Review of the Current State of Pyrolysis and Biochar Utilization in Europe: A Scientific Perspective
Abstract
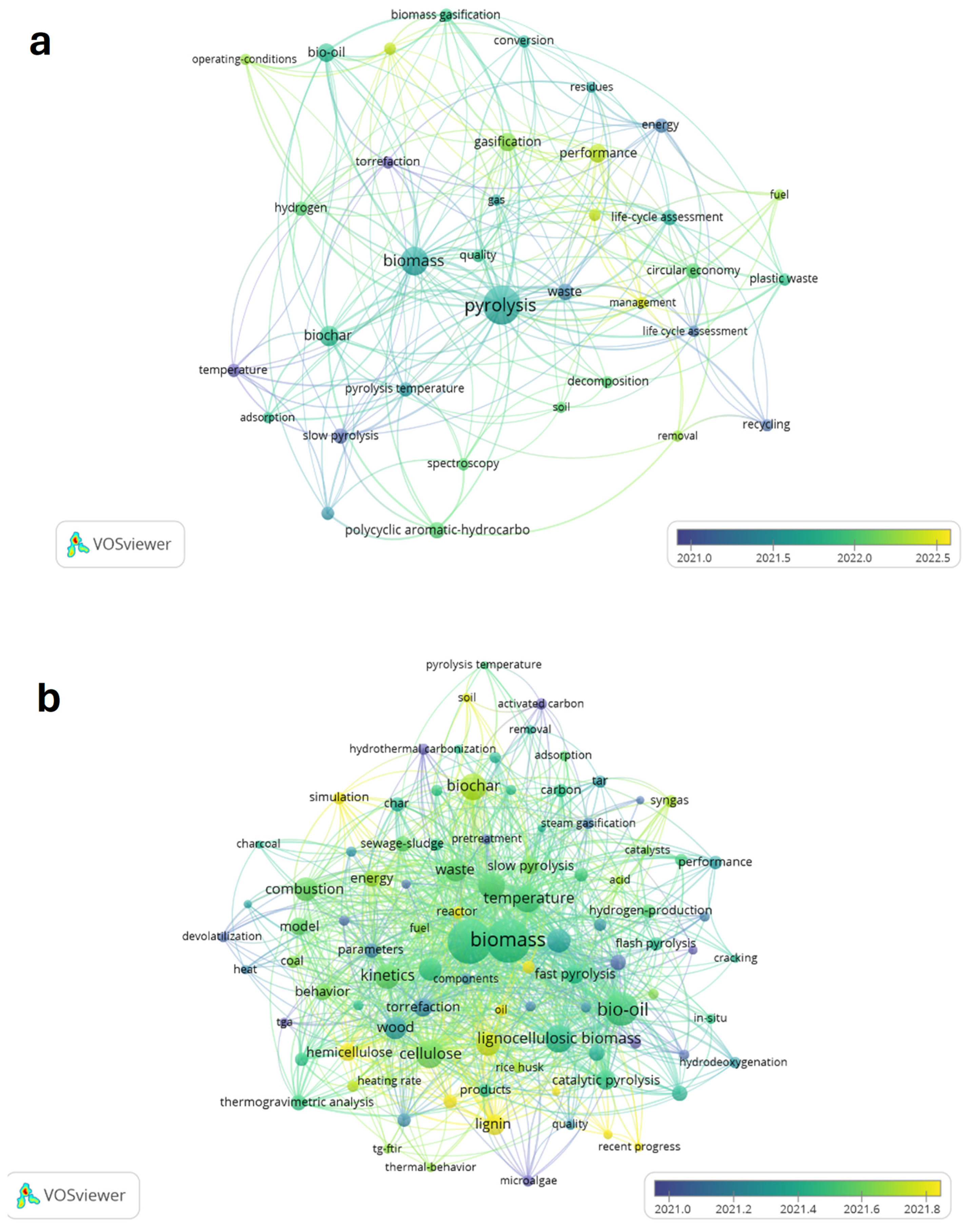
1. Introduction
2. Overview: Main Pyrolysis Studies in Europe
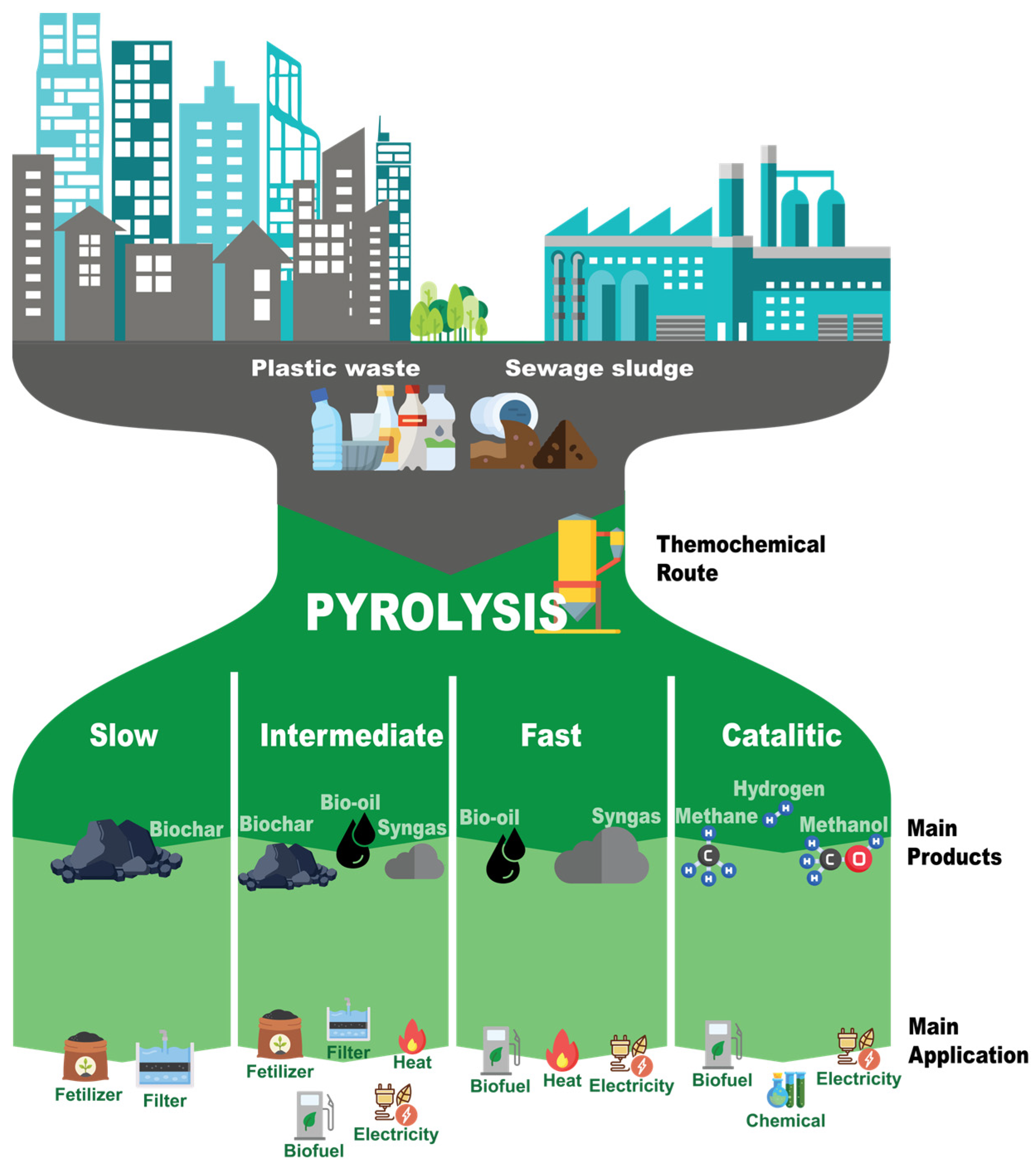
2.1. Plastic Pyrolysis
2.2. Biomass Pyrolysis
| Number in Map | Type of Pyrolisis | Feedstock | Product | Reactor Type | TRL Level * | Temperature | Reference |
|---|---|---|---|---|---|---|---|
| 9 | Slow | Refuse-derived fuel, paper, sewage sludge, and rubber, and waste wood biomass (hornbeam leaves, pine, and spruce bark) | Biochar | Tube-furnace heated; lab Scale | 2–3 | 300 °C | [27] |
| 10 | Slow | Sewage sludge | Biochar P recuperation | Pilot plant designed and operated by RE-CORD, called SPYRO (Slow Pyrolysis Reactor)—auger type reactor | 4–5 | 450 °C | [28] |
| 11 | Fast | Energy crop Miscanthus × giganteus (M × g) | Biochar Bio-oil Syngas | Muffle oven (LAC, Ht205) (a), a glass tube used as a reactor (b), cooler with flowing cold water (c), round bottom flask (d) three wash bottles filled with acetone (e) | 2–3 | 600 °C | [22] |
| 12 | Slow and Intermediate | Spruce, pine, and larch | Biochar Bio-oil Syngas | Lab-scale cylindrical fixed-bed pyrolysis chamber | 2–3 | 300, 400 and 500 °C | [23] |
| 13 | Slow | Pig manure and invasive plant Japanese knotweed | Biochar | Fixed-bed slow pyrolysis experiments were conducted with a modular stainless steel container | 2–3 | 400–700 °C | [6] |
| 14 | Slow | Disposal of waste-activated sludge from wastewater treatment of an effluent from five milk processing plant | Biochar | Quartz tube reactor (wrapped with a heating tape and high-temperature insulation) coupled with a condenser cooler (cooled through circulation of a refrigerated liquid at 0 °C) and a twin-neck round-bottom receiving flask where the pyrolysis liquid was collected | 2–3 and 4–5 | 600–700 °C | [7] |
