Abstract
Superhydrophobic materials, known for their exceptional water-repellent properties, have found widespread applications in diverse fields such as self-cleaning surfaces, anti-icing coatings, and water-resistant textiles. In recent years, researchers have explored a sustainable approach by repurposing waste materials to create superhydrophobic surfaces. This eco-friendly approach not only reduces environmental impact but also aligns with circular economy principles, contributing to a more sustainable future. Creating superhydrophobic materials from waste involves a combination of surface modification techniques and hierarchical structuring, with rigorous characterization to ensure the desired properties. These materials showcase their potential in various industries, opening doors to more environmentally friendly technologies. This review delves into the concept of superhydrophobic materials derived from waste and the methods used for their synthesis. It begins by defining superhydrophobicity and highlighting its unique characteristics. It emphasizes the pivotal role played by superhydrophobic materials across industries. The review then explores waste materials’ untapped potential, discussing the advantages of harnessing waste for superhydrophobic material development. Concrete examples of promising waste materials are provided, including agricultural residues and industrial byproducts. The review outlines five key sections that will be further developed to offer a comprehensive understanding of this innovative and sustainable approach to superhydrophobic materials.
1. Introduction
Superhydrophobic materials have gained significant attention due to their unique water-repellent properties [1,2,3,4,5,6,7,8]. They can repel water droplets and prevent water from adhering to their surfaces, leading to a range of applications in various fields, including self-cleaning surfaces, anti-icing coatings, and water-resistant textiles [6,9]. In recent years, researchers have explored the use of naturally derived hydrophobic materials [7,8,10,11] and waste materials to create superhydrophobic surfaces, providing an environmentally friendly and sustainable approach to obtaining these remarkable properties [9,12,13,14].
Superhydrophobic materials derived from waste offer an innovative and sustainable approach to obtaining remarkable water-repellent properties. By repurposing waste materials, we can reduce environmental impact, promote circular economy principles, and contribute to a greener future. The synthesis of superhydrophobic materials from waste requires a combination of surface modification techniques and hierarchical structuring, with characterization methods to validate the desired properties. The diverse applications of superhydrophobic materials demonstrate their potential for various industries and pave the way for more environmentally friendly technologies. This review offers a compilation of different research papers on the utilization of waste materials in the development of superhydrophobic surfaces. To accomplish this, we conducted a thorough search using prominent online databases, namely Web of Science (WoS) and Scopus, spanning from December 2013 to September 2023. Our methodology began with the selection of relevant publications by employing keywords such as “superhydrophobic”, “waste”, “agrowaste”, and “hydrophobic materials”. These keywords helped us identify a set of publications for our analysis. For each chosen publication, we scrutinized its content to determine its relevance to our investigation. We examined the experiments conducted, the principal findings, and the conclusions drawn. This comprehensive analysis enabled us to gain insights into various aspects and draw conclusions regarding the utilization of solid waste in the fabrication of superhydrophobic surfaces and their subsequent applications. Interestingly, we discovered several articles not initially captured through our keyword searches. These articles came to our attention while reviewing the reference lists of the publications identified during the second screening phase. These additional findings proved to be valuable contributions to our study. In the end, our study encompasses a total of more than 100 articles that collectively provide a comprehensive overview of the research landscape concerning the integration of waste materials into the production of superhydrophobic surfaces and their associated practical applications. As evident from this review spanning the past two decades, researchers on a global scale have extensively delved into the fundamentals, fabrication techniques, and applications of superhydrophobic coatings. Despite this, existing reviews have predominantly offered limited insights into the fundamentals and fabrication techniques specifically related to superhydrophobic coatings derived from waste materials. The primary objective of this review is to bridge this gap by providing a comprehensive overview of the fundamentals and fabrication techniques associated with utilizing waste materials in the production of superhydrophobic coatings. In particular, the paper is dedicated to compiling and discussing pivotal contributions in this critical domain. Furthermore, the review delves into potential applications of these coatings. It is noteworthy that recent efforts have been focused on enhancing the eco-friendliness of superhydrophobic coating formulations and processes, incorporating natural materials and waste through sustainable practices with a reduced environmental footprint. Looking ahead, the review underscores the pressing need to optimize control parameters in these processes to propel the development of superhydrophobic coatings for diverse applications.
Therefore, this comprehensive review has facilitated the derivation of several conclusions pertaining to the utilization of solid waste in the fabrication of superhydrophobic surfaces and their subsequent applications. Consequently, it presents a valuable opportunity for both academic and industrial sectors to obtain comprehensive insights into diverse waste materials that hold promise for the creation of novel superhydrophobic coatings across various substrates. Such an exploration serves to propagate the principles of sustainability and circular economy. The subsequent sections of this review are delineated as follows:
Understanding Superhydrophobicity: This section will focus on elucidating the definition and distinctive characteristics of superhydrophobicity.
Waste as a Resource for Superhydrophobic Materials: Here, we will delve into the advantages of utilizing waste materials for the creation of superhydrophobic surfaces. Additionally, we will provide noteworthy examples of waste materials, including agricultural waste like rice husks and fruit peels, industrial waste such as fly ash and silica fume, and discarded plastics like polystyrene (PS) and polyethylene terephthalate (PET), all of which are well-suited for achieving superhydrophobic properties.
Methods for Synthesizing Superhydrophobic Materials from Waste: This section will comprehensively cover the various techniques and methodologies employed in synthesizing superhydrophobic materials using waste materials as a primary resource.
Applications of Superhydrophobic Materials: Advantages Finally, we explore the practical applications of these innovative materials, highlighting the numerous advantages associated with their use in the development of new products and technologies.
2. Understanding Superhydrophobicity
Superhydrophobicity refers to an exceptional surface property characterized by its ability to repel water to an extraordinary degree [15]. Surfaces exhibiting superhydrophobic behavior typically display high contact angles (CAs), often exceeding 150°, as shown in Figure 1, where water droplets practically bead up and roll off effortlessly. This phenomenon results from the combined effects of surface roughness and low surface energy. One of the most well-known examples of superhydrophobicity in nature is the lotus effect, which has inspired scientists and engineers to create water-repellent surfaces. The lotus effect is a feature found in lotus leaves and some other plants, characterized by their unique surface structure that makes them superhydrophobic [16].
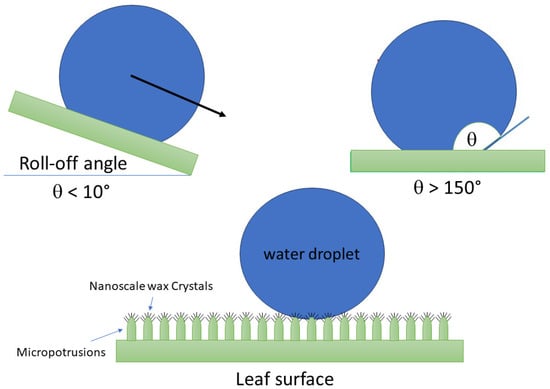
Figure 1.
Design of structure and water contact angles and roll-off angles of leaf surfaces with superhydrophobic properties [16,17].
This natural adaptation serves two main purposes for the plant:
- Self-cleaning: When water droplets touch lotus leaves, they form spherical droplets that pick-up contaminants as they roll off the leaf. This self-cleaning mechanism helps keep the leaf surface free from debris (Figure 1).
- Water repellence: Lotus leaves can stay dry even in wet conditions because water droplets are unable to adhere to the surface. This property is crucial for the lotus plant’s survival as it prevents the growth of harmful fungi and bacteria.
Superhydrophobic surfaces are typically structured at the micro or nanoscale, featuring a hierarchy of protrusions or textures that minimize the contact area between water and the surface [17]. These intricate surface structures trap air within their interstices, creating a cushioning effect that prevents water from adhering. The result is a surface that is exceptionally water repellent, self-cleaning, and resistant to wetting, finding applications in fields ranging from materials science to biology and various industrial sectors. Superhydrophobic materials play a pivotal role in a wide range of industries due to their unique properties and versatile applications [18].
Their importance in various sectors can be summarized as follows:
- Manufacturing and Engineering [19]: Superhydrophobic coatings and materials are used to reduce friction and improve the efficiency of machinery and equipment, leading to energy savings and increased lifespan of components. They are also employed to prevent corrosion and fouling on surfaces [20], enhancing the durability of industrial equipment [21].
- Transportation: In the automotive and aerospace industries, superhydrophobic materials are used to create self-cleaning and anti-icing surfaces for windshields and aircraft wings, improving visibility and safety while reducing maintenance costs [22].
- Oil and Gas: Superhydrophobic coatings are applied to pipelines and drilling equipment to repel water and prevent the buildup of ice, reducing downtime and maintenance requirements in harsh environments [23].
- Textiles: Superhydrophobic fabrics and coatings are used in the textile industry to create water-resistant and stain-resistant clothing, shoes, and outdoor gear. This enhances comfort and durability for consumers [24].
- Electronics: Superhydrophobic coatings protect electronic devices from moisture, improving their lifespan and reliability. They are also used to create self-cleaning surfaces for touchscreens and displays [25].
- Medical and Healthcare: Superhydrophobic materials are utilized in medical devices and equipment to prevent contamination, improve sterilization processes, and create water-repellent surfaces for surgical instruments [26]
- Energy: In the energy sector, superhydrophobic materials are applied to power plant components, such as condensers and heat exchangers, to improve energy efficiency by reducing heat loss due to water droplet formation [27].
- Construction: Superhydrophobic coatings are used to protect building materials from water damage, increase the lifespan of structures, and create self-cleaning facades that require minimal maintenance [28].
- Environmental Remediation: Superhydrophobic materials are used to separate oil and water in spill cleanup operations, offering an efficient and eco-friendly solution for environmental protection [24,29].
- Food and Packaging: Superhydrophobic coatings can be applied to food packaging materials to prevent moisture ingress and extend the shelf life of products. They also find use in creating anti-fouling surfaces for food processing equipment [30].
In summary, superhydrophobic materials are indispensable in various industries for their ability to enhance performance, durability, and safety while reducing maintenance costs and environmental impact. Their unique properties continue to drive innovation across multiple sectors, making them a valuable asset in modern technology and manufacturing.
3. Waste as a Resource for Superhydrophobic Materials
The utilization of waste as a resource for crafting superhydrophobic materials marks a significant stride towards sustainability and environmental stewardship [31,32].
By harnessing discarded materials such as plastics, wood fibers, and agricultural remnants, scientists and engineers have successfully developed cost-effective and environmentally friendly superhydrophobic coatings and surfaces [33]. Here are some key points regarding the utilization of waste as a resource for superhydrophobic materials:
- Sustainable Sourcing: Utilizing waste materials, such as agricultural residues, industrial byproducts, or recycled plastics, reduces the demand for virgin resources and helps in waste management and disposal. Waste-derived superhydrophobic materials can reduce the consumption of natural resources, helping to preserve finite resources and mitigate the environmental impacts associated with resource extraction [33]
- Environmental Benefits: Recycling waste into superhydrophobic materials reduces the environmental footprint associated with waste disposal and the production of traditional materials. It contributes to lower carbon emissions and resource depletion [34].
