Fluoride Removal and Recovery from Water Using Reverse Osmosis and Osmotic Membrane Crystallization
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Experimental Procedure
2.2.1. Reverse Osmosis
2.2.2. Membrane Distillation–Crystallization
2.3. Crystal Characterization
3. Results and Discussion
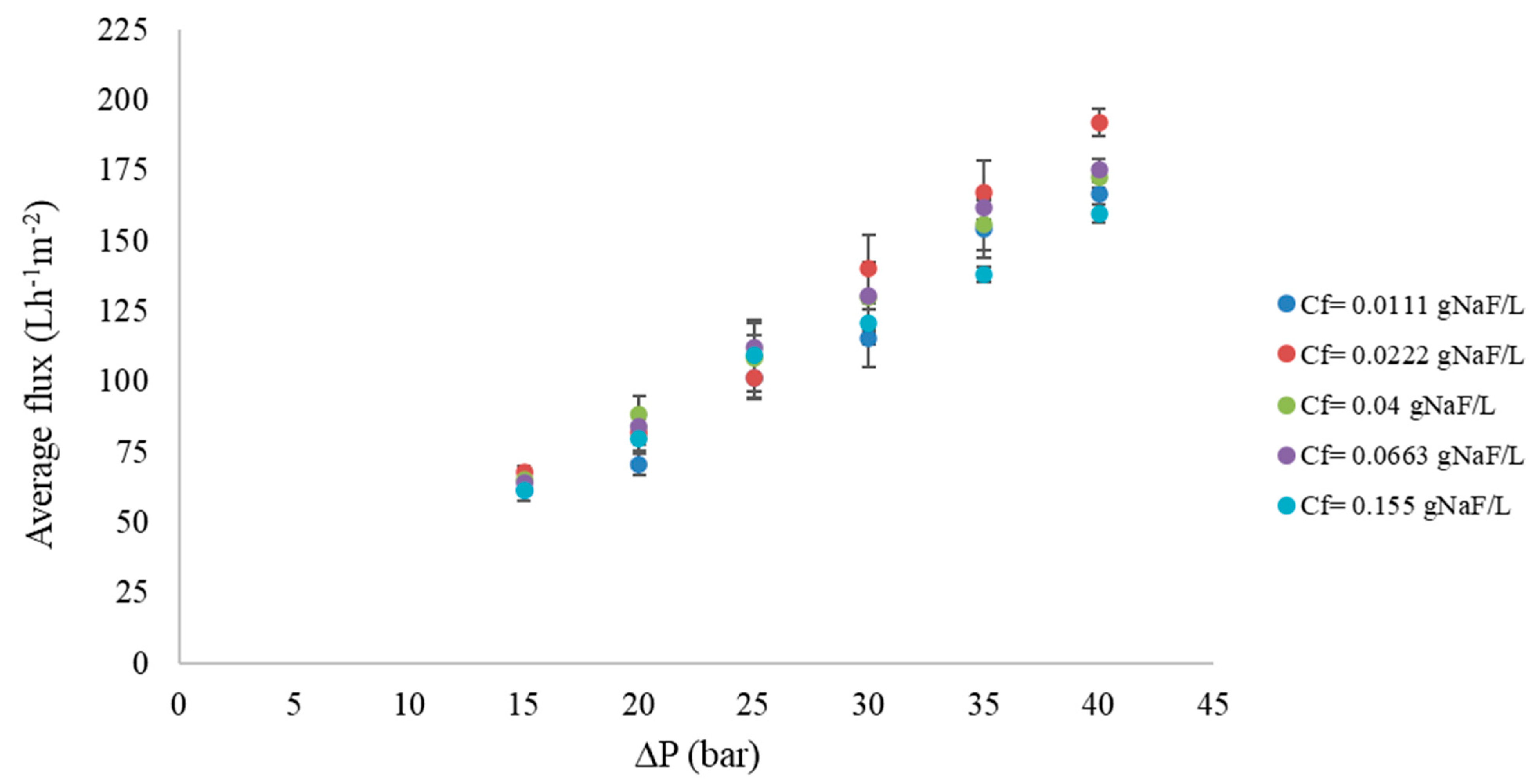
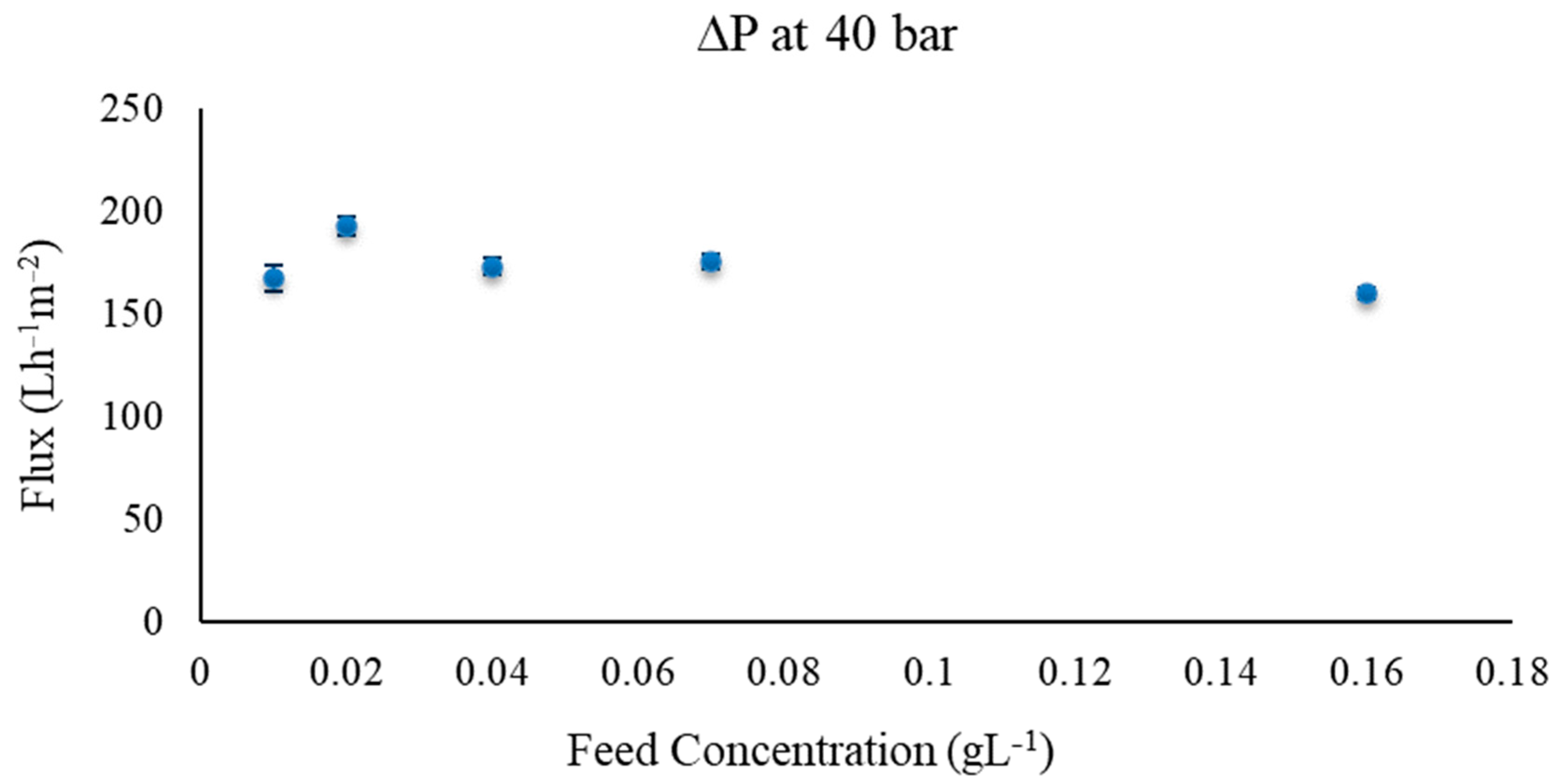
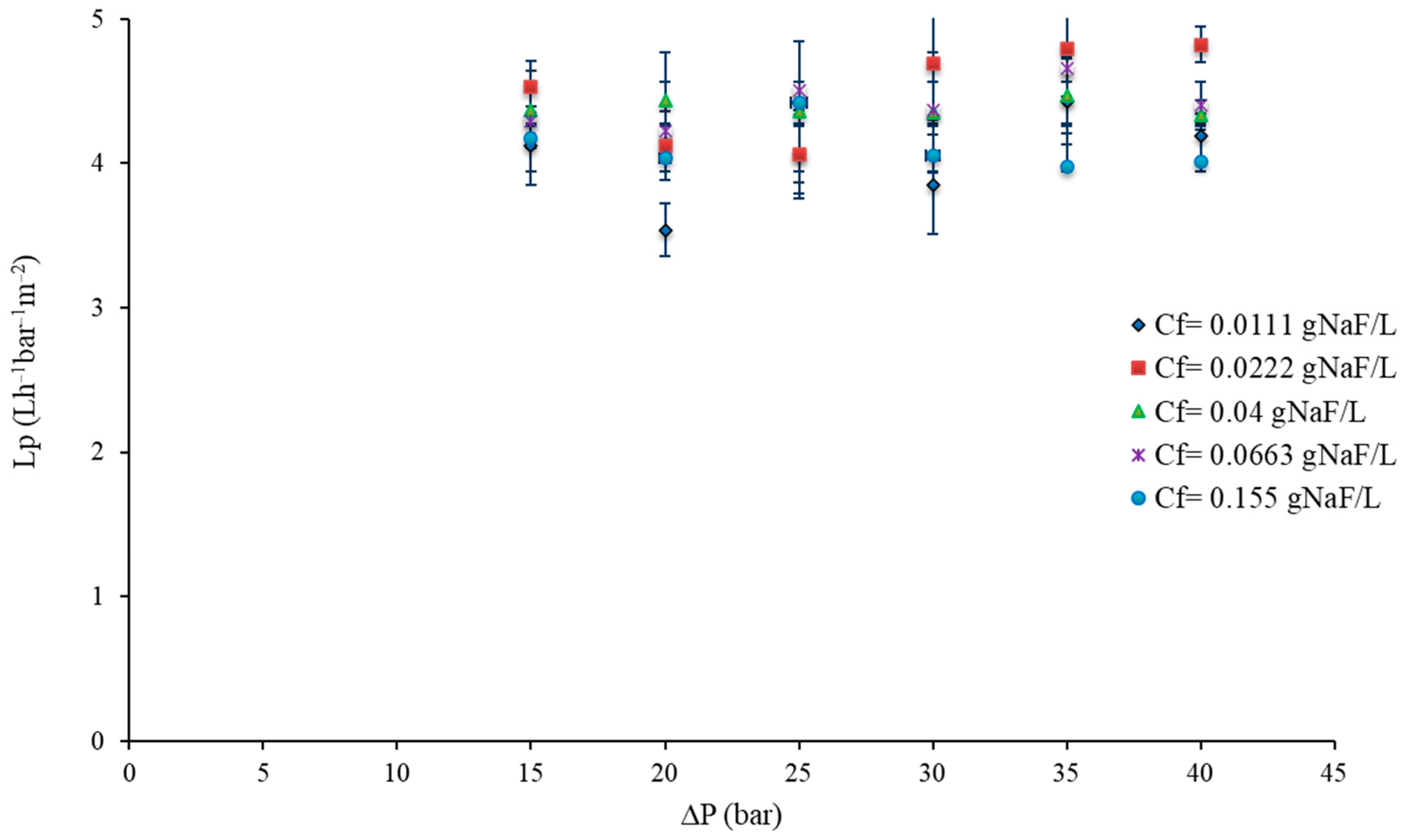
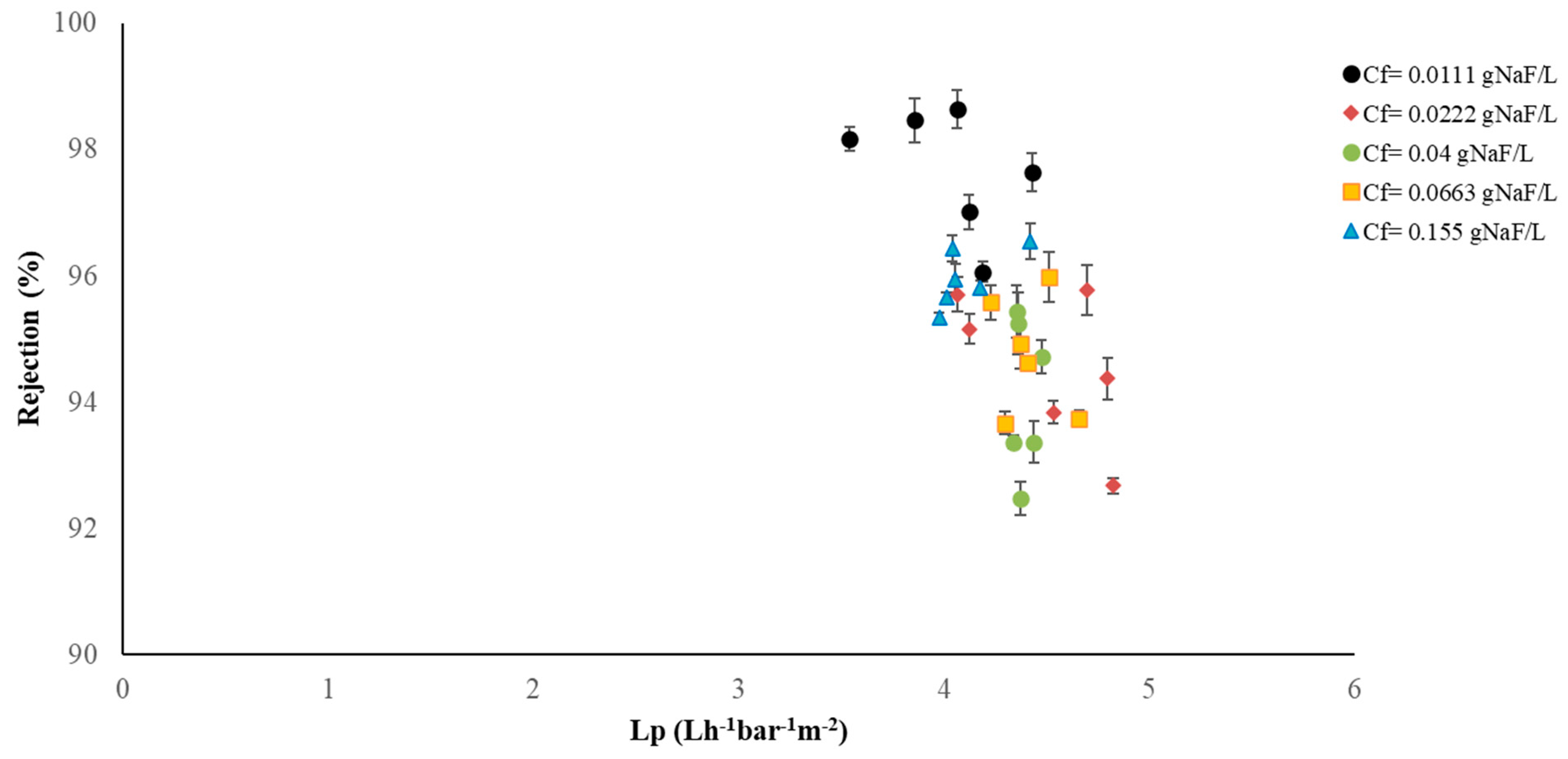
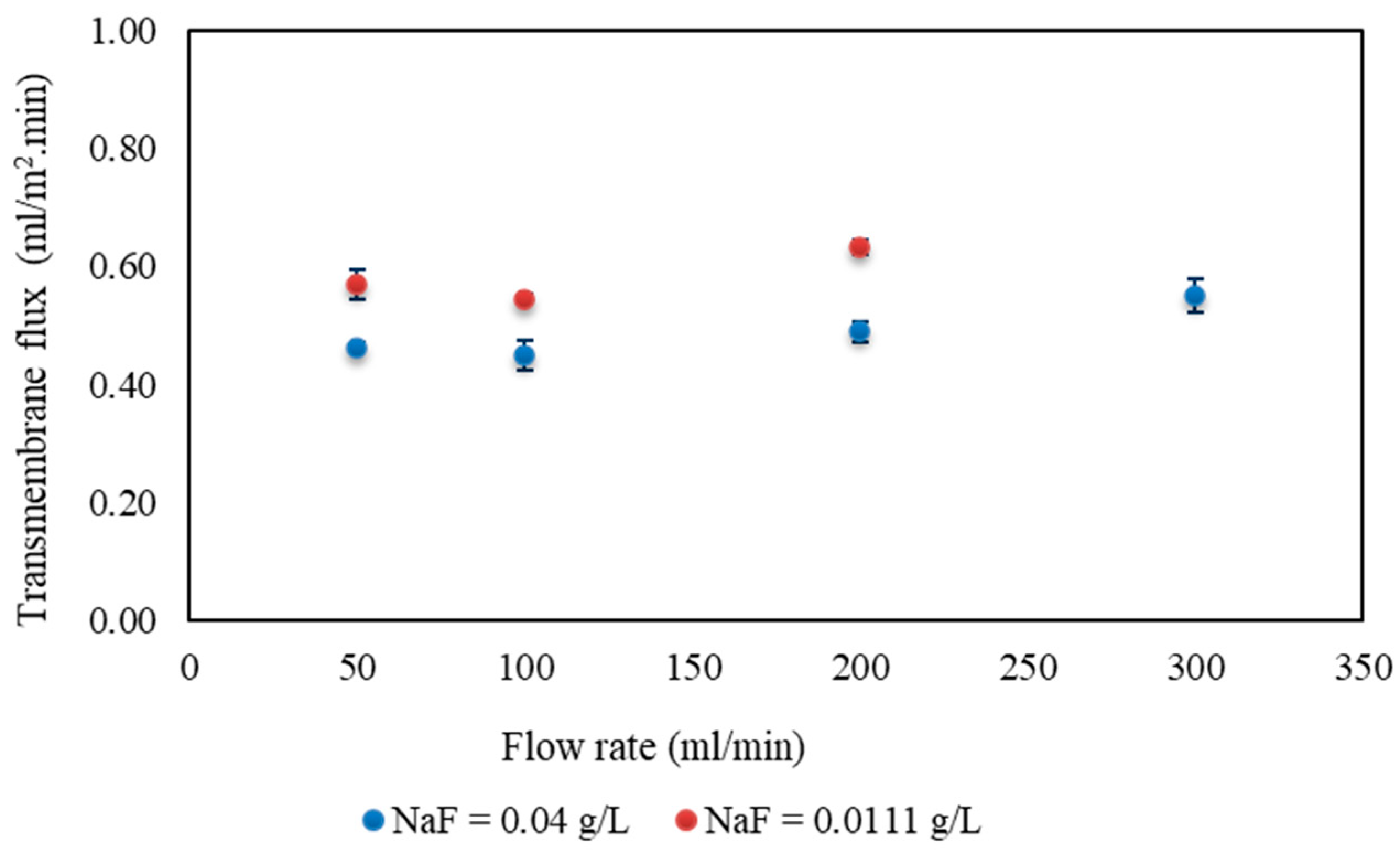
3.1. RO Results