| 15 | Intermediate | Hardwood pellets, softwood pellets, and chips | Biochar Bio-oil Syngas | TCR reactors | 2–3 | 400 °C and 500 °C. | [29] |
| 16 | Intermediate | Spent coffee grounds | Syngas (H2) Bio-oil | TCR reactors | 2–3 | 500 and 700 °C | [30] |
| 17 | Intermediate | Sewage Sludge | Bio-oil | TCR reactors | 4–5 | 450 °C pyrolysis and 700 °C post-reforming temperature | [31] |
| 18 | Intermediate | Sewage sludge | Bio-oil Biochar Syngas | TCR reactors | 4–5 | 500–600 °C and 700 °C | [32] |
| 19 | Fast | Sawdust | Biochar Bio-oil Syngas | Fluidized bed | 2–3 | 500 °C | [24] |
| 20 | Fast | Willow (Salix spp.) | Biochar Bio-oil Syngas | Abrative reactor | 4–5 | 750 °C | [25] |
| 21 | Intermediate | Pine bark | Biochar Bio-oil Syngas | Pyrolysis prototype named “Ariane” composed by an interlocking of three cylinders forming three distinct temperature zones | 2–3 | 350 °C pyrolysis zone inlet 780 °C maximum temperature | [26] |
| Number in Map | Company | Feedstock | Products | TRL * | Country |
|---|---|---|---|---|---|
| 22 | Biorizon-TNO | Lignocelullosic biomass | Bio-aromatics | 4–5 | The Netherlands |
| 23 | Project AquaGreen PCE | Sewage sludge | Syngas; biochar | 6–7 | Denmark |
| 24 | Springkildeprojektet | Agricultural waste | Syngas; Biochar | 6–7 | Denmark |
| 25 | BTGBioliquids | Biomass: sawdust, sunflower husk, roadside grass, and straw | Bio-oil | 8–9 | The Netherlands |
| 26 | GreenEco | Tire plastic | Bio-oil, biochar, syngas, steel | 8–9 | Estonia |
| 27 | Modulbg | Tire plastic | Bio-oil, biochar, syngas, steel | 8–9 | Bulgaria |