- Circular Economy: Utilizing waste in the production of superhydrophobic materials can promote the principles of a circular economy, where waste is considered a valuable resource that can be continually reused and recycled. Assessing the economic viability of using waste as a resource for superhydrophobic materials is essential. It should compete favorably with conventional materials in terms of cost and performance [35]
- Cost-Effectiveness: Repurposing waste materials can be cost effective compared to procuring new resources. It can lead to significant cost savings in material sourcing, making superhydrophobic materials more accessible for a wider range of applications [33,35].
- Diverse Waste Sources: Waste materials come from various sources, including agriculture, industry, and consumer products. These diverse sources offer a wide range of raw materials suitable for superhydrophobic applications, allowing for flexibility in material selection [33,36,37].
- Customization: Depending on the waste source and processing techniques, the properties of superhydrophobic materials can be customized to meet specific requirements, such as enhanced durability, improved hydrophobicity, or tailored surface roughness [35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54].
- Applications: Superhydrophobic materials derived from waste can be used in a variety of applications, such as self-cleaning surfaces, anti-corrosion coatings, anti-icing coatings, oil–water separation, water-repellent textiles, and more [42,44,47,50].
- Research and Development: The selection of waste materials plays a pivotal role in this context. Factors such as waste composition, structure, and availability are key determinants of their suitability for superhydrophobic applications. For example, certain plant-based waste materials may contain a wealth of hydrophobic compounds [42,43]. The augmentation of surface micro and nanostructures on waste materials serves to heighten their hydrophobic characteristics by increasing surface roughness [40,51]. This emulates the naturally occurring superhydrophobic surfaces observed in flora and fauna.
- Regulatory Considerations: Depending on the region and the nature of the waste materials, regulatory compliance may be necessary. It is crucial to adhere to relevant environmental and safety standards when working with waste materials [31].
4. Methods for Synthesizing Superhydrophobic Materials from Waste
In this section of the study, we delve into the analysis and discussion of superhydrophobic coatings derived from various waste materials, emphasizing their chemical, physical and environmental implications.
4.1. Biowaste
Table 1 presents a comprehensive list of selected primary agricultural and marine waste materials suitable for the development of novel superhydrophobic coatings and materials, thereby enhancing the value chain of waste utilization. In pursuit of this objective, agricultural waste materials, particularly rice husk ash (RHA), bagasse (BA), wheat straw (WS), corn husks (CH), and corn straw (CS) biogas residue, have emerged as the most employed resources. These materials are widely recommended for crafting superhydrophobic surfaces.

Table 1.
Summary of key agricultural waste materials utilized in superhydrophobic surface development and their principal outcomes.
4.1.1. Rice Husk Ash (RHA)
The thermal combustion of rice husk (RH) at a low temperature in an air atmosphere yields amorphous silica with a content exceeding 90%. Table 2 provides a comprehensive overview of the typical chemical composition of rice husk ash (RHA) [43,44,45]. As noted by M.U.M. Junaidi et al. [42], RHA contains 97.5 wt.% of SiO2 and 2.0 wt.% of carbon after undergoing pre-treatment with citric acid and subsequent calcination at 800 °C. This renders the waste material highly suitable for replacing commercial nanoparticles in surface modification applications. In particular, the research has led to the development of superhydrophobic coatings. These coatings are prepared by spraying a mixture of ash (6 wt.%), with particle sizes ranging from 10 nm to 700 nm, and a hydrophobic agent in an ethanol solution onto a thin layer of commercial adhesive. Two hydrophobic agents, namely fluoroalkyl silane, 1H,1H,2H,2H-perfluorodecyltriethoxysilane (HFDS), and stearic acid (SA) C18H36O2, were chosen to effectively reduce the surface energy of the coatings [42].

Table 2.
Chemical composition (wt.%) of rice husk ash [43,45].
Various chemical processes and thermal treatments have been proposed to alter the physical and chemical characteristics of rice husk (RH) [43,44]. Enhanced hydrophobicity was achieved in the coatings modified with HFDS-treated RHA, effectively reducing RHA silica agglomeration and preventing any deterioration in superhydrophobic properties. In a study conducted by J. Sathish et al., the feasibility of applying superhydrophobic ash coatings on concrete to reduce water absorption was explored [43]. Notably, RHA was subjected to modification using fluoroalkyl silane, resulting in an ethanolic solution that was applied to concrete surfaces to mitigate water absorption. This application generated water repellency similar to that of a lotus leaf, as validated by a measured water contact angle (CA) of approximately 152.3°. Furthermore, this study revealed that incorporating RHA into cement had a positive impact on its mechanical properties. The modified cement exhibited enhanced strength characteristics, particularly in terms of compressive and flexural strength, compared to conventional cement. This improvement can be attributed to the pozzolanic properties of rice husk, which facilitate the formation of additional cementitious compounds and a denser microstructure.
Given its substantial surface area and low thermal conductivity [42,43,44,45], rice husk ash can also confer hydrophobic properties onto silk or wool fabrics [44]. RHA, obtained at 700 °C, underwent alkali treatment (using a 2.5 M NaOH solution), resulting in silica nanoparticles with an average diameter of 246 nm. These nanoparticles were dispersed in an acidic solution and applied to silk/wool fabrics. The application of 1.0% nano silica notably increased the water CA values, with the highest CA of 145° achieved at a 7.5% nanoparticle concentration. Importantly, the water-repellent properties persisted even after dry cleaning and washing treatments [42,43,44,45]. Furthermore, it was demonstrated that this type of surface modification did not compromise the mechanical properties of the fabrics.
4.1.2. Bagasse
Another promising source of biodegradable raw material for the creation of bio-based superhydrophobic coatings is bagasse (BA), an agricultural waste often disposed of in landfills or through incineration, contributing to pollution concerns [46,55,56,57,58,59,60,61,62,63]. Due to its chemical composition (as detailed in Table 3) [57,58], bagasse has garnered attention from various researchers as a supplementary material in blends with Portland cement or geopolymeric binders [60]. Beyond these applications, the chemical and mineralogical composition of bagasse makes it a promising candidate for modifying the surface wettability of diverse materials. Given its inherent polarity, the modification of bagasse becomes essential to reduce its hydrophilic nature and achieve hydrophobic characteristics. For instance, Sun et al. employed N-bromosuccinimide as a catalyst to synthesize hydrophobic acetylated bagasse, primarily for oil sorption purposes [61]. In a separate study, Sait et al. enhanced bagasse’s affinity with oil through acylation, grafting it with fatty acids [62]. Another intriguing approach involves the modification of sugarcane bagasse via esterification, employing compounds like stearic acid and calcium oxide, followed by coating with a hydrophobic polymer such as polyacrylonitrile (PAN) [63]. Building on previous research, Chengrong Qin et al. undertook esterification processes to render bagasse superhydrophobic for various substrates [46]. Specifically, they subjected disintegrated and ball-milled bagasse to a heat treatment at 100 °C, followed by acylation with stearoyl chloride. This process yielded finely yellowish bagasse ester particles suspended in an ethanol suspension, which were then applied to multiple substrates using an airbrush. The result was the attainment of remarkable superhydrophobicity, as evidenced by advancing and receding contact angles (CAs) of approximately 168° and 147°, respectively, on bagasse ester-coated filter paper. In contrast, uncoated bagasse paper exhibited a CA of 0°. Notably, the surface micro/nanostructure of bagasse esters promoted the creation of air gaps between water droplets and the surface. Furthermore, the inclusion of non-polar aliphatic chains in bagasse esters contributed to the antifouling properties of the coated surfaces.

Table 3.
Chemical composition (wt.%) of bagasse [57,58].
4.1.3. Waste Wheat Straw
Numerous significant research efforts have explored the potential of utilizing modified graphene as a hydrophobic material [64,65]. However, an especially intriguing avenue of investigation involves the development of conductive superhydrophobic materials using conductive graphene or graphene-like substances obtained via the pyrolysis process of wheat straw [66]. This endeavor is driven by the hierarchical micro- and nanostructure observed in the superhydrophobic eyes of mosquitoes [47]. Yanbin Wang et al. [47] undertook a study focused on transforming agricultural waste, such as wheat straw (WS), into nanocarbon materials with a hierarchical structure. Wheat straw, like other biomass sources, can be considered a sustainable resource for producing high-value-added carbon materials with micro-nanostructures, including activated carbon, graphite, and porous carbon [67]. To convert wheat straw into nanocarbon materials with a hierarchical structure, Wang Y. et al. employed a controlled carbonization process after chemically removing siliceous and waxy impurities from natural wheat straw [47]. Specifically, they treated the biomass with an alkali solution to reduce the silicon content from approximately 2.68% to approximately 0.23%, thereby achieving electrically conductive carbon materials. The pyrolysis process was conducted at various temperatures. The surface modification of the carbon material was achieved through a polydimethylsiloxane (PDMS) coating known for its waxy and adhesive properties. The coating solution, dissolved in 0tetrahydrofuran (THF), was applied to several substrates, including copper plates, iron plates, aluminum plates, and glass slides. The results indicated that by controlling the pyrolysis conditions, a range of micro/nanohierarchical structures could be finely tuned. In general, higher annealing temperatures resulted in biochar with a higher degree of carbonization, greater aromatic substance content, reduced aliphatic substance content, increased aromaticity, and lower polarity [41,68,69]. The results by Chen. Z et al. [69] indicated that with an increase in annealing temperature to 800 °C, the coating’s morphology displayed an irregular microstructured surface, resembling carbonaceous brick-like clusters adorned with numerous nanonipples. This particular roughness was sufficient to confer superhydrophobic properties, with water CAs reaching up to 162° after treatment with PDMS.
4.1.4. Corn Husks
As previously mentioned with respect to wheat straw, corn husks (CHs) represent another valuable renewable resource that can be transformed into high-value carbon materials. A noteworthy study by Zhiyuan Liu et al. [48,49] has explored the utilization of biochar derived from a photothermal process, modified with Fe3O4, for the creation of superhydrophobic and ice-phobic coatings through spray-coating techniques. In addition to the properties discussed earlier, this study capitalized on the inherent black color of biochar to imbue it with photothermal capabilities, making it apt for de-icing applications. To achieve this, corncob biochar, produced through thermal treatment at 500 °C, underwent modification with KOH to develop a complex structure and functional groups conducive to reacting with the precursor of Fe3O4 particles. Specifically, iron nitrate nonahydrate (Fe(NO3)3⋅9H2O) was combined with biochar and subjected to heat treatment to yield Fe3O4-loaded modified residue biochar. The final structured roughness was further enhanced through PDMS modification, resulting in superhydrophobic coatings boasting CAs of approximately 156° and tilt angles of about 5°. These findings underscore that the straightforward modification of biochar using solid KOH and Fe3O4 particles promotes the creation of a micro-nano rough structure conducive to achieving a Cassie–Baxter wetting regime [70]. Consequently, the presence of air between the liquid–solid interface reduces adhesion strength between the surface and ice, thereby enhancing the anti-icing properties of biochar-based superhydrophobic coatings. The substantial improvement in de-icing properties can be attributed to the incorporation of Fe3O4 particles and their consequent photothermal capabilities [71]. Remarkably, all the coating’s properties remain stable even in alkaline and acidic environments, with CAs consistently exceeding 150° even after several cycles of wear.
