Abstract
Sustainable ingredients in cosmetics have been discussed for the past decade, and the COVID-19 pandemic has increased awareness of this significant topic. Consumers are informed and vigilant about clean labels, driving the necessity for sustainability throughout the cosmetic production chain. Moreover, nanotechnology and green chemistry approaches have emerged as innovative perspectives to develop sustainable and eco-friendly cosmetic ingredients. In this sense, in this review, we present examples and applications of sustainable ingredients derived from several types of sources (i.e., plants, animals, microorganisms, cell cultures, and recycled materials/biomaterials). The benefits and drawbacks of all classes of compounds were organized and discussed in relation to novel formulations/products. Finally, we addressed perspectives on cosmetic ingredients that prioritize sustainability and safety, with an emphasis on exploring sustainable ingredients, compounds, or molecules as promising areas for research and development.
1. Introduction
Currently, concern about environmental issues related to cosmetic ingredients is driving a growing demand for the use of sustainable alternatives [1]. Some of these ingredients may negatively impact the environment, particularly the aquatic fauna and flora, which has generated a global consumer demand for eco-friendly products [2].
This trend has generated a preference for the use of renewable products, and if possible, with all ingredients derived from natural sources—biocosmetics [3]. New labels are used to better suit consumers’ expectations of cosmetics regarding sustainable practices (Figure 1). In general, people with higher education are more worried about environmental problems related to the use of cosmetics; however, most consumers are not willing to pay more for a product that has sustainable ingredients [4]. Additionally, after the COVID-19 pandemic, a healthier life perspective has arisen, opening novel opportunities for the cosmetic industry, especially focusing on the circular economy [3,5]. Interestingly, the main drives for the beauty and personal care industries post-COVID-19 are reinforcing the skin barrier, immunity and anti-inflammatory boost features, good sustainability practices during manufacturing, local supply chain priority, upcycling cosmetics-food industries, sustainable and biodiversity-friendly ingredients, social and multicultural inclusivity, digital retailing, and healthy-related products [3].
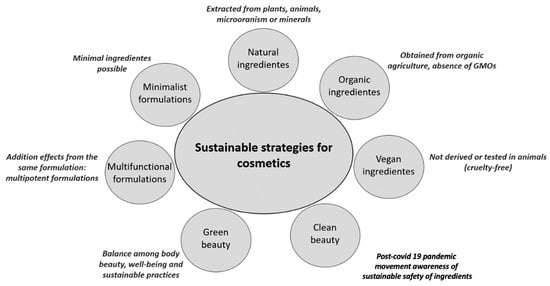
Figure 1.
Summary of sustainable strategies and trends in cosmetics. GMO = genetically modified organisms.
Environmental problems related to inappropriate cosmetic disposal have been reported. The accumulation of cosmetic residues, such as triclosan, zinc oxide, and silver nanoparticles, squalene, and micro/nanoplastics, in aquatic habitats may damage algae [6]. Toxicity caused by cosmetic residues in the aquatic environment may imbalance the presence of reactive oxygen species (ROS) in the water and the biomass composition of algae, which can damage organisms of various trophic levels [6]. Further studies need to be carried out to determine the real extent of this contamination since the environment and the complex mixing of materials can promote significant changes in the results [6].
Sunscreens prevent diseases such as skin cancer and discomfort such as sunburn, but UV filters may damage the aquatic ecosystem of lakes, rivers, groundwater, and the sea [7]. Moreover, such molecules tend to accumulate in organisms [2]. A study conducted with organic UV filters showed that these may be pollutants in the aquatic environment. Tests using Balanus amphitrite demonstrated the possibility of acute toxicity of benzophenone-8 (1) and 4-methylbenzylidene camphor (2) (Figure 2). The concentration of these compounds is higher in industrial and urbanized areas, being diluted in the underlying regions. However, with the phenomenon of bioaccumulation, long-term problems are still a possibility, even in the most distant regions [8].
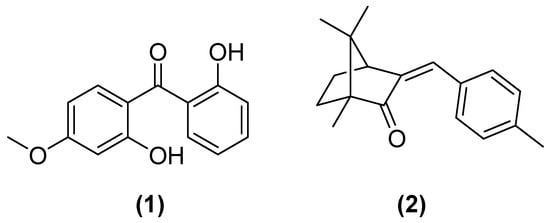
Figure 2.
Chemical structures of benzophenone-8 (1) and 4-methylbenzylidene camphor (2).
One strategy to minimize the problem of pollution derived from cosmetic ingredients is the use of products recovered from the manufacture of other industrial items. Valuing and using waste have become an economic and sustainable practice in the cosmetic industry and are known as “upcycling” or “circular beauty”. For example, the food industry produces organic waste, a known source of phenolic compounds such as flavonoids and phenolic acids, among other various derivatives that would normally be discarded [9].
Other authors have brilliantly reviewed the sustainability issues regarding the entire life cycle of the cosmetic chain: manufacturing, packaging, distribution, consumer use, and post-use [10,11,12]. In this research, we aimed to review sustainable alternatives to produce ingredients for the manufacturing of cosmetic/dermocosmetic products, mainly those based on the valuation of waste products. The focus is on current attempts to reduce the ecological impact of cosmetic products by exploring alternative, environmentally friendly methods for producing these ingredients. We compiled data regarding traditional/classic, and new ingredients, aligned with the recent trends and needs of cosmetic consumers after the COVID-19 pandemic. We structured this review according to the types of sources of renewable and/or recycled compounds/materials, like those derived from plants, microorganisms, animals, in vitro cultures, and algae.
2. Renewable Materials
2.1. From Plant Species
Plant-derived materials are widely used in cosmetics using “herbal”, “green”, and “vegan” claims. Nowadays, concerns about seasonality, responsible agriculture, and biodiversity protection are growing worldwide. Plant bioprospecting, which is the selection of wild plants with potential industrial use after genetic, chemical, and biological manipulation, focuses on exploring biodiversity ethically, improving yield, and/or accessing biological molecules [13].
Cosmetic formulations may contain extract(s), or isolated molecule(s) obtained from the extract(s). Phytoextracts are a mixture of molecules and act as multipotent ingredients. Despite being an attempt to achieve sustainability, the extraction methodology may have important impacts on the environment: the use of solvents, multi-step processes with high energy consumption, and purification necessities [14]. In cosmetics, plant extracts are used with caution, since specific odors, color-changing profiles, chemical instability, and supply-chain variations are some of the downsides of those ingredients. Nevertheless, the benefits of plant extracts are extensively investigated in the scientific community and appreciated by consumers. Antioxidant phytocompounds, for instance, in addition to acting on the degradation caused by the ROS reactions, also help to stabilize cosmetic formulations [15]. Table 1 brings together some renewable materials derived from plant extracts cited in the aforementioned text.

Table 1.
Examples of renewable materials derived from plant extracts.
Ingredients used in the formulation for sensory and stability issues may also be plant-based. Emulsions and foams require surfactants to remain stable, avoiding phase separation and ensuring adequate shelf life. Currently, most of the surfactants used are synthetic or semi-synthetic compounds (e.g., tweens, spans, or sucrose esters). However, with the trend to increase sustainability in industrial production, biosurfactants, having a renewable origin, were created. These are biocompatible and biodegradable derivatives [24] and have the advantage of being absorbed gradually [25]. Alkyl glycosides, for instance, are completely biodegradable surfactants that can be produced from a reaction of fatty alcohols from coconut or palm trees with glucose (Figure 3). These can be used to emulsify formulations, form quality foam, and may have cleaning properties [26]. Alkyl glycosides are highly stable and environmentally friendly. These are produced from the condensation of sugar with a fatty alcohol, which determines its size. Fatty alcohols can be extracted from several plant sources, including potatoes, corn, and coconut. Currently, its use is related to several products, such as shampoos, soaps, deodorants, sunscreens, and moisturizing creams, among others [27].
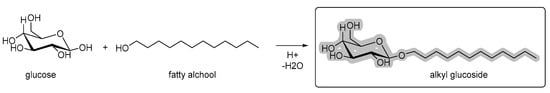
Figure 3.
Reaction of fatty alcohols with glucose results in alkyl glucoside [28].
Saponins, present in evergreen trees (Quillaja saponaria), can act as biosurfactants, showing interesting rheological characteristics. Its amphiphilic structure permits the formation of micelles beyond the critical micellar concentration, allowing emulsions and nanoemulsions [29].
The fig tree of India (Opuntia ficus-indica (L.) Mill.) grows in the region between Brazil, Mexico, and Chile. For the cosmetic industry, several compounds present in its residues are interesting, since there are phenolic compounds (e.g., gallic acid (3) and catechin (4)), as well as flavonoids with antiviral and antibacterial action (e.g., kaempferol (5) and quercetin (6)); betalains that have pigment function, being divided into two classes: betacyanins and betaxanthins. The former is red/purple, and the latter is yellow, being able to replace inorganic pigments. In addition, vitamin C (7) and carotenoids can be extracted from this plant, increasing its use [30]. The structural formulas of some compounds can be seen in Figure 4.
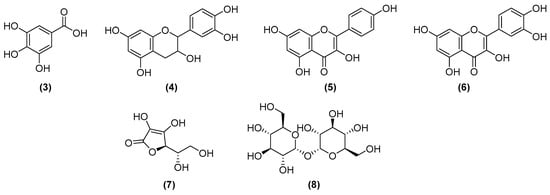
Figure 4.
Chemical structures of gallic acid (3), catechin (4), kaempferol (5), quercetin (6), vitamin C (7), and trehalose (8).
Hydroxycinnamic acids and their derivatives can develop protection against UV radiation, and they have antioxidant, anticollagenase, anti-inflammatory, antimicrobial, and antityrosinase actions, making them a set of relevant actions for products that aim to rejuvenate the skin. However, its characteristic of easy oxidation hinders its use on an industrial scale, as it generates high instability. Studies using the microencapsulation technique helped to solve this scenario and enabled the potential use of this compound in a sustainable way. These strategies can be found in various fruit and vegetable fields [31].
Another crucial aspect of the skin is sweat—a natural mechanism for temperature regulation. In several cultures, excessive sweating is regarded as a lack of personal hygiene because the bacteria in the axilla region can produce an unpleasant odor. Though several products are available on the market to reduce body odor, most of them have unknown toxicity or are harmful to the environment, such as triclosan or aluminum salts. In response to these concerns, various compounds derived from biological sources have been developed, such as essential oils, which provide a sustainable way to eliminate body odors [32].
Essential oils are usually obtained from the hydrodistillation of plant species. These are used as cosmetic ingredients because of their pleasant odor; however, their antifungal and antimicrobial properties are emphasized, allowing their use as preservatives [33]. Essential oils are composed of a mixture of complex molecules, usually containing terpenes, alcohols, esters, and ketones [34]. These components may have similar physical-chemical characteristics but different biological functionalities, including toxicity. Thus, to expand the selection and assertiveness of products and allow their use in the industrial context, it is possible to cite extraction by enzymatic methodologies, emphasizing their low energy expenditure and sustainability features [35]. Enzymatic pretreatment is used in the plant to weaken the structure of cells and facilitate and increase the extraction of metabolites. For example, this method is used in the treatment of gossip peels (Citrus bergamia Risso) with enzymes from Aspergillus sp., promoting an increase in the antimicrobial potential of the essential oil obtained.
