Insights into the Adsorption of Cr(VI) on Activated Carbon Prepared from Walnut Shells: Combining Response Surface Methodology with Computational Calculation
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemical
2.2. Preparation of Activated Carbon
2.3. Batch Adsorption Experiments
2.4. Experimental Design
2.5. Computational Methods
3. Results
3.1. Characterization of Activated Carbon
3.2. Experimental Design
3.3. Validation of Model
3.4. 3D Surfaces, Contour and Perturbation Plots
3.5. Optimization and Validation of Result
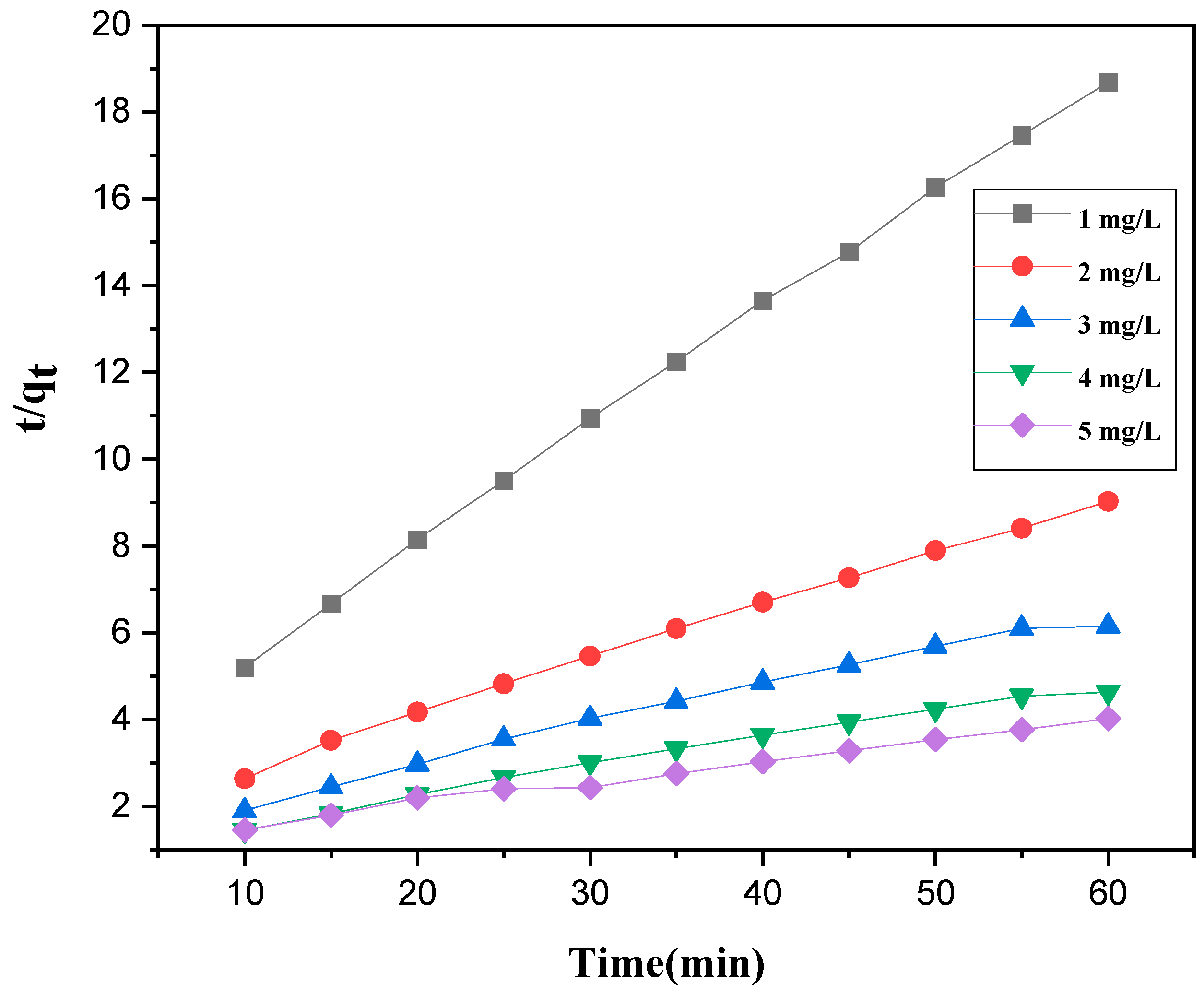
3.6. Kinetics Study
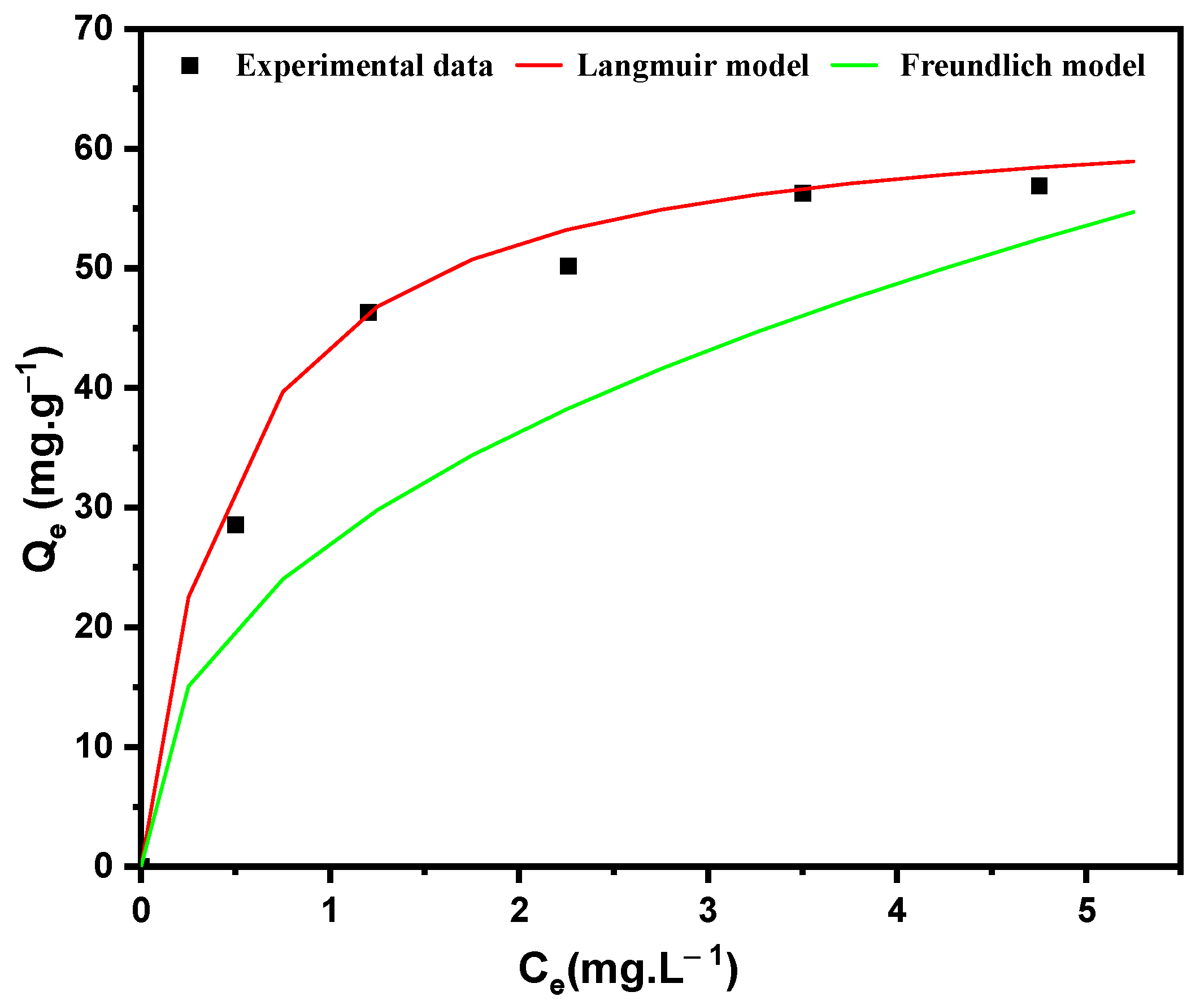
3.7. Equilibrium Study
3.8. Adsorption Process Thermodynamics
3.9. Mechanism of Adsorption of Cr(VI) on AC-Ws Surface
3.10. Reusing Test of AC-Ws
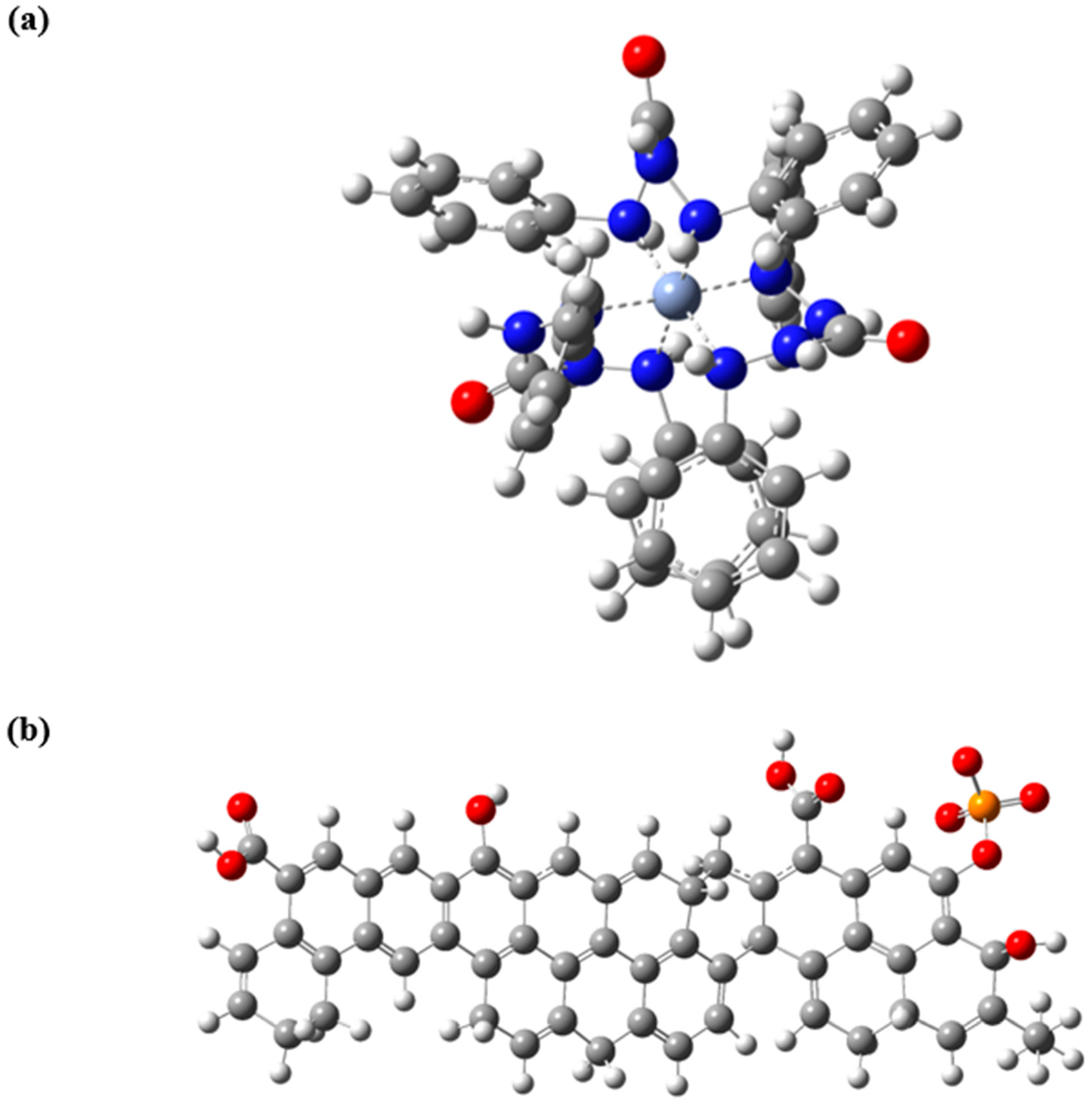
3.11. Mechanistic Study
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhang, L.; Fang, M. Nanomaterials in Pollution Trace Detection and Environmental Improvement. Nano Today 2010, 5, 128–142. [Google Scholar] [CrossRef]
- Cho, D.-W.; Chon, C.-M.; Kim, Y.; Jeon, B.-H.; Schwartz, F.W.; Lee, E.-S.; Song, H. Adsorption of Nitrate and Cr(VI) by Cationic Polymer-Modified Granular Activated Carbon. Chem. Eng. J. 2011, 175, 298–305. [Google Scholar] [CrossRef]
- Kleinberg, M.N.; Thamaraiselvan, C.; Powell, C.D.; Ronen, A.; Arnusch, C.J. Reduction of Cr(VI) to Cr(III) by Activated Carbon Cloth through Adsorption and Electrochemical Processes. ACS Appl. Eng. Mater. 2023, 1, 901–912. [Google Scholar] [CrossRef]
- Yang, J.; Yu, K.; Liu, C. Chromium Immobilization in Soil Using Quaternary Ammonium Cations Modified Montmorillonite: Characterization and Mechanism. J. Hazard. Mater. 2017, 321, 73–80. [Google Scholar] [CrossRef] [PubMed]
- Garg, U.K.; Kaur, M.P.; Garg, V.K.; Sud, D. Removal of Hexavalent Chromium from Aqueous Solution by Agricultural Waste Biomass. J. Hazard. Mater. 2007, 140, 60–68. [Google Scholar] [CrossRef] [PubMed]
- Abedi, F.; Dubé, M.A.; Kruczek, B. Adsorption of Heavy Metals on the Surface of Nanofiltration Membranes: “A Curse or Blessing”? J. Membr. Sci. 2023, 685, 121988. [Google Scholar] [CrossRef]
- Yang, J.; Yu, M.; Qiu, T. Adsorption Thermodynamics and Kinetics of Cr(VI) on KIP210 Resin. J. Ind. Eng. Chem. 2014, 20, 480–486. [Google Scholar] [CrossRef]
- Zhao, D.; Yu, Y.; Chen, J.P. Zirconium/Polyvinyl Alcohol Modified Flat-Sheet Polyvinyldene Fluoride Membrane for Decontamination of Arsenic: Material Design and Optimization, Study of Mechanisms, and Application Prospects. Chemosphere 2016, 155, 630–639. [Google Scholar] [CrossRef]
- Lv, Y.; Chang, K.; Wu, H.; Fang, P.; Chen, C.; Liao, Q. Highly Efficient Scavenging of Cr(VI) by Two-Dimensional Titanium Carbide Nanosheets: Kinetics, Isotherms and Thermodynamics Analysis. Water Sci. Technol. 2021, 84, 2446–2456. [Google Scholar] [CrossRef]