Thus, the synthesis of superhydrophobic carbon materials derived from lignocellulosic biomass waste, as discussed earlier, poses a substantial challenge. It is important to highlight that the development of an innovative, eco-friendly superhydrophobic biochar material, employed in crafting coatings resistant to water and ice, is attainable through pyrolysis. This process is facilitated with the presence of FeCl3 as a catalyst [41].
4.1.5. Milled Coral Waste Powder
The utilization of milled coral waste powder in the production of superhydrophobic composites represents another innovative and sustainable approach to addressing both environmental and material engineering challenges [50,72,73]. This emerging field combines the repurposing of coral waste, which is often a byproduct of coral reef conservation and restoration efforts, with advanced material science techniques to create composites with exceptional water-repellent properties. Coral reefs are invaluable marine ecosystems that provide habitats to a vast array of marine life. Unfortunately, they are under significant threat due to factors like climate change, ocean acidification, and human activities. Coral restoration projects generate substantial quantities of coral waste material, including fragments and rubble. Converting this waste into a valuable resource offers a dual benefit: it helps in reef conservation by reducing waste, and it provides a sustainable source of raw material for superhydrophobic composite development. The heart of this process lies in milling coral waste into a fine powder. This involves crushing and grinding the waste material to achieve the desired particle size. The resulting milled coral waste powder serves as a crucial starting point for the creation of superhydrophobic composites. To produce superhydrophobic composites, the milled coral waste powder is typically combined with polymers or other hydrophobic materials. The choice of polymer and the precise formulation may vary based on the intended application. Common polymers include polyethylene, polypropylene, or even biodegradable alternatives like polylactic acid (PLA). The mixture is carefully blended to achieve a homogenous distribution of the milled coral waste powder within the polymer matrix. Yayun Zhao et al. [73] conducted a study focused on developing an eco-friendly superhydrophobic coating using coral waste, incorporating stearic acid as a key component. The process involved milling coral waste into micro to nanoscale powder to impart superhydrophobic properties to the material. The reduction in surface energy was primarily achieved through the incorporation of stearic acid. This superhydrophobic powder was subsequently applied to uncured cement mortar, creating an effective superhydrophobic coating. The findings of surface wettability tests revealed the exceptional superhydrophobicity of the prepared coating, with a water CA of 152.6° and a water sliding angle of 1.2°. Moreover, comprehensive self-cleaning assessments demonstrated the coating’s impressive self-cleaning capabilities. Electrochemical tests confirmed that the superhydrophobic coating significantly enhanced the corrosion resistance of cement mortar. The superhydrophobic coating proposed in this study holds promising prospects for applications in marine concrete and engineering construction projects situated far from mainland islands.
4.1.6. Eggshell Waste
The production of superhydrophobic coatings using recycled eggshells has been also investigated recently [32]. The method, as proposed in [32], involves harnessing readily recyclable eggshells and ZnO to create a micro- and nano-hierarchical structure. This structure is further enhanced through modification with stearic acid (SA). To ensure a robust adhesion between the coating and the substrate, carboxymethyl cellulose (CMC) was incorporated in the mixture. The resulting superhydrophobic coating not only displays remarkable resilience against UV radiation and mechanical wear but also effectively delays the icing-over process. The entire fabrication process of this superhydrophobic coating is sustainable and cost effective, making it a highly promising option for practical everyday applications. For example, this environmentally friendly coating can be effortlessly applied to common white shoes, imparting them with a self-cleaning property ideal for outdoor use. The research by Lizbeth González-Victoriano [74] explores the repurposing of chicken eggshells, which are rich in calcium carbonate (CaCO3), to create valuable products. The primary objective of this research initiative was to assess how the incorporation of eggshell micro and nanoparticles, in combination with ZnO particles, affects the superhydrophobic properties of these coatings. The study focused extensively on establishing correlations between micro and nanometric structure characteristics and the resulting superhydrophobic behavior.
4.1.7. Seafood Shell Waste
The seafood industry’s crab shell waste poses significant environmental and health risks. The study conducted by Sunanda Roy et al. [54] aimed to develop a method for creating superhydrophobic and superoleophilic coatings using chitosan derived from waste crab shells. These coatings could then be applied to transform ordinary hydrophilic polyurethane sponges into highly efficient absorbents for organic solvents and oils in oil/water mixtures. In this context, the researchers propose an innovative, cost-effective approach to produce high-yield chitosan (44% ± 3) from discarded crab shells and utilize this processed chitosan to create a durable, versatile, and environmentally friendly superhydrophobic coating. This coating is formulated by linking the amines present in chitosan with a long-chain polymer, octadecylamine, using the cross-linking agent glutaraldehyde. When applied to inherently hydrophilic polyester fabric and standard surgical-grade cotton, it imparts superhydrophobic properties with water CAs of 158.6 and 161.4° (±3), respectively. Initial testing demonstrates that this coating can withstand repeated cycles of laundry (60 times), sandpaper abrasion (55 times), and tape peel tests (80 times) with minimal reduction in its superhydrophobicity. Abdelgalil et al. [75] studied the potential reversibility of any loss in superhydrophobic properties through a simple 2 min ironing process, underscoring the material’s resilience. In particular, the investigation revolved around ecologically responsible remediation of potentially hazardous crab shell waste with the aim of facilitating eco-friendly bacterial alkaline phosphatase (ALP) production through bioprocessing. The results highlight the synergistic biovalorization of crab shell waste and cost-effective ALP production enabled by an innovative medium formulation. It represents a significant step in seafood waste management and the bench top-scale production of ALP.
4.2. Industrial Waste
Various forms of industrial waste, including residues from anaerobic digestion, discarded polystyrene, ash from wastepaper sludge, and waste derived from palm oil fuel, are frequently encountered environmental waste sources. These materials have been explored as more sustainable alternatives for crafting novel superhydrophobic substances, as shown in Table 4. The escalating production of plastic waste has emerged as a pressing environmental issue, posing significant challenges. Microorganisms struggle to naturally break down this waste, making the recycling of plastics into value-added materials imperative.

Table 4.
Summary of the main industrial waste here analyzed.
4.2.1. Plastic Waste
Polymer waste poses a significant environmental threat. Many researchers have tried to address the issue of irresponsible production and use of plastic, promoting a circular economy. Thus, recycling polymer waste to create superhydrophobic materials offers a promising avenue to address environmental concerns and contribute to a sustainable solution.
Expanded polystyrene waste (EPSW) presents an intriguing avenue for the creation of superhydrophobic materials. EPSW, which constitutes at least 6% of global plastic production, is commonly employed in everyday items like food packaging. Unfortunately, its disposal poses significant environmental and pollution challenges, given that the styrene monomer within EPSW is linked to various diseases, including cancer [86]. Traditional disposal methods for EPSW are less than ideal: landfilling is unfavorable due to its non-biodegradable nature, incineration can result in the release of toxic emissions like furans and polychlorinated biphenyls, and mechanical recycling often yields products with higher production costs [87,88,89,90]. However, a more promising approach emerges through the development of functional coatings for cotton fabrics using spray coating techniques. Abdul Halim’s et al. [12] research explores the utilization of expanded polystyrene waste as a novel raw material to produce superhydrophobic membranes or wettability-based membranes. They explored the concept of plastic valorization, repurposing EPSW by using it as a functional coating for cotton fabrics through a simple spray coating method. Specifically, the process involves dissolving waste polystyrene in toluene and incorporating ZnO particles into the solution to enhance CAs. Multiple layers of coating are applied using the spray coating technique, with various cotton fabrics serving as substrates. This coating process is straightforward and uses cost-effective chemicals readily available in the local market. Remarkably, the application of three layers of polystyrene modified with ZnO particles onto canvas fabrics achieves high water CAs and superoleophilic surfaces. The resulting coated fabrics exhibit a superhydrophobic surface, with the highest water CA achieved by applying three layers of coating for 15 s, measuring 150°. Interestingly, these coated fabrics quickly absorb oil droplets. They then employed the ZnO EPS-coated canvas cotton fabric as a membrane for separating oil from water, both in a continuous system and a batch system. This innovative use of EPSW waste as a coating material demonstrates its potential for advanced materials and wastewater treatment applications, providing a sustainable solution to tackle plastic waste and environmental issues.
The study conducted by Eugene B. Caldona and colleagues [77] is of particular interest due to its innovative approach to creating smart composite coatings using recycled polypropylene (PP) plastics with added titanium dioxide (TiO2) particles. This method not only offers a cost-effective and environmentally friendly way to repurpose used PP plastics but also contributes to reducing plastic waste. The researchers were able to optimize the weight ratio of PP to TiO2 (2 g PP to 3.5 g TiO2) for achieving superhydrophobicity (CA of 154° ± 2) and maximum reversibility in wetting behavior (CA of 61° ± 1 after UV exposure, rising to 153° ± 3 when stored in the dark). The key feature of these PP/TiO2 composites is their extreme anti-wettability and improved thermal stability. The inclusion of TiO2 makes the wetting behavior of the surface reversible, transitioning from superhydrophobic to hydrophilic and vice versa when exposed to ultraviolet (UV) light and stored in the dark. Biomimetic electrospun nanocomposite fibers from recycled polystyrene foams exhibiting superhydrophobicity nanofibers from recycled polystyrene foam can be created by adding aluminum microparticles (Al μPs) and titanium dioxide nanoparticles (TiO2 NPs) [81]. The inclusion of Al μPs and TiO2 NPs resulted in superhydrophobic nanofibers, enhancing their resistance to water. The nanocomposite fibers with a 10% nanoparticle inclusion displayed the best superhydrophobic properties with a water CA of 152°. Furthermore, the study found that incorporating nanomaterials into waste polystyrene led to consistent fiber diameters and improved thermal stability. Heat treatment and duration significantly influenced the surface hydrophobicity. This research represents an innovative approach by integrating polymer recycling into nanotechnology processes, with potential applications in fields like fog harvesting and water-related industries. Qinglang Ma et al. [83] achieved a noteworthy accomplishment by repurposing commonly encountered plastic waste, specifically PS, into valuable functional materials. They accomplished this through an economically efficient and straightforward dip-coating technique. The resulting amalgamation of discarded PS and SiO2, referred to as PS/SiO2, forms a textile material that boasts exceptional superhydrophobic and superoleophilic properties. It demonstrates remarkable resilience to corrosive substances like acids, alkalis, and saline solutions, as well as withstanding high-temperature treatment and mechanical wear. As a practical demonstration of its utility, the PS/SiO2-coated textile effectively separates oil/water mixtures using either absorption or filtration methods. Furthermore, it possesses a self-cleaning property on its surface, making it suitable for use in garments designed to prevent dust accumulation. This work introduces/shows an innovative approach to manage waste and produce cost-effective superhydrophobic materials with diverse applications.