Enzymatic post-treatment, in turn, is a more time-consuming process with greater energy expenditure. However, this procedure increases the quality of the essential oil; for example, the olfactory characteristics of Palmarosa extract (Cymbopogon martinii) can be improved using this method since the treatment increases the concentration of terpene alcohols, adding value to this ingredient and, consequently, to the final product [35]. Moreover, essential oils, due to their volatility, are susceptible to chemical and physical decomposition. The possibility of applying lipid nanocarriers to improve topical penetration and postpone their degradation is studied. This is an interesting technology since it is safe, biocompatible, and assists in a modified release process. Lipid nanocarriers, in turn, have encapsulation efficacy, colloidal stability, and do not use organic solvents. This technology helps to overcome several challenges encountered in the large-scale manufacture of essential oil formulations. In addition, they improve the sensory perception of the formulations, an important aspect for the final consumer. To produce these nanocarriers, several lipid sources may be used, for example, the oils of parsley (Ridolfia segetum (L.) Moris), which can be obtained from hydrodistillation (azeotropic distillation) of dry leaves, a simple, low-cost process. It is interesting to note that, in addition to having structural components to produce lipid nanoparticles, the essential oils derived from parsley have antioxidant and anti-inflammatory properties [34].
Another interesting ingredient that may be obtained as a by-product of essential oil distillations are hydrolates (also known as aromatic/distillate water, floral, or hydrosols). They are polar and acidic liquids composed of volatile molecules such as aldehydes, mono- and sesquiterpene alcohols, and ketones. In cosmetics, they may be used to explore their antimicrobial, antioxidant, and anti-inflammatory properties or their aroma in fragrances and aromatherapy products [35]. Recently, there has been an international standard publication to differentiate essential oil-derived and plant-derived hydrolates in cosmetics [36]. Regarding safety issues, in vitro studies conducted using skin fibroblasts showed that at a concentration of 2.5%, hydrolates are safe [37]. However, the composition of hydrolates may vary greatly depending on the source and extraction method, which can contribute to a different toxicological profile.
As mentioned above, essential oils are commonly used for their pleasant fragrance and antimicrobial properties, while other compounds may be utilized for their moisturizing and odor-reducing abilities. Trehalose (Figure 4, (8)) is a noteworthy example. This sugar can be enzymatically hydrolyzed by various species of arthropods, plants, and fungi. Trehalose was identified as having a role in the regulation of autophagy in a grass species (Tripogon loliiformis) that, when induced, promoted desiccation tolerance [38]. As a highly hydroxilated molecule, trehalose acts as a moisturizing ingredient in several cosmetic formulations/nanoformulations available on the market. It can act as a surfactant when its lipids form [39] and prevent oxidation, which makes it effective in reducing body odors caused by the degradation of unsaturated fats [40]. However, trehalose production is limited by its high costs. To address this limitation, researchers are investigating a new method that involves a series of enzymatic reactions in cassava starch (i.e., “tapioca”) or corn starch to enable large-scale production [41].
2.2. From Microorganisms
The cultivation of microorganisms offers alternative advantages related to the lower need for space and water for its cultivation [42]. Biosynthesis has advantages because it facilitates the purification of molecules and is a low-cost, effective method to obtain contaminant-free ingredients [3]. Biocatalysis, in turn, is an interesting technique that uses microorganisms and/or enzymes to obtain cosmetic ingredients via fermentation [43]. Traditional bioprocesses may be optimized to obtain large-scale production of molecules, especially when associated with genetic manipulation [3]. For example, Saccharomyces cerevisiae can use xylose (extracted from plants) to produce fatty alcohols, acting as emulsifiers and lubricants in the cosmetic industry [25]. However, under a method of co-expression of genes, the same microorganism may be used for the biosynthesis of sinapic (9), caffeic (10), ferulic (11), and coumaric acids (12) [44] (Figure 5).
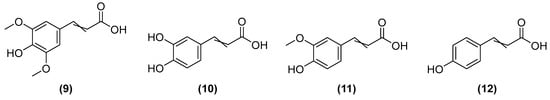
Figure 5.
Chemical structures of sinapic acid (9), caffeic acid (10), ferulic acid (11), and coumaric acid (12).
Bacterial nanocellulose is a candidate to replace synthetic polymers used in cosmetics [45]. This is a polysaccharide produced by acetic bacteria, thus being a renewable and biodegradable product. Nanocellulose is used in cosmetic formulations to improve texture and increase action in the body, and it is obtained from various sources. Nanometric cellulose, in particular, can be obtained from Komagataeibacter (considered more effective) and other strains such as Achromobacter, Aerobacter, Agrobacterium, Azotobacter, Escherichia coli, Rhizobium, Salmonella, and Sarcina. The porous structure of nanocellulose allows the incorporation of active substances, which is an interesting characteristic of producing facial masks as it maintains an elastic structure that is easy to manage, improving skin adherence and increasing the penetration of the active substances present in their pores. According to its viscosity, it is recommended for gel formulations, ensuring organoleptic properties. It can also be used to immobilize enzymes, act as an emulsion stabilizer, and replace microplastics [46]. However, problems related to its production still need to be solved. Bacteria can have genetic instability, which can alter the final product. On the other hand, nanocellulose has non-Newtonian fluid behavior, which can hinder its management, generating heterogeneity in the distribution of oxygen in the culture medium [46]. Besides, being used as ingredients, bio-based polymers are also recyclable and biodegradable alternatives for replacing plastics in cosmetic packaging [3].
Mannosylerythritol lipids (MELs) are another example of renewable materials from microorganisms, glycolipids used as biosurfactants. These are produced by the fermentative reactions of several species of fungi in the genus Pseudozyma. Different fungi generate different MELs with diverse degrees of hydrophobicity, which allows a variety of functions for these surfactants [47]. Its activity generates interesting microemulsions for the cosmetic market [48]. Other functions are related to MELs, such as anti-inflammatory and antioxidant activity, besides having moisturizing action [47]. Additionally, studies have indicated that MELs have the action for the treatment of alopecia, activating fibroblasts and follicle cells, and are also applied to improve the dispersion and water resistance of metal oxides. Therefore, MELs are an ecologically viable and biodegradable option [48].
The fragrance industry is highly reliant on the biosynthesis of volatile substances. Butyric acid is usually a petroleum-derived fatty acid that is used to intensify fruit odors or as a precursor to produce other fragrances. The mechanism of fermentative production is studied in anaerobic bacteria of the genera Butyrivibrio, Megasphaera, Roseburia, Butyribacterium, Clostridium, Sarcina, Coprococcus, and Eubacterium. Industrially, non-pathogenic species are preferred, and immobilized cell cultures are made. This process is not yet economically viable, as it has low productivity since butyric acid is toxic to bacteria. To improve production, the continuous method of product removal can be used; another option would be to use more acid-tolerant strains [49]. Other organic acids, largely used in the cosmetic industry, such as citric (13), glycolic (14), and lactic (15) acids (as highlighted in Figure 6), can be obtained from fermentative routes with good yields and are economically competitive [3].
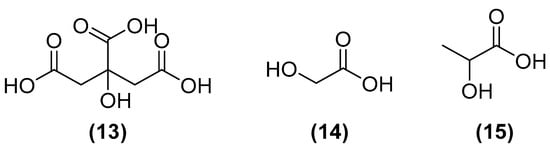
Figure 6.
Organic acids largely used in the cosmetic industry: citric (13), glycolic (14), and lactic acids (15).
Beta-ionone is a terpenoid with a floral fragrance that has gained high interest in the cosmetic industry. The sale of this terpene generated approximately €166 million between 2011 and 2015. However, due to the prolonged time for the plant growth cycle and high dilution, the direct plant extraction of beta-ionone is limited [50]. Furthermore, consumers are increasingly averse to synthetic products that contain non-natural-based ingredients. To address this issue, an alternative approach involves the use of Yarrowia lipolytica, a microorganism that is capable of producing beta-ionone through genetic manipulation. The biosynthesis of beta-ionone is completed through the cleavage of beta-carotene by carotenoid cleavage dioxygenases (CCD) (as illustrated in Figure 7). While large-scale production of beta-ionone using this method has been explored [51,52], there is still much potential for further exploitation.
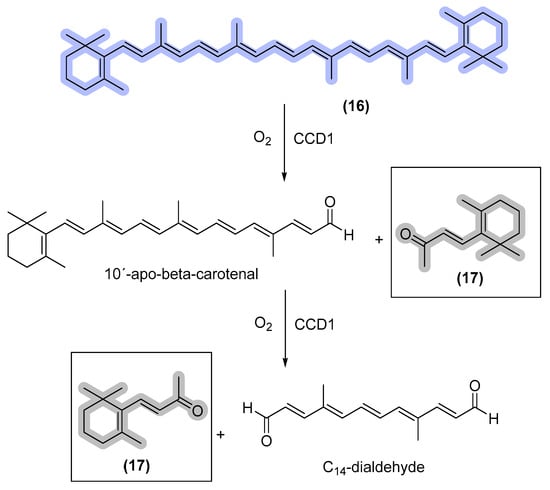
Figure 7.
Schematic representation of β-ionone biosynthesis. CCD1 = carotenoid cleavage dioxygenases; β-carotene (16); and ionone (17) [51].
Ambergris, also known as grey amber, is an aromatic wax derived from the sperm whale (Physeter macrocephalus) and has been widely used in the cosmetic industry for its unique fragrance. However, due to ethical and environmental concerns, substitutes for this fragrance have been proposed, one of which is ambroxide. Ambroxide is produced through a semi-synthesis process from isolated sage sclareol (Salvia sclarea), but the yield is very low [53]. To address this issue, an alternative method involves performing cell cultivation from a genetically modified Escherichia coli strain, which is economically viable and ecologically more sustainable [54]. By utilizing this method, the cosmetic industry can continue to produce fragrances with similar properties to ambergris while also reducing the impact on the environment and marine life.
Monoterpenoids, such as limonene (18), geraniol (19), and linalool (20) (Figure 8), may be extracted with organic solvents or by distillation due to their volatility. However, the former is polluting and the latter is costly, and both do not generate high-purity products. In this regard, microbial biosynthesis by S. cerevisiae and E. coli can be applied to obtain these molecules. The volume produced is not enough for the market; nonetheless, the production is effective. As alternatives are being studied to avoid the problems associated with synthesis, some apply: low concentrations of cofactors, enzymes, or precursors; and cytotoxicity of compounds derived from bacteria. For example, to supply the lack of endogenous enzymes, supplementation with exogenous enzymes can be made; to prevent the cytotoxicity of the compounds from leading to a decrease in the producing microorganisms, strains of microorganisms with greater tolerance to these compounds can be isolated. These approaches are interesting for fragrance features. Geraniol, for instance, has a fragrance like roses (Rosa sp.); limonene is a common critical fruit; and nerol has no characteristic fragrance, but its antimicrobial effects are of great interest to the cosmetic industry [55]. Particularly during the COVID-19 pandemic, hand sanitizers and cleaning agents with enhanced antimicrobial effects were the products that assured the survival of several cosmetic businesses. Consumers’ hygiene and wellness perceptions are changing the focus of cosmetic ingredients, and fragrances present an important impact on consumers’ priorities for value-for-money products [3].
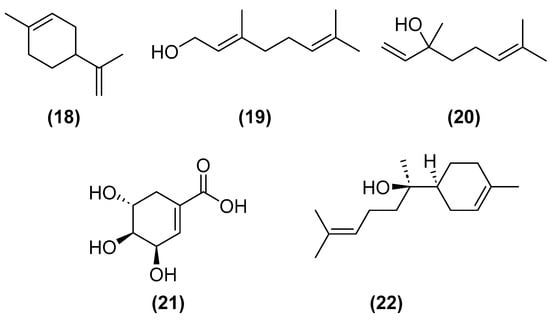
Figure 8.
Chemical structures of limonene (18), geraniol (19), linalool (20), shikimic acid (21), and bisabolol (22).