| 28 | NGE material morphing technology | Sewage sludge | Coke, Syngas | 8–9 | Austria |
| 29 | Fraunhofer | Biomass and plastic | Bio-oil; Syngas | 4–5 | Germany |
| 30 | Karlsruhe Institute of Technology | Plastic | Bio-oil; Syngas | 4–5 | Germany |
| 31 | VTT Technical research center of Finland | Biomass and plastic | Bio-oil- | 4–5 | Finland |
3. Biochar: Sewage Sludge, Thermal Conversion Processes (TCR), and Phosphorus Recovery
3.1. Sewage Sludge and Pyrolysis
3.2. Thermo-Catalytic Reforming (TCR) Approach
3.3. Importance of Phosphorus and Biochar as P Recycling and P Fertilizer
3.4. Shutdown: Possible Scenarios for Biochar Applications
- Agricultural enhancement: Biochar plays a crucial role in agriculture by enhancing soil fertility, nutrient retention, and water retention. It facilitates nutrient release to the soil, aiding in the fertilization process.
- Carbon sequestration for climate mitigation: Biochar emerges as a significant tool for carbon sequestration, contributing to climate change mitigation efforts. Its ability to capture and store carbon positions it as a promising solution in the fight against climate change.
- Industrial applications—metallurgy and construction: Biochar demonstrates promise in industrial applications, particularly in metallurgy and construction. Its use in the production of materials, such as ferroalloys, showcases its versatility and potential as a substitute for coal in various industrial processes.
- Energy production and co-firing: Biochar’s role in energy production, either through direct combustion or co-firing with other biomass sources, presents an opportunity for renewable energy generation. It can serve as both a renewable energy source and a co-substrate to control process conditions when used in conjunction with biomass.
