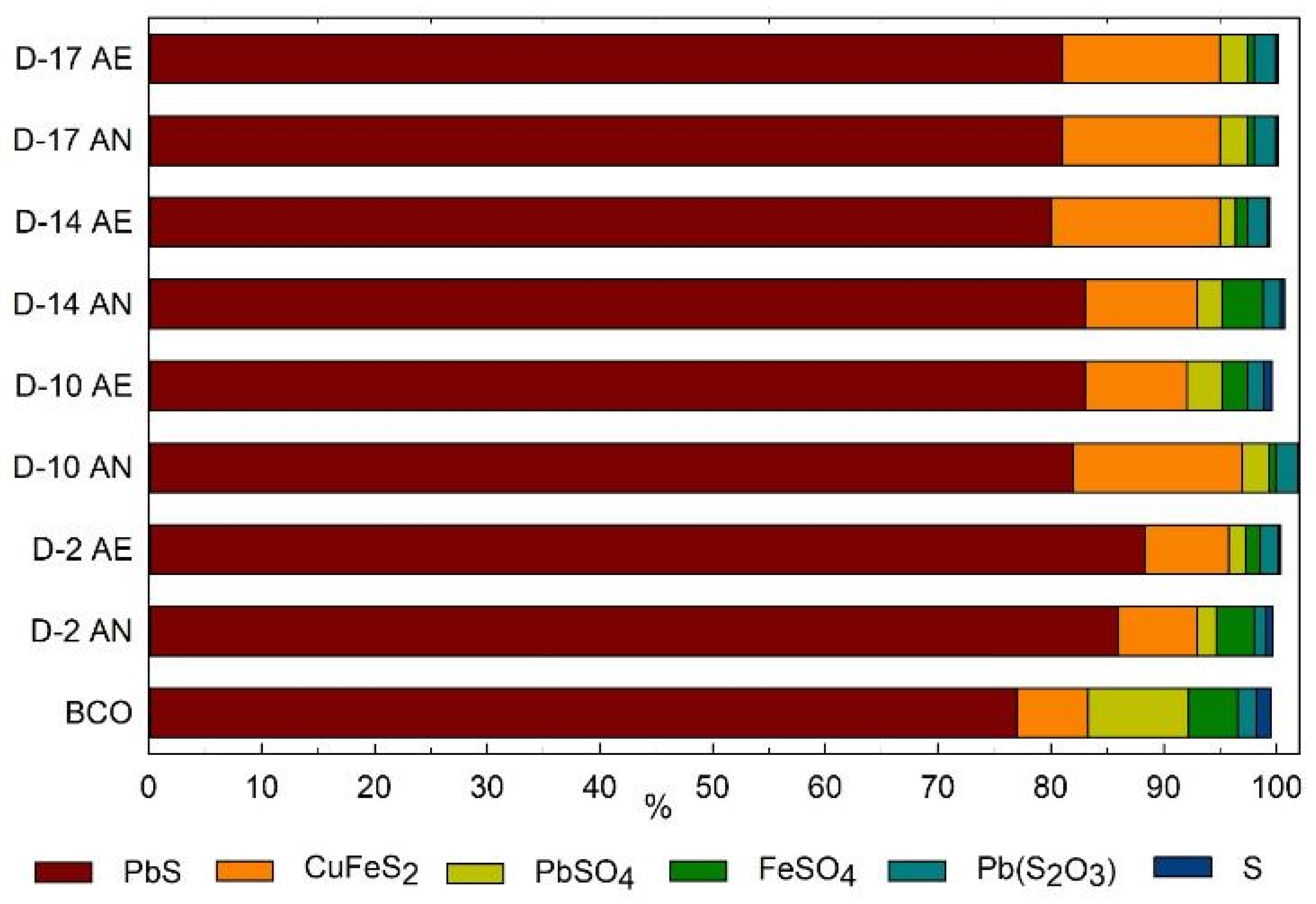
3.4. Use of Inorganic Sulphur as a Substrate
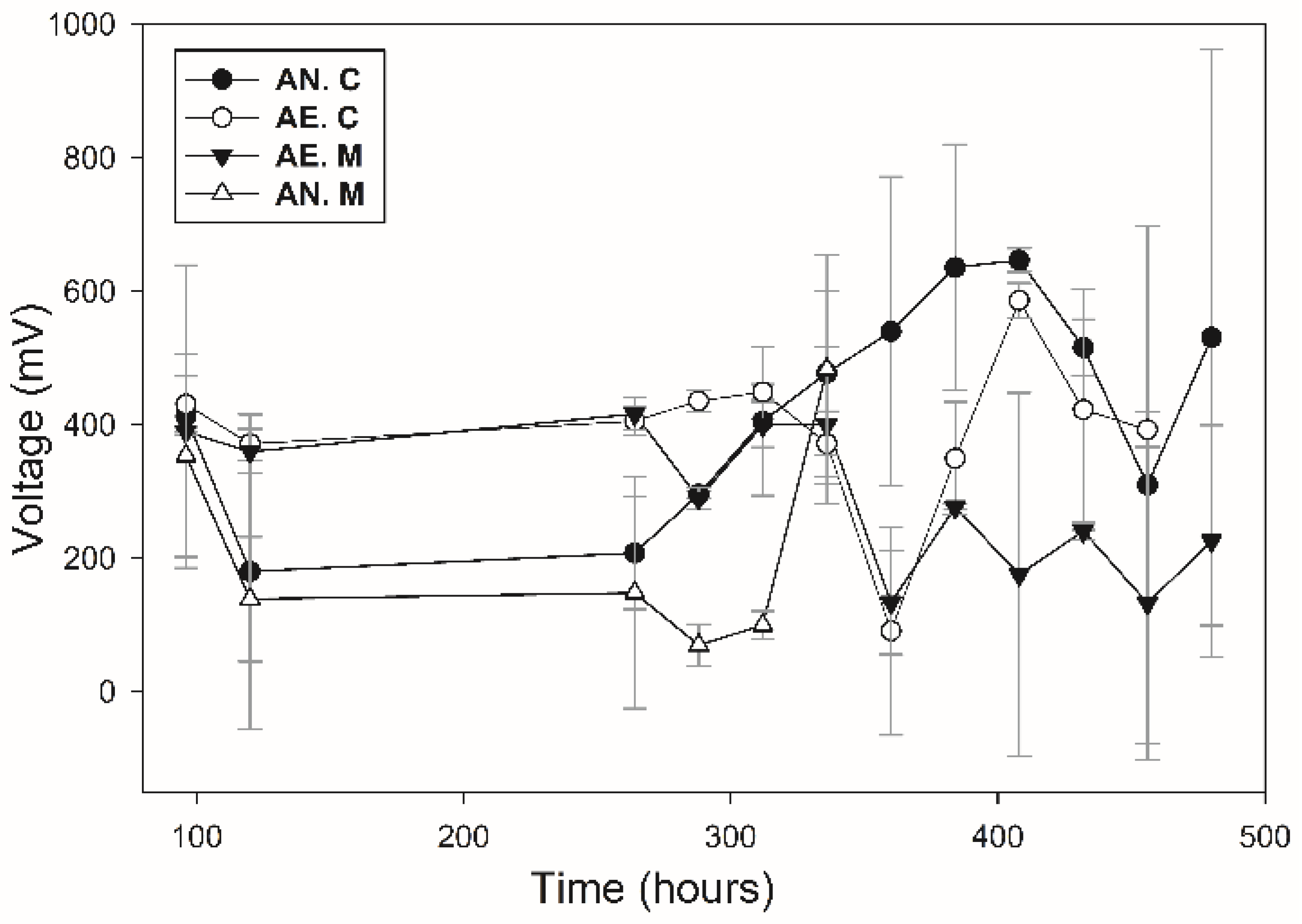
3.5. Voltage Production
| System Description | Substrate | Voltage Produced (mV) | Inoculum | References |
|---|---|---|---|---|
| Batch | PbS | 664 | Present Work | |
| Batch + salt bridge | Acetate + Fe+3 | 220–380 | Iron-reducing consortium | González-Paz et al. (2022) [52] |
| Two chambered laboratory scale microbial fuel cell | Zeolite + MgSO4 | 750 | Natural sulphate-reducing bacterium consortium | Angelov et al. (2013) [60] |
| Cylindrical SMFCs | Sulphate-rich sediments | 30–40 | L. varians GY32 | Huang et al. (2023) [53] |
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Matin, A. Organic nutrition of chemolithotrophic bacteria. Annu. Rev. Microbiol. 1978, 32, 433–468. [Google Scholar] [CrossRef] [PubMed]
- Kumar, R.; Pundir, S. Bacterial Cell, Classification and Required Essential Contents for Growth. Asian J. Pharm. Technol. 2021, 11, 181–187. [Google Scholar] [CrossRef]
- Dahl, C. A biochemical view on the biological sulphur cycle. In Environmental Technologies to Treat Sulphur Pollution: Principles and Engineering, 2nd ed.; Lens, P.N.L., Ed.; IWA Publishing: London, UK, 2020. [Google Scholar]
- Sulonen, M.L.K.; Lakaniemi, A.M.; Kokko, M.E.; Puhakka, J.A. The effect of anode potential on bioelectrochemical and electrochemical tetrathionate degradation. Bioresour. Technol. 2017, 226, 173–180. [Google Scholar] [CrossRef] [PubMed]
- Sulonen, M.L.K.; Lakaniemi, A.M.; Kokko, M.E.; Puhakka, J.A. Long-term stability of bioelectricity generation coupled with tetrathionate disproportionation. Bioresour. Technol. 2016, 216, 876–882. [Google Scholar] [CrossRef] [PubMed]
- Meulenberg, R.; Scheer, E.J.; Pronk, J.T.; Hazeu, W.; Bos, P.; Kuenen, J.G. Metabolism of tetrathionate in Thiobacillus acidophilus. FEMS Microbiol. Lett. 1993, 112, 167–172. [Google Scholar] [CrossRef]
- Sulonen, M.L.K.; Kokko, M.E.; Lakaniemi, A.M.; Puhakka, J.A. Electricity generation from tetrathionate in microbial fuel cells by acidophiles. J. Hazard. Mater. 2014, 284, 182–189. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Lin, J.Q.; Liu, X.M.; Pang, X.; Zhang, C.J.; Yang, C.L.; Gao, X.-Y.; Lin, C.-M.; Li, Y.-Q.; Li, Y.; et al. Sulphur oxidation in the acidophilic autotrophic Acidithiobacillus spp. Front. Microbiol. 2019, 10, 3290. [Google Scholar]