Shikimic acid (21) (Figure 8) is a chiral organic compound known particularly for its pharmacological action in the treatment of swine flu. However, it also has interesting actions for the cosmetic market, for instance, hair growth, since it interacts with one of the mechanisms causing alopecia. It has an anti-enzymatic action in lipases, preventing the degradation of triglycerides that release an unpleasant odor, and has the potential to be used in deodorants. The antibacterial action allows its use in antiacne preparations, in creams to control follicular hyperkeratosis, and in dandruff lotions and shampoos. Its production can be based on plant extraction. However, there is a faster and more efficient process based on glucose fermentation. More research should be carried out to improve the fermentation procedure. One should study the best fermenting microorganisms and the best biosynthetic route for their production. Considering the need for large quantities of this compound, the use of microorganisms should become the most economically viable solution, producing significant amounts of shikimic acid with a small environmental impact [56].
Bisabolol (22) (Figure 8) is a monocyclic sesquiterpene alcohol with antibacterial, anti-inflammatory, antiseptic, and moisturizing properties. It is present in chamomile (Matricaria recutita) and cadaleia (Eremanthus erythropappus). This compound can be extracted with organic solvents in addition to using polluting compounds, requiring a high amount of vegetable raw material. Its synthesis is not interesting since it would hinder the separation of its diastereoisomers, and only the alpha isomer is relevant. The alternative would be biosynthesis, using recombinant Saccharomyces cerevisiae with the gene for α-bisabolol synthesis [57]. Escherichia coli is also able to make heterologous expressions of mevalonate and bisabolol-producing genes. To increase its production, genetic alterations were performed to promote acetyl coaccumulation and secondary product reduction [58].
Several bacteria can produce sustainable pigments with bioactive properties, such as antimicrobial, antioxidant, or photoprotective action. Nonetheless, studies should be designed individually to ensure color quality and optimize cultivation conditions. It should also isolate subspecies that contain the characteristics of interest, since, in the same culture, spontaneous mutations can generate undesirable results for the product. Bacteria can also be used to produce silk biopolymers (via fermentation with E. coli) [59,60].
2.3. From Animal Species
Animal sources are as follows: (1) land animals; (2) marine animals; and (3) insects. Ingredients obtained from animal sources are controversial in the cosmetic industry. Concerns about infectious risks and ethical issues regarding animal cruelty are always discussed, and, if possible, similar ingredients from other sources are applied. Nevertheless, “vegan” and “halal” labels cannot be used if any of those ingredients are used in the composition [60].
Land animals’ ingredients are obtained directly by taking the animal’s life and/or using meat and livestock by-products. Very few companies still use this type of ingredient in their formulations since it is related to animal cruelty. Also, most of those ingredients (e.g., fats, fatty substances, keratin, collagen, and other proteins) may be obtained from vegetable or bioprocess sources. Similarly, marine products are in disuse [61].
Insects are an important source of interesting compounds for cosmetic companies. Besides, being a renewable source of raw materials, their crop is economically viable, requires little space, the energy expenditure is low, there is minimal waste during production, and a low amount of water is needed to produce its biomass. One disadvantage is the low acceptance of the final consumer [62].
Chitin, a biodegradable biopolymer produced by various organisms, including plants, fungi, and animals, is a non-toxic and biocompatible substance that can be extracted from natural sources [63]. Chitin and its derivative, chitosan, showed antimicrobial and antioxidant properties in the body. Crustaceans generate approximately 6 to 8 million tons of waste during processing each year, and chitin can be obtained from their exoskeletons [64]. In the cosmetic industry, chitin offers potential benefits for skin formulations as an antioxidant, a wetting agent, and a healing agent, making it useful for treating acne and reducing wrinkles by increasing skin softness and elasticity [62,63]. It can also be useful in hair formulations by removing sebum and providing antibacterial action. Chitin can act against caries in toothpaste by destroying the biofilm that forms on teeth and promoting dental remineralization due to its bactericidal action [63].
Chitosan, a hydrocolloid, can be formed from chitin through deacetylation. It is currently used in hair formulations due to its ability to form a film that increases elasticity and softness. This property may also be beneficial for skin care products, as the film prevents penetration of these products into deeper skin layers, ensuring the active ingredients’ topical action. Additionally, chitosan can protect the skin against the formation of free radicals [62,65]. In conclusion, chitin and chitosan offer potential as sustainable and useful ingredients in the cosmetic sector, especially in skin and hair toiletries, due to their biodegradable nature and various beneficial properties.
The cosmetic industry can benefit from other compounds that can be extracted from arthropods in addition to chitin. For example, Hermetia illucens larvae are full of amino acids such as glycine, which has moisturizing properties, and arginine, which functions as an antioxidant and enhances collagen and moisturizer production [62]. Bombyx mori, also known as silkworms, produce silk fibroin that has surfactant properties and low viscosity. When combined with capryl glycoside, fibroin from silkworms can be used as a surfactant [60]. Additionally, crustaceans, besides chitin, contain astaxanthin, a carotenoid with antioxidant action that is 10-fold greater than that of other carotenoids. This makes them a valuable and abundant source of raw materials, which can be utilized to prevent waste and create sustainable cosmetic products [64]. Beewax, a hydrophobic complex of hydrocarbons, esters, fatty acids, and propolis produced by the wax-producing glands of worker bees, may be used as a fatty thickener and rheology modifier in lipsticks and skin moisturizers. Despite its antimicrobial, anti-inflammatory, and antioxidant properties, the occlusiveness of this ingredient supports its great potential in clinical trials for psoriasis and atopic dermatitis treatments [66].
2.4. In Vitro Culture
The production of plant extracts can be optimized by applying in vitro culture [67]. This kind of cell culture is being disseminated by pharmaceutical companies but has not yet been spread in the production of cosmetics, to the best of our understanding. Since active ingredients are usually used in small amounts, in vitro crop volumes could be lower. Also, the desired plant organ could be insulated and cultured, increasing the growth rate. Another advantage of cultivation would be to isolate the plant from contaminants, allowing the acceleration of manufacturing [67]. Other benefits are the elimination of seasonality of the vegetable; ecological friendliness; use of less water volume; elimination of pest control; and use of less energy [68]. The technology can be used to produce compounds belonging to rare, protected, or endangered plants, and different tissues can be grown in aseptic environments, which favors the speed of their growth [67]. Cell culture can become an economically viable way to produce some compounds. The disadvantage is related to culture, which is affected by the plant species, genotype, culture medium, and environmental conditions, among others, requiring a qualified team for the work [69].
Major challenges from this practice are the requirements of specialized professionals for the manufacturing process, which varies from species to species, and the need for the country’s legislative adaptation to this technique, which can become a long and bureaucratic process. It should be emphasized that industrial production has challenges—the so-called “biological barriers” related to genetic instability; low metabolism; and heterogeneity of cells. There are also “technological barriers” related to the difficulty of spreading gases uniformly. The intensity of mixing movement can damage cells and increase foaming and cell aggregation, among others [69]. Thus, in vitro cultivation, in turn, may have benefits in obtaining compounds from protected, endangered, rare, and seasonal plant species [67]. The method is considered safe and can inhibit the production of toxic compounds [70]. This production can also be considered ecologically motivating, as it uses a small amount of energy and water, reducing carbon emissions [67]. In addition, the process can be made in the factory itself, where the extraction of the material will take place, saving fossil fuels that would be used in transportation [20].
Plant cell culture is an important tool for obtaining hydrosoluble and liposoluble extracts with antioxidant activity, collagen boost, and UV protection. Moreover, wall derivatives are rich in peptides and glycoproteins with anti-aging activity and modulation of extracts, such as reduction of toxic content or increase in desired molecules [71]. Nevertheless, plants may be used for molecular farming to obtain recombinant proteins with cosmetic appeal [72].
Some examples of plants with potential use for in vitro culture are Melissa officinalis and Matricaria chamomilla L. The use of chamomile in toiletries and cosmetics is quite common, which poses a challenge to sustainability with conventional planting. However, there are in vitro alternatives, such as cell suspension cultivation, aiming to obtain rapid secondary metabolites. This process does not require specific climatic or soil conditions, besides being bioinsurance. It is worth mentioning that variations in the storage medium and the number of inoculated colonies are important variables for the effectiveness of the process. Theoretically, this is an economically viable procedure, especially if there is a study of the genotype with a higher potential for callogenesis (regeneration capacity) [69]. Melissa officinalis cells showed great potential to acquire a phytocomplex rich in rosmarinic acid with high skin protection against oxidative stress, blue light, and irradiation damage [73].
2.5. From Algae
Both microalgale and macroalgale are photosynthesizing aquatic organisms. In this regard, several cosmetic ingredients can be extracted from these organisms and act as antioxidants, pigments, moisturizers, anti-inflammatory drugs, skin stain removers, antiacne, antiwrinkle, antimicrobials, and photoprotector adjuvants, among others [74,75]. It is worth mentioning that both cosmetics and cosmeceuticals require adequate organoleptic characteristics; thus, the gelling properties [76] and emulsifiers observed in algae are also of importance [77].
Macro and microalgae can serve as sources of raw materials for cosmetic ingredients. Both types of algae can be cultivated on an industrial scale by collecting small amounts from their natural habitats and growing them in large quantities. Microalgae are more advantageous for industrial production because of their fast growth, reproduction, and suitability for cultivation in controlled environments such as open or enclosed systems. As a result, they are generally easier to obtain in larger quantities than macroalgae [78]. Particularly, the closed one has benefits related to a higher concentration of recovered biomass since it has greater control [74]. Temperature variations can influence the growth of these organisms, and it is recommended to cultivate in sunny areas once high temperatures stimulate growth but limit the species that are used. Other sources of growth are the presence of CO2, nitrogen, and phosphorus. Industrial production usually dissolves nitrogen and phosphate in the culture medium [78]. The use of algae as precursors generates several benefits, such as a wide variety of compounds, large biomass production, and easy adaptation to the environment [74]. In addition, they are renewable and easily found [43].
In 2016, the seaweed market generated $10 billion for the sale of approximately 29 million tons of algae. There are almost 291 species of seaweed in 43 countries; however, the species used industrially are Eucheuma, Laminaria, Gracilaria, Undaria, Porphyra, and Kappaphycus, which make up 96% of world production [79].
Agar is composed of agarose and agaropectin [79]. Several genera of algae (e.g., Gelidiela sp., Gelidium sp., Gracilaria sp., and Pterocladiella sp.) produce agar. This is known for its emulsifying and gelling capacity and is interesting for the cosmetic sector for its improvements in the product’s rheological and organoleptic characteristics. It is noteworthy that agar can also be used as a moisturizer for the skin [77]. Table 2 shows examples of active molecules extracted from microalgae.

Table 2.
Active molecule(s) extracted from microalgae with cosmetic interest.
Waxes are commonly used in lipstick formulations, providing adequate hardness, texture, and slip characteristics. The origin of waxes can be from vegetables, animals, or petroleum derivatives. Another alternative is the use of alkenones derived from microalgae, a renewable, vegan source that allows cultivation in several geographies. It is worth mentioning that the vegetable waxes used are derived from specific plants in some regions and are dependent on the climate. For example, the availability of candelilla wax is affected by climate change, making it less available on the market [81]. Alkenones increase the softness of the formulation, an undesirable effect; however, they improve slippage and control, improving the uniformity of application of the product. Studies have shown that alkenones may be a viable alternative for microcrystalline wax (petroleum-derived) in lipsticks [81]. Euglenia gracilis, in turn, is a microalgae capable of producing palmitic acid and waxes that can be incorporated into lipsticks. This is produced on a large scale, and, depending on the luminosity, there is a production of different compounds [82].
Cyanobacteria are prokaryotic microorganisms, also known as blue algae. Its cultivation is undemanding, fast, and economical, and it has a wide variety of compounds of interest to the cosmetic industry. From the genera Cyanobium, Nodosilinea, Phormidium, Synechocystis, and Tychonema are extracts with antioxidant and anti-inflammatory capacities, containing carotenoids (beta-carotene, zeaxanthin, and lutein, among others). Extracts with potential anti-aging action are also obtained by inhibiting the digestion of hyaluronic acid and stimulating the proliferation of fibroblasts [1].
