3.1. Geopolymer Characterization
GP samples were prepared following the procedure described in the experimental section. The following amount of binder was used for the preparation of 4 prismatic samples (4 × 4 × 16 cm
3): 517.5 g MK, 899.3 g Na
2SiO
3 and 107 g 10 M NaOH. Different GW additions were considered in the study as shown in
Table 3. The GP were named as G (without H
2O
2 addition) and P (with 1% H
2O
2 addition).
3.1.1. Chemical and Microstructural Testing
Figure 1 shows FTIR spectra of geopolymer G-0, used as reference. The test was performed to assure the proper alkaline activation of the MK.
According to the literature, the band identified at 3363 cm
−1 was assigned to stretching vibration of O-H groups, and that 1645 cm
−1, were attributed to O-H bending, the water molecules presented in the chemical structure of the geopolymer [
57]. The presence of weak carbonate traces at 1397.17 cm
−1 was identified by the C-O bond stretching. [
58]. Unreacted Na
+ ions from the alkaline activator react with atmospheric CO
2 to produce NaCO
3 in a process known as carbonation. The spectra showed as well a strong band at 967 cm
−1 associated with T–O–Si (T:Al or Si) asymmetric stretching vibrations typical of geopolymer reaction [
57,
59,
60]. The feature at 674 cm
−1 were characteristic symmetric stretching vibrations of T-O-T bonds [
57,
60]. Additionally, the band positioned around 561 cm
−1 was a characteristic feature attributed to the formation of double four-membered rings in zeolite structure demonstrating the formation of this aluminosilicate as other reaction products [
56,
61].
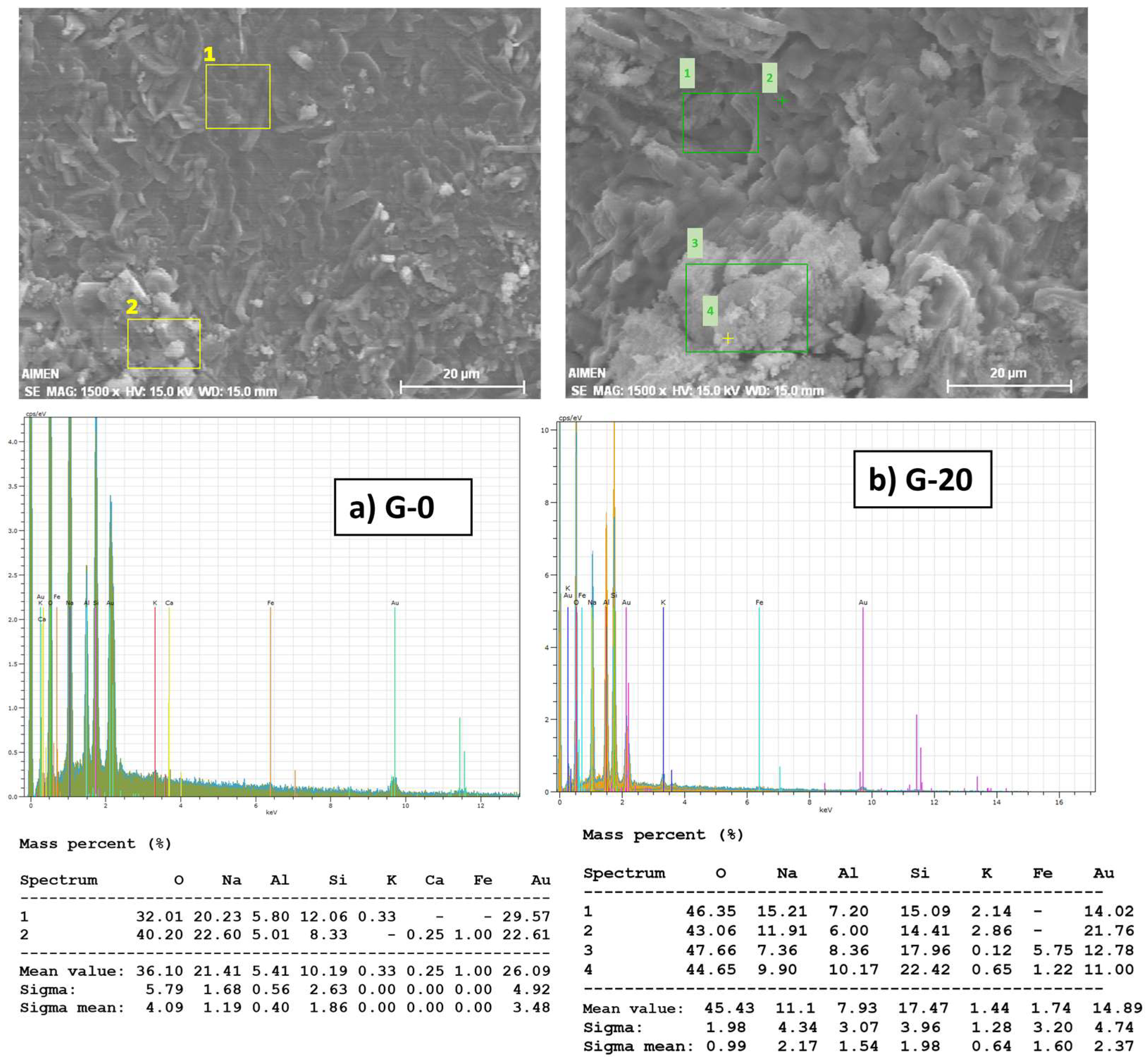
Figure 2 shows the SEM/EDX spectra for G-0 and G-20 formulations after 28 curing days. There are no significant changes in the microstructure and composition of the GP with the addition of GW. The actual composition of the geopolymer gel could not be measured but the silicon to aluminum molar ratio can be estimated to be in the range between 1 and 3, as expected from the formation of a geopolymer material [
62]. The results obtained were Si/Al = 1.88 and Si/Al= 2.2 respectively for G-0 and G-20. The increment in G-20 is due to the presence of GW particles, quartz-enriched, that are not part of the gel.
The uniformity of the geopolymer matrix is clearer in G-0 whereas unreacted GW irregular and sharp angle particles appear accumulated in the amorphous for G-20 (points 1 and 2). These heterogeneities inside the microstructure reasonably are expected to cause a strength decrease for this material. EDX spectrum collected from these particle aggregations (point 3 and 4) confirmed the attribution to GW, according to the higher Si and Al content and the presence of Fe.
EDX also confirms the presence of Na in both materials coming from the alkaline activator, which is the main responsible for the ion exchange process. Na concentration is slightly reduced in G-20 possibly due to a slower reaction progress and a higher amount of unreacted NaOH, promoting the formation of NaCO
3 (efflorescence) which is easily removed through the open pores of the material [
58].
DRX patterns for G-0, G-10 and G-20 are depicted in
Figure 3. The microstructure of G-0 is mainly amorphous, presenting the typical hump located between 20–35° 2θ which corresponds to the aluminosilicate gel that forms the main binding phase of the GP matrix, responsible for the mechanical strength. A crystalline peak is clearly revealed when GW is introduced in the mixture, at a 26° 2θ. This sharp peak is the main signal of quartz (SiO
2) which intensity increases with GW incorporation, affecting the GP gel microstructure and, in consequence, the mechanical strength as will be seen in the following section. Other mineral phases identified with the introduction of GW are biotite (K(MgFe)
3AlSi
3O
10(OH,F)
2), muscovite (KAl
2(Si
3Al)O
10(OH,F)
2) and albite (NaAlSi
3O
8), typically found in granitic rocks.
3.1.2. Mechanical Testing
Figure 4 shows the compressive and flexural strengths obtained for the different GPs after 28 days-age. The results revealed that GW additions do not significantly affect the mechanical strength of the GP up to 20% of additions. The mechanical strength is reduced by 5%, according to the slight structural changes observed by SEM. Particularly noteworthy is the value reached with 20% of GW (58 MPa) for the compressive resistance.
However, the decrease is dramatic with additions of 40% and 60% (39% and 87% respectively). The presence of unreacted GW particles and the consequent reduction of the amorphous gel fraction led to lower mechanical strengths.