Kim, T. et al. [83] embarked on a study utilizing recycled PET as a starting point to develop an environmentally conscious approach for manufacturing a superhydrophobic surface. They employed electrospinning and electrospraying techniques in their fabrication process. The concentration of the solution was regulated by adjusting the quantity of recycled PET flakes within the polymer solution. Notably, they observed that the resulting geometry could be tailored according to solution conditions, allowing for the creation of a hierarchical structure. This hierarchical structure, comprising micro and nanoscale features, effectively minimized contact with water droplets and yielded a surface endowed with superhydrophobic properties. The optimized structure exhibited an impressively high water CA (>156.6°). Significantly, this outcome holds particular importance because the researchers achieved superhydrophobicity solely through nanostructures, obviating the need for additional chemical coatings or treatments. Moreover, their inquiry extended to examining the self-cleaning capabilities and solar panel efficiency of the developed surface. Encouragingly, the findings suggest the potential of using recycled PET to produce superhydrophobic surfaces with applications in protective films for solar panels, as they demonstrated excellent self-cleaning properties and an efficiency protection rate of approximately 92%. As a result, this research stands as a substantial contribution to advancing environmentally friendly processes and the evolution of recycling technology.
4.2.2. Fly Ash and Phosphogypsum
Biogas residues resulting from anaerobic digestion typically comprise lignin, cellulose, and hemicellulose are commonly repurposed as soil enhancements or livestock feed supplements. The utilization of these biological resources plays a pivotal role in addressing energy shortages and mitigating environmental pollution. Moreover, when biochar is employed for anti-icing purposes, it contributes to eco-friendly, low-carbon development. The study conducted by Mohamed M.E. et al. [85] presents an environmentally friendly method for creating biochar (BC) and a cobalt–biochar nanocomposite (Co-BC) using rice straw biomass. The researchers developed two superhydrophobic coatings on steel surfaces by electrodepositing a nickel-modified biochar (Ni@BC) and nickel-modified cobalt-biochar nanocomposite (Ni@Co-BC), followed by treatment with stearic acid in ethanol (Ni@Co-BC@SA). The Ni@Co-BC@SA coating exhibited strong bonding to the steel surface and greater roughness, resulting in superior superhydrophobic properties, with water contact angles of 165° and 161° for Ni@Co-BC@SA and Ni@BC@SA, respectively, and water sliding angles of 1.0° and 3.0° for both coatings. Quantitative analysis showed that the Ni@Co-BC@SA coating had higher scale inhibition efficiency compared to the Ni@BC@SA coating. Furthermore, it displayed enhanced corrosion resistance, UV resistance, mechanical abrasion resistance, and chemical stability compared to the Ni@BC@SA coating. These findings suggest that the Ni@Co-BC@SA coating is a highly effective and durable superhydrophobic coating for steel substrates. This study, along with numerous other papers, underscores the critical importance of customizing specific carbonization parameters in order to enhance biochar’s porosity and surface area [20,43,48,91,92,93]. This customization significantly influences the overall effectiveness of biochar in a wide range of applications. To achieve an ice-phobic coating with photothermal conversion capabilities, surface modifications involving appropriate functional groups are necessary to alter the material’s band gap and improve its light absorption rate [94,95]. Chindaprasirt and colleagues’ [78] research focused on the importance of harnessing fly ash to develop water-repellent surfaces in construction materials, with a particular emphasis on its application in geopolymers as a substitute for traditional cement. The authors outlined a technique for achieving a superhydrophobic and self-cleaning surface on a fly ash geopolymer by employing a composite coating. This coating consists of a solution of PDMS combined with either polytetrafluoroethylene (PTFE) or calcium stearate (CS) microparticles. The incorporation of fly ash was found to enhance surface roughness. The study’s findings revealed that the CS-coated surface exhibited a CA of 140°, the PTFE-coated surface exhibited a CA of 159°, and the PTFE/fly ash-coated surface exhibited a CA of 153°, along with improved self-cleaning properties. These developments have the potential to enhance the sustainability of building construction materials. Tongyan Ren et al. [79] harnessed phosphogypsum, a significant byproduct of the phosphorus chemical industry, to fabricate a superhydrophobic material designed for efficient oil/water separation. Their method involved the use of sodium metasilicate and stearic acid as chemical modifiers, and it was notably straightforward. The resulting superhydrophobic material boasts a rough micro-nano structure with a low surface energy, featuring an impressive average water CA of 151.5° and an average water sliding angle of just 1.8°. These characteristics indicate its remarkable resistance to water adhesion. Thanks to its exceptional superhydrophobicity and superoleophilicity, this material can effectively separate various oil/water mixtures with outstanding separation efficiencies. Furthermore, the material demonstrates robust resistance to acidic solutions and can withstand repeated separation processes, highlighting its reliability even in challenging conditions and for long-term usage. In [77], Kumar Sow et al. developed a versatile, cost-effective, and fluorine-free superhydrophobic coating featuring a re-entrant surface structure. This coating was synthesized using fly ash (FA) and room-temperature-vulcanizing silicone. A comprehensive examination of the coating’s characteristics and long-term resilience was conducted. Furthermore, the outcomes of the study highlighted the coating’s efficacy in various applications such as self-cleaning, corrosion prevention, and oil–water separation.
4.2.3. Waste Paper Sludge Ash
Paper sludge ash (PSA) is an available waste material generated by the paper recycling industry which, based on its chemical composition, can be used in low-value applications, such as land spreading as a cattle bedding material and as an additive in waste effluent neutralization processes [96]. PSA is mainly composed of SiO2, Al2O3, CaO, and MgO and it is alkaline with a pH of about 12. Given the material’s composition, other works reported the possibility to use the pozzolanic reactivity of PSA to supplement cementitious material [97,98,99]. In recent years, several works have studied the feasibility to transform PSA into a super-hydrophobic powder using simple, low-cost processing [100,101,102]. Charilikleia Spathi in their PhD work [100] found that the addition of 4 wt.% of stearic acid under optimized process conditions produces superhydrophobic PSA powder with water CAs in the range of 150°. The effect of surface functionalization for several stearic acid concentrations (1, 2, 4, 8 wt.%) was studied. The as-received PSA after milling showed a fine particle structure with a median diameter (d50) between 2 and 5 mm; then, the addition of stearic acid up to 4 wt.% resulted simultaneously in the formation of a micro-particulate texture and a calcium stearate self-assembling monolayer chemically bonded to surfaces. These low-cost PSA-derived superhydrophobic powders could find use in several applications such as civil engineering infrastructure. In the same way, Wong et al. [103] studied and developed superhydrophobic paper sludges particles to achieve water-resistant coatings for concrete materials. They evaluated the feasibility of using superhydrophobic PSA powders as a surface treatment and as a partial cement replacement material to improve the resistance to water ingress and to increase the self-cleaning properties of concrete. The superhydrophobic PSA powders were obtained by dry ball milling for 8 h in the presence of a surface functionalizing agent, such as stearic acid. The results showed PSA powders with a grey color, specific gravity of 2.85 g/cm3 and CA of 153°. Thus, the addition of PSA and the applied superhydrophobic coatings reduce the absorption, diffusion, permeation, and electrical conduction, increasing the performance of concrete. The importance and the potentiality of using industrial paper waste is also demonstrated by Xuejie Yue [80]. This paper introduces a novel building envelope material called the superhydrophobic cellulose aerogel cooler (SHB-CAC) that combines several features, including self-cleaning ability, passive daytime radiative cooling, and thermal insulation, to minimize environmental heat absorption in buildings. In outdoor experiments, the prepared cellulose aerogel cooler, named SHB-CAC, demonstrated a high solar reflectance of 93% and a long-wave infrared emittance of 91%, resulting in an 8.5 °C temperature reduction compared to the ambient temperature when exposed to sunlight at 800 W/m2. Importantly, it also has low thermal conductivity (28 mW/(m K)), which helps prevent unwanted heat gain from the surrounding environment and reduces the energy needed for cooling. The superhydrophobicity of the material makes it resistant to water and dust, ensuring that it maintains its reflective and radiative properties even in different humidity conditions. Building energy simulations suggest that widespread use of SHB-CAC in China could lead to an average energy saving of 43.4% for cooling compared to traditional building materials, highlighting the potential of this approach to reduce energy consumption in buildings. Mengting Ye et al. [82] developed an eco-friendly and versatile superhydrophobic paper using silane-modified superhydrophobic nanofibrillated cellulose (M-NFC) through a straightforward spray application method. This paper offers an example of a sustainable solution to mitigate the environmental impact caused by non-biodegradable single-use plastic packaging products. Upon applying 1.5 g/m2 of M-NFC to the base paper, the resulting coated paper demonstrated exceptional superhydrophobic characteristics, boasting a water CA of 160°, impressive water repellency, and remarkable durability against various challenges. It withstood sandpaper abrasion, finger-wiping, bending, folding, and prolonged exposure to corrosive substances, such as highly acidic HCl (pH 1), highly alkaline NaOH (pH 10), high-temperature treatment at 180 °C, and intense ultraviolet irradiation. Moreover, the coated paper surface exhibited the ability to prevent the adhesion of microorganisms like S. aureus and E. coli directly, and it indirectly repelled solid contaminants when washed with water. This underscores its impressive antibacterial and anti-fouling properties. The development of this enduring and versatile superhydrophobic paper not only presents a promising solution to combat white pollution but also opens up new avenues for exploring potential applications of paper-based materials.
4.2.4. Blast Furnace Slags
As stated for waste paper sludge ashes, another industrial waste suitable to prepare super-hydrophobic powders is blast furnace slags (BFSs). BFS is an industrial waste produced in the steel manufacturing industry which occupies a large amount of land resources, causing environmental pollution in water, soil, and the atmosphere. Its composition primarily consists of silicates, aluminate, and calcium, and it is used in concrete as a substitute for aggregates and for clinker [104,105]. To decrease the environmental impact and to provide an economical and green route to reuse it, Qu.Z [106] studied the possibility to functionalize ground granulated blast furnace slag (GGBFS) by providing hydrophobic properties to and improving the leaching properties of concrete. In particular, the functionalization of fine particles by stearic acid was performed and different experimental conditions including the milling time, speed, and stearic acid dosage were optimized to achieve a higher performance, in terms of water CAs. The stearic acid in different concentrations (0.5, 1, 2, and 4 wt.%) and the GGBFS were milled together and the influence of milling conditions on the hydrophobicity was studied. The analysis of the results makes it clear that the optimal conditions for producing GGBS with CAs of approximately 156° involve milling for 0.5 h and maintaining a concentration of 1 wt.% of stearic acid. Furthermore, it was shown that the addition of these superhydrophobic GGBSs in lightweight aggregate concrete can decrease the capillary absorption.