3. Recycled Materials
Food waste is increasingly being recovered and used to produce high-value compounds. This process generates economic and environmental benefits as it reduces the number of pollutants eliminated by the food industry. Many secondary products can be reevaluated and processed to generate interesting compounds, including cosmetic ingredients [9]. Based on the United Nations Food and Agriculture Organization (FAO), the priority for the environment is to avoid waste production; therefore, the recovery of food excess is a potential solution [83]. Naturally, the logistics of this action have some points of attention: (1) food waste is highly perishable, and special conditions of storage and transport may be required; (2) the seasonality of food supply must be incorporated into the ingredient schedule; (3) food and cosmetic grade ingredients may have very different specifications; and (4) extraction methods sometimes require multi-step and/or solvent processes. Nevertheless, the relationship between different industries and commodity production may involve government policies such as tax exemptions on production and specific taxation of final products. Therefore, a detailed analysis of the reuse of food waste for cosmetics is needed to evaluate its cost-effectiveness. The circular economy regarding the food and cosmetic industries is shown in Figure 9 and several examples of potential reuses are listed.
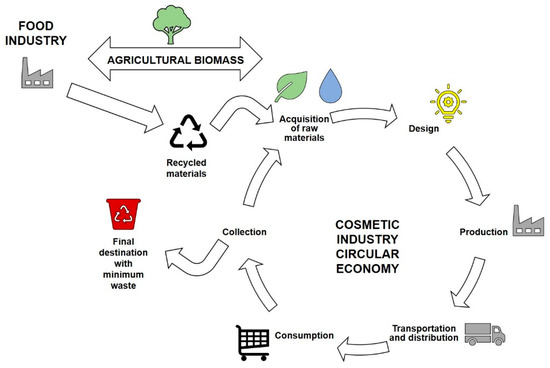
Figure 9.
Circular economy between food and cosmetic sectors.
The wine industry is an example of a high generation of effluents estimated at approximately 14.5 million tons annually in Europe alone, which contain numerous compounds of interest (such as polyphenols) that can be recovered and used in cosmetic formulations [84,85]. The liquid-solid extraction method is suitable for industrial-scale applications but utilizes substances that can be harmful to the environment. Recent studies suggest the extraction and purification of polar compounds from organic matrices with natural deep eutectic solvents (NaDESs), but there is still a lack of information regarding the effect of bioactive formulations. Preliminary findings indicate that extracts prepared using NaDESs can enhance the bioavailability of molecules in topical products [86].
Therefore, the extraction methods employed for the recovery of wine compounds are a crucial determinant of the extract’s composition. The dry pomace of white grapes (Vitis sp.) yields higher amounts of antioxidants and tyrosinase inhibitors. In contrast, wet pomace enables the extraction of anti-inflammatory agents, which cannot be extracted when the pomace is dried due to the degradation of such substances during the drying process [87]. Furthermore, the phenolic compounds found in grapes have the potential to inhibit enzymes involved in skin aging, making this approach a potential method for developing new products [84,88,89]. In light of this, several interesting formulations and/or nanoformulations passed through development by beauty brands, such as Pelegrims, which uses by-products from British vineyards, and Le Domaine Skincare from the Rhône Valley vineyard.
More than 3 million bottles of myrtle (Myrtus communis) liquor are produced every year in Sardinia (Italy), and approximately 200,000 tons of waste are discarded annually. However, its pericarps and seeds have high concentrations of linoleic acid, a fatty acid with protective antioxidant activities on the skin that could potentially be used as ingredients in cosmetics. Its characteristics are maintained when its residues are used after hydroalcoholic infusions, thus their viable reuse [9].
From 8 to 20 million tons of melon (Cucumis melo L.) shells and seeds are discarded annually. Such residues have interesting compounds for the cosmetic industry, such as polyphenols, flavonoids, orthodiphenols, and carotenoids, among others. All derivatives with potential antioxidant properties can be used in cosmetic preparations [90].
Coffee is consumed daily globally; however, only 10% of the fruit is used to prepare the drink, with 90% being discarded and generating up to approximately 823,740 kg/year of waste. From this material, a relevant amount of pigment could be extracted and revalued [83]. Additionally, the most abundant by-products of coffee are chlorogenic acid and caffeine, which have the potential to act as antioxidant compounds and adjuvants to improve the efficacy of sunscreens [25,91,92].
Walnut and hazelnut shells can be used as a source of lignin, an ingredient capable of absorbing UVA/UVB radiation. Currently, sunscreens are based on synthetic compounds, and their consumption is high. On the other hand, today there is a concern regarding its accumulation in aquatic organisms, and further investigation could clarify the effects in humans [2], since zinc oxide and titanium dioxide can generate free radicals in water, and zinc nanoparticles were found to be toxic in an experiment with zebrafish embryos. However, the concentration used in this study was higher than that found in the environment [7]. Therefore, further investigation could determine the relationship between sunscreen residues and coral damage [93]. Although experiments using UV filters based on lignin polymers from nut shells and hazelnuts indicated low sun protection factor (SPF), when isolated, the substitution of part of the formulation by these sustainable compounds could benefit the environment with the reduction of the synthetic UV filter concentration, a strategy investigated by our research group through studying bioactive multifunctional sunscreens [2,94,95,96,97,98,99].
Mango leaves (Mangifera indica L.) are traditionally used in Chinese medicine and show a variety of isolable bioactive compounds but are discarded by the food industry. The main compound of interest is tyrosinase, which catalyzes melanin synthesis, increasing the skin’s protection from UV rays and darkening the skin. Flavonoids are also present and can act as antioxidants [23].
Similarly to the case of mango leaves, approximately 60% of the mass of each pineapple (Ananas comosus L.) is discarded. In 2016, more than 435,000 tons of pineapple waste were generated. Its barks and stems, however, can be used to produce extracts with bioactive compounds, whose polysaccharides could be separated by precipitation. An enzyme of interest is bromelain, which shows anti-inflammatory action. In addition, several other by-products can be used as intermediates to produce citric and lactic acids, among others. The fibers of the juice contain polyphenols, which are useful in cosmetic production [100].
Pomegranate (Punica granatum) is a source of several polyphenolic antioxidants. Still, generally, industries use only their juice, discarding the other parts of the fruit, even those containing compounds such as anthocyanins, hydrolyzable tannins, and ellagic acid, for example [101]. The ellagic and punicic acids are inhibitors of tyrosinase, making it interesting to decrease spots on the skin. Also, they have great interest as antifungals [20].
The olive tree (Olea europaea L.) is a determinant of olive oil production. More than 8 million hectares are occupied by this species [102], and since the market for its derivatives is growing, consequently, waste production is also increasing [103]. Its residues (especially leaves) have potential compounds for the cosmetic industry, such as hydroxytyrosol (23), with antioxidant and inhibitory action of melanin production, avoiding the formation of spots on the skin; tyrosol (24), showing antioxidant and anti-inflammatory activities; palmitic acid (25), a fatty acid acting as a moisturizer; beta-carotene (16), an antioxidant, anti-inflammatory, and regenerating agent of the epidermis; and verbascoside (26), a glycoside with antioxidant and anti-inflammatory activity. These compounds can be obtained by nanofiltration or reverse osmosis, and some of the chemical structures can be seen in Figure 10 [104].
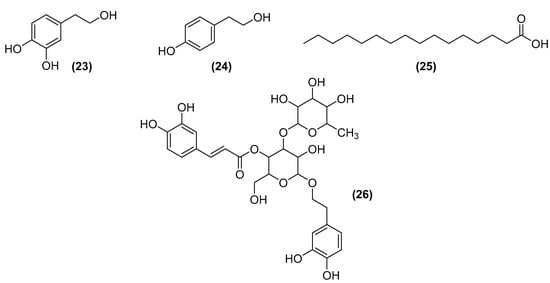
Figure 10.
Potential compounds for the cosmetic industry from olive trees include hydroxytyrosol (23), tyrosol (24), palmitic acid (25), and verbascoside (26).
Approximately 20% of fruit and vegetable production goes to waste. In the case of kiwi (several species of the Actinidiaceae family), the waste comes from leaves, barks, flowers, roots, seeds, and fruits that do not follow market patterns, leading to close to 106 tons of residuesbeing discarded annually. These residues have bioactive components, such as proanthocyanidine, which can be used as an ecological pigment. It also has components with antioxidant, anti-inflammatory, and antimicrobial activities [20,105].
Camellia sinensis and C. assamica are responsible for the green, black, and oolong teas, which are some of the most consumed teas, being the waste produced considerably. Its polyphenols (epigallocatechin gallate, catechin, epicatechin, glycoside-3-O-quercetin, and kaempferol, among others) have anti-inflammatory and antioxidant properties. These also have very attractive pigments for cosmetic formulations [106].
Almonds (Prunus dulcis Mill.) are the most consumed walnuts in the world nowadays. These are treated industrially to serve as highly nutritious food; however, their peel, bark, leaves, and branches are not used, generating abundant residues (just the shells of almonds generate 0.8 to 1.7 tons of discard/year). These residues preserve part of the plant’s bioactive compounds, including polyphenols (such as catechins and kaempferol) and polyunsaturated fatty acids (PUFAs) that can act as antioxidants and as a source of lipids [107].
Approximately 4.84 million tons of American cocoa (Theobroma cacao L.) are produced annually, representing a market of more than 24 billion dollars (2019 data). However, only 20% of cocoa is used, and the other 80% is discarded or applied as biodiesel. In these residues, there are several polyphenols with antioxidant action (procyanidins (27) and catechins (4), anti-inflammatory epicatechin (28), protective against tooth degradation theobromine (29), sunscreen epicatechin and catechins, and promoter of collagen synthesis catechins) (Figure 11) [108].
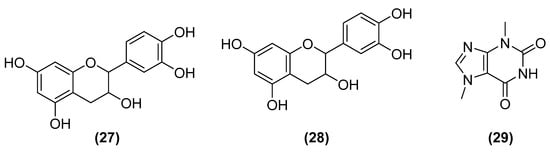
Figure 11.
Polyphenols with antioxidant action from American cocoa include procyanidins (27), epicatechin (28), and theobromine (29).
Lignocellulose is a compound widely found in plant biomass that belongs to the cell walls. Its complex structure consists of cellulose, hemicellulose, and lignin. One of the most common hemicelluloses is known as xylan. Its potential uses in cosmetic products include antioxidants, emulsifiers, stabilizers, and moisturizers, besides acting as prebiotics. The compound’s hydrolysis can form a series of arabino-oligosaccharides with high potential as an antioxidant agent [109].
Mushrooms (Agaricus blazei Murill) are high-waste generator ingredients in the food industry. However, ethanolic extracts of these residues are safe for cosmetic use [110]. A. blazei Murill, in turn, is used medicinally since it contains polysaccharides. For the cosmetic industry, the presence of ergosterol and phenolic acids may have antioxidant and anti-aging functions; assist in normalizing collagen synthesis; suppress inflammatory responses; and normalize the amount of lipids in the skin. Another interesting factor of this fungus is the high concentration of mannitol, a compound useful as a preservative and wetting agent [110]. Cordyceps militaris has applications in the cosmetic industry for its moisturizing properties for skin and hair. These typically undergo solid-state fermentation, and their culture media are discarded after use. However, studies suggest that there is the possibility of reusing components present in the media, such as polyphenols, rutin, and quercetin with antioxidant action, as well as tyrosinase inhibitors and photoprotectors [15]. Tremella fuciformis is rich in bioactive compounds. This mushroom can be grown in sustainable biomass (decomposition of wood or sawdust) and has a polysaccharide called glucuronoxylomanan, showing anti-aging and anti-inflammatory properties. Another characteristic of this compound is its ability to act as a thickener and moisturizer [29].