4. Comparation between Pyrolysis from Plastic and Biomass: Impacts and Overview
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Paz-Ferreiro, J.; Nieto, A.; Méndez, A.; Askeland, M.P.J.; Gascó, G. Biochar from Biosolids Pyrolysis: A Review. Int. J. Environ. Res. Public Health 2018, 15, 956. [Google Scholar] [CrossRef]
- Pires, A.P.P.; Arauzo, J.; Fonts, I.; Domine, M.E.; Fernández Arroyo, A.; Garcia-Perez, M.E.; Montoya, J.; Chejne, F.; Pfromm, P.; Garcia-Perez, M. Challenges and Opportunities for Bio-Oil Refining: A Review. Energy Fuels 2019, 33, 4683–4720. [Google Scholar] [CrossRef]
- Yogalakshmi, K.N.; Devi, T.P.; Sivashanmugam, P.; Kavitha, S.; Kannah, R.Y.; Varjani, S.; AdishKumar, S.; Kumar, G. Lignocellulosic Biomass-Based Pyrolysis: A Comprehensive Review. Chemosphere 2022, 286, 131824. [Google Scholar] [CrossRef]
- Leng, L.; Huang, H. An Overview of the Effect of Pyrolysis Process Parameters on Biochar Stability. Bioresour. Technol. 2018, 270, 627–642. [Google Scholar] [CrossRef]
- Yapıcı, E.; Akgün, H.; Özkan, K.; Günkaya, Z.; Özkan, A.; Banar, M. Prediction of Gas Product Yield from Packaging Waste Pyrolysis: Support Vector and Gaussian Process Regression Models. Int. J. Environ. Sci. Technol. 2023, 20, 461–476. [Google Scholar] [CrossRef]
- Qiu, J.; de Souza, M.F.; Robles-Aguilar, A.A.; Ghysels, S.; Ok, Y.S.; Ronsse, F.; Meers, E. Improving Biochar Properties by Co-Pyrolysis of Pig Manure with Bio-Invasive Weed for Use as the Soil Amendment. Chemosphere 2023, 312, 137229. [Google Scholar] [CrossRef] [PubMed]
- Kwapinska, M.; Pisano, I.; Leahy, J.J. Preliminary Assessment of Pyrolysis Biochar Derived from Milk/Dairy Processing Sludge as a Potential Component of Fertilizers. ACS Sustain. Chem. Eng. 2023, 11, 2345–2353. [Google Scholar] [CrossRef]
- EPRO. Plastics-the Facts 2019 An Analysis of European Plastics Production, Demand and Waste Data; EPRO: Bristol, UK, 2019. [Google Scholar]
- Zhou, N.; Dai, L.; Lyu, Y.; Li, H.; Deng, W.; Guo, F.; Chen, P.; Lei, H.; Ruan, R. Catalytic Pyrolysis of Plastic Wastes in a Continuous Microwave Assisted Pyrolysis System for Fuel Production. Chem. Eng. J. 2021, 418, 129412. [Google Scholar] [CrossRef]
- Sekar, M.; Ponnusamy, V.K.; Pugazhendhi, A.; Nižetić, S.; Praveenkumar, T.R. Production and Utilization of Pyrolysis Oil from Solidplastic Wastes: A Review on Pyrolysis Process and Influence of Reactors Design. J. Environ. Manag. 2022, 302, 114046. [Google Scholar] [CrossRef] [PubMed]
- Ortega, F.; Martín-Lara, M.Á.; Pula, H.J.; Zamorano, M.; Calero, M.; Blázquez, G. Characterization of the Products of the Catalytic Pyrolysis of Discarded COVID-19 Masks over Sepiolite. Appl. Sci. 2023, 13, 3188. [Google Scholar] [CrossRef]
- Ligero, A.; Calero, M.; Pérez, A.; Solís, R.R.; Muñoz-Batista, M.J.; Martín-Lara, M.Á. Low-Cost Activated Carbon from the Pyrolysis of Post-Consumer Plastic Waste and the Application in CO2 Capture. Process Saf. Environ. Prot. 2023, 173, 558–566. [Google Scholar] [CrossRef]
- Palomar-Torres, A.; Torres-Jimenez, E.; Kegl, B.; Bombek, G.; Volmajer-Valh, J.; Lešnik, L. Catalytic Pyrolysis of Plastic Wastes for Liquid Oils’ Production Using ZAP USY Zeolite as a Catalyst. Int. J. Environ. Sci. Technol. 2023, 20, 17–30. [Google Scholar] [CrossRef]
- Aminu, I.; Nahil, M.A.; Williams, P.T. Pyrolysis-Plasma/Catalytic Reforming of Post-Consumer Waste Plastics for Hydrogen Production. Catal. Today 2023, 420, 114084. [Google Scholar] [CrossRef]