As expected, the foaming agent addition promoted a dramatic drop of the mechanical strength as well. Porous samples presented smaller values of both flexural and compressive resistance. For the case of 20% of GW, the compressive strength decreases down to 6.7 MPa (P-20), 93% lower than the non-porous G-20. The pores act as the stress concentration points and are prone to failure when load is applied [
63]. Therefore, it is expected a strong linking between mechanical performance and density and porosity depicted in
Table 4.
3.1.3. Physical Testing
Bulk density increased with GW additions; the filler occupied the empty voids and promoted a decrease in the porosity values. In contrast, with the 1% introduction H
2O
2, the porosity % increased by a factor of 10 and the bulk density decreased due to the air voids formation obtaining a lighter material [
64].
Additionally, porous GPs with different GW additions were immersed in water for 24 h to validate the chemical integrity of the formed gel matrix. This test allowed to confirm that GP (non-porous and porous) with GW additions up to 20% maintained the consolidation after the immersion time. On the contrary, GPs with higher GW content (>20%) were turned into dust after testing revealing a non-acceptable GP gel reaction due to the high crystalline contribution of the GW. Therefore, they were discarded, and P-20 formulation was selected as the material for further adsorption studies.
Figure 5 presents the aspect of the GP P-20 and
Table 5 summarizes the synthesis parameters and their properties.
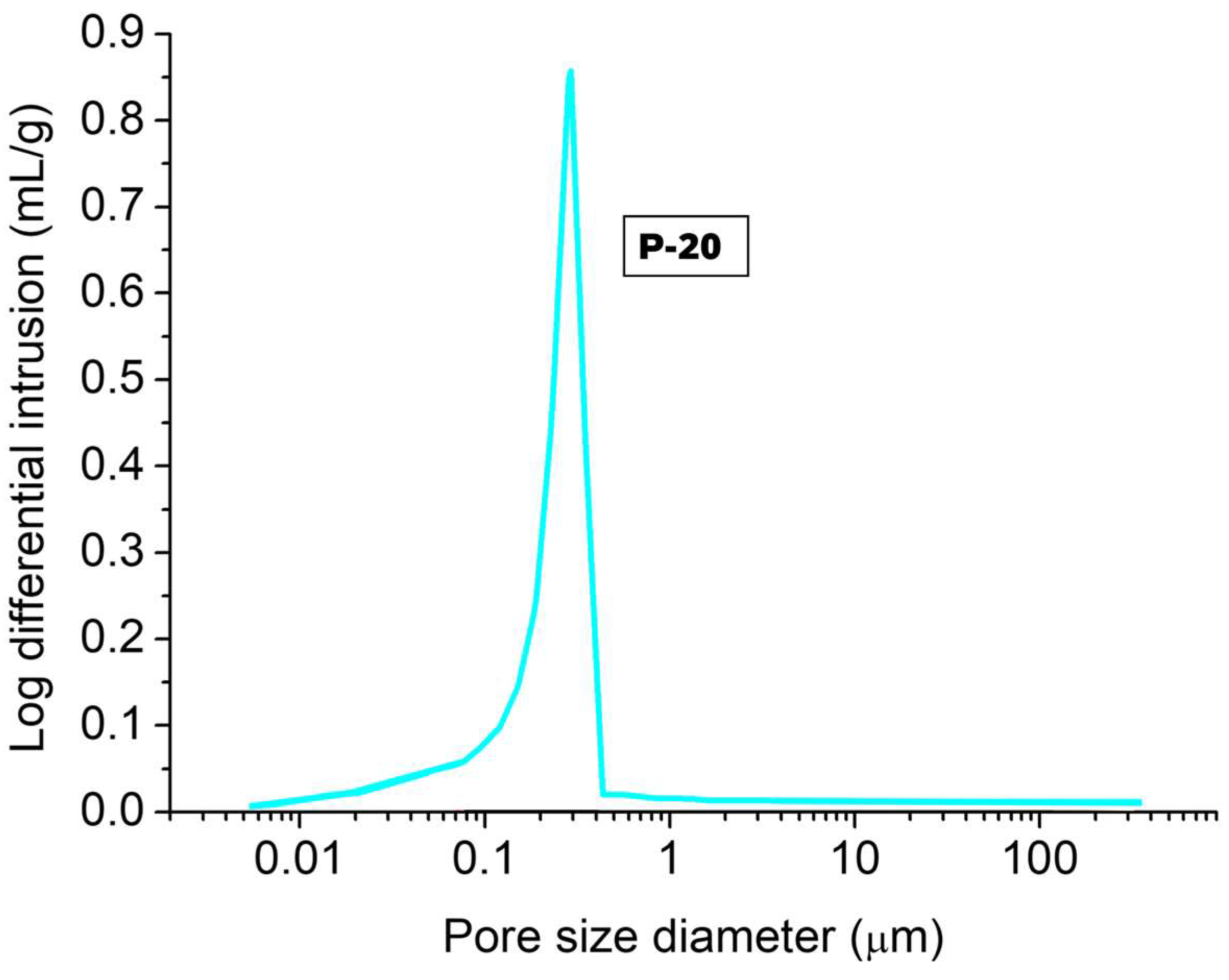
The pore size distribution of P-20 is illustrated in
Figure 6 by a differential volume intrusion graph with the corresponding critical pore size. The critical pore size is located between 0.1–0.4 µm for this material at 28 days-age. The results indicated that mesopores (6–50 nm), macropores (0.05–5 µm) and air voids (>5 µm) [
65] dominated the pore distribution, resulting in an average pore size of 0.27 µm.
Larger pores (air voids) were microscopically identified formed by a phenomenon called coalescence. With the introduction of oxygen bubbles by chemical foaming, the nucleation particles become weaker, and the bubbles start to coalesce, thus increasing the overall pore size and interconnectivity [
66].
3.1.4. Behavior in Aqueous Media
Leaching tests of P-20 (10 g/L) were performed to determine the release of ionic species in aqueous solution. The aim is to guarantee the absence of NH
4+ over time and identify the potential pH increase due to the alkalinity (OH
− release) of the material. The results are detailed in
Table 6.
It can be observed that within the first hour of contact there was a considerable increase in pH from 5.6 (initial condition) to 9.1. This was due to the unreacted NaOH which was physically adsorbed or weakly bonded and easily leached through the GP microstructure [
10,
30]. pH increased over time until it reached a value of 10.1 after 24 h of immersion. Alkalis leaching could also be demonstrated by the increase of [Na
+] over time. Same time-dependent behavior was verified by Aly et al. [
67]. As expected, an increase in the electrical conductivity was also obtained due to the leaching of ions, especially the Na
+. Small traces of Cl
− and SO
4−2 were also found in this test, but NH
4+ ion leaching was not detected.