The canned fish industry generates a significant amount of liquid waste. Usually, before disposal, treatment is made to decrease the amount of organic matter. Solvent extraction processes (recovered) or mechanical or enzymatic extraction could transform aqueous residues into PUFAs [111]. PUFAs are important for skin homeostasis, and their deficiency in the body tends to damage the skin’s barrier function. In formulations, PUFAs may act against photoaging by decreasing the production of pro-inflammatory compounds, in addition to disabling mechanisms to produce prostaglandins. Furthermore, studies have suggested that fish oils may improve the symptoms of various dermatitis (inflammatory conditions) by moisturizing the skin [112].
Mussels (Mytilus galloprovincialis) are a source of proteins with low environmental impact. Damaged mussels are rejected by consumers, generating waste (approximately 27% of the mussels are discarded by such criteria). Thus, the use of its proteins and peptides as a source of antioxidants can be ecologically feasible for the cosmetic industry, for example, aiming at the production of anti-aging creams [113].
Calcium phosphates are biodegradable and biocompatible compounds. These can be obtained from natural sources such as eggshells, fish scales, shells, and milk, among others. They can also be synthesized. In the cosmetic area, calcium phosphates can be used in toothpastes as well as in sunscreens as an inorganic UV filter. They are also useful for formulations to decrease skin shine, absorb sweat and sebum, and create an opaque effect with mild sensory As for deodorants, they can absorb smelly, volatile compounds [114].
Iberian ham is a traditional food in Spain. Its fat is usually discarded. Recent studies have suggested a potential use in the cosmetic industry focused on the composition rich in monounsaturated fatty acids, such as ellagic, gallic, and oleic acids. Those have antioxidant potential and can act as protectors against UV radiation damage. Interestingly, the extraction method is independent of organic solvents [115].
Corn residues (Zea mays L.) from biorefineries also have interesting compounds, especially after fermentation. Squalenes, carotenoids, omega-3, and other steroids with antioxidant potential have already been obtained [116].
Pine wood (Pine sp.) has crude sulfate turpentine, a terpene with a pleasant odor with potential use as a fragrance. The pulp formed in the paper industry presents such a compound, which is considered a residue. Recent studies have shown that this material can be used to isolate alpha and beta-pinene, which have pleasant scents [117].
Moreover, packaging materials have a great impact on the sustainability of cosmetic products. Most marketed cosmetics are available in plastic derived from petrochemicals since they are cost-effective and resistant. However, the substitution for recyclable or biodegradable plastics and reusable packages is a trend to meet consumers’ expectations regarding sustainable practices. Some companies are also combining environmental and social policies with packaging recycling programs, aligned with post-COVID-19 trends [12]. Plastic materials such as poly(ethylene terephthalate) (PET) retain volatile compounds impacting the aroma of products, while other sources of plastic materials such as ethylene-vinyl alcohol copolymers retain water-impacting mechanical properties. Those incompatibilities with plastics must be addressed by packaging design substituting for aluminum or glass containers (which are more recyclable than plastics) [118]. Besides environmental issues, the toxicological effects of plastic packaging on human health are gaining consumer attention, especially for endocrine disruptors’ release from packaging [119]. Industries should have responsibility over the post-use phase of cosmetic products and invest time to design sustainable strategies for the packaging life cycle, alongside concerns about toxicity issues regarding these materials.
4. Other Ingredients
Nanotechnology may be used to improve the properties of all the ingredients mentioned above. When encapsulated into nanovehicles, ingredients can be used in much smaller concentrations, therefore contributing to green labels. Examples of nanovehicles are liposomes, polymer nanoparticles, lipid nanoparticles, nanoemulsions, nanocrystals, and others. However, the process to obtain these nanoformulations must be carefully designed to use a few natural resources [120]. Another example is organoclays, which are interesting materials composed of mineral clay and organic surfactants to obtain biocomposites/nanocomposites. For cosmetics, the potential use of this technology is still under discussion; however, targeted delivery in the skin, rheology control of formulations, and safety increase in ingredients are the main advantages [3].
5. Future Perspectives on Sustainable Ingredients for Cosmetics
Cosmetic industries are required not only to choose safe and sustainable ingredients but also to guarantee that the entire cosmetic supply chain and production are following sustainable practices. Validation of new suppliers aligned with sustainable principles, ensuring that carbon footprints have been tracked and compensated throughout the process. Sustainability is also observed in the social context through more inclusive policies and the promotion of communities that are intimately dependent on biodiversity. Besides being environmentally friendly, cosmetics must be safe throughout their shelf lives. Zero-waste cosmetics includes responsibility for the acquisition, production, and destination of products and packages, including recycling opportunities [3].
The cosmetic industry is encouraged to reduce toxic and non-renewable ingredients and use fewer synthetic ones. On the other hand, organizations have been developing green metrics to track sustainable practices and promote consumer transparency [121].
Future cosmetics must address issues to ensure the viability of “going green“: velocity of natural production versus synthetic production; accessibility of natural products due to their high cost; shelf life of natural ingredients; seasonability issues of natural ingredients; awareness of the environmental impact of competing with agriculture materials; and international certification of “green manufactures” [3].
6. Conclusions
Sustainability is the key to the new era for cosmetic ingredients amongst the current informed and vigilant consumers. Sustainable ingredients can be plant, animal, microorganism, in vitro culture-based, and/or obtained from recycled materials. Each class has its benefits and drawbacks. While plant-derived ingredients are widely used and easily obtained, in vitro-cultured ones require special conditions to achieve good yields. Animal-derived ingredients are controversial and borderline with consumers’ necessities. Otherwise, recycled/upcycled materials showed interesting potential to benefit the environment and several sectors under circular economy logistics. Nevertheless, cosmetic industries must be careful when choosing ingredients, ensuring that they are aligned with their specific target’s needs and obtained through cost-effective processes. The future of cosmetics seems to be based on the use of sustainable ingredients and companies with social and environmental concerns.
Author Contributions
Conceptualization, R.S. and A.R.B.; methodology, R.S. and A.R.B.; formal analysis, A.R.B.; investigation, R.S., R.M.M., A.M.L., J.G., C.R., W.V.M., M.V.R.V. and A.R.B.; writing—original draft preparation, R.S., R.M.M., A.M.L., C.R., W.V.M. and A.R.B.; writing—review and editing, R.S., R.M.M., A.M.L., J.G., C.R., W.V.M. and A.R.B.; supervision, A.R.B.; project administration, A.R.B.; funding acquisition, A.R.B. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq, Process 303862/2022-0); Coordenação de Aperfeiçoamento de Pessoal de Nível Superior-Brasil (CAPES, Finance Code 001); Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP, processes 2017/10789-1 and 2018/10799-0); PrInt USP—PAME (call 2015/2023); and by national funds through FCT—Foundation for Science and Technology, I.P., under the EXPL/BTM-MAT/0112/2021, UIDB/04567/2020, and UIDP/04567/2020 projects attributed to CBIOS.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available upon request from the corresponding author.
Acknowledgments
R.M.M. acknowledges deeply the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior-Brasil, CAPES, for the doctoral Ars. A.M.L. acknowledges financial support from FAPESP. A.R.B. is highly thankful to the Conselho Nacional de Desenvolvimento Científico e Tecnológico, CNPq, for the Research Productivity Scholarship, and to the PrInt USP/CAPES Program.
Conflicts of Interest
W.V.M. was employed by the company Chemyunion Ltd. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
- Morone, J.; Lopes, G.; Preto, M.; Vasconcelos, V.; Martins, R. Exploitation of Filamentous and Picoplanktonic Cyanobacteria for Cosmetic Applications: Potential to Improve Skin Structure and Preserve Dermal Matrix Components. Mar. Drugs 2020, 18, 486. [Google Scholar] [CrossRef]
- Gordobil, O.; Olaizola, P.; Banales, J.M.; Labidi, J. Lignins from Agroindustrial By-Products as Natural Ingredients for Cosmetics: Chemical Structure and in Vitro Sunscreen and Cytotoxic Activities. Molecules 2020, 25, 1131. [Google Scholar] [CrossRef]
- Goyal, N.; Jerold, F. Biocosmetics: Technological Advances and Future Outlook. Environ. Sci. Pollut. Res. 2021, 30, 25148–25169. [Google Scholar] [CrossRef] [PubMed]