4.2.5. Palm Oil Fuel Ash (POFA) Waste
POFA is one the main agro-industrial wastes produced from empty fruit branches, fibers, and kernels [107]. Since 1990, the possibility of using POFA as a replacement to Portland cement in a concrete material has been studied. In 2018, Saharudin K. and colleagues conducted pioneering research to investigate the feasibility of utilizing POFA as a source of silica to produce super-hydrophobic coatings [84]. This study involved the conversion of POFA into silica through a heat treatment process in the presence of a sodium hydroxide (NaOH) solution as a substitute for tetraethylorthosilane. Subsequently, this solution was modified by adding PDMS as the surface functionalizing agent to impart hydrophobic characteristics. The resulting silica material was employed to create chemically stable and UV-resistant super-hydrophobic surfaces using a spray coating method. A comprehensive investigation was conducted to explore the impact of varying the weight ratio of PDMS to silica solution (SS) (1:0, 1:1, 1:2, 1:3, 1:4, 1:5) on the super-hydrophobic properties. To assess the stability of the coating, pH testing and UV irradiation experiments were conducted. Remarkably, a water CA of 156 ± 1° was attained when the glass substrate was coated with a PDMS:SS ratio of 1:2. Furthermore, exposure to UV aging significantly enhanced the water CA of PDMS:SS (1:2) to 171 ± 2. The results demonstrated that the PDMS:SS (1:2)-coated glass substrate exhibited outstanding stability and durability even in acidic and basic environments. This innovative approach offers a potential solution for repurposing agricultural waste and creating water-repellent surfaces with promising durability and chemical stability. In a study by Sreekantan S. et al. documented in reference [85], the researchers explored the biocompatibility of coatings made from PDMS and silica derived from POFA for potential biomedical applications. They successfully developed an environmentally sustainable super-hydrophobic coating by synthesizing silica from POFA and incorporating PDMS. The manufacturing process involved employing isopropanol as a solvent and applying the resulting coating onto a glass substrate. A water CA measuring 151° serves as confirmation of the material’s super-hydrophobic characteristics. Furthermore, comprehensive cytotoxicity assessments were carried out. These assessments included an examination of cell viability and cell morphology using two different cell lines, namely the mouse fibroblast cell line (L929) and the hamster lung fibroblast cell line (V79). The outcomes of these investigations demonstrated that the toxicity of PDMS:SS coatings is contingent upon the concentration of the super-hydrophobic coating. Concentrations exceeding 12.5 mg/mL resulted in cell toxicity. These findings unequivocally endorse the potential application of the synthesized super-hydrophobic coating in the field of biomedical science.
5. Applications of Superhydrophobic Materials: Advantages
Several recent studies present innovative ideas for utilizing agricultural waste in the field of anti-icing/de-icing for wind energy [48,49]. Wind energy turbine blade surfaces can be damaged at low temperatures and high humidities by ice cover, decreasing the output power of the wind turbine. Traditional de-icing methods, including mechanical, liquid, and thermal approaches, can be replaced or bypassed through the application of biochar-based ice-phobic coatings (Figure 2).
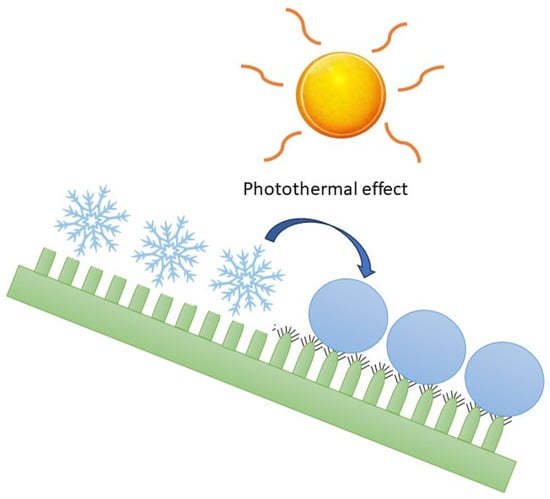
Figure 2.
The rolling of supercooled water droplets on the superhydrophobic ice-phobic coating with a photothermal conversion effect [41].
According to the studies of Liu et al. [49], superhydrophobic ice-phobic coatings with a photothermal conversion effect can be prepared from biochar-based corn straw biogas residues. Photothermal conversion materials are important to obtain self-deicing capabilities, and, for this purpose, biochar could be an interesting sustainable option. In fact, it is black, and the lattice vibrations of the material absorb optical energy, generating heat and giving them a high optical absorption capacity over a long wavelength [75,107,108]. Ice-phobic surfaces, drawing inspiration from the attributes observed in animals and plants, offer a range of advantages. These advantages encompass enabling the smooth movement of supercooled water droplets along the surface of the blade, diminishing the adhesion between water droplets and the coating and postponing the commencement of icing. These attributes hold particular significance for wind turbines, given their frequent placement in locales prone to winter icing, such as high-altitude and high-humidity regions. During the rotation of wind turbine blades, they accumulate super-cooled water droplets, leading to the formation of ice. This ice poses significant, albeit concealed, hazards to the safe operation of wind turbines. The concept of a super-hydrophobic surface holds great promise in the realm of anti-icing research.
Some interesting applications are those derived from pretreated silica-rich RHA to produce antifouling coatings that reduce the cumulative water uptake in the concrete materials and described by M.U.M. Junaidi et al. [42]. In this research the use of rice husk ash as a silica precursor substituting commercial nanoparticles which are more costly was suggested [42]. The near superhydrophobic coating could be formed easily by spraying a mixture of ash (6 wt.%) and a hydrophobic agent in ethanol solution on a thin layer of commercial adhesive. The findings show how RHA can be transformed into near-superhydrophobic ceramics for antifouling coatings through a successful chemical modification process utilizing HFDS and SA. FTIR analysis demonstrated the effective grafting of HFDS and SA onto RHA through Si-OH. In recent years, there has been a growing interest in developing coatings for concrete substrates with luminescent properties, and RHA has been utilized as a silica source to produce photoluminescent superhydrophobic coatings. These photoluminescent coatings can be valuable in detecting and analyzing corrosion in reinforced concrete [109]. Using agricultural waste as a modifier for concrete offers an environmentally friendly approach to improving construction material properties while reducing agricultural waste, thus contributing to sustainability and a lower environmental impact in the construction sector. Wong et al. [103] demonstrated that superhydrophobic paper sludge ash, when used as a surface coating, can remarkably reduce water absorption and sorptivity in concrete by up to 85–99%. This approach of using low-cost superhydrophobic materials as water-repellent coatings for concrete holds the potential to enhance concrete durability and contribute to greater sustainability in the construction industry [103]. Together with its superhydrophobic properties, the interest in recent years for the development of coatings for concrete substrates with luminescent properties has led to the use of RHA as a silica resource to produce photoluminescent (PL) superhydrophobic coatings [109]. Then, the use of agrowaste as a modifier for concrete can provide an environmentally friendly approach to increase construction materials’ properties while reducing agricultural waste. However, these studies contribute to greater sustainability and a low-impact material for the construction sector.
Taking again the valorization of RHA waste, other works explored the use of nano silica extracted from RHA to fabricate hydrophobic surfaces on Eri Silk/Wool Fabric [44]. This study demonstrates the feasibility of creating a cost-effective process using natural nanoparticles to achieve effective hydrophobic finishing on textiles, with satisfactory washing durability. This addresses the societal demand for high-quality hydrophobic treatments on technical textiles for premium applications. Superhydrophobic coatings with anti-fouling, oil absorption performance and high time/temperature/pH stability can be obtained by a high-yield agricultural waste, such as the modified bagasse ester. Bagasse is an ideal biodegradable raw material with a low cost and is readily available [46,61,63] for the development of bio-based superhydrophobic coatings applied by spray coating onto several substrates (glass slide, aluminum flake, and filter paper) and used as a hydrophobic sorbent for diesel oil removal from seawater [46,61,63]. Interesting is the study related to the conversion of waste wheat straw to conductive superhydrophobic nanocarbon materials, inspired by mosquito’s compound eyes [47]. In fact, superhydrophobic surfaces often have a low surface energy but are electrically insulated, impeding mass production in different applications. To overcome this, Wang et al. [47] developed a simple and economical protocol to fabricate conductive superhydrophobic surfaces based on bio-char of waste wheat straw. The feasibility to use waste to produce low-cost superhydrophobic powders for concrete materials could be also attained by industrial waste as PSA or BFS [96,104,105,106,107]. Regarding PP waste, the exceptional thermal stability observed in composite coatings derived from recycled polypropylene (PP) plastics combined with titanium dioxide (TiO2) particles [76] suggests promising prospects not only for smart coatings but also for diverse barrier-related purposes. The results suggest opportunities for the scalable and cost-efficient manufacturing of these PP/TiO2 composites. Their potential applications span everyday life scenarios and encompass industrial contexts like corrosion prevention, de-icing methods, and oil/water separation processes.
6. Summary
In the context of this review, researchers face a significant challenge: exploring the potential of utilizing several kinds of waste to develop exceptionally efficient superhydrophobic materials. These materials, summarized in Figure 3, exhibit significant potential in furnishing adaptable solutions that span a wide range of industries. They present effective solutions to address issues concerning corrosion, contamination, efficiency, and environmental preservation. Furthermore, these materials are characterized by their sustainability and cost-effectiveness, endowing them with strong appeal for an extensive array of applications.
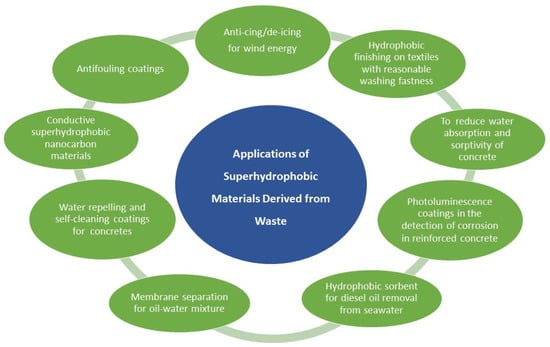
Figure 3.
Summary of the several applications of superhydrophobic materials derived from waste.
This review has highlighted the concept of superhydrophobicity and its unique characteristics, emphasizing the pivotal role played by superhydrophobic materials. By harnessing waste materials, such as agricultural residues and industrial byproducts, it is possible to reduce waste and environmental impact and also develop innovative and sustainable solutions. The integration of waste materials into the production of superhydrophobic surfaces offers a promising avenue for sustainable and environmentally friendly technologies. Through a comprehensive analysis of relevant research papers, it is evident that waste materials offer untapped potential for the fabrication of superhydrophobic surfaces. These materials can be modified and combined with other substances to achieve desirable properties, such as water repellency and self-cleaning capabilities promoting sustainability in various sectors.
To sum up, harnessing waste as a resource for superhydrophobic materials represents an environmentally responsible and economically viable approach. This practice not only tackles crucial sustainability issues but also diminishes waste generation while unlocking fresh avenues for inventive material solutions across multiple industries.