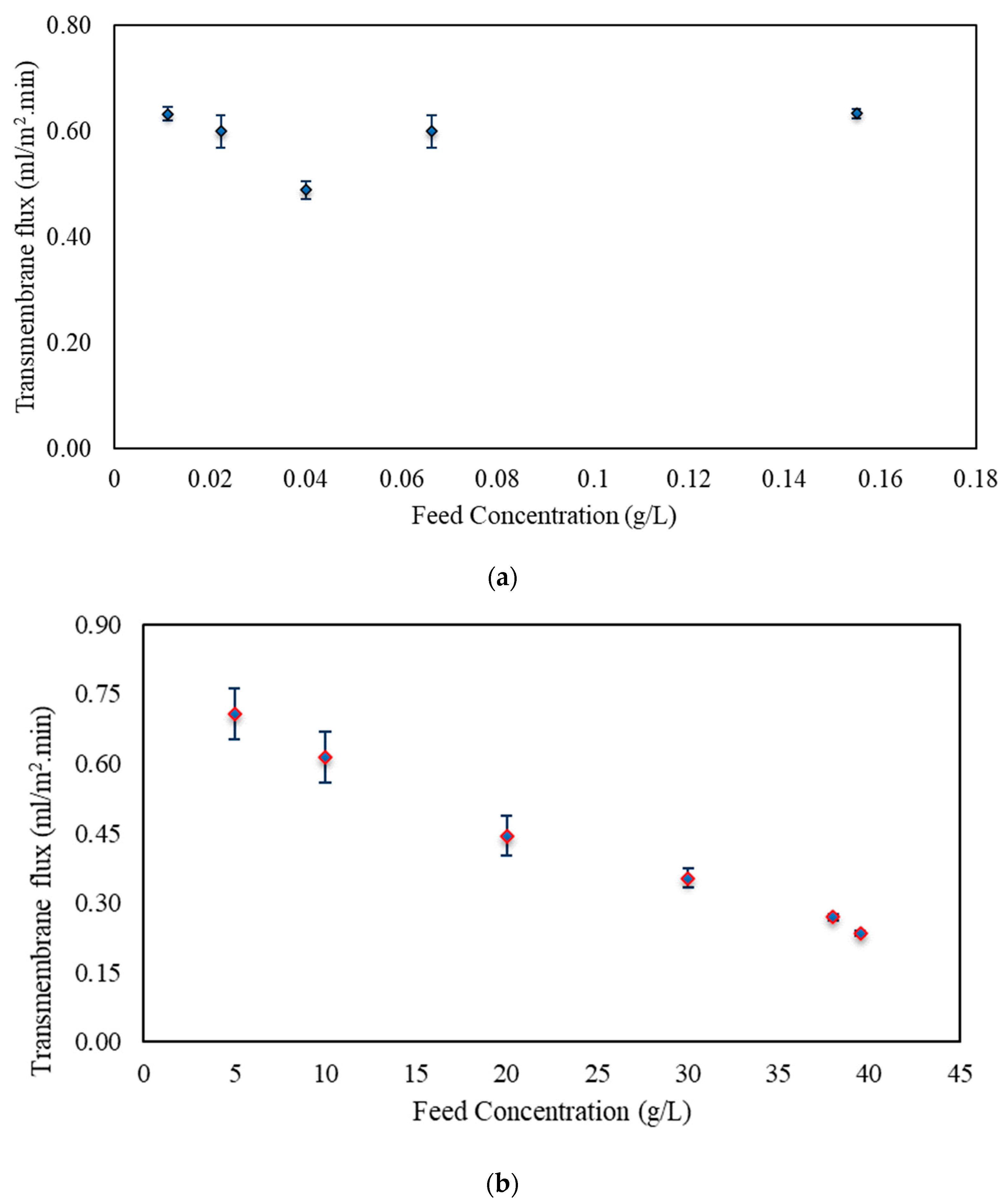
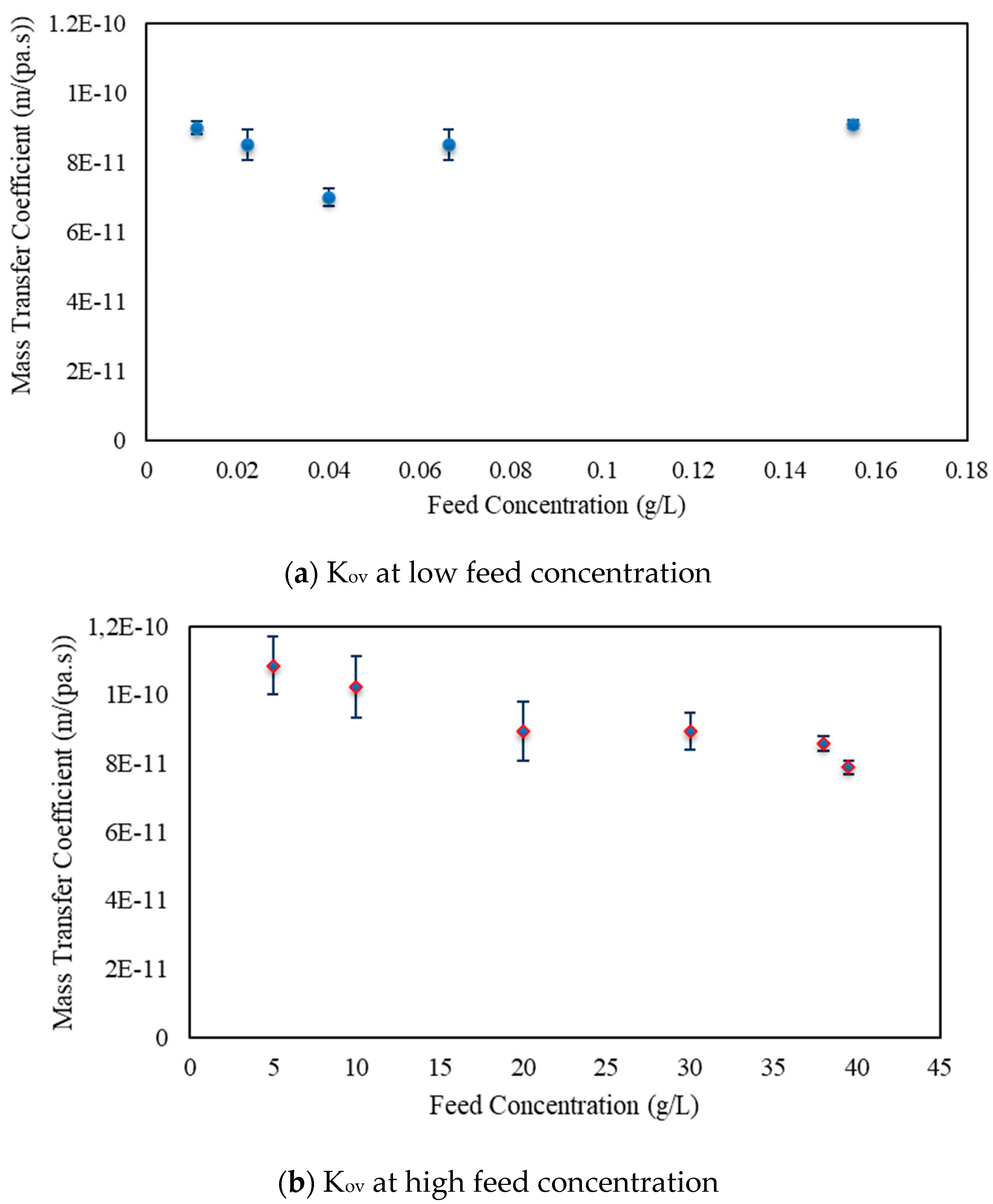
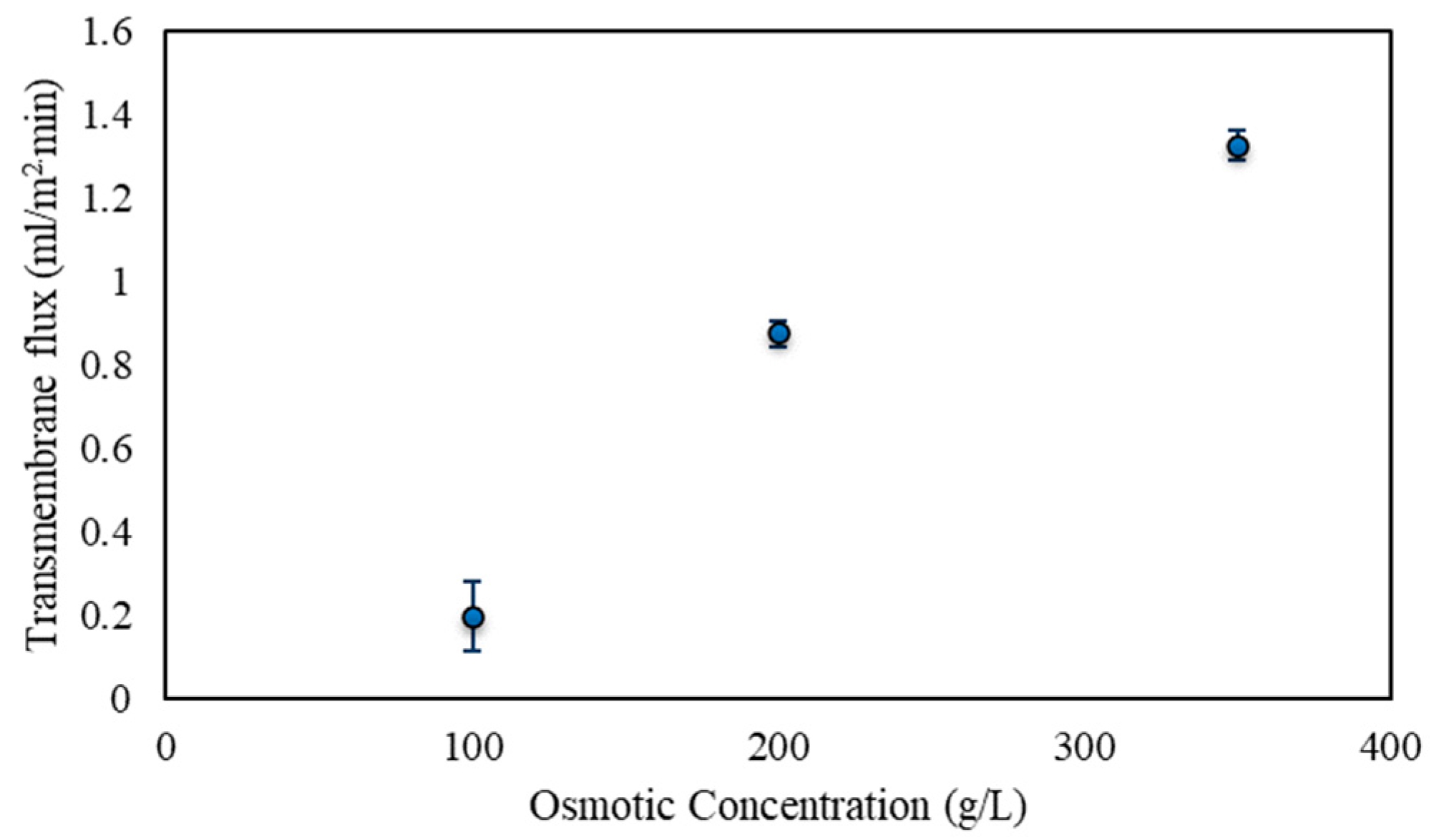
3.2. Osmotic Membrane Distillation–Crystallization Results
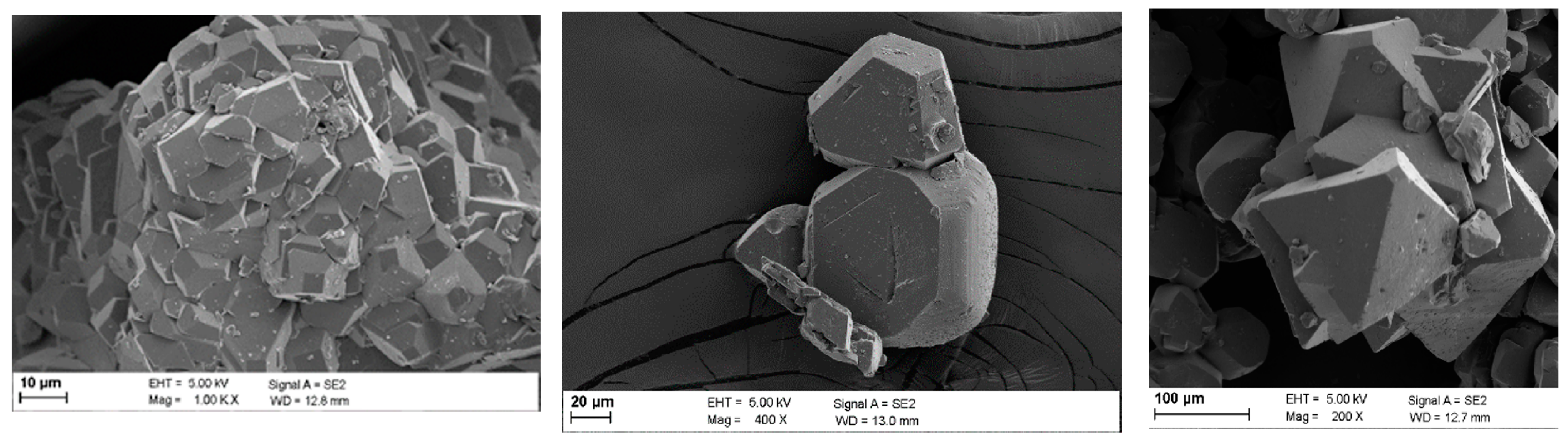
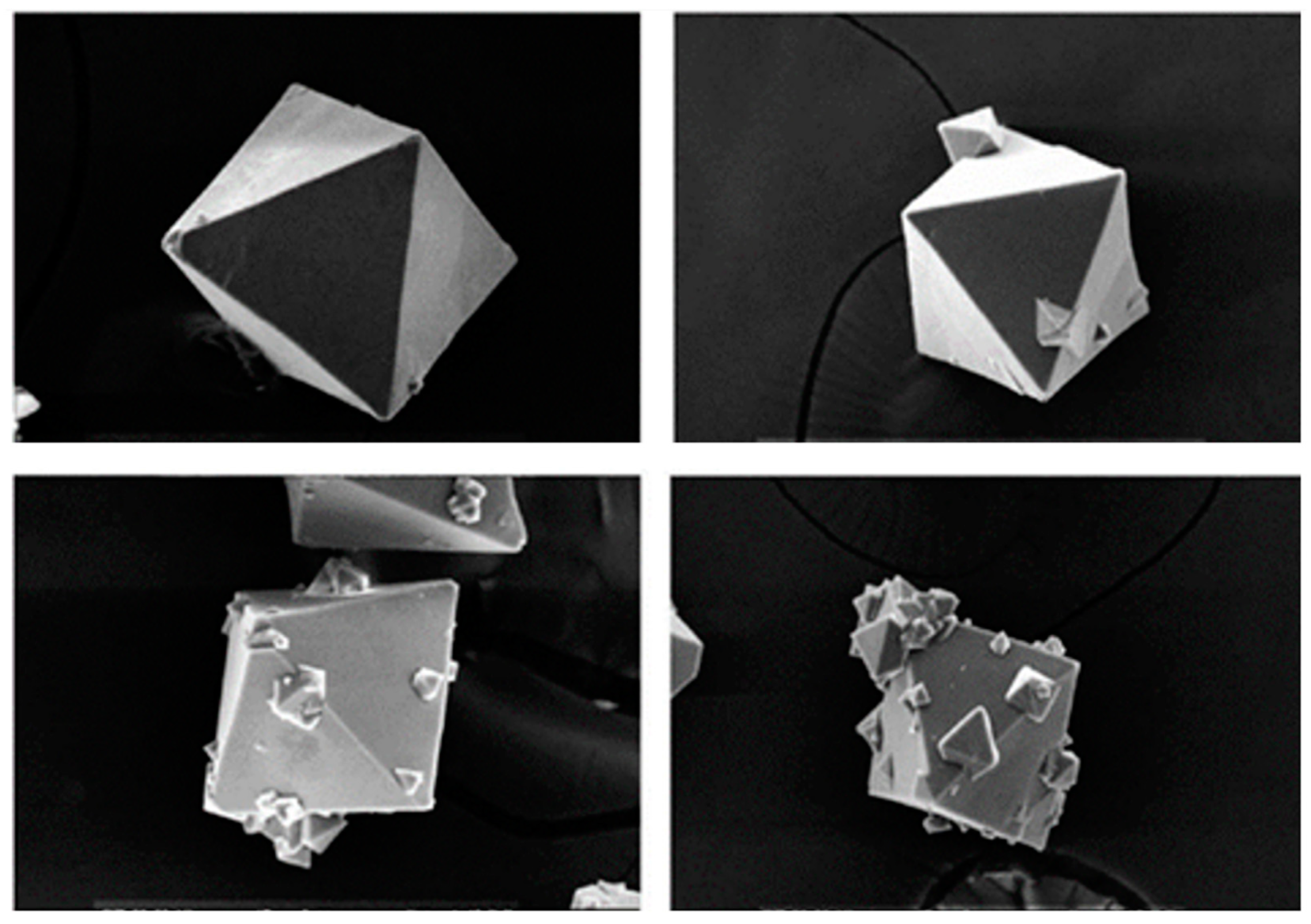
3.3. Crystal Characterization
4. Conclusions
- Reverse osmosis
- Osmotic membrane distillation–crystallization system
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A

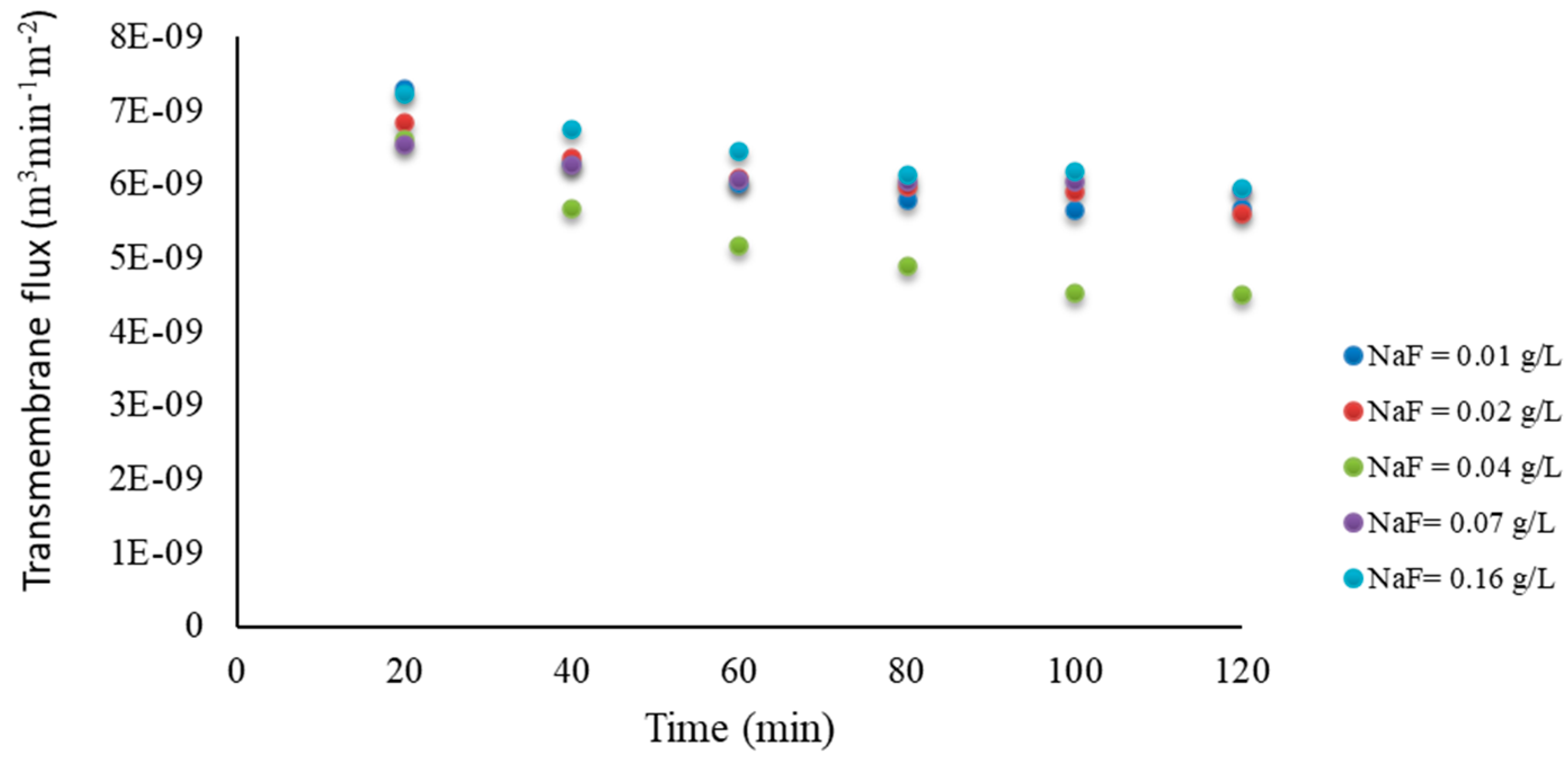
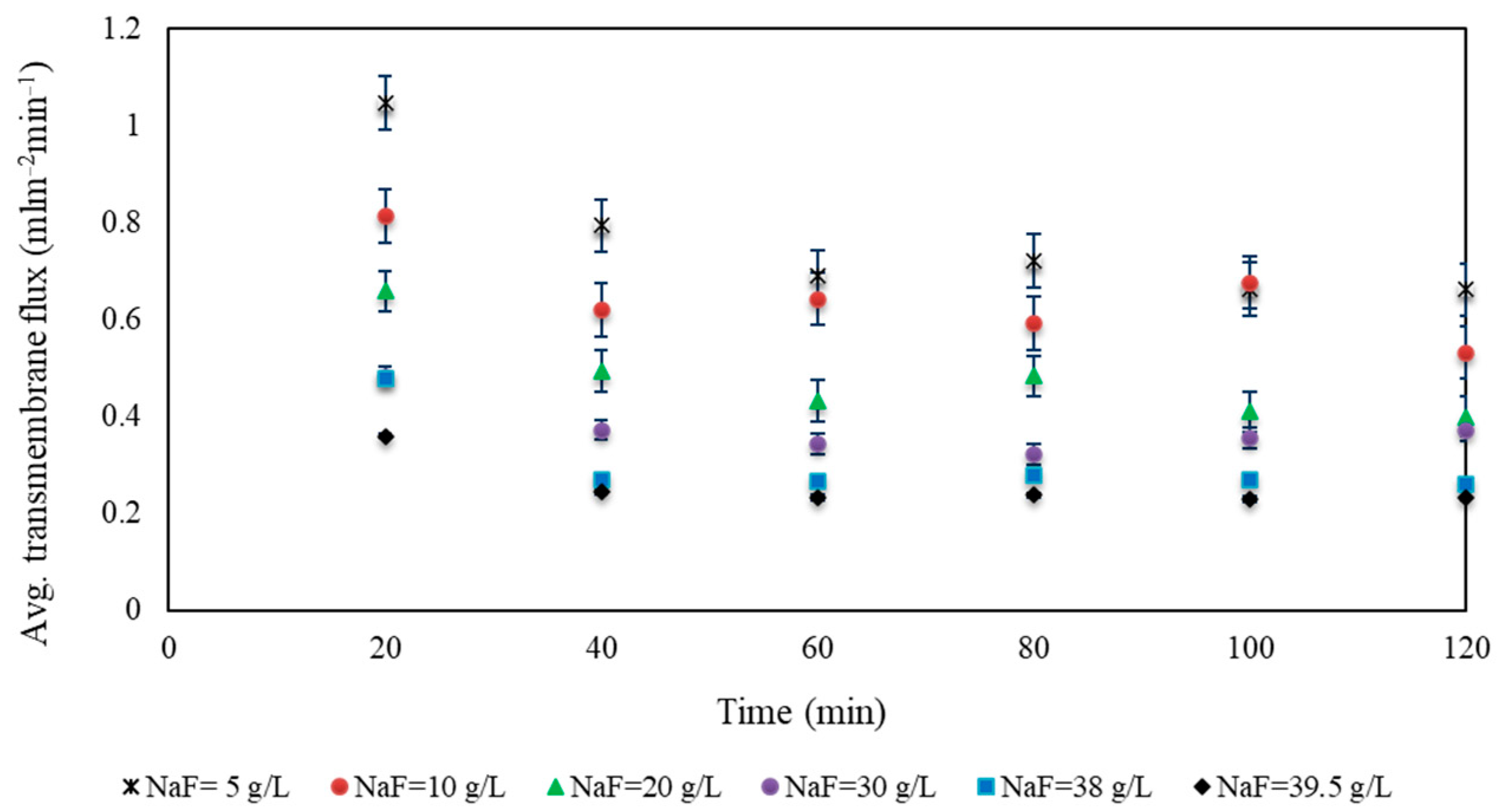