- Kaliyadan, F.; Al Dhafiri, M.; Aatif, M. Attitudes toward Organic Cosmetics: A Cross-Sectional Population-Based Survey from the Middle East. J. Cosmet. Dermatol. 2021, 20, 2552–2555. [Google Scholar] [CrossRef] [PubMed]
- Esposti, P.D.; Mortara, A.; Roberti, G. Sharing and Sustainable Consumption in the Era of Covid-19. Sustainability 2021, 13, 1903. [Google Scholar] [CrossRef]
- Xin, X.; Huang, G.; Zhang, B. Review of Aquatic Toxicity of Pharmaceuticals and Personal Care Products to Algae. J. Hazard. Mater. 2021, 410, 124619. [Google Scholar] [CrossRef] [PubMed]
- Hanigan, D.; Truong, L.; Schoepf, J.; Nosaka, T.; Mulchandani, A.; Tanguay, R.L.; Westerhoff, P. Trade-Offs in Ecosystem Impacts from Nanomaterial versus Organic Chemical Ultraviolet Filters in Sunscreens. Water Res. 2018, 139, 281–290. [Google Scholar] [CrossRef]
- Tsui, M.M.P.; Chen, L.; He, T.; Wang, Q.; Hu, C.; Lam, J.C.W.; Lam, P.K.S. Organic Ultraviolet (UV)Filters in the South China Sea Coastal Region: Environmental Occurrence, Toxicological Effects and Risk Assessment. Ecotoxicol. Env. Saf. 2019, 181, 26–33. [Google Scholar] [CrossRef]
- Correddu, F.; Maldini, M.; Addis, R.; Petretto, G.L.; Palomba, M.; Battacone, G.; Pulina, G.; Nudda, A.; Pintore, G. Myrtus Communis Liquor Byproduct as a Source of Bioactive Compounds. Foods 2019, 8, 237. [Google Scholar] [CrossRef]
- Bom, S.; Jorge, J.; Ribeiro, H.M.; Marto, J. A Step Forward on Sustainability in the Cosmetics Industry: A Review. J. Clean Prod. 2019, 225, 270–290. [Google Scholar] [CrossRef]
- Rocca, R.; Acerbi, F.; Fumagalli, L.; Taisch, M. Sustainability Paradigm in the Cosmetics Industry: State of the Art. Clean. Waste Syst. 2022, 3, 100057. [Google Scholar] [CrossRef]
- Martins, A.M.; Marto, J.M. A Sustainable Life Cycle for Cosmetics: From Design and Development to Post-Use Phase. Sustain. Chem. Pharm. 2023, 35, 101178. [Google Scholar] [CrossRef]
- Manjari, K.S.; Chakraborty, D.; Kumar, A.; Singh, S. Biodiversity and Importance of Plant Bioprospecting in Cosmetics. In Bioprospecting of Plant Biodiversity for Industrial Molecules; Upadhyay, S.K., Singh, S.P., Eds.; John Wiley & Sons Ltd.: Hoboken, NJ, USA, 2021; pp. 189–210. ISBN 9781119718017. [Google Scholar]
- Kapadia, P.; Newell, A.S.; Cunningham, J.; Roberts, M.R.; Hardy, J.G. Extraction of High-Value Chemicals from Plants for Technical and Medical Applications. Int. J. Mol. Sci. 2022, 23, 10334. [Google Scholar] [CrossRef]
- Pintathong, P.; Chomnunti, P.; Sangthong, S.; Jirarat, A.; Chaiwut, P. The Feasibility of Utilizing Cultured Cordyceps Militaris Residues in Cosmetics: Biological Activity Assessment of Their Crude Extracts. J. Fungi 2021, 7, 973. [Google Scholar] [CrossRef]
- Elloumi, W.; Maalej, A.; Ortiz, S.; Michel, S.; Chamkha, M.; Boutefnouchet, S.; Sayadi, S. Pistacia lentiscus, L. Distilled Leaves as a Potential Cosmeceutical Ingredient: Phytochemical Characterization, Transdermal Diffusion, and Anti-Elastase and Anti-Tyrosinase Activities. Molecules 2022, 27, 855. [Google Scholar] [CrossRef]
- Liu, J.K. Natural Products in Cosmetics. Nat. Prod. Bioprospect. 2022, 12, 1–43. [Google Scholar] [CrossRef]
- Wang, S.; Zhu, F. Chemical Composition and Biological Activity of Staghorn Sumac (Rhus Typhina). Food Chem. 2017, 237, 431–443. [Google Scholar] [CrossRef] [PubMed]
- Lin, T.K.; Zhong, L.; Santiago, J.L. Anti-Inflammatory and Skin Barrier Repair Effects of Topical Application of Some Plant Oils. Int. J. Mol. Sci. 2018, 19, 70. [Google Scholar] [CrossRef] [PubMed]
- Dini, I.; Laneri, S. The New Challenge of Green Cosmetics: Natural Food Ingredients for Cosmetic Formulations. Molecules 2021, 26, 3921. [Google Scholar] [CrossRef] [PubMed]
- Baldisserotto, A.; Buso, P.; Radice, M.; Dissette, V.; Lampronti, I.; Gambari, R.; Manfredini, S.; Vertuani, S. Moringa Oleifera Leaf Extracts as Multifunctional Ingredients for “Natural and Organic” Sunscreens and Photoprotective Preparations. Molecules 2018, 23, 664. [Google Scholar] [CrossRef] [PubMed]
- Valanciene, E.; Jonuskiene, I.; Syrpas, M.; Augustiniene, E.; Matulis, P.; Simonavicius, A.; Malys, N. Advances and Prospects of Phenolic Acids Production, Biorefinery and Analysis. Biomolecules 2020, 10, 874. [Google Scholar] [CrossRef]
- Shi, F.; Xie, L.; Lin, Q.; Tong, C.; Fu, Q.; Xu, J.; Xiao, J.; Shi, S. Profiling of Tyrosinase Inhibitors in Mango Leaves for a Sustainable Agro-Industry. Food Chem. 2020, 312, 126042. [Google Scholar] [CrossRef]
- Xu, X.; Chen, A.; Ge, X.; Li, S.; Zhang, T.; Xu, H. Chain Conformation and Physicochemical Properties of Polysaccharide (Glucuronoxylomannan) from Fruit Bodies of Tremella Fuciformis. Carbohydr. Polym. 2020, 245, 116354. [Google Scholar] [CrossRef]
- Mellou, F.; Varvaresou, A.; Papageorgiou, S. Renewable Sources: Applications in Personal Care Formulations. Int. J. Cosmet. Sci. 2019, 41, 517–525. [Google Scholar] [CrossRef]
- Alfalah, M.; Loranger, C.; Sasseville, D. Alkyl Glucosides. Dermatitis 2017, 28, 3–4. [Google Scholar] [CrossRef] [PubMed]
- Loranger, C.; Alfalah, M.; Le Bouedec, M.; Sasseville, D. Alkyl Glucosides in Contact Dermatitis. Dermatitis 2017, 28, 5–13. [Google Scholar] [CrossRef] [PubMed]
- Delavault, A.; Grüninger, J.; Kapp, D.; Hollenbach, R.; Rudat, J.; Ochsenreither, K.; Syldatk, C. Enzymatic Synthesis of Alkyl Glucosides by β-Glucosidases in a 2-in-1 Deep Eutectic Solvent System. Chem. Ing. Technol. 2022, 94, 417–426. [Google Scholar] [CrossRef]
- Xu, M.; Wan, Z.; Yang, X. Recent Advances and Applications of Plant-Based Bioactive Saponins in Colloidal Multiphase Food Systems. Molecules 2021, 26, 75. [Google Scholar] [CrossRef] [PubMed]
- Silva, M.A.; Albuquerque, T.G.; Pereira, P.; Ramalho, R.; Vicente, F.; Oliveira, M.B.P.P.; Costa, H.S. Opuntia Ficus-Indica (L.) Mill.: A Multi-Benefit Potential to Be Exploited. Molecules 2021, 26, 951. [Google Scholar] [CrossRef] [PubMed]
- Taofiq, O.; González-Paramás, A.M.; Barreiro, M.F.; Ferreira, I.C.F.R.; McPhee, D.J. Hydroxycinnamic Acids and Their Derivatives: Cosmeceutical Significance, Challenges and Future Perspectives, a Review. Molecules 2017, 22, 281. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, E.C.V.d.; Salvador, D.S.; Holsback, V.; Shultz, J.D.; Michniak-Kohn, B.B.; Leonardi, G.R. Deodorants and Antiperspirants: Identification of New Strategies and Perspectives to Prevent and Control Malodor and Sweat of the Body. Int. J. Dermatol. 2021, 60, 613–619. [Google Scholar] [CrossRef]
- Antoniotti, S. Tuning of Essential Oil Properties by Enzymatic Treatment: Towards Sustainable Processes for the Generation of New Fragrance Ingredients. Molecules 2014, 19, 9203–9214. [Google Scholar] [CrossRef]
- Miranda, M.; Cruz, M.T.; Vitorino, C.; Cabral, C. Nanostructuring Lipid Carriers Using Ridolfia segetum (L.) Moris Essential Oil. Mater. Sci. Eng. C 2019, 103, 109804. [Google Scholar] [CrossRef]
- Aćimović, M.; Tešević, V.; Smiljanić, K.; Cvetković, M.; Stanković, J.; Kiprovski, B.; Sikora, V. Hydrolates: By-Products of Essential Oil Distillation: Chemical Composition, Biological Activity and Potential Uses. Adv. Technol. 2020, 9, 54–70. [Google Scholar] [CrossRef]
- Tavares, C.S.; Gameiro, J.A.; Roseiro, L.B.; Figueiredo, A.C. Hydrolates: A Review on Their Volatiles Composition, Biological Properties and Potential Uses. Phytochem. Rev. 2022, 21, 1661–1737. [Google Scholar] [CrossRef]
- Smiljanić, K.; Prodić, I.; Trifunovic, S.; Krstić Ristivojević, M.; Aćimović, M.; Stanković Jeremić, J.; Lončar, B.; Tešević, V. Multistep Approach Points to Compounds Responsible for the Biological Activity and Safety of Hydrolates from Nine Lamiaceae Medicinal Plants on Human Skin Fibroblasts. Antioxidants 2023, 12, 1988. [Google Scholar] [CrossRef]
- Williams, B.; Njaci, I.; Moghaddam, L.; Long, H.; Dickman, M.B.; Zhang, X.; Mundree, S. Trehalose Accumulation Triggers Autophagy during Plant Desiccation. PLoS Genet 2015, 11, e1005705. [Google Scholar] [CrossRef] [PubMed]
- Janek, T.; Krasowska, A.; Czyznikowska, Z.; Łukaszewicz, M. Trehalose Lipid Biosurfactant Reduces Adhesion of Microbial Pathogens to Polystyrene and Silicone Surfaces: An Experimental and Computational Approach. Front. Microbiol. 2018, 9, 2441. [Google Scholar] [CrossRef]
- Liu, Z.; Vermillion, K.; Jin, C.; Wang, X.; Zhao, W. NMR Study on the Oxidation of Vegetable Oils for Assessing the Antioxidant Function of Trehalose. Biocatal. Agric. Biotechnol. 2021, 36, 102134. [Google Scholar] [CrossRef]
- Ohtake, S.; Wang, J. Trehalose: Current Use and Future Applications. J. Pharm. Sci. 2011, 100, 2020–2053. [Google Scholar] [CrossRef]
- Hussain, M.H.; Mohsin, M.Z.; Zaman, W.Q.; Yu, J.; Zhao, X.; Wei, Y.; Zhuang, Y.; Mohsin, A.; Guo, M. Multiscale Engineering of Microbial Cell Factories: A Step Forward towards Sustainable Natural Products Industry. Synth. Syst. Biotechnol. 2022, 7, 586–601. [Google Scholar] [CrossRef]
- Heath, R.S.; Ruscoe, R.E.; Turner, N.J. The Beauty of Biocatalysis: Sustainable Synthesis of Ingredients in Cosmetics. Nat. Prod. Rep. 2022, 39, 335–388. [Google Scholar] [CrossRef]
- Grajales-Hernández, D.A.; Armendáriz-Ruiz, M.A.; Gallego, F.L.; Mateos-Díaz, J.C. Approaches for the Enzymatic Synthesis of Alkyl Hydroxycinnamates and Applications Thereof. Appl. Microbiol. Biotechnol. 2021, 105, 3901–3917. [Google Scholar] [CrossRef]
- Sharma, A.; Thakur, M.; Bhattacharya, M.; Mandal, T.; Goswami, S. Commercial Application of Cellulose Nano-Composites—A Review. Biotechnol. Rep. 2019, 21, e00316. [Google Scholar] [CrossRef] [PubMed]