- Wang, X.; Chen, M.; Nayanathara, R.M.O.; Zhang, Z.; Zhang, X. Tune Wastepaper to Hydrophobic Membranes through Metal-Ion-Induced Lignocellulose Nanofibril Crosslinking for Oil-Water Separation. Carbohydr. Polym. Technol. Appl. 2023, 6, 100377. [Google Scholar] [CrossRef]
- Paziresh, S.; Dehqan, A.; Zinadini, S.; Zinatizadeh, A.A.; Vatanpour, V. Rosemary Particle as a New Green Additive to Improve Polysulfone Membrane Separation Performance in Removal of Organic Pollutants. Sep. Purif. Technol. 2024, 334, 126015. [Google Scholar] [CrossRef]
- He, G.; Xiao, Y.; Du, H. An Overview and Recent Progress in Photocatalytic Cr(VI) Reduction and Hydrogen Evolution. Int. J. Hydrogen Energy 2024, 53, 633–646. [Google Scholar] [CrossRef]
- Tzou, Y.M.; Wang, S.L.; Wang, M.K. Fluorescent Light Induced Cr(VI) Reduction by Citrate in the Presence of TiO2 and Ferric Ions. Colloids Surf. A Physicochem. Eng. Asp. 2005, 253, 15–22. [Google Scholar] [CrossRef]
- Basnet, P.; Ojha, P.K.; Gyawali, D.; Ghimire, K.N.; Paudyal, H. Thermochemical Study of Cr(VI) Sequestration onto Chemically Modified Areca Catechu and Its Recovery by Desorptive Precipitation Method. Heliyon 2022, 8, e10305. [Google Scholar] [CrossRef] [PubMed]
- Cao, J.; Shi, Y.; Xin, J.; Kong, S.; Wang, X. Application of Microbial Fuel Cells with Tungsten-Based Semiconductor Modified Electrode in the Treatment of Cr (VI) Pollutions. Biochem. Eng. J. 2023, 198, 109034. [Google Scholar] [CrossRef]
- Youssef, H.M.; Abdulhamed, Y.K.; Abu El-Reash, G.M.; Yousef, T.A. Cr(III) and Ni(II) Complexes of Isatin-Hydrazone Ligand: Preparation, Characterization, DFT Studies, Biological Activity, and Ion-Flotation Separation of Ni(II). Inorg. Chem. Commun. 2022, 138, 109278. [Google Scholar] [CrossRef]
- Kubra, K.; Pavithra, V.; Gopal, B.; Janaswamy, S. An Integrated Chemical Approach for the Removal of Hazardous Cr(VI) and Other Contaminants of Tannery Wastewater: Biopolymer-Assisted Adsorption Separation and Conversion into Valuable Materials. Sep. Purif. Technol. 2024, 330, 125220. [Google Scholar] [CrossRef]
- Su, K.; Hu, G.; Zhao, T.; Dong, H.; Yang, Y.; Pan, H.; Lin, Q. The Ultramicropore Biochar Derived from Waste Distiller’s Grains for Wet-Process Phosphoric Acid Purification: Removal Performance and Mechanisms of Cr(VI). Chemosphere 2024, 349, 140877. [Google Scholar] [CrossRef] [PubMed]
- Jahanban-Esfahlan, A.; Jahanban-Esfahlan, R.; Tabibiazar, M.; Roufegarinejad, L.; Amarowicz, R. Recent Advances in the Use of Walnut (Juglans regia L.) Shell as a Valuable Plant-Based Bio-Sorbent for the Removal of Hazardous Materials. RSC Adv. 2020, 10, 7026–7047. [Google Scholar] [CrossRef]