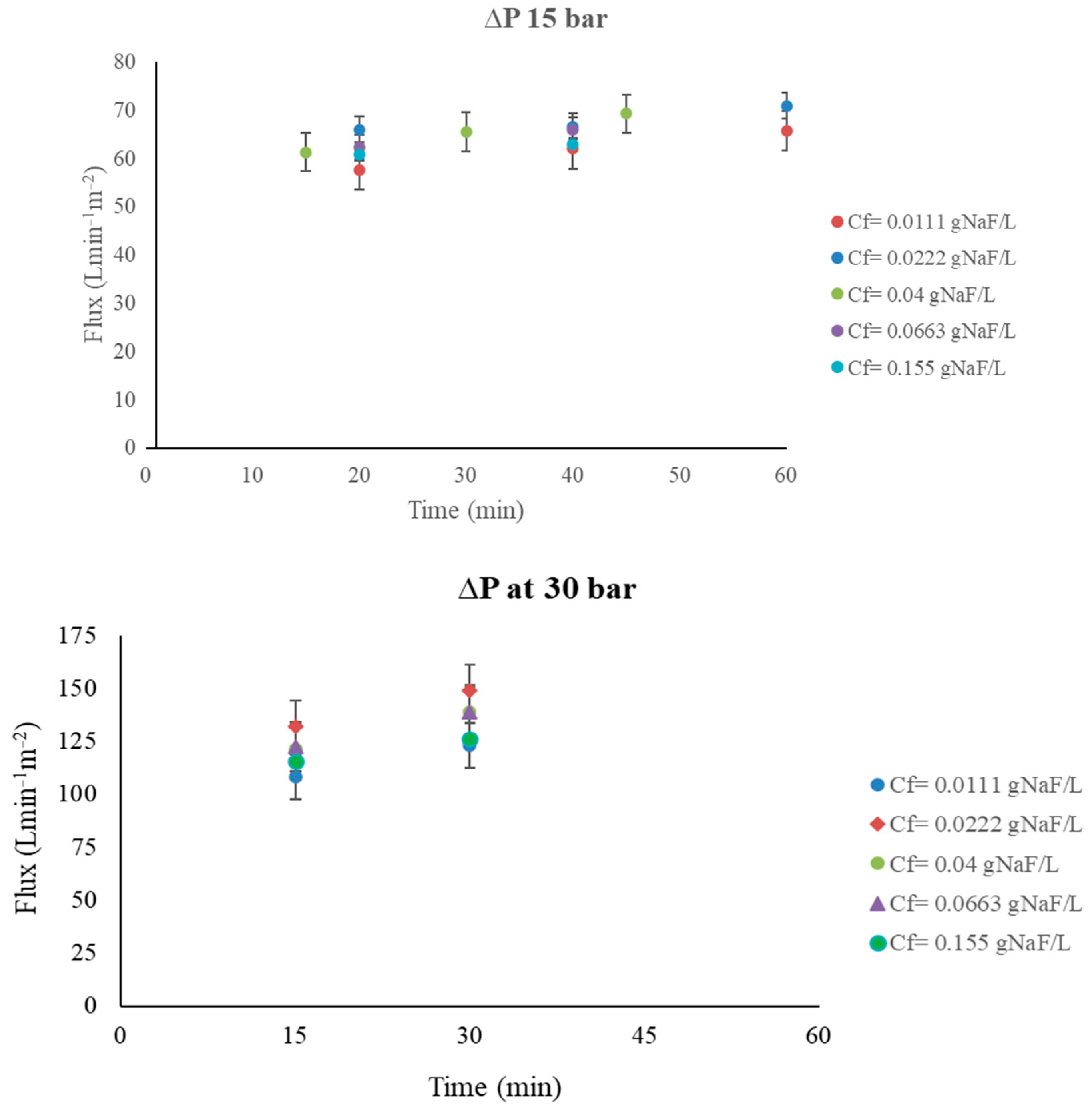

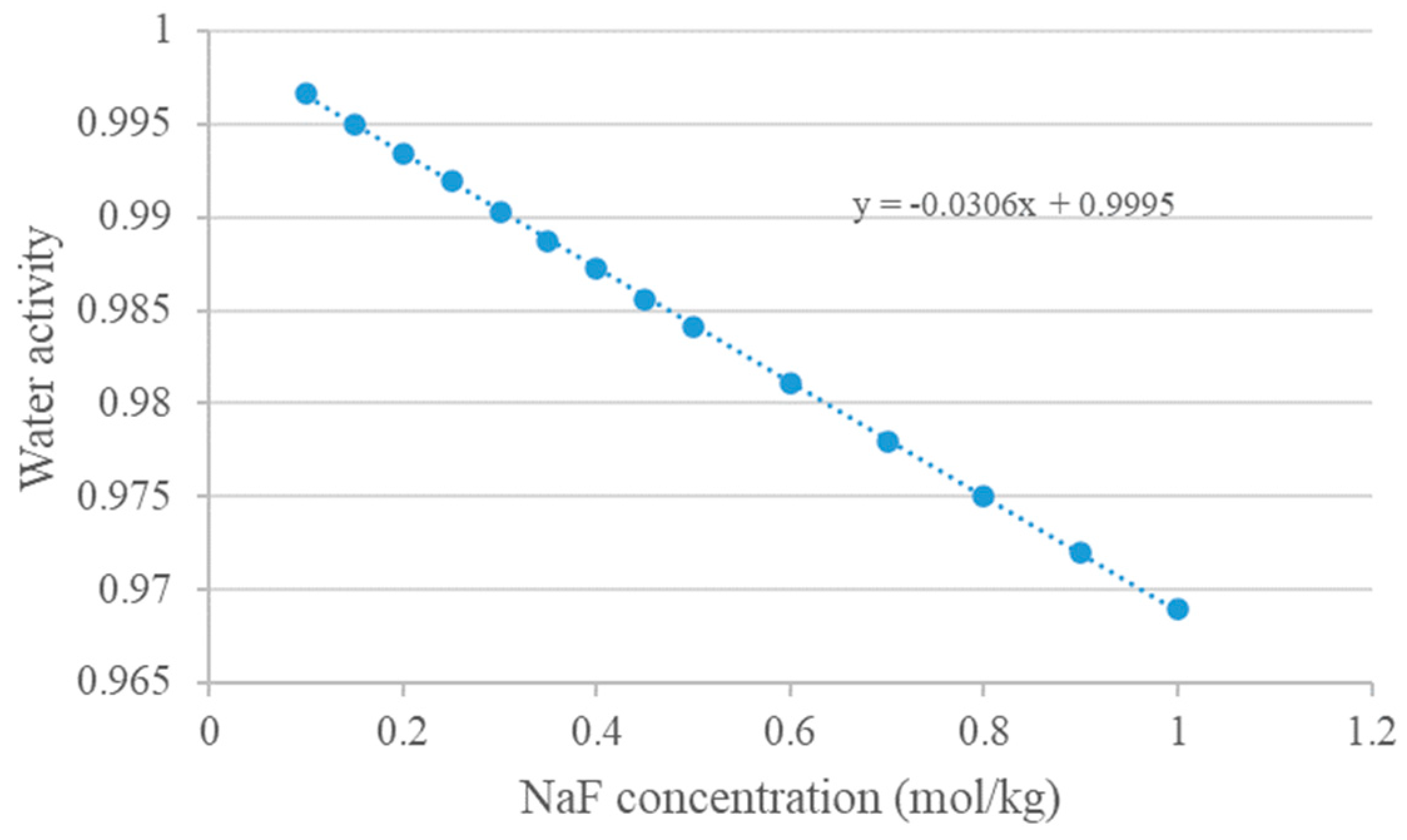
Appendix B. Water Activity
References
- Brindha, K.; Elango, L. Fluoride in groundwater: Causes, implications and mitigation measures. Fluoride Prop. Appl. Environ. Manag. 2011, 1, 111–136. [Google Scholar]
- Waghmare, S.S.; Arfin, T. Fluoride removal from water by various techniques. Int. J. Innov. Sci. Eng. Technol. 2015, 2, 560–571. [Google Scholar]
- Bejaoui, I.; Mnif, A.; Hamrouni, B. Performance of Reverse Osmosis and Nanofiltration in the Removal