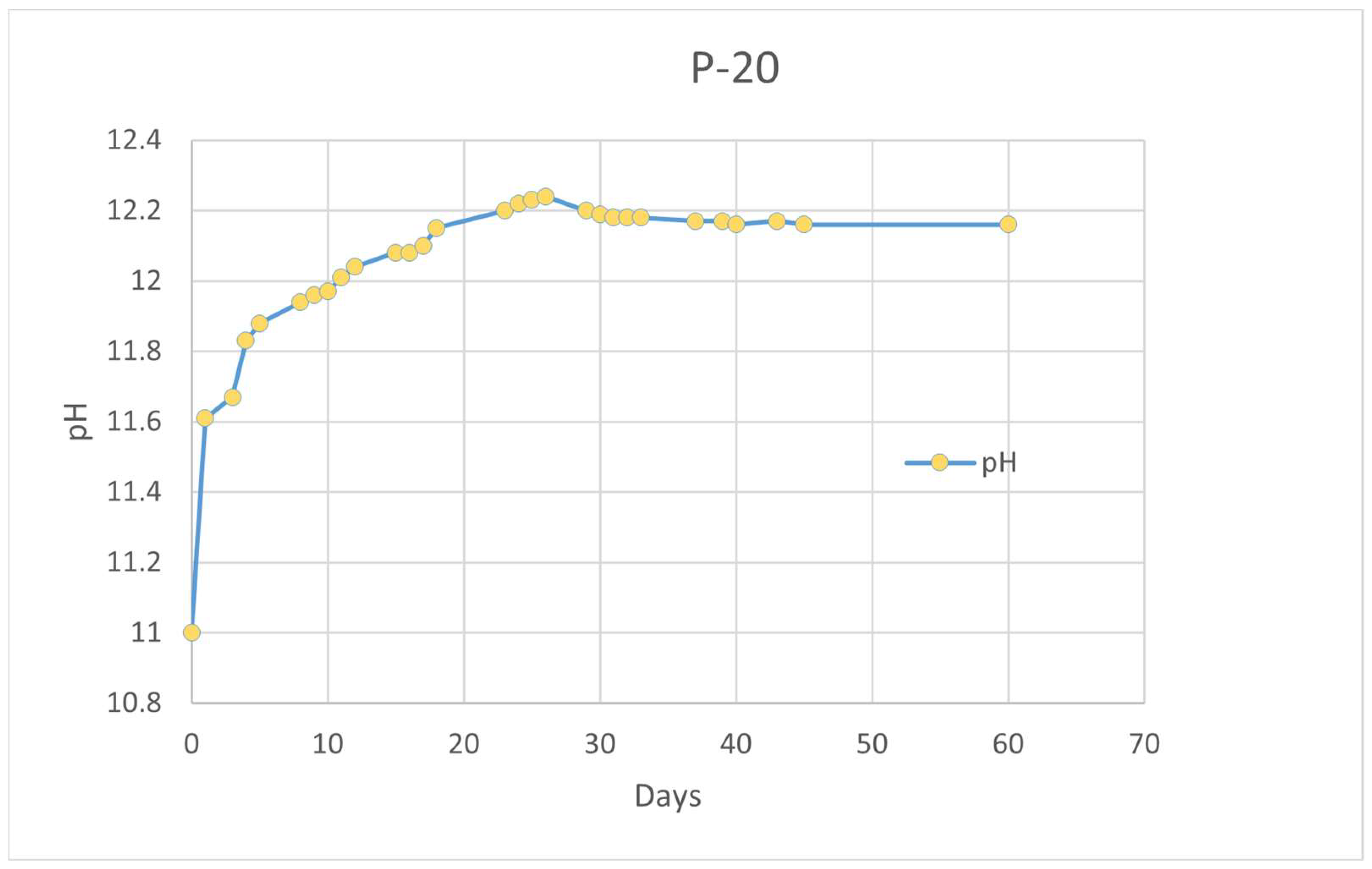
A pH control test was carried out in deionized water in a solution with 120 g/L of P-20 and for 60 days. The results are shown in
Figure 7.
The initial pH was 11 according to the higher concentration of P-20 in comparison with the previous test (10 g/L). A significant increase was detected during the first 24 h up to pH = 11.6. The trend continued to increase until 25 days, where a maximum of pH = 12.27 was reached. In good agreement with the previous leaching tests, the initial increment can be explained due to the presence of unreacted NaOH release.
Then, an asymptotic behavior was achieved up to 60 days. A similar tendency was found by Novais et al. [
10,
68] suggesting the potential pH-buffering effect of the porous MK-based GP since they could enclose free leachable alkalis in the pores. The mechanical integrity of P-20 remained intact after 60 days.
It is clear that this increasing pH trend is going to be dependent on factors such as the nature and concentration of the activator [
69], geopolymers porosity [
68], H
2O
2 content [
68] and liquid-to-solid ratio [
70].