- Faussone, G.C.; Seljak, T.; Jasiukaitytė-Grojzdek, E.; Baškovič, U.Ž.; Katrašnik, T.; Grilc, M. Pyrolysis Oil from Post-Consumer Packaging and Its Ageing: Physical and Chemical Properties and Drop-in Performance in a Power Generating Unit. Energy Rep. 2023, 10, 613–627. [Google Scholar] [CrossRef]
- Januszewicz, K.; Hunicz, J.; Kazimierski, P.; Rybak, A.; Suchocki, T.; Duda, K.; Mikulski, M. An Experimental Assessment on a Diesel Engine Powered by Blends of Waste-Plastic-Derived Pyrolysis Oil with Diesel. Energy 2023, 281, 128330. [Google Scholar] [CrossRef]
- Timken, H. Circular Economy for Plastic Waste to Polypropylene via Oil Refinery with Filtering and Metal Oxide Treatment of Pyrolysis Oil. U.S. Patent EP20905131.7A, 23 December 2020. [Google Scholar]
- Chakraborty, S.; Fernald, D.T.; David, L.G.; Herbanek, R.; Richard, J.J.; Johnny, D.C.; Boddie, L.L. Conversion of Waste Plastics to Petrochemicals. U.S. Patent No. EP22740153.6A, 14 January 2022. [Google Scholar]
- Hassibi, N.; Quiring, Y.; Carré, V.; Aubriet, F.; Vernex-Loset, L.; Mauviel, G.; Burklé-Vitzthum, V. Analysis and Control of Products Obtained from Pyrolysis of Polypropylene Using a Reflux Semi-Batch Reactor and GC-MS/FID and FT-ICR MS. J. Anal. Appl. Pyrolysis 2023, 169, 105826. [Google Scholar] [CrossRef]
- Laghezza, M.; Papari, S.; Fiore, S.; Berruti, F. Techno-Economic Assessment of the Pyrolysis of Rubber Waste. Energy 2023, 278, 127841. [Google Scholar] [CrossRef]
- Fahmy, T.Y.A.; Fahmy, Y.; Mobarak, F.; El-Sakhawy, M.; Abou-Zeid, R.E. Biomass Pyrolysis: Past, Present, and Future. In Environment, Development and Sustainability; Springer: Berlin/Heidelberg, Germany, 2020; pp. 17–32. [Google Scholar] [CrossRef]
- Burdová, H.; Kwoczynski, Z.; Nebeská, D.; Souki, K.S.A.; Pilnaj, D.; Grycová, B.; Klemencová, K.; Leštinský, P.; Kuráň, P.; Trögl, J. The Influence of Diesel Contaminated Soil on Miscanthus × giganteus Biomass Thermal Utilization and Pyrolysis Products Composition. J. Clean. Prod. 2023, 406, 136984. [Google Scholar] [CrossRef]
- da Costa, T.P.; Murphy, F.; Roldan, R.; Mediboyina, M.K.; Chen, W.; Sweeney, J.; Capareda, S.; Holden, N.M. Technical and Environmental Assessment of Forestry Residues Valorisation via Fast Pyrolysis in Ireland. Biomass Bioenergy 2023, 173, 106766. [Google Scholar] [CrossRef]
- Shi, Z.; Jin, Y.; Svanberg, R.; Han, T.; Minidis, A.B.E.; Ann-Sofi, K.D.; Kjeldsen, C.; Jönsson, P.G.; Yang, W. Continuous Catalytic Pyrolysis of Biomass Using a Fluidized Bed with Commercial-Ready Catalysts for Scale-Up. Energy 2023, 273, 127288. [Google Scholar] [CrossRef]
- Johansson, A.C.; Bergvall, N.; Molinder, R.; Wikberg, E.; Niinipuu, M.; Sandström, L. Comparison of Co-Refining of Fast Pyrolysis Oil from Salix via Catalytic Cracking and Hydroprocessing. Biomass Bioenergy 2023, 172, 106753. [Google Scholar] [CrossRef]
- Chataigner, V.; Tarlet, D.; Ricoul, F.; Bellettre, J. Experimental and Theoretical Study of Heat and Mass Transfer in a Continuous, Vertical and Coaxial Pyrolysis Reactor for High Porosity Biochar Production. Fuel 2023, 351, 128848. [Google Scholar] [CrossRef]
- Dziok, T. Production of Low-Mercury Solid Fuel by Mild Pyrolysis Process. Energies 2023, 16, 3046. [Google Scholar] [CrossRef]
- Salimbeni, A.; Di Bianca, M.; Lombardi, G.; Rizzo, A.M.; Chiaramonti, D. Opportunities of Integrating Slow Pyrolysis and Chemical Leaching for Extraction of Critical Raw Materials from Sewage Sludge. Water 2023, 15, 1060. [Google Scholar] [CrossRef]
- Gill, M.; Kurian, V.; Kumar, A.; Stenzel, F.; Hornung, A.; Gupta, R. Thermo-Catalytic Reforming of Alberta-Based Biomass Feedstock to Produce Biofuels. Biomass Bioenergy 2021, 152, 106203. [Google Scholar] [CrossRef]