of Fluoride from Model Water and Metal Packaging Industrial Effluent. Sep. Sci. Technol. 2014, 49, 1135–1145. [Google Scholar] [CrossRef]
- Barathi, M.; Kumar, A.S.K.; Rajesh, N. Impact of fluoride in potable water—An outlook on the existing defluoridation strategies and the road ahead. Coord. Chem. Rev. 2019, 387, 121–128. [Google Scholar] [CrossRef]
- World Health Organization. Fluoride in Drinkingwater, Background Document for Development of WHO Guidelines for Drinking-Water Quality; World Health Organization: Geneva, Switzerland, 2004. [Google Scholar]
- Fawell, J.; Bailey, K.; Chilton, J.; Dahi, E.; Fewtrell, L.; Magara, Y. Fluoride in Drinking-Water; IWA Publishing: London, UK, 2006. [Google Scholar]
- Shen, J.; Schafer, A.I. Factors affecting fluoride and natural organic matter (NOM) removal from natural waters in Tanzania by nanofiltration/reverse osmosis. Sci. Total Environ. 2015, 527–528, 520–529. [Google Scholar] [CrossRef]
- Malago, J. Fluoride Levels in Surface and Groundwater in Africa: A Review. Am. J. Water Sci. Eng. 2017, 3, 1–17. [Google Scholar] [CrossRef]