However, initial alkali leaching supposes the most critical issue to implement the material in wastewater treatments since legislation pH limits (pH between 5.5 and 9) represent a fundamental requirement for waste-derived products. Thus, rinsing treatments for pH decreasing were evaluated before the material implementation.
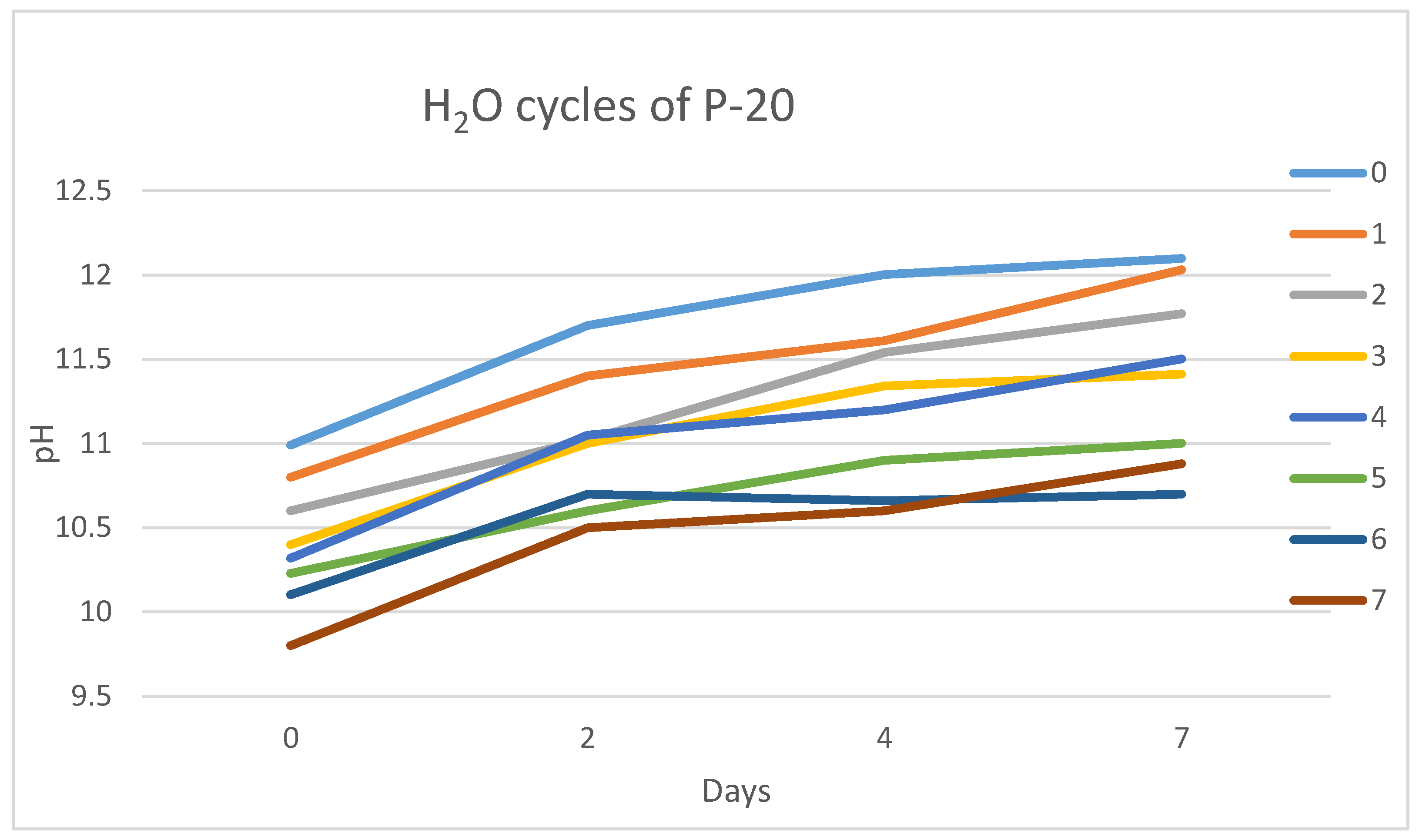
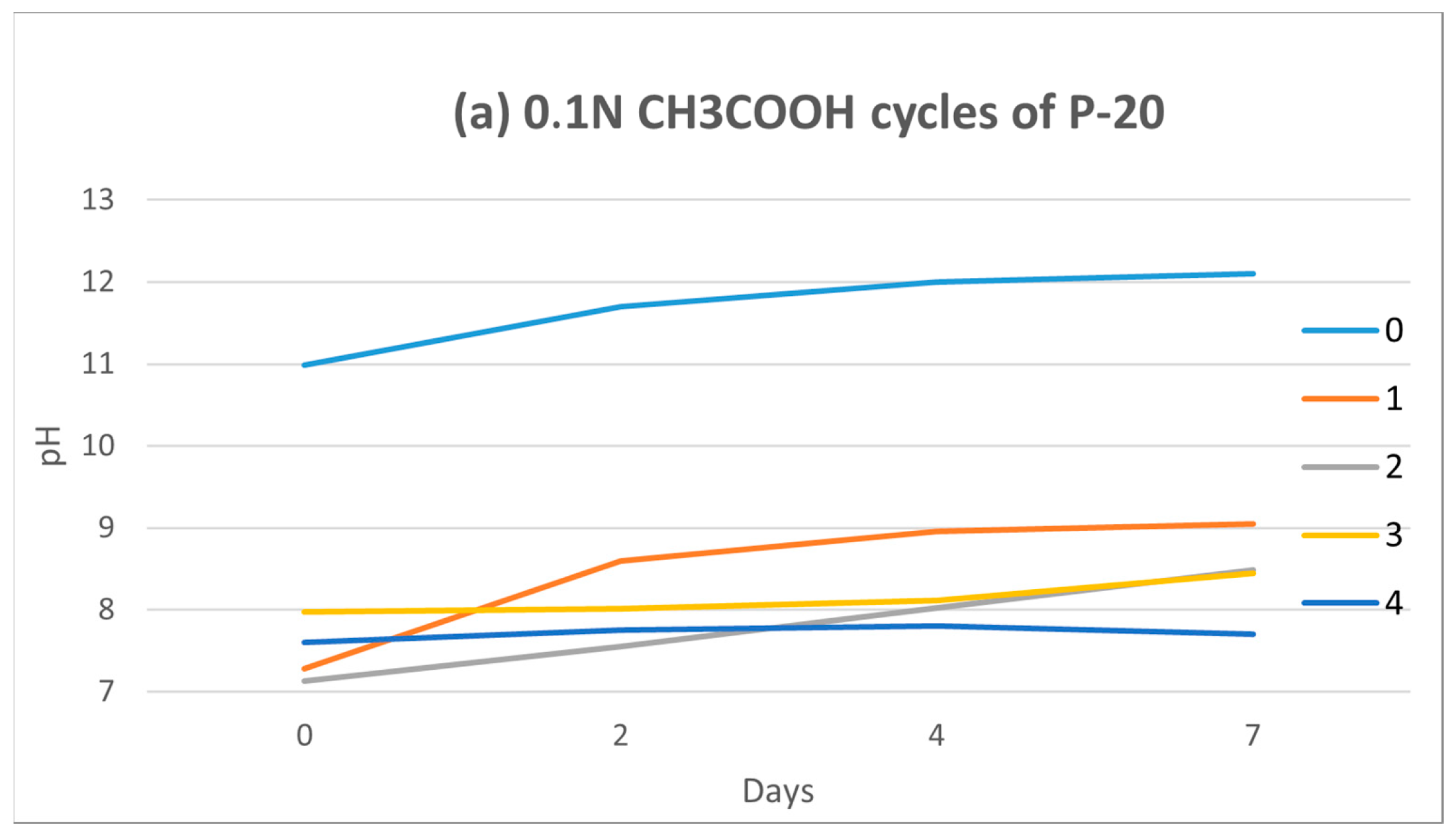
Figure 8 shows the effect of the cyclic immersion of P-20 granules in water.
The proposed cleansing treatment induced a slow pH drop after each cycle. The pH value was reduced by one unit each monitored day (0, 2, 4 and 7). However, the tendency is increasing over time (from 0 to 7 days), so P-20 continued to leach alkalis at lower concentrations.
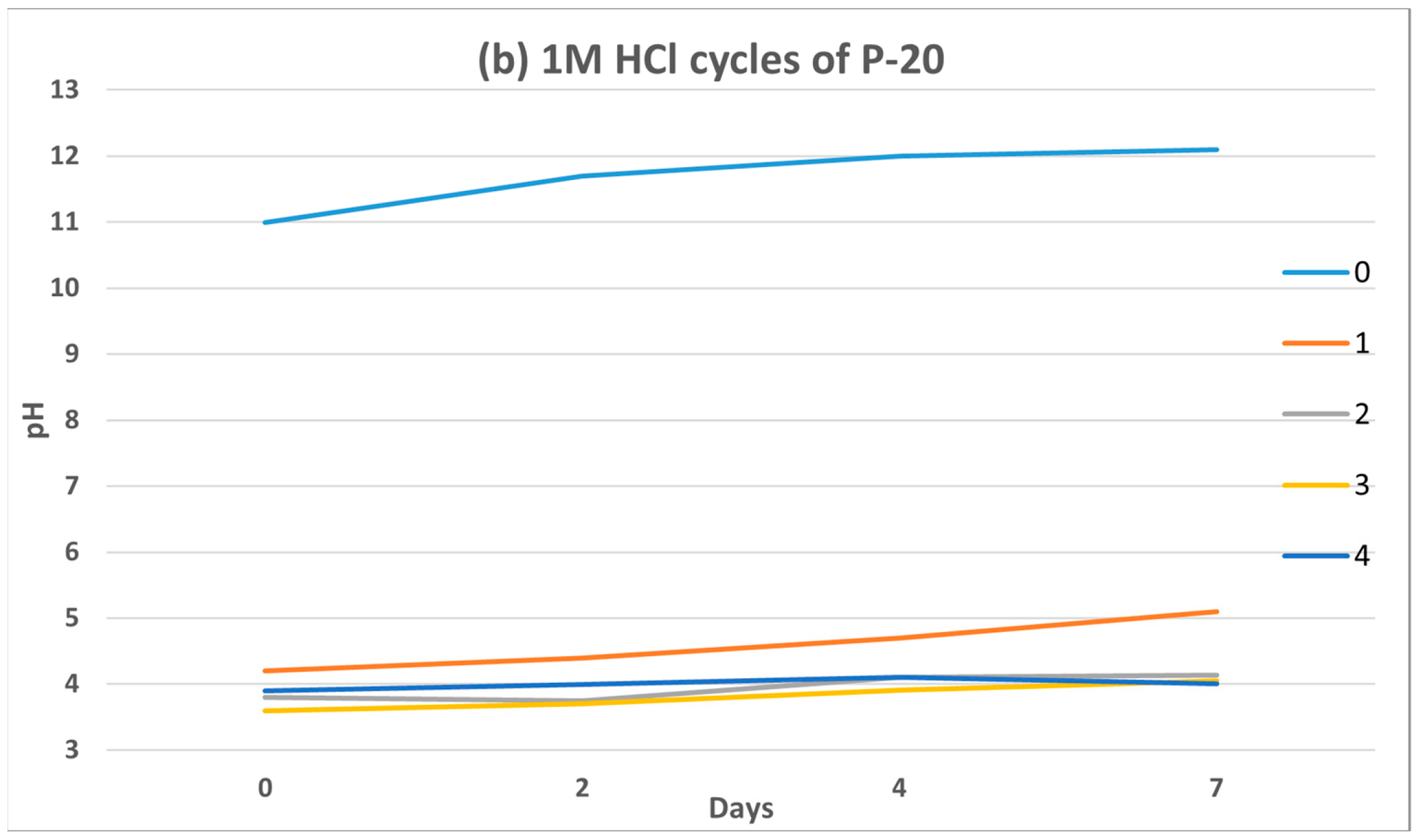
In order to diminish the P-20 effect on the leachate pH, two alternative acid treatments have been considered. It is well-known the good acid resistance properties of GP [
71], maintaining their consistency and mechanical integrity after acid attack. Based on this, P-20 was immersed for 2 h in (a) 0.1 N glacial acetic acid (weak acid) and (b) 1 M hydrochloric acid (strong acid) during 4 cycles. Results obtained after 7 days are depicted in
Figure 9.