- Elmously, M.; Jäger, N.; Apfelbacher, A.; Daschner, R.; Hornung, A. Thermo-Catalytic Reforming of Spent Coffee Grounds. Bioresour. Bioprocess. 2019, 6, 44. [Google Scholar] [CrossRef]
- Bashir, M.A.; Lima, S.; Jahangiri, H.; Majewski, A.J.; Hofmann, M.; Hornung, A.; Ouadi, M. A Step Change towards Sustainable Aviation Fuel from Sewage Sludge. J. Anal. Appl. Pyrolysis 2022, 163, 105498. [Google Scholar] [CrossRef]
- Marazza, D.; Macrelli, S.; D’Angeli, M.; Righi, S.; Hornung, A.; Contin, A. Greenhouse Gas Savings and Energy Balance of Sewage Sludge Treated through an Enhanced Intermediate Pyrolysis Screw Reactor Combined with a Reforming Process. Waste Manag. 2019, 91, 42–53. [Google Scholar] [CrossRef] [PubMed]
- Lauridsen Carsten. Friction Heated Pyrolysis Unit and Method for Friction Heating a Biomass Pyrolysis Unit. European Patent EP 4253504 A1, 29 March 2023.
- Paradowski, H. Method and installation for fractionating gas derived from pyrolysis of hydrocarbons. U.S. Patent US20040237581, 28 August 2002. [Google Scholar]
- Surup, G.R.; Trubetskaya, A.; Tangstad, M. Charcoal as an Alternative Reductant in Ferroalloy Production: A Review. Processes 2020, 8, 1432. [Google Scholar] [CrossRef]
- Mousa, E.; Sjöblom, K. Modeling and Optimization of Biochar Injection into Blast Furnace to Mitigate the Fossil CO2 Emission. Sustainability 2022, 14, 2393. [Google Scholar] [CrossRef]
- Djandja, O.S.; Wang, Z.C.; Wang, F.; Xu, Y.P.; Duan, P.G. Pyrolysis of Municipal Sewage Sludge for Biofuel Production: A Review. Ind. Eng. Chem. Res. 2020, 59, 16939–16956. [Google Scholar] [CrossRef]
- Yu, B.; Luo, J.; Xie, H.; Yang, H.; Chen, S.; Liu, J.; Zhang, R.; Li, Y.Y. Species, Fractions, and Characterization of Phosphorus in Sewage Sludge: A Critical Review from the Perspective of Recovery. Sci. Total Environ. 2021, 786, 147437. [Google Scholar] [CrossRef] [PubMed]
- Cieślik, B.; Konieczka, P. A Review of Phosphorus Recovery Methods at Various Steps of Wastewater Treatment and Sewage Sludge Management. The Concept of “No Solid Waste Generation” and Analytical Methods. J. Clean. Prod. 2017, 142, 1728–1740. [Google Scholar] [CrossRef]
- Frišták, V.; Bošanská, D.; Turčan, V.; Pipíška, M.; Pfeifer, C.; Soja, G. Relevance of Pyrolysis Products Derived from Sewage Sludge for Soil Applications. Agriculture 2023, 13, 89. [Google Scholar] [CrossRef]
- Hornung, A.; Jahangiri, H.; Ouadi, M.; Kick, C.; Deinert, L.; Meyer, B.; Grunwald, J.; Daschner, R.; Apfelbacher, A.; Meiller, M.; et al. Thermo-Catalytic Reforming (TCR)–An Important Link between Waste Management and Renewable Fuels as Part of the Energy Transition. Appl. Energy Combust. Sci. 2022, 12, 100088. [Google Scholar] [CrossRef]
- Sajdak, M.; Majewski, A.; Di Gruttola, F.; Gałko, G.; Misztal, E.; Rejdak, M.; Hornung, A.; Ouadi, M. Evaluation of the Feasibility of Using TCR-Derived Chars from Selected Biomass Wastes and MSW Fractions in CO2 Sequestration on Degraded and Post-Industrial Areas. Energies 2023, 16, 2964. [Google Scholar] [CrossRef]
- Kick, C.; Uchaikina, A.; Apfelbacher, A.; Daschner, R.; Helmreich, B.; Hornung, A. Aqueous Phase of Thermo-Catalytic Reforming of Sewage Sludge—Quantity, Quality, and Its Electrooxidative Treatment by a Boron-Doped Diamond Electrode. Sep. Purif. Technol. 2022, 286, 120392. [Google Scholar] [CrossRef]