- Ndoun, M.C.; Knopf, A.; Preisendanz, H.E.; Vozenilek, N.; Elliott, H.A.; Mashtare, M.L.; Velegol, S.; Veith, T.L.; Williams, C.F. Fixed Bed Column Experiments Using Cotton Gin Waste and Walnut Shells-Derived Biochar as Low-Cost Solutions to Removing Pharmaceuticals from Aqueous Solutions. Chemosphere 2023, 330, 138591. [Google Scholar] [CrossRef]
- Banerjee, M.; Basu, R.K.; Das, S.K. Cr(VI) Adsorption by a Green Adsorbent Walnut Shell: Adsorption Studies, Regeneration Studies, Scale-up Design and Economic Feasibility. Process Saf. Environ. Prot. 2018, 116, 693–702. [Google Scholar] [CrossRef]
- Soto-Maldonado, C.; Caballero-Valdés, E.; Santis-Bernal, J.; Jara-Quezada, J.; Fuentes-Viveros, L.; Zúñiga-Hansen, M.E. Potential of Solid Wastes from the Walnut Industry: Extraction Conditions to Evaluate the Antioxidant and Bioherbicidal Activities. Electron. J. Biotechnol. 2022, 58, 25–36. [Google Scholar] [CrossRef]
- Jiang, Y. Preparation of Activated Carbon from Walnut Shell and Its Application in Industrial Wastewater. AIP Conf. Proc. 2017, 1839, 020063-1–020063-4. [Google Scholar] [CrossRef]
- Gao, X.; Wu, L.; Wan, W.; Xu, Q.; Li, Z. Preparation of Activated Carbons from Walnut Shell by Fast Activation with H3PO4: Influence of Fluidization of Particles. Int. J. Chem. React. Eng. 2018, 16, 20170074. [Google Scholar] [CrossRef]
- Sanati, A.M.; Kamari, S.; Ghorbani, F. Application of Response Surface Methodology for Optimization of Cadmium Adsorption from Aqueous Solutions by Fe3O4@SiO2@APTMS Core–Shell Magnetic Nanohybrid. Surf. Interfaces 2019, 17, 100374. [Google Scholar] [CrossRef]
- El Mouchtari, E.M.; Daou, C.; Rafqah, S.; Najjar, F.; Anane, H.; Piram, A.; Hamade, A.; Briche, S.; Wong-Wah-Chung, P. TiO2 and Activated Carbon of Argania Spinosa Tree Nutshells Composites for the Adsorption Photocatalysis Removal of Pharmaceuticals from Aqueous Solution. J. Photochem. Photobiol. A Chem. 2020, 388, 112183. [Google Scholar] [CrossRef]
- Yazid, H.; Bouzid, T.; El Himri, M.; Regti, A.; El Haddad, M. Bisphenol A (BPA) Remediation Using Walnut Shell as Activated Carbon Employing Experimental Design for Parameter Optimization and Theoretical Study to Establish the Adsorption Mechanism. Inorg. Chem. Commun. 2024, 161, 112064. [Google Scholar] [CrossRef]
- Regti, A.; Lakbaibi, Z.; Ben El Ayouchia, H.; El Haddad, M.; Laamari, M.R.; El Himri, M.; Haounati, R. Hybrid Methods Combining Computational and Experimental Measurements for the Uptake of Eriochrome Black T Dye Utilising Fish Scales. Int. J. Environ. Anal. Chem. 2023, 103, 4549–4568. [Google Scholar] [CrossRef]
- Suryati, L.; Sulistyarti, H.; Atikah, A. Development of Spectrophotometric Method for Determination of Chromium Species Using Hypochlorite Agent Based on the Formation of Cr(VI)-Diphenylcarbazide Complex. J. Pure App. Chem. Res. 2015, 4, 34–41. [Google Scholar] [CrossRef]