The acetic acid (pH = 2.9) produced a complete neutralization of the structure from the beginning of the test, reaching a pH close to 7. In the case of 1 M HCl (pH = 0) the drop was even more appreciable. According to Aly et al. [
67], when GP is introduced into an strong acid solution (pH = 0), almost the 100% of the Na
+ ions are released. It is possible to promote an ion exchange between Na
+ and H
+, and the protons to become part of the sialate structure of P-20. However, it is observed that the predominant trend over time (0→7 days) is an increase in pH for both acids, so alkalis leaching is still present. As demonstrated by Zechynska-Hebda et al. [
72], one month after hydrochloric acid immersion is required to stabilize at pH~7.
The results suggested that P-20 can be rinsed to drop the leachate pH without GP degradation in line with the environmental regulations for different wastewater streams, although further studies will be carried out on this regard.
3.2. Adsorption Testing
The effect of different variables in the adsorption process was measured, including the initial water pH, adsorbent dose, contact time and initial adsorbate concentration.
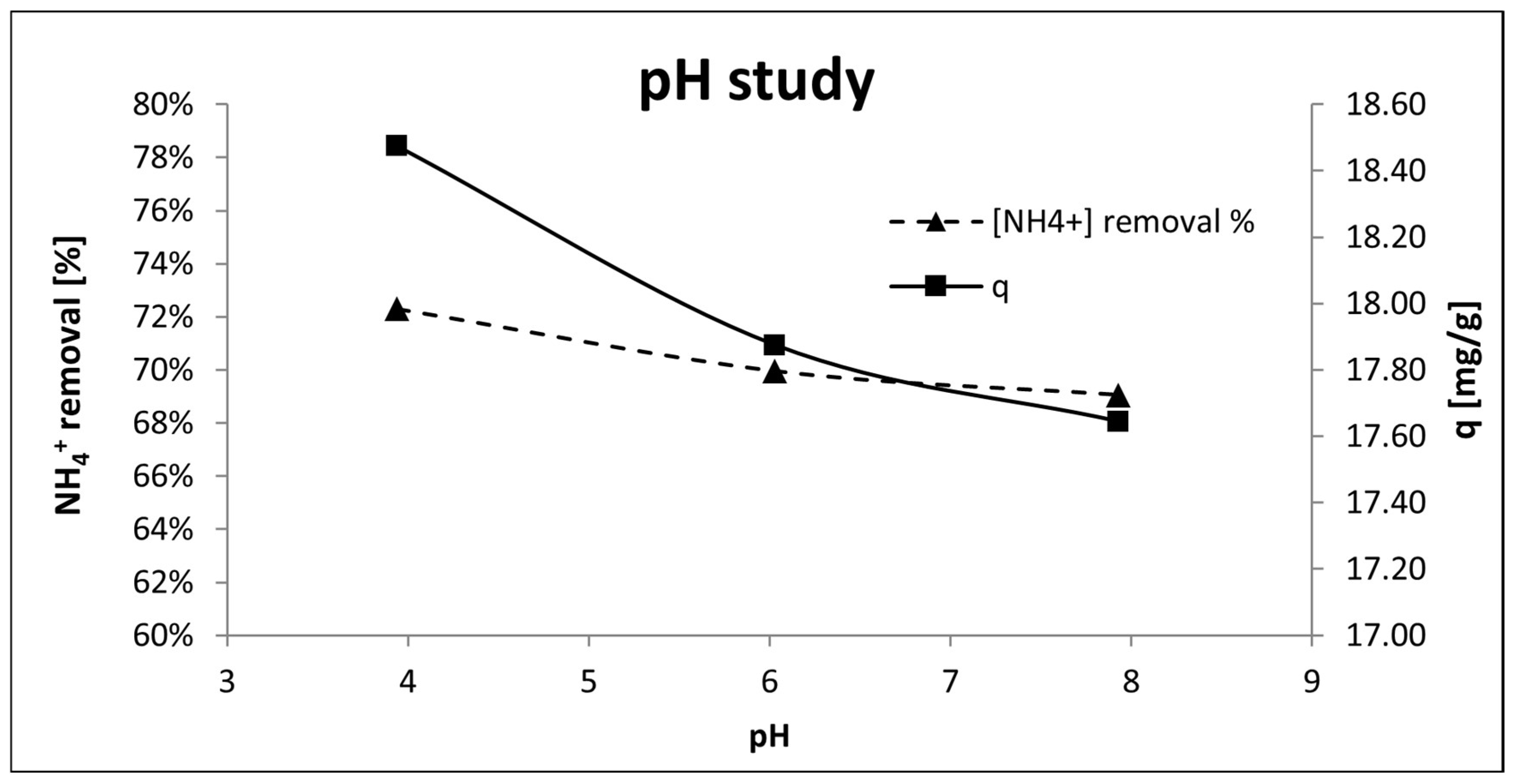
Figure 10 illustrates the initial pH effect on the adsorption capacity of P-20. There were no significant differences between the range of study (pH 4 to pH 8), however the more acid media suggested a slight improvement in the adsorption capacity (q) and NH
4+ removal. There was a twofold explanation for this phenomenon: firstly, the more acidic medium promoted the greater Na
+ release and thus, the more active sites available for NH
4+ adsorption [
67]. Secondly, the equilibrium ammonium-ammonia (NH
3 ⇌ NH
4+) is pH-dependent. A decrease in pH caused a displacement of the equilibrium favoring ammonium production generating a higher amount of adsorbate available for the adsorption process. However, it should not be ruled out that H
+ ions may also act as competing cations, as Yi Liu et al. [
73] showed with a phosphoric acid-based geopolymer that decreased its efficiency in acidic medium to remove Pb(II), Cd(II) and Ni(II). Therefore, further studies are needed to clarify the effect of pH on NH
4+ removal.
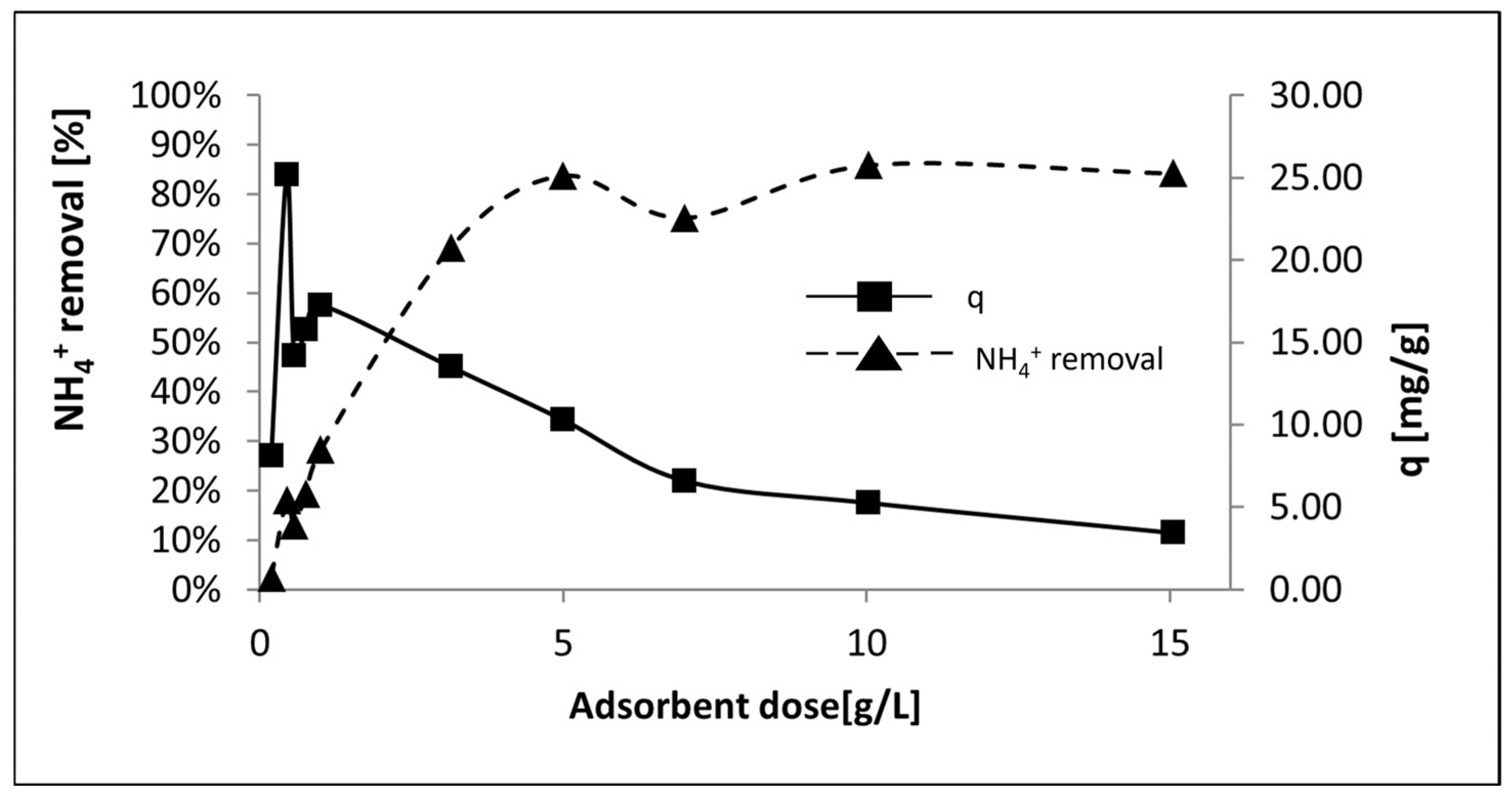
The optimal adsorbent dose (g P-20/ L) was also evaluated in
Figure 11. On one hand, the results showed that the increase of the adsorbent dose up to 5 g/L promoted an increment of the NH
4+ removal up to 80%. From this point, at higher GP dose, the removal levels kept similar according to Luukkonen et al. [
44]. On the other hand, adsorption capacity reached a maximum of 25.17 mg/g at 0.44 g/L and then decreased gradually. This trend was attributable to over-crowding of the adsorption sites and more unsaturated active sites on the adsorbent surface as the adsorbent dose increased [
74].