- Alam, M.; Fernandes, S.; Mandal, S.; Rameez, M.J.; Bhattacharya, S.; Peketi, A.; Mazumdar, A.; Ghosh, W. 34S enrichment as a signature of thiosulfate oxidation in the ‘Proteobacteria’. FEMS Microbiol. Lett. 2021, 368, fnab073. [Google Scholar] [CrossRef]
- Doyle, L.E.; Marsili, E. Weak electricigens: A new avenue for bioelectrochemical research. Bioresour. Technol. 2018, 258, 354–364. [Google Scholar] [CrossRef]
- Wing, B.A.; Halevy, I. Intracellular metabolite levels shape sulphur isotope fractionation during microbial sulfate respiration. Proc. Natl. Acad. Sci. USA 2014, 111, 18116–18125. [Google Scholar] [CrossRef]
- Chabert, N.; Bonnefoy, V.; Achouak, W. Quorum sensing improves current output with Acidithiobacillus ferrooxidans. Microb. Biotechnol. 2018, 11, 136–140. [Google Scholar] [CrossRef]
- Ohmura, N.; Sasaki, K.; Matsumoto, N.; Saiki, H. Anaerobic Respiration Using Fe3+, S0, and H2 in the Chemolithoautotrophic Bacterium Acidithiobacillus ferrooxidans. J. Bacteriol. 2002, 184, 2081–2087. [Google Scholar] [CrossRef] [PubMed]
- González, A.; Bellenberg, S.; Mamani, S.; Ruiz, L.; Echeverría, A.; Soulère, L.; Doutheau, A.; Demergasso, C.; Sand, W.; Queneau, Y.; et al. AHL signalling molecules with a large acyl chain enhance biofilm formation on sulphur and metal sulfides by the bioleaching bacterium Acidithiobacillus ferrooxidans. Appl. Microbiol. Biotechnol. 2013, 97, 3729–3737. [Google Scholar] [CrossRef] [PubMed]
- Keller, K.L.; Wall, J.D. Genetics and molecular biology of the electron flow for sulfate respiration in Desulfovibrio. Front. Microbiol. 2011, 2, 135. [Google Scholar] [CrossRef] [PubMed]
- Koch, T.; Dahl, C. A novel bacterial sulphur oxidation pathway provides a new link between the cycles of organic and inorganic sulphur compounds. ISME J. 2018, 12, 2479–2491. [Google Scholar] [CrossRef] [PubMed]
- Boon, M.; Snijder, M.; Hansford, G.S.; Heijnen, J.J. The oxidation kinetics of zinc sulphide with Thiobacillus ferrooxidans. Hydrometallurgy 1998, 48, 171–186. [Google Scholar] [CrossRef]
- Baba, A.A.; Adekola, F.A.; Atata, R.F.; Ahmed, R.N.; Panda, S. Bioleaching of Zn(II) and Pb(II) from Nigerian sphalerite and galena ores by mixed culture of acidophilic bacteria. Trans. Nonferrous Met. Soc. China (Engl. Ed.) 2011, 21, 2535–2541. [Google Scholar] [CrossRef]