Author Contributions
Conceptualization, M.C. and R.T.; data curation, M.C. and R.T.; writing—original draft preparation, M.C. and R.T.; review and editing, M.C., D.N.B., S.C. and R.T. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
No new data were created or analyzed in this study. Data sharing is not applicable to this article.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
| Al µPs | Aluminum microparticles |
| ALP | Bacterial alkaline phosphatase |
| BA | Bagasse |
| BFS | Blast furnace slags |
| BC | Biochar |
| CS | Calcium stearate |
| Co-BC | Cobalt-biochar nanocomposite |
| CAs | Contact angles |
| CH | Corn husks |
| CS | Corn straw |
| EDX | Energy Dispersive X-Ray Analysis |
| EPS | Expanded polystyrene |
| EPSW | Expanded polystyrene waste |
| FA | Fly ash |
| FTIR | Fourier-transform infrared spectroscopy |
| GGBFS | Ground granulated blast furnace slag |
| HFDS | 1H,1H,2H,2H-perfluorodecyltriethoxysilane |
| Ni@BC | Nickel-modified biochar |
| Ni@BC@SA | Nickel-modified biochar followed by treatment with stearic acid in ethanol |
| Ni@Co-BC | Nickel-modified cobalt-biochar nanocomposite |
| Ni@Co-BC@SA | Nickel-modified cobalt-biochar nanocomposite followed by treatment with stearic acid in ethanol |
| POFA | Palm oil fuel ash |
| PSA | Paper sludge ash |
| PDMS | Polydimethylsiloxane |
| PET | Polyethylene terephthalate |
| PLA | Polylactic acid |
| PP | Polypropylene |
| PS | Polystyrene |
| PSW | Polystyrene waste |
| PTFE | Polytetrafluoroethylene |
| RH | Rice hush |
| RHA | Rice husk ash |
| SEM | Scanning Electron Microscopy |
| M-NFC | Silane-modified superhydrophobic nanofibrillated cellulose |
| SS | Silica solution |
| NaOH | Sodium hydroxide |
| SA | Stearic acid |
| SHB-CAC | Superhydrophobic cellulose aerogel cooler |
| THF | Tetrahydrofuran |
| TGA | Thermal Gravimetric Analysis |
| TiO2 NPs | Titanium dioxide nanoparticles |
| PSAW | Waste paper sludge ash |
| WS | Wheat straw |
| WoS | Web of Science |
References
- Darmanin, T.; Guittard, F. Superhydrophobic and superoleophobic properties in nature. Mater. Today 2015, 18, 273–285. [Google Scholar] [CrossRef]
- Parvate, S.; Dixit, P.; Chattopadhyay, S. Superhydrophobic Surfaces: Insights from Theory and Experiment. Phys. Chem. B 2020, 124, 1323–1360. [Google Scholar] [CrossRef]
- Mukhopadhyay, R.D.; Vedhanarayanan, B.; Ajayaghosh, A. Creation of “Rose Petal” and “Lotus Leaf” Effects on Alumina by Surface Functionalization and Metal-Ion Coordination. Angew. Chem. Int. 2017, 56, 16018. [Google Scholar] [CrossRef]
- Taurino, R.; Cannio, M.; Boccaccini, D.N.; Messori, M.; Bondioli, F. Preliminary study on the design of superhydrophobic surface by 3D inkjet printing of a sol-gel solution. J. Sol-Gel Sci. Technol. 2023, 108, 368–376. [Google Scholar] [CrossRef]
- Taurino, R.; Fabbri, E.; Messori, M.; Pilati, F.; Pospiech, D.; Synytska, A. Facile preparation of superhydrophobic coatings by sol–gel processes. J. Colloid. Int. Sci. 2008, 325, 149–156. [Google Scholar] [CrossRef] [PubMed]
- Hooda, A.; Goyat, M.S.; Pandey, J.K.; Kumar, A.; Gupta, R. A review on fundamentals, constraints and fabrication techniques of superhydrophobic coatings. Prog. Org. Coat. 2020, 142, 10557. [Google Scholar] [CrossRef]
- Mishra, V.K.; Saini, R.; Kumar, N. A review on superhydrophobic materials and coating techniques. IOP Conf. Ser. Mater. Sci. Eng. 2021, 1168, 012026. [Google Scholar] [CrossRef]
- Simpson, J.T.; Hunter, S.R.; Aytug, T. Superhydrophobic materials and coatings: A review. Rep. Prog. Phys. 2015, 78, 086501. [Google Scholar] [CrossRef] [PubMed]
- Wei, X.; Niu, X. Recent Advances in Superhydrophobic Surfaces and Applications on Wood. Polymers 2023, 15, 1682. [Google Scholar] [CrossRef] [PubMed]
- Razavi, S.M.; Oh, J.; Sett, S.; Feng, L.; Yan, X.; Hoque, M.J.; Liu, A.; Haasch, R.T.; Masoomi, M.; Bagheri, R.; et al. Superhydrophobic Surfaces Made from Naturally Derived Hydrophobic Materials. ACS Sustain. Chem. Eng. 2017, 5, 11362–11370. [Google Scholar] [CrossRef]
- Shayesteh, H.; Khosrowshahi, M.S.; Mashhadimoslem, H.; Maleki, F.; Rabbani, Y.; Emrooz, H.B.M. Durable superhydrophobic/superoleophilic melamine foam based on biomass-derived porous carbon and multi-walled carbon nanotube for oil/water separation. Sci. Rep. 2023, 13, 4515–4531. [Google Scholar] [CrossRef] [PubMed]
- Halim, A.; Gabriel, A.A.; Ismayati MRayhan, P.L.N.; Azizah, U. Expanded Polystyrene Waste Valorization as a Superhydrophobic Membrane for Oil Spill Remediation. Waste Biomass Valor 2023, 14, 2025–2036. [Google Scholar] [CrossRef]
- Bayer, I.S. Superhydrophobic Coatings from Ecofriendly Materials and Processes: A Review. Adv. Mater. Interfaces 2020, 7, 2000095. [Google Scholar] [CrossRef]
- Saji, S.V. Superhydrophobic surfaces and coatings by electrochemical methods—A review. J. Adhes. Sci. Technol. 2022, 37, 137–161. [Google Scholar] [CrossRef]
- Shirtcliffe, N.J.; McHale, G.; Atherton, S.; Newton, M.I. An introduction to superhydrophobicity. Adv. Colloid Interface Sci. 2010, 161, 124–138. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, M.; Nishikawa, N.; Mayama, H.; Nonomura, Y.; Yokojima, S.; Nakamura, S.; Uchida, K. Theoretical Explanation of the Lotus Effect: Superhydrophobic Property Changes by Removal of Nanostructures from the Surface of a Lotus Leaf. Langmuir 2015, 31, 7355–7363. [Google Scholar] [CrossRef]
- Elzaabalawy, A.; Meguid, S.A. Advances in the development of superhydrophobic and icephobic surfaces. Int. J. Mech. Mater. Des. 2022, 18, 509–547. [Google Scholar] [CrossRef]
- Yong, H.; Li, Z.; Huang, X.; Wang, K.; Zhou, Y.-N.; Li, Q.; Shi, J.; Liu, M.; Zhou, D. Superhydrophobic Materials: Versatility and Translational Applications. Adv. Mater. Interfaces 2022, 9, 2200435. [Google Scholar] [CrossRef]
- Wang, D.; Sun, Q.; Hokkanen, M.J.; Zhang, C.; Lin, F.-Y.; Liu, Q.; Zhu, S.-P.; Zhou, T.; Chang, Q.; He, B.; et al. Design of robust superhydrophobic surfaces. Nature 2020, 582, 55–59. [Google Scholar] [CrossRef]
- Mohamed, M.E.; Adel, O.; Khamis, E. Fabrication of biochar-based superhydrophobic coating on steel substrate and its UV resistance, anti-scaling, and corrosion resistance performance. Sci. Rep. 2023, 13, 9453. [Google Scholar] [CrossRef]
- Zhang, L.; Zhou, A.G.; Sun, B.R.; Chen, K.S.; Yu, H.-Z. Functional and versatile superhydrophobic coatings via stoichiometric silanization. Nat. Commun. 2021, 12, 982. [Google Scholar] [CrossRef] [PubMed]
- Brown, S.; Lengaigne, J.; Sharifi, N.; Pugh, M.; Moreau, C.; Dolatabadi, A.; Martinu, L.; Klemberg-Sapieha, J.E. Durability of superhydrophobic duplex coating systems for aerospace applications. Surf. Coat. Technol. 2020, 401, 126249. [Google Scholar] [CrossRef]
- Ijaola, A.O.; Farayibi, P.K.; Asmatulu, E. Superhydrophobic coatings for steel pipeline protection in oil and gas industries: A comprehensive review. J. Nat. Gas. Sci. Eng. 2020, 83, 103544. [Google Scholar] [CrossRef]
- Ye, Z.; Li, S.; Zhao, S.; Deng, L.; Zhang, J.; Dong, A. Textile coatings configured by double-nanoparticles to optimally couple superhydrophobic and antibacterial properties. Chem. Eng. J. 2021, 420, 127680. [Google Scholar] [CrossRef]
- Wei, J.; Zhang, J.; Cao, X.; Jinhui, H.; Huang, X.; Zhang, J. Durable superhydrophobic coatings for prevention of rain attenuation of 5G/weather radomes. Nat. Commun. 2023, 14, 2862. [Google Scholar] [CrossRef]
- Falde, E.J.; Yohe, S.T.; Colson, Y.L.; Grinstaff, M.W. Superhydrophobic materials for biomedical applications. Biomaterials 2016, 104, 87–103. [Google Scholar] [CrossRef]
- Ramakrishna, S.; Santhosh Kumar, K.; Mathew, D.; Reghunadhan Nair, C.P. A robust, melting class bulk superhydrophobic material with heat-healing and self-cleaning properties. Sci. Rep. 2016, 5, 18510. [Google Scholar] [CrossRef]
- Liu, H.; Jiang, T.; Wang, F.; Ou, J.; Li, W. Thermochromic superhydrophobic coatings for building energy conservation. Energy Build. 2021, 251, 111374. [Google Scholar] [CrossRef]
- Liu, Y.; Lin, Z.; Luo, Y.; Wu, R.; Fang, R.; Umar, A.; Zhang, Z.; Zhao, Z.; Yao, J.; Zhao, S. Superhydrophobic MOF based materials and their applications for oil-water separation. J. Clean. Prod. 2023, 420, 138347. [Google Scholar] [CrossRef]
- Li, J.; Tian, J.; Gao, Y.; Qin, R.; Pi Hemu Li, M.; Yang, P. All-natural superhydrophobic coating for packaging and blood-repelling materials. Chem. Eng. J. 2021, 410, 128347. [Google Scholar]
- Hyman, M.; Turner, B.; Carpintero, A. Guidelines for National Waste Management Strategies. United Nations Environment Programme. Available online: https://wedocs.unep.org/bitstream/handle/20.500.11822/8669/-ies_%20moving%20from%20challenges%20to%20opportunities-2013UNEP%20NWMS%20English.pdf?sequence=3&isAllowed=y (accessed on 20 April 2023).