- Almeida, T.; Silvestre, A.J.D.; Vilela, C.; Freire, C.S.R. Bacterial Nanocellulose toward Green Cosmetics: Recent Progresses and Challenges. Int. J. Mol. Sci. 2021, 22, 2836. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, M.; Morita, T.; Fukuoka, T.; Imura, T.; Kitamoto, D. Glycolipid Biosurfactants, Mannosylerythritol Lipids, Show Antioxidant and Protective Effects against H2O2-Induced Oxidative Stress in Cultured Human Skin Fibroblasts. J. Oleo. Sci. 2012, 61, 457–464. [Google Scholar] [CrossRef] [PubMed]
- Morita, T.; Fukuoka, T.; Imura, T.; Kitamoto, D. Production of Mannosylerythritol Lipids and Their Application in Cosmetics. Appl. Microbiol. Biotechnol. 2013, 97, 4691–4700. [Google Scholar] [CrossRef] [PubMed]
- Jiang, L.; Fu, H.; Yang, H.K.; Xu, W.; Wang, J.; Yang, S.T. Butyric Acid: Applications and Recent Advances in Its Bioproduction. Biotechnol. Adv. 2018, 36, 2101–2117. [Google Scholar] [CrossRef] [PubMed]
- Paparella, A.; Shaltiel-harpaza, L.; Ibdah, M. Β-Ionone: Its Occurrence and Biological Function and Metabolic Engineering. Plants 2021, 10, 754. [Google Scholar] [CrossRef] [PubMed]
- Cataldo, V.F.; López, J.; Cárcamo, M.; Agosin, E. Chemical vs. Biotechnological Synthesis of C13-Apocarotenoids: Current Methods, Applications and Perspectives. Appl. Microbiol. Biotechnol. 2016, 100, 5703–5718. [Google Scholar] [CrossRef]
- Czajka, J.J.; Nathenson, J.A.; Benites, V.T.; Baidoo, E.E.K.; Cheng, Q.; Wang, Y.; Tang, Y.J. Engineering the Oleaginous Yeast Yarrowia Lipolytica to Produce the Aroma Compound β-Ionone. Microb. Cell Fact. 2018, 17, 136. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Xie, X.; Tang, H.; Peng, C.; Peng, F. The Bioactivities of Sclareol: A Mini Review. Front. Pharmacol. 2022, 13, 1014105. [Google Scholar] [CrossRef] [PubMed]
- Schalk, M.; Pastore, L.; Mirata, M.A.; Khim, S.; Schouwey, M.; Deguerry, F.; Pineda, V.; Rocci, L.; Daviet, L. Toward a Biosynthetic Route to Sclareol and Amber Odorants. J. Am. Chem. Soc. 2012, 134, 18900–18903. [Google Scholar] [CrossRef]
- Liu, Y.; Ma, X.; Liang, H.; Stephanopoulos, G.; Zhou, K. Monoterpenoid Biosynthesis by Engineered Microbes. J. Ind. Microbiol. Biotechnol. 2021, 48, kuab065. [Google Scholar] [CrossRef]
- Rawat, G.; Tripathi, P.; Saxena, R.K. Expanding Horizons of Shikimic Acid: Recent Progresses in Production and Its Endless Frontiers in Application and Market Trends. Appl. Microbiol. Biotechnol. 2013, 97, 4277–4287. [Google Scholar] [CrossRef]
- Kim, T.Y.; Park, H.; Kim, S.K.; Kim, S.J.; Park, Y.C. Production of (−)-α-Bisabolol in Metabolically Engineered Saccharomyces Cerevisiae. J. Biotechnol. 2021, 340, 13–21. [Google Scholar] [CrossRef]
- Lim, H.S.; Kim, S.K.; Woo, S.G.; Kim, T.H.; Yeom, S.J.; Yong, W.; Ko, Y.J.; Kim, S.J.; Lee, S.G.; Lee, D.H. (−)-α-Bisabolol Production in Engineered Escherichia Coli Expressing a Novel (−)-α-Bisabolol Synthase from the Globe Artichoke Cynara Cardunculus Var. Scolymus. J. Agric. Food Chem. 2021, 69, 8492–8503. [Google Scholar] [CrossRef]
- Choksi, J.; Vora, J.; Shrivastava, N. Bioactive Pigments from Isolated Bacteria and Its Antibacterial, Antioxidant and Sun Protective Application Useful for Cosmetic Products. Indian J. Microbiol. 2020, 60, 379–382. [Google Scholar] [CrossRef]
- Gomes, V.; Salgueiro, S.P. From Small to Large-Scale: A Review of Recombinant Spider Silk and Collagen Bioproduction. Discov. Mater. 2022, 2, 3. [Google Scholar] [CrossRef]
- Cristiano, L.; Guagni, M. Zooceuticals and Cosmetic Ingredients Derived from Animals. Cosmetics 2022, 9, 13. [Google Scholar] [CrossRef]
- Almeida, C.; Rijo, P.; Rosado, C. Bioactive Compounds from Hermetia Illucens Larvae as Natural Ingredients for Cosmetic Application. Biomolecules 2020, 10, 976. [Google Scholar] [CrossRef]
- Casadidio, C.; Peregrina, D.V.; Gigliobianco, M.R.; Deng, S.; Censi, R.; Di Martino, P. Chitin and Chitosans: Characteristics, Eco-Friendly Processes, and Applications in Cosmetic Science. Mar. Drugs 2019, 17, 369. [Google Scholar] [CrossRef]
- Pinheiro, A.C.A.S.; Mart, F.J.; Barba, F.J.; Tappi, S.; Rocculi, P. Innovative Non-Thermal Technologies for Recovery and Valorization of Value-Added Products from Crustacean Economy Approach. Foods 2021, 10, 2030. [Google Scholar] [CrossRef]
- Farias, J.M.; Stamford, T.C.M.; Resende, A.H.M.; Aguiar, J.S.; Rufino, R.D.; Luna, J.M.; Sarubbo, L.A. Mouthwash Containing a Biosurfactant and Chitosan: An Eco-Sustainable Option for the Control of Cariogenic Microorganisms. Int. J. Biol. Macromol. 2019, 129, 853–860. [Google Scholar] [CrossRef] [PubMed]
- Nong, Y.; Maloh, J.; Natarelli, N.; Gunt, H.B.; Tristani, E.; Sivamani, R.K. A Review of the Use of Beeswax in Skincare. J. Cosmet. Dermatol. 2023, 22, 2166–2173. [Google Scholar] [CrossRef] [PubMed]
- Krasteva, G.; Georgiev, V.; Pavlov, A. Recent Applications of Plant Cell Culture Technology in Cosmetics and Foods. Eng. Life Sci. 2021, 21, 68–76. [Google Scholar] [CrossRef] [PubMed]
- Eibl, R.; Meier, P.; Stutz, I.; Schildberger, D.; Hühn, T.; Eibl, D. Plant Cell Culture Technology in the Cosmetics and Food Industries: Current State and Future Trends. Appl. Microbiol. Biotechnol. 2018, 102, 8661–8675. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, A.; ul Qamar, M.T.; Shoukat, A.; Aslam, M.M.; Tariq, M.; Hakiman, M.; Joyia, F.A. The Effects of Genotypes and Media Composition on Callogenesis, Regeneration and Cell Suspension Culture of Chamomile (Matricaria chamomilla L.). PeerJ 2021, 9, e11464. [Google Scholar] [CrossRef] [PubMed]
- Marchev, A.S.; Georgiev, M.I. Plant in Vitro Systems as a Sustainable Source of Active Ingredients for Cosmeceutical Application. Molecules 2020, 25, 2006. [Google Scholar] [CrossRef] [PubMed]
- Barbulova, A.; Apone, F.; Colucci, G. Plant Cell Cultures as Source of Cosmetic Active Ingredients. Cosmetics 2014, 1, 94–104. [Google Scholar] [CrossRef]
- Georgiev, V.; Slavov, A.; Vasileva, I.; Pavlov, A. Plant Cell Culture as Emerging Technology for Production of Active Cosmetic Ingredients. Eng. Life Sci. 2018, 18, 779–798. [Google Scholar] [CrossRef] [PubMed]
- Pressi, G.; Bertaiola, O.; Guarnerio, C.; Barbieri, E.; Guzzo, F.; Durand, C.; Peno-mazzarino, L.; Cocetta, V.; Giacomini, I.; Semenzato, A. In Vitro Cultured Melissa Officinalis Cells as Effective Ingredient to Protect Skin against Oxidative Stress, Blue Light, and Infrared Irradiations Damages. Cosmetics 2021, 8, 23. [Google Scholar] [CrossRef]
- Aslam, A.; Bahadar, A.; Liaquat, R.; Saleem, M.; Waqas, A.; Zwawi, M. Algae as an Attractive Source for Cosmetics to Counter Environmental Stress. Sci. Total Environ. 2021, 772, 144905. [Google Scholar] [CrossRef]
- Morocho-Jácome, A.L.; Ruscinc, N.; Martinez, R.M.; Carvalho, J.C.M.; Santos de Almeida, T.; Rosado, C.; Costa, J.G.; Velasco, M.V.R.; Baby, A.R. (Bio)Technological Aspects of Microalgae Pigments for Cosmetics. Appl. Microbiol. Biotechnol. 2020, 104, 9513–9522. [Google Scholar] [CrossRef]
- Lopez-Hortas, L.; Florez-Fernandez, N.; Torres, M.D.; Ferreira-Anta, T.; Casas, M.P.; Balboa, E.M.; Falque, E.; Domínguez, H. Applying Seaweed Compounds in Cosmetics, Cosmeceuticals and Nutricosmetics. Mar. Drugs 2021, 19, 552. [Google Scholar] [CrossRef]
- Kalasariya, H.S.; Yadav, V.K.; Yadav, K.K.; Tirth, V.; Algahtani, A.; Islam, S.; Gupta, N.; Jeon, B.H. Seaweed-Based Molecules and Their Potential Biological Activities: An Eco-Sustainable Cosmetics. Molecules 2021, 26, 5313. [Google Scholar] [CrossRef] [PubMed]
- Chauton, M.S.; Forbord, S.; Mäkinen, S.; Sarno, A.; Slizyte, R.; Mozuraityte, R.; Standal, I.B.; Skjermo, J. Sustainable Resource Production for Manufacturing Bioactives from Micro- and Macroalgae: Examples from Harvesting and Cultivation in the Nordic Region. Physiol. Plant 2021, 173, 495–506. [Google Scholar] [CrossRef] [PubMed]
- Hentati, F.; Tounsi, L.; Djomdi, D.; Pierre, G.; Delattre, C.; Ursu, A.V.; Fendri, I.; Abdelkafi, S.; Michaud, P. Bioactive Polysaccharides from Seaweeds. Molecules 2020, 25, 3152. [Google Scholar] [CrossRef]
- Alves, A.; Sousa, E.; Sousa, E.; Kijjoa, A.; Pinto, M.; Pinto, M. Marine-Derived Compounds with Potential Use as Cosmeceuticals and Nutricosmetics. Molecules 2020, 25, 2536. [Google Scholar] [CrossRef]
- Huynh, A.; Maktabi, B.; Reddy, C.M.; O’Neil, G.W.; Chandler, M.; Baki, G. Evaluation of Alkenones, a Renewably Sourced, Plant-Derived Wax as a Structuring Agent for Lipsticks. Int. J. Cosmet. Sci. 2020, 42, 146–155. [Google Scholar] [CrossRef]
- Harada, R.; Nomura, T.; Yamada, K.; Mochida, K.; Suzuki, K. Genetic Engineering Strategies for Euglena Gracilis and Its Industrial Contribution to Sustainable Development Goals: A Review. Front. Bioeng. Biotechnol. 2020, 8, 790. [Google Scholar] [CrossRef] [PubMed]
- Iriondo-Dehond, A.; Iriondo-Dehond, M.; Del Castillo, M.D. Applications of Compounds from Coffee Processing By-Products. Biomolecules 2020, 10, 1219. [Google Scholar] [CrossRef]
- Matos, M.S.; Romero-Díez, R.; Álvarez, A.; Bronze, M.R.; Rodríguez-Rojo, S.; Mato, R.B.; Cocero, M.J.; Matias, A.A. Polyphenol-Rich Extracts Obtained from Winemakingwaste Streams as Natural Ingredients with Cosmeceutical Potential. Antioxidants 2019, 8, 355. [Google Scholar] [CrossRef] [PubMed]
- Hübner, A.d.A.; de Aguiar, M.M.G.B.; Demarque, D.P.; Rosado, C.; Velasco, M.V.R.; Kikuchi, I.S.; Baby, A.R.; Pessoa, F.V.L.S. Technological Aspects and Potential Cutaneous Application of Wine Industry by-Products. Appl. Sci. 2023, 13, 9068. [Google Scholar] [CrossRef]