- Saratale, G.D.; Saratale, R.G.; Shahid, M.K.; Zhen, G.; Kumar, G.; Shin, H.-S.; Choi, Y.-G.; Kim, S.-H. A comprehensive overview on electro-active biofilms, role of exo-electrogens and their microbial niches in microbial fuel cells (MFCs). Chemosphere 2017, 178, 534–547. [Google Scholar] [CrossRef]
- Selim, H.M.M.; Kamal, A.M.; Ali, D.M.M.; Hassan, R.Y.A. Bioelectrochemical Systems for Measuring Microbial Cellular Functions. Electroanalysis 2017, 29, 1498–1505. [Google Scholar] [CrossRef]
- Visser, P.M.; Verspagen, J.M.; Sandrini, G.; Stal, L.J.; Matthijs, H.C.; Davis, T.W.; Paerl, H.W.; Huisman, J. How rising CO2 and global warming may stimulate harmful cyanobacterial blooms. Harmful Algae 2016, 54, 145–159. [Google Scholar] [CrossRef]
- Ramaraj, R.; Tsai, D.D.W.; Chen, P.H. Carbon dioxide fixation of freshwater microalgae growth on natural water medium. Ecol. Eng. 2015, 75, 86–92. [Google Scholar] [CrossRef]
- Singh, S.K.; Rahman, A.; Dixit, K.; Nath, A.; Sundaram, S. Evaluation of promising algal strains for sustainable exploitation coupled with CO2 fixation. Environ. Technol. 2016, 37, 613–622. [Google Scholar] [CrossRef] [PubMed]
- Rossi, F.; Olguín, E.J.; Diels, L.; De Philippis, R. Microbial fixation of CO2 in water bodies and in drylands to combat climate change, soil loss and desertification. New Biotechnol. 2015, 32, 109–120. [Google Scholar] [CrossRef] [PubMed]
- Lovley, D.R. The microbe electric: Conversion of organic matter to electricity. Curr. Opin. Biotechnol. 2008, 19, 564–571. [Google Scholar] [CrossRef] [PubMed]
- Ge, X.; Cao, X.; Song, X.; Wang, Y.; Si, Z.; Zhao, Y.; Wang, W.; Tesfahunegn, A.A. Bioenergy generation and simultaneous nitrate and phosphorus removal in a pyrite-based constructed wetland-microbial fuel cell. Bioresour. Technol. 2020, 296, 122350. [Google Scholar] [CrossRef]
- Eaktasang, N.; Kang, C.S.; Lim, H.; Kwean, O.S.; Cho, S.; Kim, Y.; Kim, H.S. Production of electrically-conductive nanoscale filaments by sulfate-reducing bacteria in the microbial fuel cell. Bioresour. Technol. 2016, 210, 61–67. [Google Scholar] [CrossRef] [PubMed]
- Du, Y.; Feng, Y.; Teng, Q.; Li, H. Effect of inorganic salt in the culture on microbial fuel cells performance. Int. J. Electrochem. Sci. 2015, 10, 1316–1325. [Google Scholar] [CrossRef]