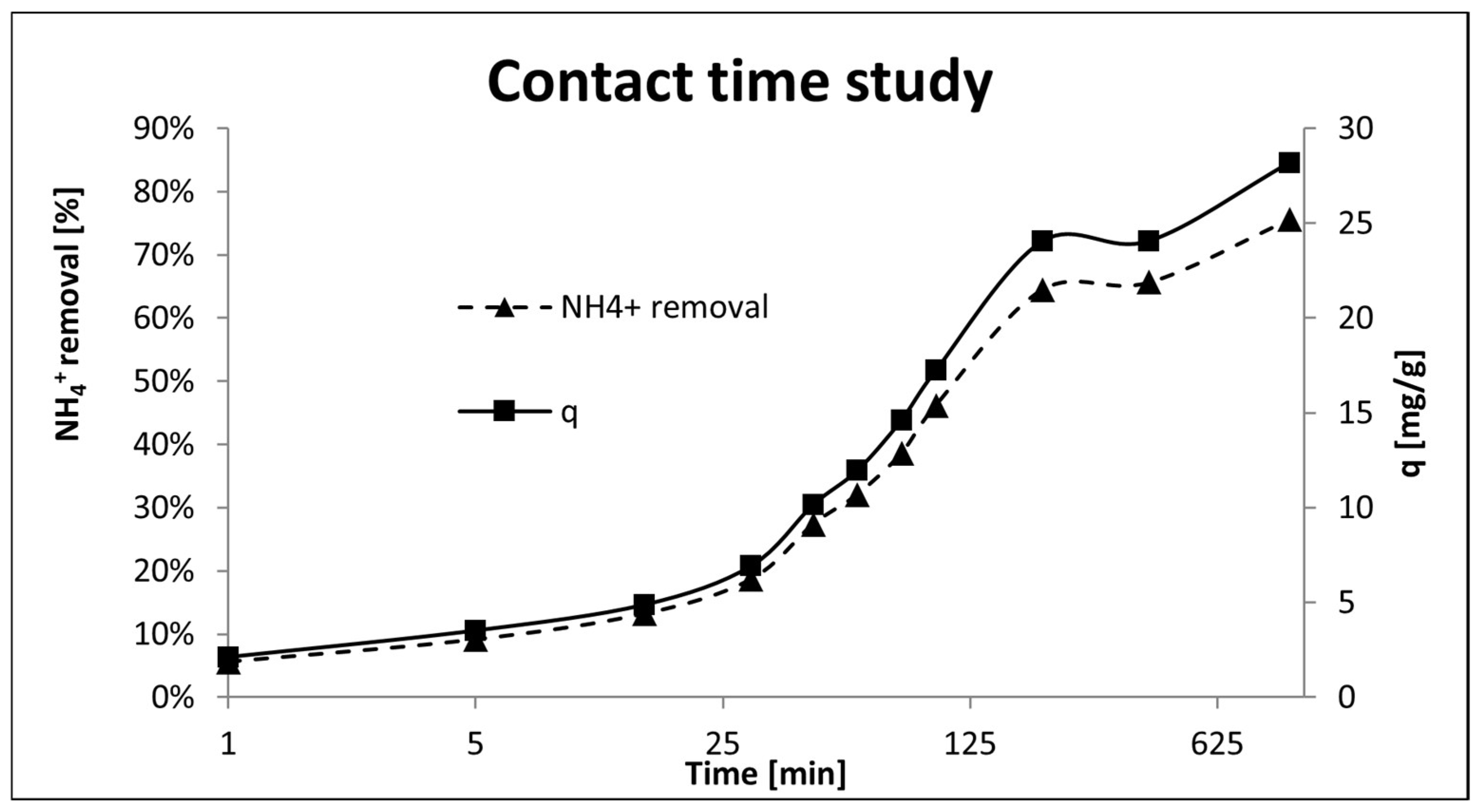
The effect of the contact time (1→1000 min) between the adsorbent and the adsorbate is depicted in
Figure 12. The removal efficiency and adsorption capacity increased rapidly in the first 200 min obtaining 65.5% of NH
4+ removal and
q = 24 mg/g. Thereafter, the adsorption efficiency increased slowly reaching maximum adsorption capacity of 28.18 mg/g at 1000 min. Therefore, the general trend was growing, being faster at the beginning of the procedure. These results improved the results reported in [
43,
44] reaching a stabilization/saturation at 30–90 min and 120 min respectively whereas P-20 started to partially saturate after 200 min.
In the same experiment, the drop in [NH
4+] and the increase in [Na
+] were monitored (
Figure 13). The NH
4+ concentration decreased from the initial 65 mg/g to 15.9 mg/g whereas [Na
+] increased from 14.3 to 89.50 mg/g following a similar correlation. These results clearly showed that the ion exchange process between NH
4+ and Na
+ was taking place.