- Punzo, A.; Porru, E.; Silla, A.; Simoni, P.; Galletti, P.; Roda, A.; Tagliavini, E.; Samorì, C.; Caliceti, C. Grape Pomace for Topical Application: Green Nades Sustainable Extraction, Skin Permeation Studies, Antioxidant and Anti-Inflammatory Activities Characterization in 3d Human Keratinocytes. Biomolecules 2021, 11, 1181. [Google Scholar] [CrossRef]
- Ferri, M.; Rondini, G.; Calabretta, M.M.; Michelini, E.; Vallini, V.; Fava, F.; Roda, A.; Minnucci, G.; Tassoni, A. White Grape Pomace Extracts, Obtained by a Sequential Enzymatic plus Ethanol-Based Extraction, Exert Antioxidant, Anti-Tyrosinase and Anti-Inflammatory Activities. N. Biotechnol. 2017, 39, 51–58. [Google Scholar] [CrossRef]
- Baroi, A.M.; Sieniawska, E.; Świątek, Ł.; Fierascu, I. Grape Waste Materials—An Attractive Source for Developing Nanomaterials with Versatile Applications. Nanomaterials 2023, 13, 836. [Google Scholar] [CrossRef]
- Hoss, I.; Rajha, H.N.; Khoury, R.E.; Youssef, S.; Manca, M.L.; Manconi, M.; Louka, N.; Maroun, R.G. Valorization of Wine-Making By-Products’ Extracts in Cosmetics. Cosmetics 2021, 8, 109. [Google Scholar] [CrossRef]
- Vella, F.M.; Cautela, D.; Laratta, B. Characterization of Polyphenolic Compounds in Cantaloupe Melon By-Products. Foods 2019, 8, 196. [Google Scholar] [CrossRef]
- Rodrigues, F.; Gaspar, C.; Palmeira-De-Oliveira, A.; Sarmento, B.; Amaral, M.H.; Oliveira, M.B.P.P. Application of Coffee Silverskin in Cosmetic Formulations: Physical/Antioxidant Stability Studies and Cytotoxicity Effects. Drug Dev. Ind. Pharm. 2016, 42, 99–106. [Google Scholar] [CrossRef]
- Rosado, C.; Tokunaga, V.K.; Sauce, R.; De Oliveira, C.A.; Sarruf, F.D.; Parise-Filho, R.; Maurício, E.; De Almeida, T.S.; Velasco, M.V.R.; Baby, A.R. Another Reason for Using Caffeine in Dermocosmetics: Sunscreen Adjuvant. Front. Physiol. 2019, 10, 519. [Google Scholar] [CrossRef]
- Burns, E.E.; Davies, I.A. Coral Ecotoxicological Data Evaluation for the Environmental Safety Assessment of Ultraviolet Filters. Env. Toxicol. Chem. 2021, 40, 3441–3464. [Google Scholar] [CrossRef] [PubMed]
- Tomazelli, L.C.; de Assis Ramos, M.M.; Sauce, R.; Cândido, T.M.; Sarruf, F.D.; de Oliveira Pinto, C.A.S.; de Oliveira, C.A.; Rosado, C.; Velasco, M.V.R.; Baby, A.R. SPF Enhancement Provided by Rutin in a Multifunctional Sunscreen. Int. J. Pharm. 2018, 552, 401–406. [Google Scholar] [CrossRef] [PubMed]
- Hübner, A.A.; Demarque, D.P.; Lourenço, F.R.; Rosado, C.; Baby, A.R.; Kikuchi, I.S.; Bacchi, E.M. Phytocompounds Recovered from the Waste of Cabernet Sauvignon (Vitis vinifera L.) Vinification: Cytotoxicity (in Normal and Stressful Conditions) and In Vitro Photoprotection Efficacy in a Sunscreen System. Cosmetics 2022, 10, 2. [Google Scholar] [CrossRef]
- De Oliveira Bispo, M.; Morocho-Jácome, A.L.; Escudeiro, C.C.; Martinez, R.M.; de Oliveira Pinto, C.A.S.; Rosado, C.; Velasco, M.V.R.; Baby, A.R. Photoprotective Efficacy of the Association of Rosmarinic Acid 0.1% with Ethylhexyl Methoxycinnamate and Avobenzone. Cosmetics 2023, 10, 11. [Google Scholar] [CrossRef]
- Hubner, A.; Sobreira, F.; Neto, A.V.; de Oliveira Pinto, C.A.S.; Dario, M.F.; Díaz, I.E.C.; Lourenço, F.R.; Rosado, C.; Baby, A.R.; Bacchi, E.M. The Synergistic Behavior of Antioxidant Phenolic Compounds Obtained Fromwinemaking Waste’s Valorization, Increased the Efficacy of a Sunscreen System. Antioxidants 2019, 8, 530. [Google Scholar] [CrossRef] [PubMed]
- Hübner, A.A.; Sarruf, F.D.; Oliveira, C.A.; Neto, A.V.; Fischer, D.C.H.; Kato, E.T.M.; Lourenço, F.R.; Baby, A.R.; Bacchi, E.M. Safety and Photoprotective Efficacy of a Sunscreen System Based on Grape Pomace (Vitis vinifera L.) Phenolics from Winemaking. Pharmaceutics 2020, 12, 1148. [Google Scholar] [CrossRef] [PubMed]
- Peres, D.D.A.; Sarruf, F.D.; de Oliveira, C.A.; Velasco, M.V.R.; Baby, A.R. Ferulic Acid Photoprotective Properties in Association with UV Filters: Multifunctional Sunscreen with Improved SPF and UVA-PF. J. Photochem. Photobiol. B 2018, 185, 46–49. [Google Scholar] [CrossRef]
- Campos, D.A.; Ribeiro, T.B.; Teixeira, J.A.; Pastrana, L.; Pintado, M.M. Integral Valorization of Pineapple (Ananas comosus L.) by-Products through a Green Chemistry Approach towards Added Value Ingredients. Foods 2020, 9, 60. [Google Scholar] [CrossRef]
- Boggia, R.; Turrini, F.; Villa, C.; Lacapra, C.; Zunin, P.; Parodi, B. Green Extraction from Pomegranate Marcs for the Production of Functional Foods and Cosmetics. Pharmaceuticals 2016, 9, 63. [Google Scholar] [CrossRef]
- Clodoveo, M.L.; Crupi, P.; Annunziato, A.; Corbo, F. Innovative Extraction Technologies for Development of Functional Ingredients Based on Polyphenols from Olive Leaves. Foods 2022, 11, 103. [Google Scholar] [CrossRef]
- Foti, P.; Pino, A.; Romeo, F.V.; Vaccalluzzo, A.; Caggia, C.; Randazzo, C.L. Olive Pomace and Pâté Olive Cake as Suitable Ingredients for Food and Feed. Microorganisms 2022, 10, 237. [Google Scholar] [CrossRef]
- Madureira, J.; Margaça, F.M.A.; Santos-Buelga, C.; Ferreira, I.C.F.R.; Verde, S.C.; Barros, L. Applications of Bioactive Compounds Extracted from Olive Industry Wastes: A Review. Compr. Rev. Food Sci. Food Saf. 2022, 21, 453–476. [Google Scholar] [CrossRef]
- Chamorro, F.; Carpena, M.; Fraga-Corral, M.; Echave, J.; Riaz Rajoka, M.S.; Barba, F.J.; Cao, H.; Xiao, J.; Prieto, M.A.; Simal-Gandara, J. Valorization of Kiwi Agricultural Waste and Industry By-Products by Recovering Bioactive Compounds and Applications as Food Additives: A Circular Economy Model. Food Chem. 2022, 370, 131315. [Google Scholar] [CrossRef]
- Wang, S.; Zeng, T.; Zhao, S.; Zhu, Y.; Feng, C.; Zhan, J.; Li, S.; Ho, C.T.; Gosslau, A. Multifunctional Health-Promoting Effects of Oolong Tea and Its Products. Food Sci. Hum. Wellness 2022, 11, 512–523. [Google Scholar] [CrossRef]
- Barral-Martinez, M.; Fraga-Corral, M.; Garcia-Perez, P.; Simal-Gandara, J.; Prieto, M.A. Almond By-Products: Valorization for Sustainability and Competitiveness of the Industry. Foods 2021, 10, 1793. [Google Scholar] [CrossRef]
- Agudelo, C.; Bravo, K.; Ramírez-Atehortúa, A.; Torres, D.; Osorio, E.; Carrillo-Hormaza, L. Chemical and Skincare Property Characterization of the Main Cocoa Byproducts: Extraction Optimization by Rsm Approach for Development of Sustainable Ingredients. Molecules 2021, 26, 7429. [Google Scholar] [CrossRef] [PubMed]
- Capetti, C.C.d.M.; Vacilotto, M.M.; Dabul, A.N.G.; Sepulchro, A.G.V.; Pellegrini, V.O.A.; Polikarpov, I. Recent Advances in the Enzymatic Production and Applications of Xylooligosaccharides. World J. Microbiol. Biotechnol. 2021, 37, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Taofiq, O.; Rodrigues, F.; Barros, L.; Peralta, R.M.; Barreiro, M.F.; Ferreira, I.C.F.R.; Oliveira, M.B.P.P. Agaricus Blazei Murrill from Brazil: An Ingredient for Nutraceutical and Cosmeceutical Applications. Food Funct. 2019, 10, 565–572. [Google Scholar] [CrossRef] [PubMed]
- Monteiro, A.; Paquincha, D.; Martins, F.; Queirós, R.P.; Saraiva, J.A.; Švarc-Gajić, J.; Nastić, N.; Delerue-Matos, C.; Carvalho, A.P. Liquid By-Products from Fish Canning Industry as Sustainable Sources of Ω3 Lipids. J. Env. Manag. 2018, 219, 9–17. [Google Scholar] [CrossRef] [PubMed]
- Huang, T.H.; Wang, P.W.; Yang, S.C.; Chou, W.L.; Fang, J.Y. Cosmetic and Therapeutic Applications of Fish Oil’s Fatty Acids on the Skin. Mar. Drugs 2018, 16, 256. [Google Scholar] [CrossRef]
- Cunha, S.A.; de Castro, R.; Coscueta, E.R.; Pintado, M. Hydrolysate from Mussel Mytilus Galloprovincialis Meat: Enzymatic Hydrolysis, Optimization and Bioactive Properties. Molecules 2021, 26, 5228. [Google Scholar] [CrossRef]
- Carella, F.; Esposti, L.D.; Adamiano, A.; Iafisc, M. The Use of Calcium Phosphates in Cosmetics, State of the Art and Future Perspectives. Materials 2021, 14, 6398. [Google Scholar] [CrossRef]
- Bruna-García, E.; Redondo, B.I.; Castro, M.M. New Method for Obtaining a Bioactive Essence Extracted from Iberian Ham Fat Rich in MUFA and Antioxidants. Molecules 2022, 27, 428. [Google Scholar] [CrossRef] [PubMed]
- Cairone, F.; Cesa, S.; Ciogli, A.; Fabrizi, G.; Goggiamani, A.; Iazzetti, A.; Di Lena, G.; Sanchez Del Pulgar, J.; Lucarini, M.; Cantò, L.; et al. Valorization of By-Products from Biofuel Biorefineries: Extraction and Purification of Bioactive Molecules from Post-Fermentation Corn Oil. Foods 2022, 11, 153. [Google Scholar] [CrossRef] [PubMed]
- Sagorin, G.; Cazeils, E.; Basset, J.; Reiter, M. From Pine to Perfume. Chimia 2021, 75, 780–787. [Google Scholar] [CrossRef] [PubMed]
- Briasco, B.; Capra, P.; Cozzi, A.C.; Mannucci, B.; Perugini, P. Packaging Evaluation Approach to Improve Cosmetic Product Safety. Cosmetics 2016, 3, 32. [Google Scholar] [CrossRef]
- Bou-Maroun, E.; Dahbi, L.; Dujourdy, L.; Ferret, P.-J.; Chagnon, M.-C. Migration Studies and Endocrine Disrupting Activities: Chemical Safety of Cosmetic Plastic Packaging. Polymers 2023, 15, 4009. [Google Scholar] [CrossRef] [PubMed]
- Gupta, V.; Mohapatra, S.; Mishra, H.; Farooq, U.; Kumar, K.; Ansari, M.J.; Aldawsari, M.F.; Alalaiwe, A.S.; Mirza, M.A.; Iqbal, Z. Nanotechnology in Cosmetics and Cosmeceuticals—A Review of Latest Advancements. Gels 2022, 8, 173. [Google Scholar] [CrossRef] [PubMed]
- Eckelman, M.J.; Moroney, M.S.; Zimmerman, J.B.; Anastas, P.T.; Thompson, E.; Scott, P.; McKeever-Alfieri, M.; Cavanaugh, P.F.; Daher, G. Applying Green Chemistry to Raw Material Selection and Product Formulation at The Estée Lauder Companies. Green Chem. 2021, 24, 2397–2408. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).