- Ni, G.; Christel, S.; Roman, P.; Wong, Z.L.; Bijmans, M.F.M.; Dopson, M. Electricity generation from an inorganic sulphur compound containing mining wastewater by acidophilic microorganisms. Res. Microbiol. 2016, 167, 568–575. [Google Scholar] [CrossRef]
- AGreene, C.; Patel, B.K.C.; Sheehy, A.J. Deferribacter thermophilus gen. nov., a Novel Thermophilic Manganese and Iron Reducing Bacterium Isolated from a Petroleum Reservoir. Strain 1997, 47, 505–509. [Google Scholar]
- Nandhana, G.; Rajasulochana, P. Genome Sequence Analysis of the Bacillus Cereus Isolated From Soil Sample. Ann. Rom. Soc. Cell Biol. 2021, 25, 2625–2636. [Google Scholar]
- Hubbard, C.R.; Snyder, R.L. RIR—Measurement and Use in Quantitative XRD. Powder Diffr. 1988, 3, 74–77. [Google Scholar] [CrossRef]
- Mirahati, R.Z.; Amalia, Y.; Juliyanto, M.; Larasati, L.; Putri, A. Mineral Preparation Using Rod Mill for Mineral Galena Characterization Silika Galena Pirit. RSF Conf. Ser. Eng. Technol. 2021, 1, 355–362. [Google Scholar]
- Rice, E.W.; Bridgewater, L.; American Public Health Association (Eds.) Standard Methods for the Examination of Water and Wastewater; American Public Health Association: Washington, DC, USA, 2012; Volume 10, p. 541. [Google Scholar]
- Deval, A.; Dikshit, A.K. Construction, Working and Standardization of Microbial Fuel Cell. APCBEE Procedia 2013, 5, 59–63. [Google Scholar] [CrossRef]
- Min, B.; Cheng, S.; Logan, B.E. Electricity generation using membrane and salt bridge microbial fuel cells. Water Res. 2005, 39, 1675–1686. [Google Scholar] [CrossRef]
- Trevors, J.T. Sterilization and inhibition of microbial activity in soil. J. Microbiol. Methods 1996, 26, 53–59. [Google Scholar] [CrossRef]
- Liberman, L.; Kleinerman, O.; Davidovich, I.; Talmon, Y. Micrograph contrast in low-voltage SEM and cryo-SEM. Ultramicroscopy 2020, 218, 113085. [Google Scholar] [CrossRef] [PubMed]
- Rueden, C.T.; Schindelin, J.; Hiner, M.C.; Dezonia, B.E.; Walter, A.E.; Arena, E.T.; Eliceiri, K.W. ImageJ2: ImageJ for the next generation of scientific image data. BMC Bioinform. 2017, 18, 529. [Google Scholar] [CrossRef]
- Nichele, L.; Persichetti, V.; Lucidi, M.; Cincotti, G. Quantitative evaluation of ImageJ thresholding algorithms for microbial cell counting. OSA Contin. 2020, 3, 1417. [Google Scholar] [CrossRef]