- Wildman, J.; Repiščák, P.; Paterson, M.J.; Galbraith, I. General Force-Field Parametrization Scheme for Molecular Dynamics Simulations of Conjugated Materials in Solution. J. Chem. Theory Comput. 2016, 12, 3813–3824. [Google Scholar] [CrossRef]
- Perdew, J.P.; Burke, K.; Ernzerhof, M. Generalized Gradient Approximation Made Simple. Phys. Rev. Lett. 1996, 77, 3865–3868. [Google Scholar] [CrossRef] [PubMed]
- Lu, T.; Chen, F. Multiwfn: A Multifunctional Wavefunction Analyzer. J. Comput. Chem. 2012, 33, 580–592. [Google Scholar] [CrossRef] [PubMed]
- Humphrey, W.; Dalke, A.; Schulten, K. VMD: Visual Molecular Dynamics. J. Mol. Graph. 1996, 14, 33–38. [Google Scholar] [CrossRef] [PubMed]
- Johnson, E.R.; Keinan, S.; Mori-Sánchez, P.; Contreras-García, J.; Cohen, A.J.; Yang, W. Revealing Noncovalent Interactions. J. Am. Chem. Soc. 2010, 132, 6498–6506. [Google Scholar] [CrossRef] [PubMed]
- Nyberg, J.; Karlsson, M.O.; Hooker, A.C. Simultaneous Optimal Experimental Design on Dose and Sample Times. J. Pharmacokinet. Pharmacodyn. 2009, 36, 125–145. [Google Scholar] [CrossRef] [PubMed]
- Ahmad Isiyaka, H.; Jumbri, K.; Soraya Sambudi, N.; Uba Zango, Z.; Ain Fathihah Binti Abdullah, N.; Saad, B. Effective Adsorption of Metolachlor Herbicide by MIL-53(Al) Metal-Organic Framework: Optimization, Validation and Molecular Docking Simulation Studies. Environ. Nanotechnol. Monit. Manag. 2022, 18, 100663. [Google Scholar] [CrossRef]
- Goertzen, S.L.; Thériault, K.D.; Oickle, A.M.; Tarasuk, A.C.; Andreas, H.A. Standardization of the Boehm Titration. Part I. CO2 Expulsion and Endpoint Determination. Carbon 2010, 48, 1252–1261. [Google Scholar] [CrossRef]
- Koyuncu, F.; Güzel, F.; İnal, İ.I.G. High Surface Area and Supermicroporous Activated Carbon from Capsicum (Capsicum annuum L.) Industrial Processing Pulp via Single-Step KOH-Catalyzed Pyrolysis: Production Optimization, Characterization and Its Some Water Pollutants Removal and Supercapacitor Performance. Diam. Relat. Mater. 2022, 124, 108920. [Google Scholar] [CrossRef]
- Rose, P.K.; Poonia, V.; Kumar, R.; Kataria, N.; Sharma, P.; Lamba, J.; Bhattacharya, P. Congo Red Dye Removal Using Modified Banana Leaves: Adsorption Equilibrium, Kinetics, and Reusability Analysis. Groundw. Sustain. Dev. 2023, 23, 101005. [Google Scholar] [CrossRef]
- Huang, T.; Song, D.; Yang, C.; Zhang, S. Nonthermal Plasma-Irradiated Polyvalent Ferromanganese Binary Hydro(Oxide) for the Removal of Uranyl Ions from Wastewater. Environ. Res. 2023, 217, 114911. [Google Scholar] [CrossRef]
- Zafar, F.F.; Barati, B.; Rasoulzadeh, H.; Sheikhmohammadi, A.; Wang, S.; Chen, H. Adsorption Kinetics Analysis and Optimization of Bisphenol A onto Magnetic Activated Carbon with Shrimp Shell Based Precursor. Biomass Bioenergy 2022, 166, 106604. [Google Scholar] [CrossRef]