- Hornung, A.; Daschner, R.; Ouadi, M.; Zhou, J.; Lieftink Hygear, D.; Grassi, A.; Capaccioli, S.; Contin, A.; Righi, S.; Marazza, D.; et al. To-Syn-Fuel Project to Convert Sewage Sludge in Value-Added Products. In Proceedings of the 28th European Biomass Conference and Exhibition, Virtual, 6–9 July 2020. [Google Scholar] [CrossRef]
- Li, H.; Li, Y.; Xu, Y.; Lu, X. Biochar Phosphorus Fertilizer Effects on Soil Phosphorus Availability. Chemosphere 2020, 244, 125471. [Google Scholar] [CrossRef]
- Gul, S.; Whalen, J.K. Biochemical Cycling of Nitrogen and Phosphorus in Biochar-Amended Soils. Soil Biol. Biochem. 2016, 103, 1–15. [Google Scholar] [CrossRef]
- Qian, T.; Yang, Q.; Jun, D.C.F.; Dong, F.; Zhou, Y. Transformation of Phosphorus in Sewage Sludge Biochar Mediated by a Phosphate-Solubilizing Microorganism. Chem. Eng. J. 2019, 359, 1573–1580. [Google Scholar] [CrossRef]
- Wang, F.; Yu, F.; Wei, Y.; Li, A.; Xu, S.; Lu, X. Promoting Hydrocarbon Production from Fatty Acid Pyrolysis Using Transition Metal or Phosphorus Modified Al-MCM-41 Catalyst. J. Anal. Appl. Pyrolysis 2021, 156, 105146. [Google Scholar] [CrossRef]
- Novotný, M.; Marković, M.; Raček, J.; Šipka, M.; Chorazy, T.; Tošić, I.; Hlavínek, P. The Use of Biochar Made from Biomass and Biosolids as a Substrate for Green Infrastructure: A Review. Sustain. Chem. Pharm. 2023, 32, 100999. [Google Scholar] [CrossRef]
- Xu, Q.; Tang, S.; Wang, J.; Ko, J.H. Pyrolysis Kinetics of Sewage Sludge and Its Biochar Characteristics. Process Saf. Environ. Prot. 2018, 115, 49–56. [Google Scholar] [CrossRef]
- Figueiredo, C.C.d.; Reis, A.d.S.P.J.; Araujo, A.S.d.; Blum, L.E.B.; Shah, K.; Paz-Ferreiro, J. Assessing the Potential of Sewage Sludge-Derived Biochar as a Novel Phosphorus Fertilizer: Influence of Extractant Solutions and Pyrolysis Temperatures. Waste Manag. 2021, 124, 144–153. [Google Scholar] [CrossRef]
- Adhikari, S.; Gascó, G.; Méndez, A.; Surapaneni, A.; Jegatheesan, V.; Shah, K.; Paz-Ferreiro, J. Influence of Pyrolysis Parameters on Phosphorus Fractions of Biosolids Derived Biochar. Sci. Total Environ. 2019, 695, 133846. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Chen, C.; Gray, E.M.; Boyd, S.E.; Yang, H.; Zhang, D. Roles of Biochar in Improving Phosphorus Availability in Soils: A Phosphate Adsorbent and a Source of Available Phosphorus. Geoderma 2016, 276, 1–6. [Google Scholar] [CrossRef]
- Qureshi, M.S.; Oasmaa, A.; Pihkola, H.; Deviatkin, I.; Tenhunen, A.; Mannila, J.; Minkkinen, H.; Pohjakallio, M.; Laine-Ylijoki, J. Pyrolysis of Plastic Waste: Opportunities and Challenges. J. Anal. Appl. Pyrolysis 2020, 152, 104804s. [Google Scholar] [CrossRef]
- Surup, G.R.; Nielsen, H.K.; Großarth, M.; Deike, R.; Van den Bulcke, J.; Kibleur, P.; Müller, M.; Ziegner, M.; Yazhenskikh, E.; Beloshapkin, S.; et al. Effect of Operating Conditions and Feedstock Composition on the Properties of Manganese Oxide or Quartz Charcoal Pellets for the Use in Ferroalloy Industries. Energy 2020, 193, 116736. [Google Scholar] [CrossRef]


| Number in Map | Type of Pyrolysis | Feedstock | Product | Reactor Type | TRL Level * | Temperature | Reference |
|---|---|---|---|---|---|---|---|
| 1 | Fast and Catalytic | Surgical and FFP2 masks | Syngas Bio-oil Biochar | Horizontal tubular furnace | 2–3 | 450, 500, and 550 °C | [11] |
| 2 | Fast | Dirty and wet mixture of post-consumer plastic waste | Activated carbon form biochar | Tubular furnace | 2–3 | 500 °C | [12] |
| 3 | Catalytic with ZAP USY zeolite | Plastic from Municipal waste | Bio-oil | Single-batch fixed-bed pyrolysis reactor | 2–3 | 400 °C±30 °C | [13] |