- Zeng, L.; Huang, J.; Zhang, Y.; Qiu, G.; Tong, J.; Chen, D.; Zhou, J.; Luo, X. An effective method of DNA extraction for bioleaching bacteria from acid mine drainage. Appl. Microbiol. Biotechnol. 2008, 79, 881–888. [Google Scholar] [CrossRef]
- Obata, F.; Murota, H.; Shibata, S.; Ozuru, R.; Fujii, J. Investigation of Bacteria from Spoiled Bottled Salad Dressing Leading to Gas Explosion. Yonago Acta Med. 2022, 65, 207–214. [Google Scholar] [CrossRef]
- Kumar, C.G.; Joo, H.S.; Koo, Y.M.; Paik, S.R.; Chang, C.S. Thermostable alkaline protease from a novel marine haloalkalophilic Bacillus clausii isolate. World J. Microbiol. Biotechnol. 2004, 20, 351–357. [Google Scholar] [CrossRef]
- Ghosh, A.; Bhardwaj, M.; Satyanarayana, T.; Khurana, M.; Mayilraj, S.; Jain, R.K. Bacillus lehensis sp. nov., an alkalitolerant bacterium isolated from soil. Int. J. Syst. Evol. Microbiol. 2007, 57, 238–242. [Google Scholar] [CrossRef] [PubMed]
- Aslan, C.; Aulia, N.I.; Devianto, H.; Harimawan, A. Influence of axenic culture of Bacillus clausii and mixed culture on biofilm formation, carbon steel corrosion, and methyl ester degradation in B30 storage tank system. J. Environ. Chem. Eng. 2022, 10, 108013. [Google Scholar] [CrossRef]
- Ng, D.H.P.; Kumar, A.; Cao, B. Microorganisms meet solid minerals: Interactions and biotechnological applications. Appl. Microbiol. Biotechnol. 2016, 100, 6935–6946. [Google Scholar] [CrossRef] [PubMed]
- Ye, J.; Joseph, S.D.; Ji, M.; Nielsen, S.; Mitchell, D.R.G.; Donne, S.; Horvat, J.; Wang, J.; Munroe, P.; Thomas, T. Chemolithotrophic processes in the bacterial communities on the surface of mineral-enriched biochars. ISME J. 2017, 11, 1087–1101. [Google Scholar] [CrossRef]
- Gupta, D.; Guzman, M.S.; Bose, A. Extracellular electron uptake by autotrophic microbes: Physiological, ecological, and evolutionary implications. J. Ind. Microbiol. Biotechnol. 2020, 47, 863–876. [Google Scholar] [CrossRef]
- Cullity, B.D.; Stock, S.R. Elements of X-ray Diffraction, 3rd ed.; Pearson Prentice Hall: Old Bridge, NJ, USA, 2014; Volume 1. [Google Scholar]
- Zhao, H.; Zhang, Y.; Zhang, X.; Qian, L.; Sun, M.; Yang, Y.; Zhang, Y.; Wang, J.; Kim, H.; Qiu, G. The dissolution and passivation mechanism of chalcopyrite in bioleaching: An overview. Miner. Eng. 2019, 136, 140–154. [Google Scholar] [CrossRef]