- Zerhouni, J.; Rhazi Filali, F.; Naciri Bennani, M.; Qabaqous, O.; Bouymajane, A.; Houssaini, J.; Allaoui, S.; Benhallam, F. Study of the Effect of Acid-Base Character of the Lamellar Double Hydroxides “Zn3Al-CO3” and of the “Ghassoul” Clay on Their Redox Potential and Antimicrobial Activities. Iran. J. Mater. Sci. Eng. 2021, 18, 1–13. [Google Scholar] [CrossRef]
- Chen, S.; Yue, Q.; Gao, B.; Li, Q.; Xu, X. Removal of Cr(VI) from Aqueous Solution Using Modified Corn Stalks: Characteristic, Equilibrium, Kinetic and Thermodynamic Study. Chem. Eng. J. 2011, 168, 909–917. [Google Scholar] [CrossRef]
- Wu, Y.; Luo, H.; Wang, H.; Wang, C.; Zhang, J.; Zhang, Z. Adsorption of Hexavalent Chromium from Aqueous Solutions by Graphene Modified with Cetyltrimethylammonium Bromide. J. Colloid Interface Sci. 2013, 394, 183–191. [Google Scholar] [CrossRef] [PubMed]
- Ltifi, I.; Ayari, F.; Hassen, C.; Ayadi, M.T. Study of the Adsorption of Bright Green by a Natural Clay and Modified. J. Mater. Sci. Eng. 2016, 5, 317–324. [Google Scholar] [CrossRef]
- Grich, A.; Bouzid, T.; Naboulsi, A.; Regti, A.; Tahiri, A.A.; El Himri, M.; El Haddad, M. Preparation of Low-Cost Activated Carbon from Doum Fiber (Chamaerops humilis) for the Removal of Methylene Blue: Optimization Process by DOE/FFD Design, Characterization, and Mechanism. J. Mol. Struct. 2024, 1295, 136534. [Google Scholar] [CrossRef]
- Khan, A.A.; Iqbal, J.; Bashir, M.T.; Amin, M.T.; Sikandar, M.A.; Rahman, M.M.; Arifuzzman, M. Synthesis, Characterization, and Performance of Nano-Metal-Oxide (Al2O3) Blended Biochar for the Removal of Iron from Contaminated Water for Enhanced Kinetic and Adsorption Studies. Processes 2023, 11, 3423. [Google Scholar] [CrossRef]
- Karthikeyan, T.; Rajgopal, S.; Miranda, L. Chromium(VI) Adsorption from Aqueous Solution by Sawdust Activated Carbon. J. Hazard. Mater. 2005, 124, 192–199. [Google Scholar] [CrossRef]
- Yang, J.; Yu, M.; Chen, W. Adsorption of Hexavalent Chromium from Aqueous Solution by Activated Carbon Prepared from Longan Seed: Kinetics, Equilibrium and Thermodynamics. J. Ind. Eng. Chem. 2015, 21, 414–422. [Google Scholar] [CrossRef]
- Noufel, K.; Djebri, N.; Boukhalfa, N.; Boutahala, M.; Dakhouche, A. Removal of Bisphenol A and Trichlorophenol from Aqueous Solutions by Adsorption with Organically Modified Bentonite, Activated Carbon Composites: A Comparative Study in Single and Binary Systems. Groundw. Sustain. Dev. 2020, 11, 100477. [Google Scholar] [CrossRef]
- Yazid, H.; Achour, Y.; El Kassimi, A.; Nadir, I.; El Himri, M.; Laamari, M.; El Haddad, M. Removal of Congo Red from Aqueous Solution Using Cuttlefish Bone Powder. PCR 2021, 9, 565–577. [Google Scholar] [CrossRef]