| 4 | Catalytic with Ni/MCM-41 | HDPE, PP, OS, PET plastics which were recycled waste plastics donated by Regain Polymers Castleford, UK | Gas, liquid, and char, but the main one is gas (H2) | Two-stage experimental reactor system 1st stage pyrolysis reactor and a 2nd stage non-thermal plasma reactor | 2–3 | 750 °C | [14] |
| 5 | Intermediate | Polypropylene—PP | Biochar Bio-oil | Glass semi-batch reactor | 2–3 | 480 °C | [19] |
| 6 | Slow | Rubber | Char Oil (mainly composed of C10, benzene, D-limonene, cyclohexadiene, and cyclo-heptane) Gas, mainly H2 and CH4 | Horizontal batch mechanically fluidized reactor (MFR) | 2–3 | 300–500 °C | [20] |
| 7 | Intermediate | Plastic from Municipal waste | Bio-oil Biochar | Rotary kiln | 6–7 | 400 °C | [15] |
| 8 | Slow | Polypropylene—PP Polystyrene—PS | Bio-oil | Single-batch fixed-bed pyrolysis reactor | 2–3 | 400–500 °C | [16] |
| Characteristic | Plastic | Biomass |
|---|---|---|
| Feedstock | Pyrolysis involves the thermal decomposition of plastic waste | Pyrolysis involves the conversion of organic materials such as wood, agricultural residues, and energy crops. |
| Composition | Primarily made of carbon and hydrogen | Contains a mixture of carbon, hydrogen, oxygen, and other elements, including nitrogen and sulphur |
| Energy Content | Higher energy content compared to biomass (more energy-rich products) | Lower energy content compared to plastic |
| Process and Products | Plastics are typically heated in the absence of oxygen, leading to the production of liquid fuels, gases, and a solid residue (char). | Follows a similar plastic process, but due to the varied composition of biomass, the product yield and composition can differ. Biomass pyrolysis can produce bio-oils, gases (including syngas), and biochar. |
| Environmental Impact | Both plastic and biomass pyrolysis can contribute to reducing waste and mitigating greenhouse gas emissions. However, plastic pyrolysis can release harmful pollutants due to the presence of chlorine and other additives in plastics, which requires proper emission control systems | On the other hand, it is generally considered more environmentally friendly due to the renewable nature of biomass and its potential to be carbon neutral |
| Applications | The products derived from plastic pyrolysis, such as pyrolysis oil, can be used as a feedstock in refineries or as an alternative fuel | Biomass pyrolysis products, such as biochar, can be use as fertilizer in agriculture and syngas can be applied as biofuel |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Volpi, M.P.C.; Silva, J.C.G.; Hornung, A.; Ouadi, M. Review of the Current State of Pyrolysis and Biochar Utilization in Europe: A Scientific Perspective. Clean Technol. 2024, 6, 152-175. https://doi.org/10.3390/cleantechnol6010010
Volpi MPC, Silva JCG, Hornung A, Ouadi M. Review of the Current State of Pyrolysis and Biochar Utilization in Europe: A Scientific Perspective. Clean Technologies. 2024; 6(1):152-175. https://doi.org/10.3390/cleantechnol6010010
Chicago/Turabian StyleVolpi, Maria P. C., Jean C. G. Silva, Andreas Hornung, and Miloud Ouadi. 2024. "Review of the Current State of Pyrolysis and Biochar Utilization in Europe: A Scientific Perspective" Clean Technologies 6, no. 1: 152-175. https://doi.org/10.3390/cleantechnol6010010
APA StyleVolpi, M. P. C., Silva, J. C. G., Hornung, A., & Ouadi, M. (2024). Review of the Current State of Pyrolysis and Biochar Utilization in Europe: A Scientific Perspective. Clean Technologies, 6(1), 152-175. https://doi.org/10.3390/cleantechnol6010010