- Zhong, H.; Fujii, K.; Nakano, Y.; Jin, F. Effect of CO2 bubbling into aqueous solutions used for electrochemical reduction of CO2 for energy conversion and storage. J. Phys. Chem. C 2015, 119, 55–61. [Google Scholar] [CrossRef]
- Arndt, C.; Gaill, F.; Felbeck, H. Anaerobic sulphur metabolism in thiotrophic symbioses. J. Exp. Biol. 2001, 204, 741–750. [Google Scholar] [CrossRef]
- Singh, R.; Chaudhary, S.; Yadav, S.; Patil, S.A. Bioelectrocatalytic sulfide oxidation by a haloalkaliphilic electroactive microbial community dominated by Desulfobulbaceae. Electrochim. Acta 2022, 423, 140576. [Google Scholar] [CrossRef]
- González-Paz, J.R.; Becerril-Varela, K.; Guerrero-Barajas, C. Iron reducing sludge as a source of electroactive bacteria: Assessing iron reduction in biofilm bacteria, planktonic cells and isolates from a microbial fuel cell. Arch. Microbiol. 2022, 204, 632. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.; Yang, Y.; Yang, S.; Yang, X.; Huang, Y.; Dong, M.; Zhou, S.; Xu, M. Filamentous electroactive microorganisms promote mass transfer and sulfate reduction in sediment microbial electrochemical systems. Chem. Eng. J. 2023, 466, 143214. [Google Scholar] [CrossRef]
- Kuznetsova, L.S.; Arlyapov, V.A.; Plekhanova, Y.A.; Tarasov, S.E.; Kharkova, A.S.; Saverina, E.A.; Reshetilov, A.N. Conductive Polymers and Their Nanocomposites: Application Features in Biosensors and Biofuel Cells. Polymers 2023, 15, 3783. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Y.; Zhou, S.; Xu, N.; Zhuang, L. Electrochemical characterization of anodic biofilms enriched with glucose and acetate in single-chamber microbial fuel cells. Colloids Surf. B Biointerfaces 2011, 82, 641–646. [Google Scholar] [CrossRef]
- Su, W.; Zhang, L.; Tao, Y.; Zhan, G.; Li, D.; Li, D. Sulfate reduction with electrons directly derived from electrodes in bioelectrochemical systems. Electrochem. Commun. 2012, 22, 37–40. [Google Scholar] [CrossRef]
- Shields, P.; Cathcart, L. Oxidase Test Protocol–Library. Am. Soc. Microbiol. ASM MicrobeLibrary 2013, 1–5. Available online: http://www.microbelibrary.org/library/laboratory-test/3229-oxidase-test-protocol. (accessed on 21 December 2023).