- Mejbar, F.; Miyah, Y.; Benjelloun, M.; Ssouni, S.; Saka, K.; Lahrichi, A.; Zerrouq, F. High-Performance of Cu@eggshells for Toxic Dyes Catalytic Wet Peroxide Oxidation: Kinetics, Design of Experiments, Regeneration, and Cost Analysis. Case Stud. Chem. Environ. Eng. 2024, 9, 100572. [Google Scholar] [CrossRef]
- Wang, C.; Xie, J.; Zheng, M.; Zhu, J.; Shi, C. Preparation of Mesoporous Biochar from Cornstalk for the Chromium (VI) Elimination by Using One-Step Hydrothermal Carbonation. J. Anal. Methods Chem. 2021, 2021, 3418887. [Google Scholar] [CrossRef]
- Chandra Joshi, N.; Singh, A. Adsorptive Performances and Characterisations of Biologically Synthesised Zinc Oxide Based Nanosorbent (ZOBN). Groundw. Sustain. Dev. 2020, 10, 100325. [Google Scholar] [CrossRef]
- Atieh, M.A. Removal of Chromium (VI) from Polluted Water Using Carbon Nanotubes Supported with Activated Carbon. Procedia Environ. Sci. 2011, 4, 281–293. [Google Scholar] [CrossRef]
- Tang, Q.; Wu, H.; Zhou, M.; Yang, D. Preparation of a Novel High-Performance Lignin-Based Anionic Adsorption Resin for Efficient Removal of Cr(VI) in Aqueous Solutions. Ind. Crop. Prod. 2023, 199, 116720. [Google Scholar] [CrossRef]
- Owlad, M.; Aroua, M.K.; Wan Daud, W.M.A. Hexavalent Chromium Adsorption on Impregnated Palm Shell Activated Carbon with Polyethyleneimine. Bioresour. Technol. 2010, 101, 5098–5103. [Google Scholar] [CrossRef] [PubMed]
- Liang, S.; Jiao, W.; Zhang, D.; Zhang, H.; Qiao, R.; Liu, H.; Wang, M.; Chen, Y.; Zou, M.; Huang, Y.; et al. Phosphoramidic Acid Functionalized Silica Microspheres for Simultaneous Removal of Cr(VI), As(V) and Se(VI) from Aqueous Solutions Based on Molecular Geometry Match. J. Environ. Chem. Eng. 2023, 11, 110300. [Google Scholar] [CrossRef]
- Giri, R.; Kumari, N.; Behera, M.; Sharma, A.; Kumar, S.; Kumar, N.; Singh, R. Adsorption of Hexavalent Chromium from Aqueous Solution Using Pomegranate Peel as Low-Cost Biosorbent. Environ. Sustain. 2021, 4, 401–417. [Google Scholar] [CrossRef]
- Jayasuriya, D.M.N.H.; Nadarajah, K. Understanding Association between Methylene Blue Dye and Biosorbent: Palmyrah Sprout Casing in Adsorption Process in Aqueous Phase. Water Sci. Eng. 2023, 16, 154–164. [Google Scholar] [CrossRef]
- Balakrishnan, A.; Jacob, M.M.; Dayanandan, N.; Chinthala, M.; Ponnuchamy, M.; Vo, D.-V.N.; Appunni, S.; Gajendhran, A.S. Chitosan/Metal Organic Frameworks for Environmental, Energy, and Bio-Medical Applications: A Review. Mater. Adv. 2023, 4, 5920–5947. [Google Scholar] [CrossRef]
- Sellaoui, L.; Gómez-Avilés, A.; Dhaouadi, F.; Bedia, J.; Bonilla-Petriciolet, A.; Rtimi, S.; Belver, C. Adsorption of Emerging Pollutants on Lignin-Based Activated Carbon: Analysis of Adsorption Mechanism via Characterization, Kinetics and Equilibrium Studies. Chem. Eng. J. 2023, 452, 139399. [Google Scholar] [CrossRef]