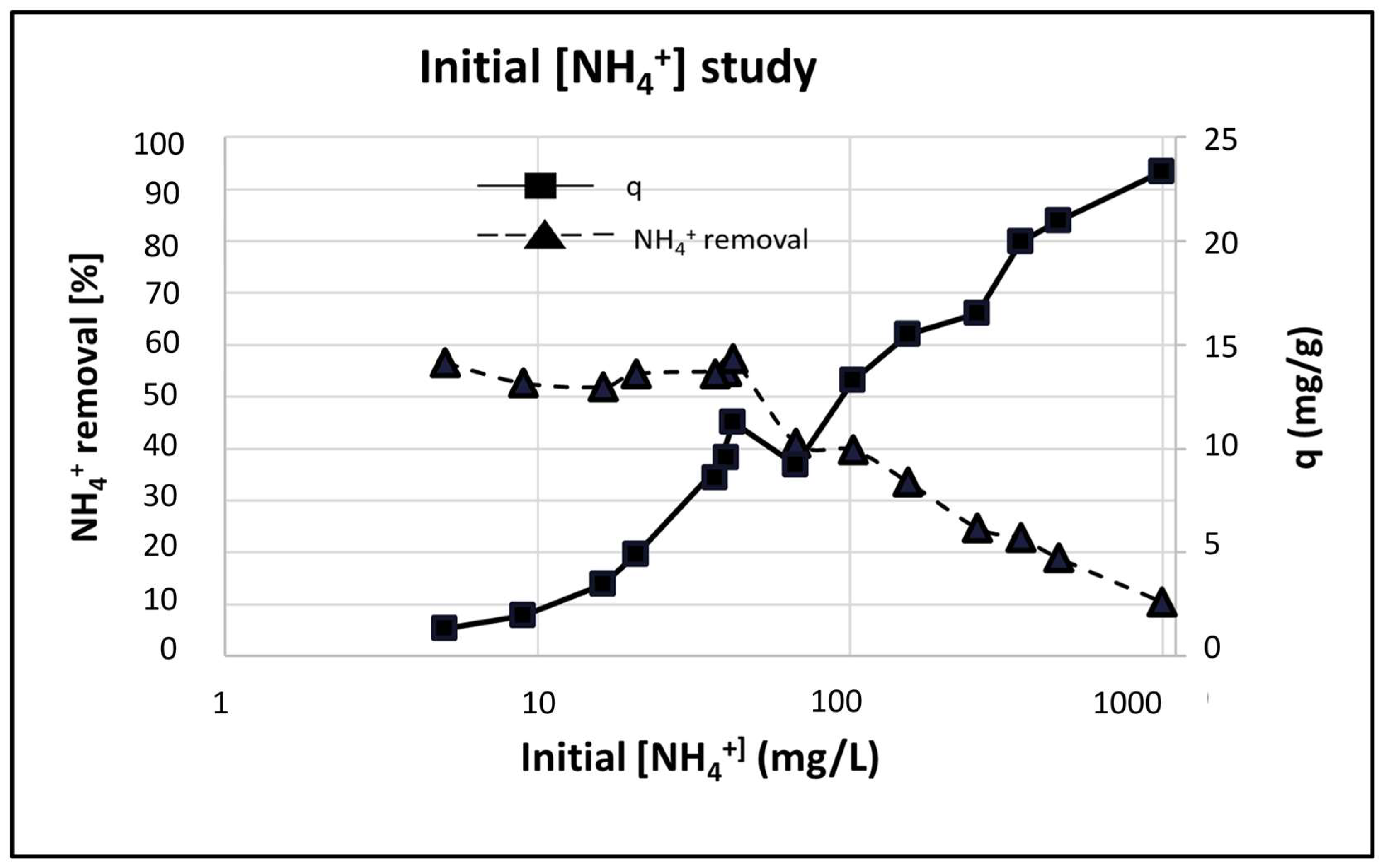
Adsorbent characteristics of P-20 were measured by varying the initial ammonium concentration from 10 mg/L to 1000 mg/L and was represented in logarithmic scale in
Figure 14. When the initial NH
4+ concentration increased, the NH
4+ removal efficiency decreased from 60 to 10%. However, the adsorption capacity increased from 1 to 24.8 mg/g as the initial NH
4+ concentration increased. Sanguanpak et al. [
43] obtained the same effect and determined that higher initial NH
4+ concentrations enhanced the adsorption uptake as the mass transfer driving force of NH
4+ between the geopolymer adsorbent and the aqueous solution increased.
These experimental data were used to carry out the adsorption mechanism characterization (isotherm and kinetics).
Typically, adsorption processes are studied from equilibrium equations denominated adsorption isotherms. These equations allow to know, at equilibrium, the amount of adsorbed ion, as a function ion concentration at a constant temperature. The data from the initial contaminant concentration effect test will be used to fit the different isothermal models using the iteration method. Five isotherm models (two parameters: Langmuir [
48] and Freundlich [
49]; three parameters: Langmuir-Freundlich [
50], Redlich-Peterson [
51] and Tóth [
52]) were tested for modelling the adsorption mechanisms of P-20. The parameters shown in these isotherms are mostly common to the different models: (a) the maximum capacity (
qm), (b) the dimensionless parameter
b, indicative of the adsorption energy and (c) the dimensionless parameter
n which represents the heterogeneity of the adsorbent surface.
The isotherms models were evaluated by RSME (error measure) and by R
2 (goodness). The best fit was achieved with the Redlich-Peterson (RP) equation according to the high value of correlation coefficient as detailed in
Table 7 and
Figure 15.
RP is an empirical isotherm incorporating three parameters and combines elements from both Langmuir and Freundlich equations; therefore, the mechanism of adsorption is a mix and does not follow ideal monolayer adsorption. With this model, equation number 10 was obtained with the following values for the parameters:
qm = 18.35 mg/g, lower than if compared to the experimental data (28.18 mg/g and 24.8 mg/g in contact time and initial [NH
4+] experiments respectively);
b = 0.02, indicative of the affinity of the binding sites, and
n = 0.9 (this value, close to 1, indicated that the adsorption on the surface was homogeneous which supported Langmuir assumption [
75]).
Previous reported works on heavy metal adsorption with GPs [
34,
76] have shown the good fit of the RP isotherm to explain the adsorption process.
These results contrast with those reported by Sanguanpak et al. [
43] and Luukkonen et al. [
44]. The former obtained that the best isotherm was the Freundlich model while P-20 did not show an acceptable fit. The latter obtained the best fit with the Langmuir-Freundlich (i.e., Sips) model. In this work, Sips isotherm could also explain the adsorption process, as well as Tóth. With these two models,
qm = 26.098 mg/g and 27.645 mg/g were obtained respectively, as summarized in
Table 8. These values were higher than the capacity obtained with RP model and closer to the experimental data. The shape of the curve in the three best models (RP, Toth and Sips) indicated that the NH
4+ was disposed on the GP surface as a monolayer and with strong attraction between molecules. However, it did not become asymptotic at high concentrations, so a physical-chemical adsorption mechanism was suggested [
77].
The value of
qm obtained with the RP isotherm model was higher than that of most natural zeolites: clinoptilolite (2.27 mg/g and 6.96 mg/g) [
78,
79], clinoptilolite-morderite (1.65 mg/g) [
80], clinoptilolite-heulandite zeolite (14.42 mg/g) [
42] and morderite (14.56 mg/g) [
81] and some of synthetic zeolites: gismondite (3.17 mg/g) [
82], NaP1, K–F and K-phillipsite/K-chabazite (7.02 mg/g) [
83], zeolite 13× (8.61 mg/g) [
84], thermal-treated clinoptilolite (10.61 mg/g) [
79], microwave-treated clinoptilolite (11.59 mg/g) [
79] and Na–P zeolite (16.36 mg/g) [
85].
The equilibrium obtained with the above isotherm requires some time to be reached. The ions go through a series of processes until they reach the adsorbent surface, and the slowest ones determined the kinetics of the reaction. Adsorption kinetics provide insight into the reaction rate and the sorption mechanism involving mass transfer, diffusion, and reaction on the adsorbent surface during adsorption. The adsorption kinetics include 3 mass transfer processes [
86,
87,
88]:
- (1)
External diffusion (or film diffusion): the transfer of adsorbate in the liquid film around the adsorbent.
- (2)
Internal diffusion (or intraparticle diffusion): the transfer of adsorbate (NH4+) through the pores of the adsorbent.
- (3)
Adsorption onto active sites.
Various adsorption kinetic models such as pseudo-first-order, pseudo-second order, Elovich and Weber-Morris [
53,
54,
55,
56] were tested in this case.