- Wen, G.; Huang, J.X.; Guo, Z.G. Energy-effective superhydrophobic nanocoating based on recycled eggshell. Colloids Surf. A Physicochem. Eng. Asp. 2019, 568, 20–28. [Google Scholar] [CrossRef]
- Castillo, J.; Galarza-Acosta, G.L. Superhydrophobic silica nanoparticles produced from rice husks, wettability at the macro- and nanoscale. Appl. Phys. A 2024, 130, 102. [Google Scholar] [CrossRef]
- Peng, X.; Jiang, Y.; Chen, Z.; Osman, A.I.; Farghali, M.; Rooney, D.W.; Yap, P.-S. Recycling municipal, agricultural and industrial waste into energy, fertilizers, food and construction materials, and economic feasibility: A review. Environ. Chem. Lett. 2023, 21, 765–801. [Google Scholar] [CrossRef]
- Malobi, S.; Sunirmal, J. Development of superhydrophobic coating from biowaste and natural wax. Mater. Today: Proc. 2022, 52, 1422–1428. [Google Scholar]
- Ncube, A.; Mtetwa, S.; Bukhari, M.; Fiorentino, G.; Passaro, R. Circular Economy and Green Chemistry: The Need for Radical Innovative Approaches in the Design for New Products. Energies 2023, 16, 1752. [Google Scholar] [CrossRef]
- Liu, Z.; de Souza, T.S.P.; Holland, B.; Dunshea, F.; Barrow, C.; Suleria, H.A.R. Valorization of Food Waste to Produce Value-Added Products Based on Its Bioactive Compounds. Processes 2023, 11, 840. [Google Scholar] [CrossRef]
- Vilas-Boas, A.A.; Pintado, M.; Oliveira, A.L.S. Natural Bioactive Compounds from Food Waste: Toxicity and Safety Concerns. Foods. 2021, 10, 1564. [Google Scholar] [CrossRef]
- Ma, Q.; Wu, Z.; Neacșu, V.A.; Zhao, S.; Chai, Y.; Zhang, H. Recycling plastic waste into multifunctional superhydrophobic textiles. Nano Res. 2022, 15, 9921–9925. [Google Scholar] [CrossRef]
- Lu, J.; Jiang, S.Y.; Chen, J.; Lee, C.-H.; Cai, Z.; Ruan, H.D. Fabrication of superhydrophobic soil stabilizers derived from solid wastes applied for road construction: A review. Transp. Geotech. 2023, 40, 100974. [Google Scholar] [CrossRef]
- Li, D.-C.; Xu, W.-F.; Cheng, H.-Y.; Xi, K.-F.; Xu, B.-D.; Jiang, H. One-Step Thermochemical Conversion of Biomass Waste into Superhydrophobic Carbon Material by Catalytic Pyrolysis. Glob. Chall. 2020, 4, 1900085. [Google Scholar] [CrossRef]
- Junaidi, M.U.M.; Ahmad, N.N.R.; Leo, C.P.; Yee, H.M. Near superhydrophobic coating synthesized from rice husk ash: Anti-fouling evaluation. Prog. Org. Coat. 2016, 99, 140–146. [Google Scholar] [CrossRef]
- Sathish, J.; Selvakumar, P. Rice husk modified cement strength-An environmental approach. J. Environ. Biol. 2019, 40, 807–811. [Google Scholar] [CrossRef]
- Borah, M.P.; Kalita, B.B.; Jose, S.; Baruah, S. Fabrication of Hydrophobic Surface on Eri Silk/Wool Fabric Using Nano Silica Extracted from Rice Husk. Silicon 2023, 15, 7039–7046. [Google Scholar] [CrossRef]
- Rafiee, E.; Shahebrahimi, S.; Feyzi, M.; Shaterzadeh, M. Optimization of synthesis and characterization of nano silica produced from rice husk a common waste material. Int. Nano Lett. 2012, 2, 29. [Google Scholar] [CrossRef]
- Qin, C.; Wang, W.; Li, W.; Zhang, S.; Li, Z. Developing bagasse towards superhydrophobic coatings. Cellulose 2021, 28, 3617–3630. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, D.; Deng, J.; Zhou, F.; Duan, Z.; Su, Q.; Pang, S. Mosquito’s Compound Eyes as Inspiration for Fabrication of Conductive Superhydrophobic Nanocarbon Materials from Waste Wheat Straw. ACS Sustain. Chem. Eng. 2019, 7, 3883–3894. [Google Scholar] [CrossRef]
- Liu, Z.; Feng, F.; Li, Y.; Sun, Y.; Tagawa, K. A corncob biochar-based superhydrophobic photothermal coating with micro-nano-porous rough-structure for ice-phobic properties. Surf. Coat. Technol. 2023, 457, 129299. [Google Scholar] [CrossRef]
- Liu, Z.; Li, Y.; Sun, Y.; Feng, F.; Tagawa, K. Preparation of biochar-based photothermal superhydrophobic coating based on corn straw biogas residue and blade anti-icing performance by wind tunnel test. Renew. Energ. 2023, 210, 618–626. [Google Scholar] [CrossRef]
- Topić Popović, N.; Lorencin, V.; Strunjak-Perović, I.; Čož-Rakovac, R. Shell Waste Management and Utilization: Mitigating Organic Pollution and Enhancing Sustainability. Appl. Sci. 2023, 13, 623. [Google Scholar] [CrossRef]
- Saleem, J.; Moghal, Z.K.; Sun, L.; McKay, G. Valorization of mixed plastics waste for the synthesis of flexible superhydrophobic films. Adv. Compos. Hybrid Mater. 2024, 7, 11. [Google Scholar] [CrossRef]
- Pang, B.; Zheng, H.; Jin, Z.; Hou, D.; Zhang, Y.; Song, X.; Sun, Y.; Liu, Z.; She, W.; Yang, L.; et al. Inner superhydrophobic materials based on waste fly ash: Microstructural morphology of microetching effects. Compos. Part B Eng. 2024, 268, 111089. [Google Scholar] [CrossRef]
- Wang, B.; Yu, P.; Yang, Q.; Jing, Z.; Wang, W.; Li, P.; Tong, X.; Lin, F.; Wang, D.; Lio, G.E.; et al. Upcycling of biomass waste into photothermal superhydrophobic coating for efficient anti-icing and deicing. Mater. Today Phys. 2022, 24, 100683. [Google Scholar] [CrossRef]
- Roy, S.; Goh, K.-L.; Verma, C.; Ghosh, B.D.; Sharma, K.; Maji, P.K. A Facile Method for Processing Durable and Sustainable Superhydrophobic Chitosan-Based Coatings Derived from Waste Crab Shell. ACS Sustain. Chem. Eng. 2022, 10, 4694–4704. [Google Scholar] [CrossRef]
- Antunes, F.A.F.; Santos, J.C.; Chandel, A.K.; Carrier, D.J.; Peres, G.F.D.; Milessi, T.S.S.; da Silva, S.S. Repeated batches as a feasible industrial process for hemicellulosic ethanol production from sugarcane bagasse by using immobilized yeast cells. Cellulose 2019, 26, 3787–3800. [Google Scholar] [CrossRef]
- Thai, Q.B.; Nguyen, S.T.; Ho, D.K.; Tran, T.D.; Huynh, D.M.; Do, N.H.N.; Luu, T.P.; Le, P.K.; Le, D.K.; Phan-Thien, N.; et al. Cellulose-based aerogels from sugarcane bagasse for oil spill-cleaning and heat insulation applications. Carbohyd Polym. 2020, 228, 115365. [Google Scholar] [CrossRef]
- Rezende, C.A.; Lima, M.A.; Maziero, P.; Azevedo, E.R.; Garcia, W.; Polikarpov, I. Chemical and morphological characterization of sugarcane bagasse submitted to a delignification process for enhanced enzymatic digestibility. Biotechnol. Biofuels 2011, 4, 1–18. [Google Scholar] [CrossRef]
- Huang, C.; Wu, H.; Li, R.F.; Zong, M.H. Improving lipid production from bagasse hydrolysate with Trichosporon fermentans by response surface methodology. New Biotechnol. 2012, 29, 372–378. [Google Scholar] [CrossRef]
- Mulinari, D.R.; Voorwald, H.J.C.; Cioffi, M.O.H.; Rocha, G.J.; Da Silva, M.L.C.P. Surface modification of sugarcane bagasse cellulose and its effect on mechanical and water absorption properties of sugarcane bagasse cellulose/HDPE composites. BioResources 2010, 5, 661–671. [Google Scholar] [CrossRef]
- Payá, J.; Monzó, J.; Borrachero, M.V.; Tashima, M.M.; Soriano, L. 17—Bagasse ash. In Woodhead Publishing Series in Civil and Structural Engineering, Waste and Supplementary Cementitious Materials in Concrete; Siddique, R., Cachim, P., Eds.; Woodhead Publishing: Cambridge, UK, 2018; pp. 559–598. [Google Scholar]
- Sun, X.F.; Sun, R.C.; Sun, J.X. Acetylation of sugarcane bagasse using NBS as a catalyst under mild reaction conditions for the production of oil sorption-active materials. Bioresour. Technol. 2004, 95, 343–350. [Google Scholar] [CrossRef]
- Said, A.E.A.A.; Ludwick, A.G.; Aglan, H.A. Usefulness of raw bagasse for oil absorption: A comparison of raw and acylated bagasse and their components. Bioresour. Technol. 2009, 100, 2219–2222. [Google Scholar] [CrossRef]
- Abdelwahab, N.A.; Shukry, N.; El-kalyoubi, S.F. Preparation and characterization of polymer coated partially esterified sugarcane bagasse for separation of oil from seawater. Environ. Technol. 2017, 38, 1905–1914. [Google Scholar] [CrossRef]
- Lei, W.W.; Li, H.; Shi, L.Y.; Diao, Y.F.; Zhang, Y.L.; Ran, R.; Ni, W. Achieving enhanced hydrophobicity of graphene membranes by covalent modification with polydimethylsiloxane. Appl. Surf. Sci. 2017, 404, 230–237. [Google Scholar] [CrossRef]
- Guo, Y.; Xu, G.; Yang, X.; Ruan, K.; Ma, T.; Zhang, Q.; Gu, J.; Wu, Y.; Liu, H.; Guo, Z. Significantly enhanced and precisely modelled thermal conductivity in polyimide nanocomposites with chemically modified graphene via in situ polymerization and electrospinning-hot press technology. J. Mater. Chem. C 2018, 6, 3004–3015. [Google Scholar] [CrossRef]
- Ezejiofor TI, N.; Enebaku, U.E.; Ogueke, C. Waste to wealthvalue recovery from agro-food processing wastes using biotechnology: A review. Br. Biotechnol. J. 2014, 4, 418–481. [Google Scholar] [CrossRef]
- Sevilla, M.; Al-Jumialy AS, M.; Fuertes, A.B.; Mokaya, R. Optimization of the Pore Structure of Biomass-Based Carbons in Relation to Their Use for CO2 Capture under Low-and High-Pressure Regimes. ACS Appl. Mater. Interfaces 2018, 10, 1623–1633. [Google Scholar] [CrossRef]
- Akbarian, A.; Andooz, A.; Kowsari, E.; Ramakrishna, S.; Asgari, S.; Cheshmeh, Z.A. Challenges and opportunities of lignocellulosic biomass gasification in the path of circular bioeconomy. Bioresour. Technol. 2022, 362, 127774. [Google Scholar] [CrossRef]
- Chen, Z.; Chen, B.; Zhou DChen, W. Bisolute sorption and thermodynamic behavior of organic pollutants to biomass-derived biochars at two pyrolytic temperatures. Environ. Sci. Technol. 2012, 46, 12476–12483. [Google Scholar] [CrossRef]