- Zhu, K.; Ma, J.; Cong, J.; Zhang, T.; Lei, H.; Xu, H.; Luo, Z.; Li, M. The Road to Reuse of Walnut By-Products: A Comprehensive Review of Bioactive Compounds, Extraction and Identification Methods, Biomedical and Industrial Applications. Trends Food Sci. Technol. 2024, 143, 104264. [Google Scholar] [CrossRef]
- Ebisike, K.; Elvis Okoronkwo, A.; Kanayo Alaneme, K.; Jeremiah Akinribide, O. Thermodynamic Study of the Adsorption of Cd2+ and Ni2+ onto Chitosan—Silica Hybrid Aerogel from Aqueous Solution. Results Chem. 2023, 5, 100730. [Google Scholar] [CrossRef]
- Inglezakis, V.J.; Zorpas, A.A. Heat of Adsorption, Adsorption Energy and Activation Energy in Adsorption and Ion Exchange Systems. Desalin. Water Treat. 2012, 39, 149–157. [Google Scholar] [CrossRef]
- Garg, R.; Garg, R.; Sillanpää, M.; Alimuddin; Khan, M.A.; Mubarak, N.M.; Tan, Y.H. Rapid Adsorptive Removal of Chromium from Wastewater Using Walnut-Derived Biosorbents. Sci. Rep. 2023, 13, 6859. [Google Scholar] [CrossRef]
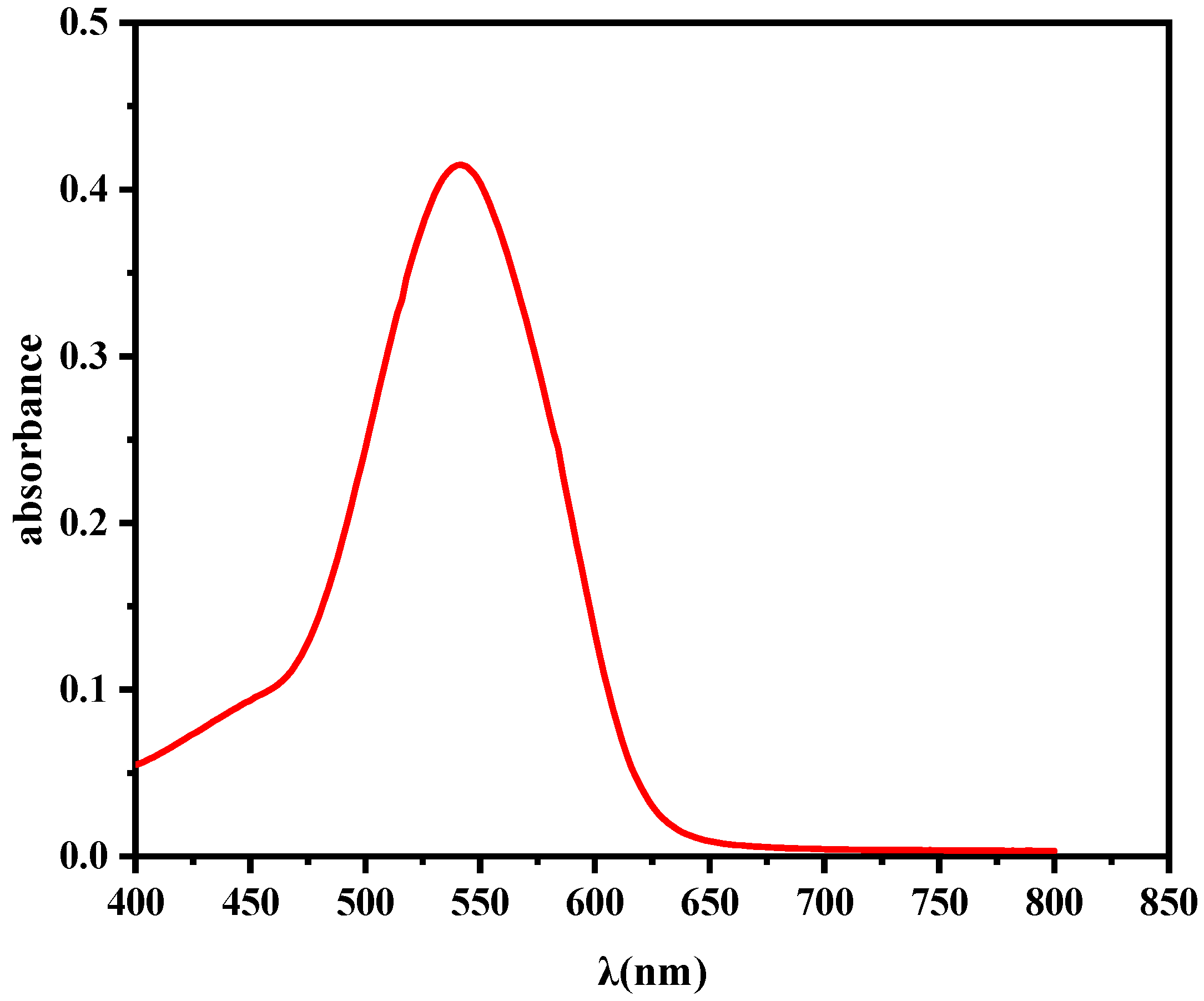
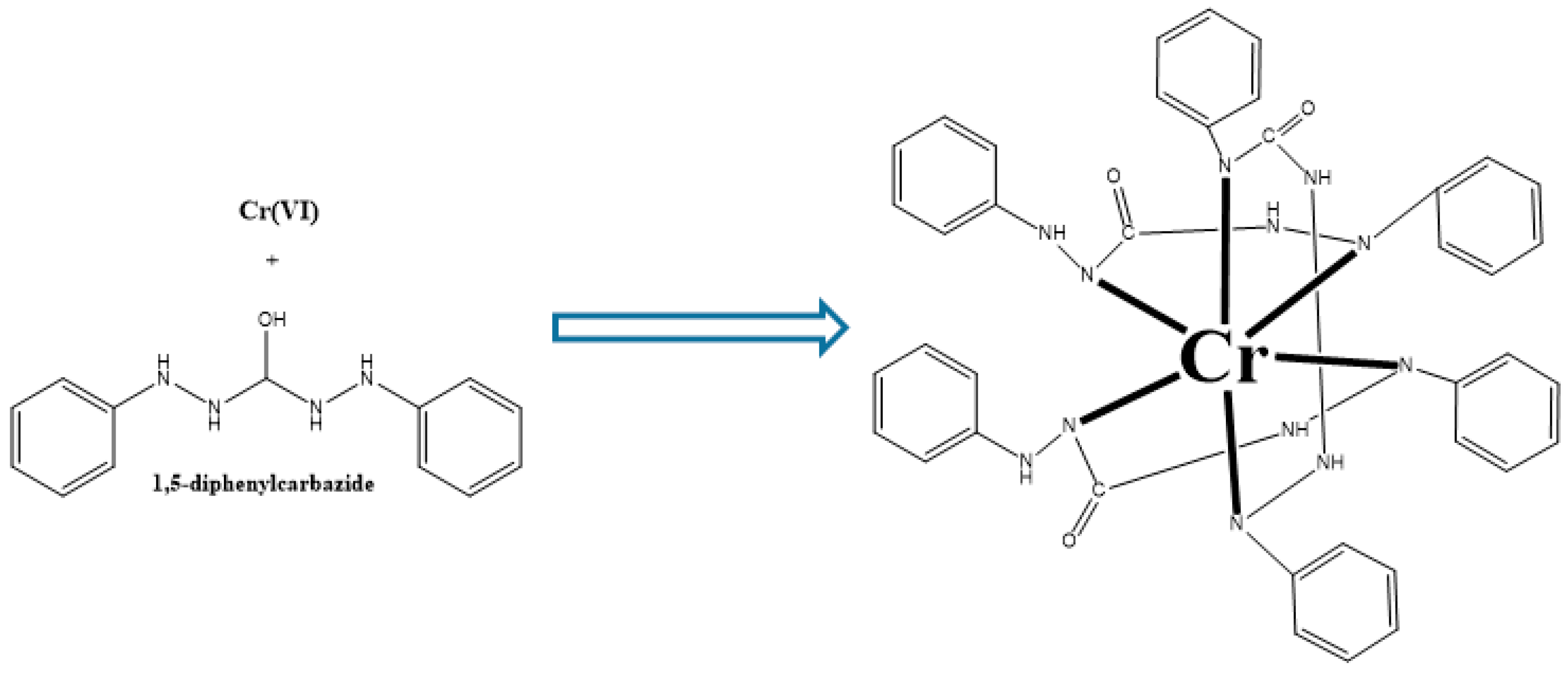

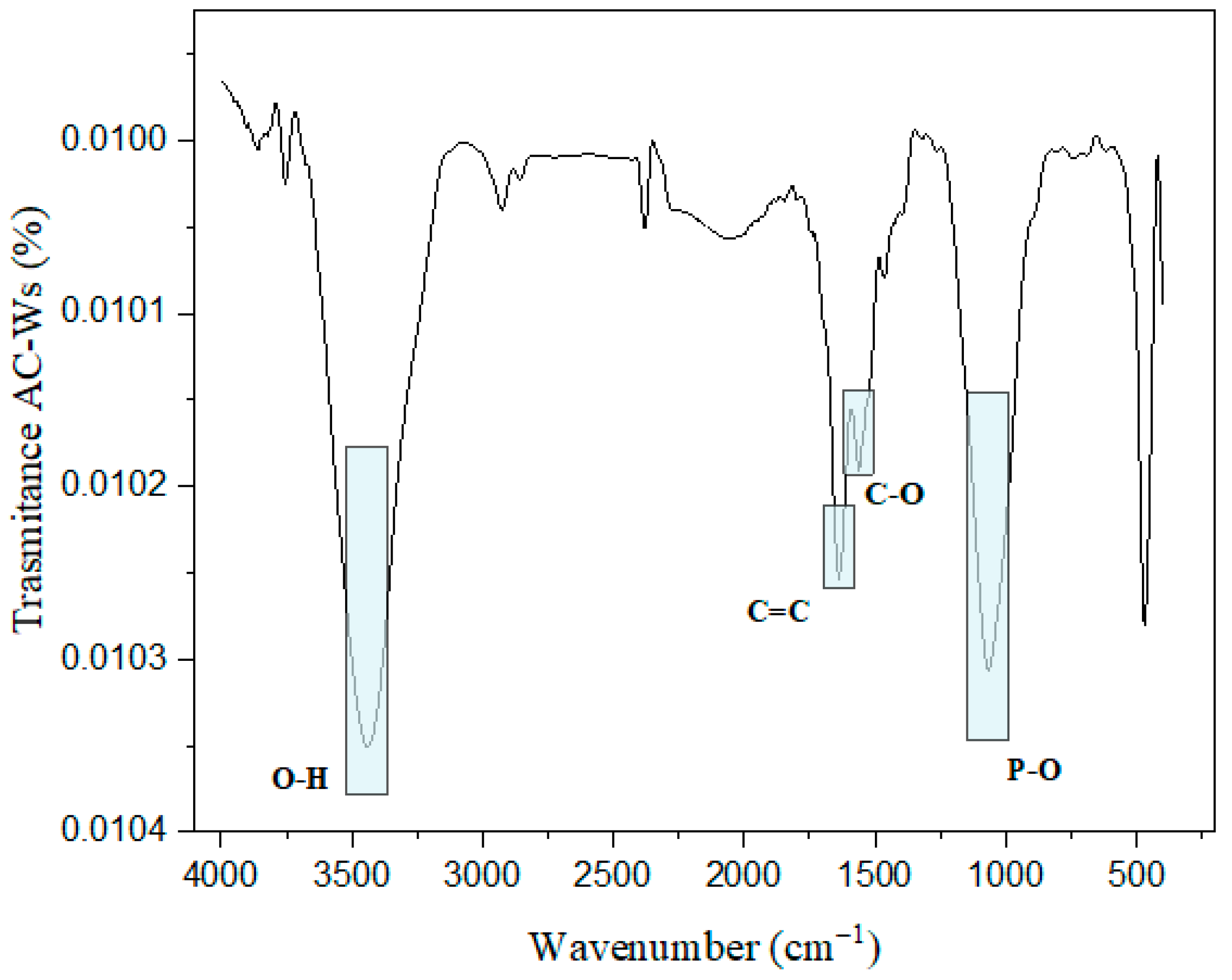
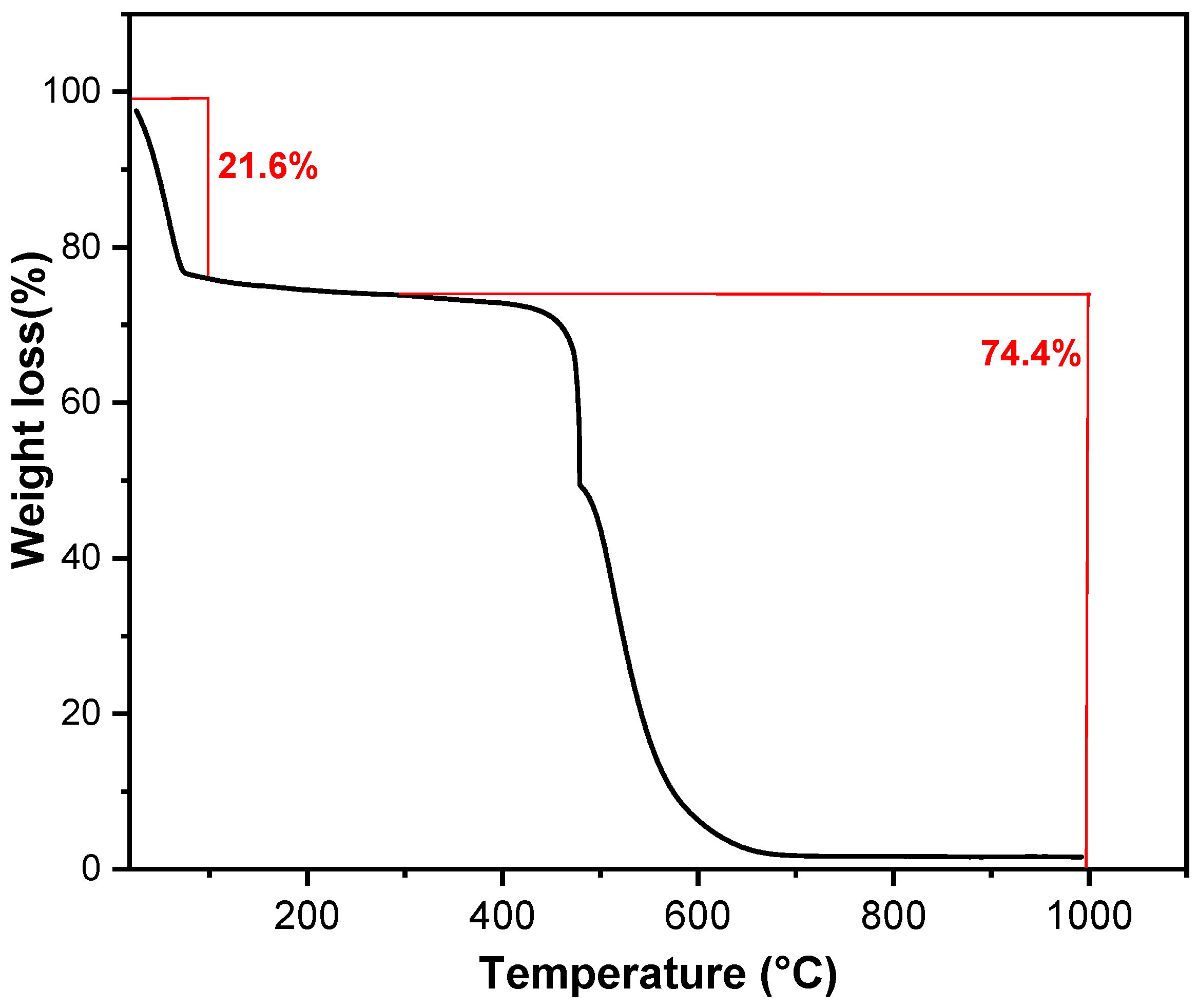




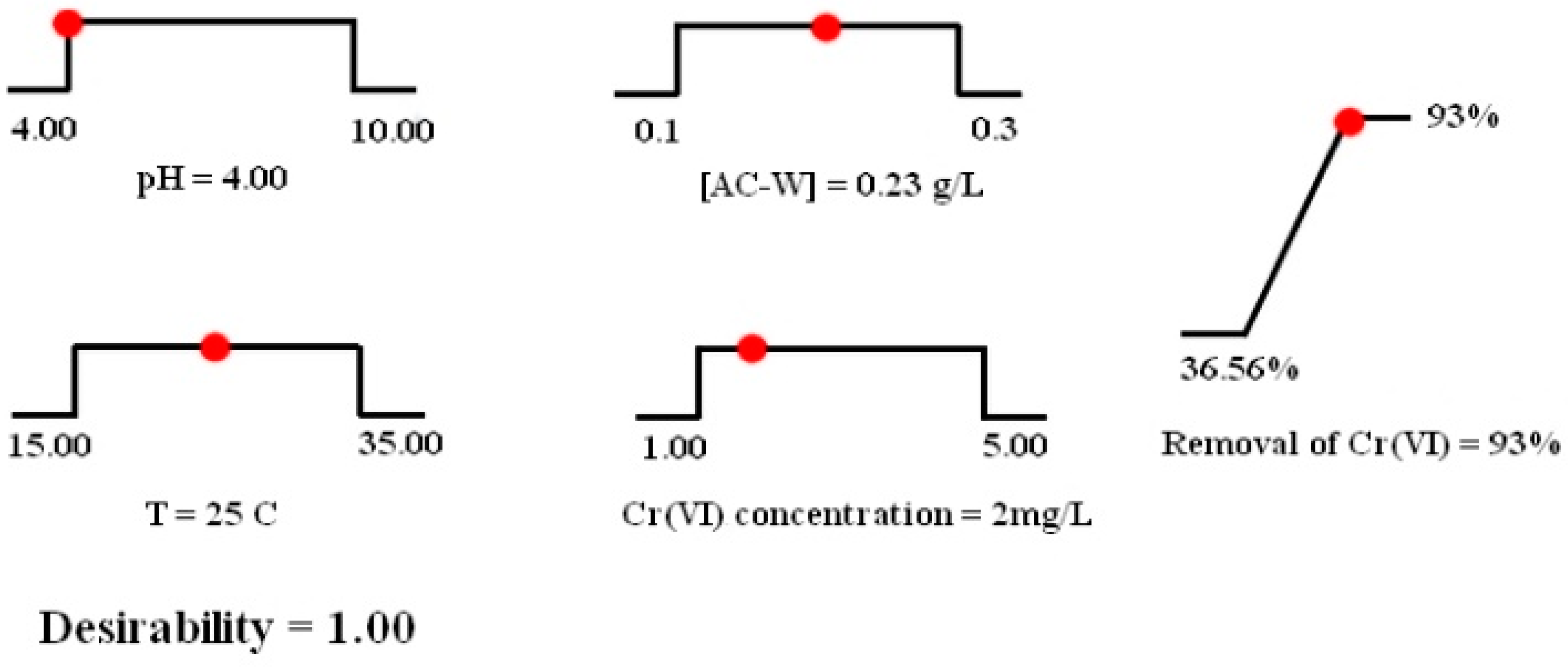




| Factor | Name | Unit | Central Value | Range of Variation |
|---|---|---|---|---|
| X1 | Dose of AC-Ws | mg | 10 | 5 |
| X2 | pH | 7 | 3 | |
| X3 | T | °C | 25 | 10 |
| X4 | Concentration of Cr (VI) | mg/L | 3 | 2 |
| Experiment | Mass of AC-Ws (mg) | pH | T (°C) | Concentration of Cr(VI) (mg/L) | Y (%) |
|---|---|---|---|---|---|
| 1 | 5 | 4 | 15 | 1 | 95.0 |
| 2 | 15 | 4 | 15 | 1 | 100 |
| 3 | 5 | 10 | 15 | 1 | 55.6 |
| 4 | 15 | 10 | 15 | 1 | 55.6 |
| 5 | 5 | 4 | 35 | 1 | 88.65 |
| 6 | 15 | 4 | 35 | 1 | 97.0 |
| 7 | 5 | 10 | 35 | 1 | 41.5 |
| 8 | 15 | 10 | 35 | 1 | 45.6 |
| 9 | 5 | 4 | 15 | 5 | 70.26 |
| 10 | 15 | 4 | 15 | 5 | 85.56 |
| 11 | 5 | 10 | 15 | 5 | 30.56 |
| 12 | 15 | 10 | 15 | 5 | 35.65 |
| 13 | 5 | 4 | 35 | 5 | 63.0 |
| 14 | 15 | 4 | 35 | 5 | 80.23 |
| 15 | 5 | 10 | 35 | 5 | 35.23 |
| 16 | 15 | 10 | 35 | 5 | 48.6 |
| 17 | 5 | 7 | 25 | 3 | 55.5 |
| 18 | 5 | 7 | 25 | 3 | 54.9 |
| 19 | 5 | 7 | 25 | 3 | 55.3 |
| 20 | 15 | 7 | 25 | 3 | 60.3 |
| 21 | 10 | 4 | 25 | 3 | 90.0 |
| 22 | 10 | 10 | 25 | 3 | 38.0 |
| 23 | 10 | 7 | 15 | 3 | 50.0 |
| 24 | 10 | 7 | 35 | 3 | 45.6 |
| 25 | 10 | 7 | 25 | 1 | 60.0 |
| 26 | 10 | 7 | 25 | 5 | 40.6 |
| 27 | 10 | 7 | 25 | 3 | 50.6 |
| Squares Sum | Freedom Degrees | Mean Square | Ratio | p-Value % | R2 | R2Adj | |
|---|---|---|---|---|---|---|---|
| Regression | 11.007 | 14 | 786.220 | 8423.78 | 0.000 *** | 0.98 | 0.96 |
| Residuals | 197.687 | 12 | 16.474 | ||||
| Lack of fit | 197.501 | 10 | 19.750 | 211.608 | 0.471 ** | ||
| Error | 0.187 | 2 | 0.093 | ||||
| Total | 11.204 | 26 |