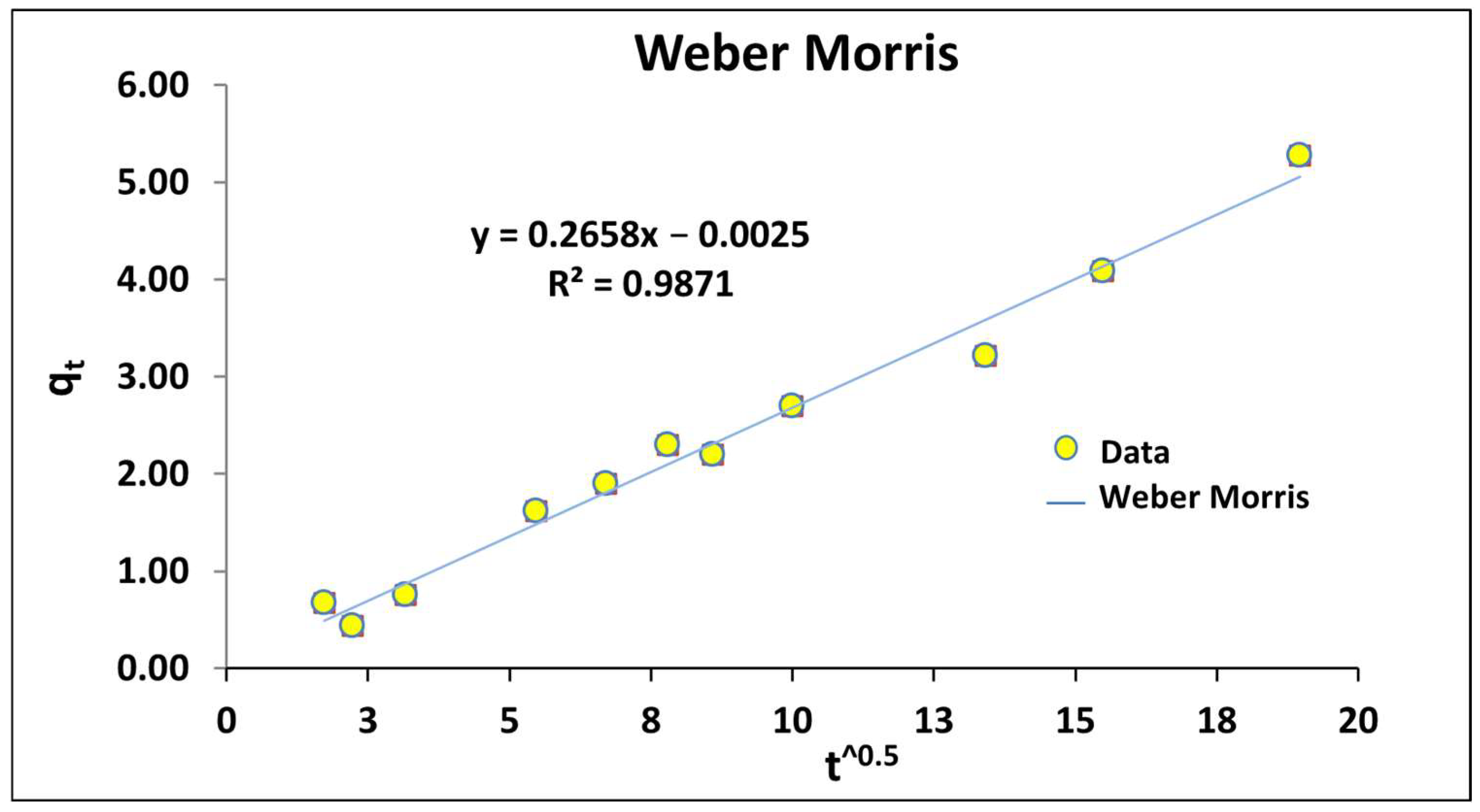
Figure 16 and
Table 8 detail the R
2 of the fit with Weber Morris equation presenting the best results.
The Webber-Morris equation is the following:
According to the data of this study, the Equation (12) is obtained:
The value of
C (value of the equation for
t = 0) close to 0 revealed that the limiting process was the intraparticle diffusion. Furthermore, the fit of the data did not present multilinearity. Considering the high porosity identified in P-20, the internal diffusion through porosity was identified as the limiting process [
86,
88].
Figure 17 shows the results obtained of the column test carried out for 160 h with an initial concentration of 250 mg/L of NH
4+. It can be observed that the concentration of NH
4+ ions dropped sharply at the beginning of the test, around 17 mg/L, obtaining an outstanding NH
4+ removal of 93% during the first hours. However, this value grew up to 123 mg/L at 25 h, being 50% the NH
4+ removal. From then on, the output solution had an increasing concentration of contaminant, reaching almost an asymptote at 110 h indicating that the GP hardly retained NH
4+ from the solution becoming saturated. Therefore, a regeneration process of P-20 for reuse should be considered after 110 h of adsorption treatment. The adsorption capacity obtained in this experiment was 25.24 mg/g similar to Langmuir-Freundlich and Tóth isotherms.
3.3. Validation in a Real Wastewater Pilot Plant
The pilot plant for wastewater treatment was designed at Xiloga S.L. landfill. The plant consisted of three key points as it can be seen in
Figure 18.
The pilot plant started from the
(a) tank containing the leachate detailed in
Table 9. The composition presented a large content of suspended solids, organic matter and possible competitive ions. The temperature, pH and EC conditions were also very changeable as shown by the high standard deviation. The [NH
4+] was high and variable with a mean value of 1978 mg/L. The
(b) Air stripping system, the first filter of the plant, required high pH levels for high ammonium removal efficiency [
89,
90]. Ammonium reacts with water to form ammonium hydroxide. Thus, in ammonia stripping, lime or caustic is added to the wastewater until the pH reaches to 11.5–12.0 which converts ammonium hydroxide ions to ammonia gas (NH
4OH → NH
3 + H
2O). The last area of the designed plant consisted of a
(c) vertical wetland where 125 L of P-20 were deposited.
The results were monitored for 5 days at those three outlets points (a), (b) and (c). The NH
4+ removal efficiencies and the pH and electric conductivity (EC) variations were measured as detailed in
Figure 19 and
Figure 20.
NH4+ concentration decreased from 91 mg/L to 17 mg/L at day 0 which supposed an initial removal of 81% of NH4+. After one day, P-20 still showed a good performance reducing the NH4+ concentration from 140 mg/L to 53.45 mg/L, which represented a total elimination of 61.82%. It is observed that the efficacy decreased with time as the active sites of the P-20 begin to be saturated. After 2 days, P-20 adsorption decreased from [NH4+] 223.2 mg/L to 172.4 mg/L which represented an efficiency of 22.75%. The contaminant removals on day 3 and 4 are 10.95 and 5.92% respectively. P-20 showed a similar performance in column tests which reflected the suitability and high selectivity to NH4+ even under real operating conditions.
The NH
4+ removal at the beginning was similar or even higher than that reported for natural zeolites: mesolite (55%) [
91], Turkish sepiolite (60%) [
92], Romanian volcanic tuff (83%) [
93] and Gordes clinoptilolite (85%) [
94]; and it is even similar to many artificial zeolites: ceramic adsorbent (19.4%) [
95], NaOH modified mordenite (81%) [
96], ultrasound modified zeolite (84.6%) [
97] and magnetic zeolite NaA (85%) [
98]. However, these results reported for zeolites correspond to tests carried out in synthetic solutions.
It is worth to note the increment in the electrical conductivity and pH during the treatment. As shown in
Figure 20, the pH increased sharply when passing through the stripping system (from 8 to 12 units). After passing through P-20 the pH > 13 which represented a gradient of approximately 1 unit with respect to the stripping. This was due to the alkalis leaching to the wastewater effluent. This effect could be modified as demonstrated in the results obtained in
Section 3.1.4. According to the same reason, the EC also increased in values around 10–28 mS/cm.
An economic assessment of the adsorbent developed was carried out for comparative purposes. Considering the composition of P-20, the final cost was estimated on 0.4Ꞓ kg
−1/ 0.43 USD kg
−1. The most expensive component was the foaming agent (11Ꞓ kg
−1) followed by the alkaline activator (0.9 Ꞓ kg
−1) and finally the MK (0.3 Ꞓ kg
−1). However, the H
2O
2 has a low impact on the total cost (13%). The Na-silicate was the product with the highest impact (59%). Considering the current adsorption technologies, P-20 was found less expensive than reported MOFs (~70 k USD kg
−1) [
99] and activated carbon (1.8–2.1 USD kg
−1) [
100] and in the price range reported for biochar (0.35–1.2 USD kg
−1) [
100]. Clay-based adsorbents are still the cheapest option (0.04 USD kg
−1) [
29]. Alkaline activating solutions in geopolymer manufacturing are not only one of the most expensive components but also the main responsible for the material carbon footprint according to reported LCAs [
101]. Thus, recent researchers are focused on replacing traditional sodium silicates with other waste-based activators to alleviate both the cost and emissions of the final GP [
102,
103,
104].