- Lee, E.; Kim, D.H. Simple fabrication of asphalt-based superhydrophobic surface with controllable wetting transition from Cassie-Baxter to Wenzel wetting state. Colloids Surf. Physicochem. Eng. Aspects 2021, 625, 126927. [Google Scholar] [CrossRef]
- Zhu, Q.; Song, J.; Liu, Z.; Wu, K.; Li, X.; Chen, Z.; Pang, H. Photothermal catalytic degradation of textile dyes by laccase immobilized on Fe3O4@SiO2 nanoparticles. J. Colloid. Interface Sci. 2022, 623, 992–1001. [Google Scholar] [CrossRef]
- Amiri, H.; Aghbashlo, M.; Sharma, M.; Gaffey, J.; Manning, L.; Basri SM, M.; Kennedy, J.F.; Gupta, V.K.; Tabatabaei, M. Chitin and chitosan derived from crustacean waste valorization streams can support food systems and the UN Sustainable Development. Goals. Nat. Food. 2022, 3, 822–828. [Google Scholar] [CrossRef]
- Zhao, Y.; Wang, Q.; Li, X.; Shi, Z.; Zhang, H.; Song, Q. Study of Self-Cleaning and Anticorrosion Superhydrophobic Coating on Cement Mortar Using Milled Coral Waste Powder. J. Mater. Civ. Eng. 2023, 35, 04023346. [Google Scholar] [CrossRef]
- González-Victoriano, L.; Chanona-Pérez, J.J.; Arredondo-Tamayo, B.; Gallegos-Cerda, S.D.; Hernández-Varela, J.D.; Galvan-Colorado, C. Superhydrophobic Coatings from Eggshell Waste Micro and Nanoparticles, Surface Characterization using Image Texture Analysis, Light, and Confocal Microscopy. Microsc. Microanal. 2023, 29, 815–817. [Google Scholar] [CrossRef]
- Abdelgalil, S.A.; Abo-Zaid, G.A. Bioprocess development as a sustainable platform for eco-friendly alkaline phosphatase production: An approach towards crab shells waste management. Microb. Cell Fact. 2022, 21, 141. [Google Scholar] [CrossRef]
- Caldona, E.B.; Albayalde, J.M.C.; Aglosolos, A.M.P.; Bautista, K.S.; Tavora, M.D.; Cabalza SA, P.; Diaz JR, O.; Mulato Michelle, D. Titania-Containing Recycled Polypropylene Surfaces with Photo-Induced Reversible Switching Wettability. J. Polym. Environ. 2019, 27, 1564–1571. [Google Scholar] [CrossRef]
- Sow, P.K.; Singhal, R.; Sahoo, P.; Radhakanth, S. Fabricating low-cost, robust superhydrophobic coatings with re-entrant topology for self-cleaning, corrosion inhibition, and oil-water separation. J. Colloid. Interface Sci. 2021, 600, 358–372. [Google Scholar] [CrossRef]
- Chindaprasirt, P.; Jitsangiam, P.; Pachana, P.K.; Rattanasak, U. Self-cleaning superhydrophobic fly ash geopolymer. Sci. Rep. 2023, 13, 44. [Google Scholar] [CrossRef]
- Ren, T.; Wang, Y.; Fu, X.; Jiang, L.; Yuan, A.; Wei, Z.; Xu, H.; Lei, J.; He, P.; Xiao, Y. A superhydrophobic material based on an industrial solid waste for oil/water separation. Can. J. Chem. Eng. 2022, 100, 1771. [Google Scholar] [CrossRef]
- Yue, X.; Wu, H.; Zhang, T.; Yang, D.; Qiu, F. Superhydrophobic waste paper-based aerogel as a thermal insulating cooler for building. Energy 2022, 245, 123287. [Google Scholar] [CrossRef]
- Uddin, M.N.; Desai, F.J.; Asmatulu, E. Biomimetic electrospun nanocomposite fibers from recycled polystyrene foams exhibiting superhydrophobicity. Energ. Ecol. Environ. 2020, 5, 1–11. [Google Scholar] [CrossRef]
- Ye, M.; Tian, Z.; Wang, S.; Ji, X.; Wang, D.; Ci, X. Simple preparation of environmentally friendly and durable superhydrophobic antibacterial paper. Cellulose 2023, 30, 2427–2440. [Google Scholar] [CrossRef]
- Kim, T.; Song, M.G.; Kim, K.; Jeon, H.; Kim, G.H. Recyclable Superhydrophobic Surface Prepared via Electrospinning and Electrospraying Using Waste Polyethylene Terephthalate for Self-Cleaning Applications. Polymers 2023, 15, 3810. [Google Scholar] [CrossRef]
- Saharudin, K.A.; Sreekantan, S.; Basiron, N.; Chun, L.K.; Kumaravel, V.; Abdullah, T.K.; Ahmad Zainal, A. Improved super-hydrophobicity of eco-friendly coating from palm oil fuel ash (POFA) waste. Surf. Coat. Technol. 2018, 337, 126–135. [Google Scholar] [CrossRef]
- Sreekantan, S.; Hassan, M.; Sundera Murthe, S.; Seeni, A. Biocompatibility and Cytotoxicity Study of Polydimethylsiloxane (PDMS) and Palm Oil Fuel Ash (POFA) Sustainable Super-Hydrophobic Coating for Biomedical Applications. Polymers 2020, 12, 3034. [Google Scholar] [CrossRef]
- Gil-Jasso, N.D.; Giles-Mazón, E.A.; Soriano-Giles, G.; Reinheimer, E.W.; Varela-Guerrero, V.; Ballesteros-Rivas, M.F. A methodology for recycling waste expanded polystyrene using flower essential oils. Fuel 2022, 307, 121835. [Google Scholar] [CrossRef]
- Bläsing, M.; Amelung, W. Plastics in soil: Analytical methods and possible sources. Sci. Total Environ. 2018, 612, 422–435. [Google Scholar] [CrossRef]
- Hakur, S.; Verma, A.; Sharma, B.; Chaudhary, J.; Tamulevicius, S.; Thakur, V.K. Recent developments in recycling of polystyrene based plastics. Curr. Opin. Green. Sustain. Chem. 2018, 13, 32–38. [Google Scholar]
- Hamad, K.; Kaseem, M.; Deri, F. Recycling of waste from polymer materials: An overview of the recent works. Polym. Degrad. Stab. 2013, 98, 2801–2812. [Google Scholar] [CrossRef]
- Verma, R.; Vinoda, K.S.; Papireddy, M.; Gowda, A.N.S. Toxic pollutants from plastic waste-a review. Procedia Environ. Sci. 2016, 35, 701–708. [Google Scholar] [CrossRef]
- Gul, E.; Alrawashdeh, K.A.; Masek, O.; Skreiberg, Ø.; Corona, A.; Zampilli, M.; Wang, L.; Samaras, P.; Yang, Q.; Zhou, H.; et al. Production and use of biochar from lignin and lignin-rich residues (such as digestate and olive stones) for wastewater treatment. J. Anal. Appl. Pyrolysis 2021, 158, 105263. [Google Scholar] [CrossRef]
- Oni, B.A.; Oziegbe, O.; Olawole, O.O. Significance of biochar application to the environment and economy. Ann. Agric. Sci. 2019, 64, 222–236. [Google Scholar] [CrossRef]
- Anand, A.; Gautam, S.; Ram, L.C. Feedstock and pyrolysis conditions affect suitability of biochar for various sustainable energy and environmental applications. J. Anal. Appl. Pyrolysis 2023, 170, 105881. [Google Scholar] [CrossRef]
- Guo, H.; Liu, M.; Xie, C.; Zhu, Y.; Sui, X.; Wen, C.; Li, Q.; Zhao, W.; Yang, J.; Zhang, L. A sunlight-responsive and robust anti-icing/deicing coating based on the amphiphilic materials. Chem. Eng. J. 2020, 402, 126161. [Google Scholar] [CrossRef]
- Liu, Y.; Huang, R.; Hu, W.; Lin, L.; Liu, J.; Wang, Q.; Wang, D.; Wu, Z.; Zhang, J. High performance photothermal conversion of sludge derived biochar and its potential for peroxydisulfate-based advanced oxidation processes. Sep. Purif. Technol. 2022, 303, 122214. [Google Scholar] [CrossRef]
- Dunster, A.M. Paper Sludge and Paper Sludge Ash in Portland Cement Manufacture; MinRes Case Study; Building Research Establishment (BRE): Watford, UK, 2007. [Google Scholar]
- Azrizal, M.F.; Noorsuhada, M.N.; Latif, M.F.P.M.; Arshad, M.F.; Sulaiman, H. The properties of wastepaper sludge ash and its generic applications. In Proceedings of the International Conference on Nanomaterials: Science, Engineering and Technology (ICoNSET), Penang Island, Malaysia, 5–6 August 2019; Volume 1349. [Google Scholar]
- Ahmad, S.; Malik, M.I.; Wani, M.B.; Ahmad, R. Study of Concrete Involving Use of Waste Paper Sludge Ash as Partial Replacement of Cement. IOSR J. Eng. 2013, 3, 6–15. [Google Scholar] [CrossRef]
- Segui, P.; Aubert, J.E.; Husson, B.; Measson, M. Characterisation of wastepaper sludge ash for its valorisation as a component of hydraulic binders. Appl. Clay Sci. 2012, 57, 79–85. [Google Scholar] [CrossRef]
- Spathi, C. Resource Efficient Reuse Applications for Paper Sludge Ash. Ph.D. Thesis, Imperial College, London, UK, 2013. [Google Scholar]
- Young, N. Development of Hydrophobic Coatings from Paper Sludge Ash. Master’s Thesis, Imperial College, London, UK, 2013. [Google Scholar]
- Spathi, C.; Young, N.; Heng, J.Y.Y.; Vandeperre, L.J.M.; Cheeseman, C.R. A simple method for preparing super-hydrophobic powder using paper sludge ash. Mater. Lett. 2015, 142, 80–83. [Google Scholar] [CrossRef]
- Wong, H.S.; Barakat, R.; Alhilali, A.; Saleh, M.; Cheeseman, C.R. Hydrophobic concrete using waste paper sludge ash. Cem. Concr. Res. 2015, 70, 9–20. [Google Scholar] [CrossRef]
- Carvalho, S.; Vernilli, F.; Almeida, B.; Oliveira, M.; Silva, S. Reducing environmental impacts: The use of basic oxygen furnace slag in portland cement. J. Clean. Prod. 2018, 172, 385–390. [Google Scholar] [CrossRef]
- Al-Jabari, M. 1—Introduction to concrete chemistry. In Woodhead Publishing Series in Civil and Structural Engineering, Integral Waterproofing of Concrete Structures; Woodhead Publishing: Cambridge, UK, 2022; pp. 1–36. ISBN 9780128243541. [Google Scholar]
- Qu, Z. Design and Performance of Water Resistant Cementitious Materials: Mechanisms, Evaluation and Applications. Ph.D. Thesis, 1 (Research TU/e/Graduation TU/e), Built Environment. Technische Universiteit Eindhoven, Eindhoven, The Netherlands, 2020. [Google Scholar]
- Hamada, H.M.; Jokhio, G.A.; Yahaya, F.M.; Humada, A.M.; Gul, Y. The present state of the use of palm oil fuel ash (POFA) in concrete. Constr. Build Mater. 2018, 175, 26–40. [Google Scholar] [CrossRef]
- Ma, Y.; Zhang, J.; Zhu, G.; Gong, X.; Wu, M. Robust photothermal self-healing superhydrophobic coating based on carbon nanosphere/carbon nanotube composite. Mater. Des. 2022, 221, 110897. [Google Scholar] [CrossRef]
- Wang, R.; Wang, X.; Sun, Y. One-step synthesis of self-doped carbon dots with highly photoluminescence as multifunctional biosensors for detection of iron ions and pH. Sens. Actuators B-Chem. 2017, 241, 73–79. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).