| Concentration of Cr(VI) (mg/L) | Pseudo First-Order | Pseudo Second-Order | |||||
|---|---|---|---|---|---|---|---|
| Qe.exp (mg/g) | Qe,cal (mg/g) | K1 (min−1) | R2 | Qe,cal (mg/g) | k2 × 10−2 (g/mg·min) | R2 | |
| 1 | 3.212 | 2.6 | 0.0599 | 0.952 | 20.94 | 3.584 | 0.995 |
| 2 | 6.645 | 6.223 | 0.0598 | 0.894 | 44.2 | 7.692 | 0.992 |
| 3 | 9.748 | 7.261 | 0.0391 | 0.961 | 22.66 | 10.75 | 0.991 |
| 4 | 12.944 | 10.185 | 0.0414 | 0.937 | 12.11 | 14.705 | 0.993 |
| 5 | 14.905 | 11.634 | 0.0598 | 0.959 | 16.814 | 19.607 | 0.995 |
| Temperature | Langmuir Model | Freundlich Model | ||||
|---|---|---|---|---|---|---|
| Qm (mg/g) | RL | R2 | KF | 1/n | R2 | |
| 293K | 38.46 | 0.641 | 0.99 | 22.13 | 0.42 | 0.96 |
| 303K | 41.56 | 0.472 | 0.98 | 32.25 | 0.312 | 0.95 |
| 313K | 48.23 | 0.373 | 0.99 | 41.65 | 0.224 | 0.97 |
| 323K | 52.42 | 0.308 | 0.99 | 57.81 | 0.202 | 0.94 |
| Adsorbent | Qmax (mg·g−1) | pH | Reference |
|---|---|---|---|
| Walnut Shell AC-Ws | 59.76 | 4 | This study |
| Palm Kernel Shell | 8.2 | 4 | [55] |
| ACSBB | 52.5 | 4 | [56] |
| Palm shell AC | 12.6 | 4 | [57] |
| Coconut Shell charcoal | 10.8 | 4 | [58] |
| Almond Shell | 2.4 | 4 | [59] |
| T (K) | ΔG (kJ·mol−1) | ΔS (J·mol−1·K−1) | ΔH (kJ·mol−1) |
|---|---|---|---|
| 293 | −36.20 | 212.45 | −28.596676 |
| 303 | −41.99 | ||
| 313 | −43.77 | ||
| 323 | −45.56 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yazid, H.; Bouzid, T.; El mouchtari, E.m.; Bahsis, L.; El Himri, M.; Rafqah, S.; El haddad, M. Insights into the Adsorption of Cr(VI) on Activated Carbon Prepared from Walnut Shells: Combining Response Surface Methodology with Computational Calculation. Clean Technol. 2024, 6, 199-220. https://doi.org/10.3390/cleantechnol6010012
Yazid H, Bouzid T, El mouchtari Em, Bahsis L, El Himri M, Rafqah S, El haddad M. Insights into the Adsorption of Cr(VI) on Activated Carbon Prepared from Walnut Shells: Combining Response Surface Methodology with Computational Calculation. Clean Technologies. 2024; 6(1):199-220. https://doi.org/10.3390/cleantechnol6010012
Chicago/Turabian StyleYazid, Hicham, Taoufiq Bouzid, El mountassir El mouchtari, Lahoucine Bahsis, Mamoune El Himri, Salah Rafqah, and Mohammadine El haddad. 2024. "Insights into the Adsorption of Cr(VI) on Activated Carbon Prepared from Walnut Shells: Combining Response Surface Methodology with Computational Calculation" Clean Technologies 6, no. 1: 199-220. https://doi.org/10.3390/cleantechnol6010012
APA StyleYazid, H., Bouzid, T., El mouchtari, E. m., Bahsis, L., El Himri, M., Rafqah, S., & El haddad, M. (2024). Insights into the Adsorption of Cr(VI) on Activated Carbon Prepared from Walnut Shells: Combining Response Surface Methodology with Computational Calculation. Clean Technologies, 6(1), 199-220. https://doi.org/10.3390/cleantechnol6010012