- Angelov, A.; Bratkova, S.; Loukanov, A. Microbial fuel cell based on electroactive sulfate-reducing biofilm. Energy Convers. Manag. 2013, 67, 283–286. [Google Scholar] [CrossRef]


| Factors | Categorical Levels | |
|---|---|---|
| Substrate | Culture medium MR (MC) | MR+ inorganic mineral (C) |
| Gas fraction | Atmospheric (Atm) | CO2 (AN) |
| Compound | Formula | Concentration (%) |
|---|---|---|
| Galena | PbS | 77 |
| Sulphur species of lead | PbS | 14.5 |
| Sulphur | S | 5 |
| Others | -- | 19.5 |
| Condition | Substrate | H2S (ppm) | SO4−2 (ppm) | PbS (%) | Voltage (mV) | |
|---|---|---|---|---|---|---|
| Ti | TF | |||||
| AE | C | 479 | 32.22 ±1.71 | 86 | 81 | 586 ± 26.1 |
| AE | MC | 35.15 | 10.90 ± 0.28 | * | * | 438 ± 29.6 |
| AN | MC | 171.5 | 10.00 ± 1.57 | * | * | 383.5 ± 57.2 |
| AN | C | 1150.0 | 1344.44 ± 116.27 | 88 | 80 | 647 ± 17.6 |
| Condition | Substrate | H2S (ppm) | SO4−2 (ppm) | Voltage (mV) |
|---|---|---|---|---|
| AE | C | 0 | 20 | 48.1 |
| AE | MC | 0 | 10 | 42.3 |
| AN | MC | 0 | 10 | 40.2 |
| AN | C | 0 | 260 | 27 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gaucin Gutiérrez, S.C.; Rojas-Contreras, J.A.; Zazueta-Álvarez, D.E.; Delgado, E.; Vázquez Ortega, P.G.; Medrano Roldán, H.; Reyes Jáquez, D. Exploration of In Vitro Voltage Production by a Consortium of Chemolithotrophic Microorganisms Using Galena (PbS) as a Sulphur Source. Clean Technol. 2024, 6, 62-76. https://doi.org/10.3390/cleantechnol6010005
Gaucin Gutiérrez SC, Rojas-Contreras JA, Zazueta-Álvarez DE, Delgado E, Vázquez Ortega PG, Medrano Roldán H, Reyes Jáquez D. Exploration of In Vitro Voltage Production by a Consortium of Chemolithotrophic Microorganisms Using Galena (PbS) as a Sulphur Source. Clean Technologies. 2024; 6(1):62-76. https://doi.org/10.3390/cleantechnol6010005
Chicago/Turabian StyleGaucin Gutiérrez, Susana Citlaly, Juan Antonio Rojas-Contreras, David Enrique Zazueta-Álvarez, Efren Delgado, Perla Guadalupe Vázquez Ortega, Hiram Medrano Roldán, and Damián Reyes Jáquez. 2024. "Exploration of In Vitro Voltage Production by a Consortium of Chemolithotrophic Microorganisms Using Galena (PbS) as a Sulphur Source" Clean Technologies 6, no. 1: 62-76. https://doi.org/10.3390/cleantechnol6010005
APA StyleGaucin Gutiérrez, S. C., Rojas-Contreras, J. A., Zazueta-Álvarez, D. E., Delgado, E., Vázquez Ortega, P. G., Medrano Roldán, H., & Reyes Jáquez, D. (2024). Exploration of In Vitro Voltage Production by a Consortium of Chemolithotrophic Microorganisms Using Galena (PbS) as a Sulphur Source. Clean Technologies, 6(1), 62-76. https://doi.org/10.3390/cleantechnol6010005