- Craig, L.; Lutz, A.; Berry, K.A.; Yang, W. Recommendations for fluoride limits in drinking water based on estimated daily fluoride intake in the Upper East Region, Ghana. Sci. Total Environ. 2015, 532, 127–137. [Google Scholar] [CrossRef]
- Kheradpisheh, Z.; Mirzaei, M.; Mahvi, A.H.; Mokhtari, M.; Azizi, R.; Fallahzadeh, H.; Ehrampoush, M.H. Impact of drinking water fluoride on human thyroid hormones: A case-control study. Sci. Rep. 2018, 8, 2674. [Google Scholar] [CrossRef] [PubMed]
- Meenakshi; Maheshwari, R.C. Fluoride in drinking water and its removal. J. Hazard. Mater. 2006, 137, 456–463. [Google Scholar] [CrossRef]
- Bhatnagar, A.; Kumar, E.; Sillanpää, M. Fluoride removal from water by adsorption—A review. Chem. Eng. J. 2011, 171, 811–840. [Google Scholar] [CrossRef]
- United Nations. United Nations Sustainable Develepment Goals; United Nations: New York, NY, USA, 2015. [Google Scholar]
- World Health Organization. Drinking Water; World Health Organization: Geneva, Switzerland, 2022. [Google Scholar]
- He, J.; Yang, Y.; Wu, Z.; Xie, C.; Zhang, K.; Kong, L.; Liu, J. Review of fluoride removal from water environment by adsorption. J. Environ. Chem. Eng. 2020, 8, 104516. [Google Scholar] [CrossRef]
- Hou, D.; Wang, J.; Zhao, C.; Wang, B.; Luan, Z.; Sun, X. Fluoride removal from brackish groundwater by direct contact membrane distillation. J. Environ. Sci. 2010, 22, 1860–1867. [Google Scholar] [CrossRef] [PubMed]
- Moran Ayala, L.I.; Paquet, M.; Janowska, K.; Jamard, P.; Quist-Jensen, C.A.; Bosio, G.N.; Mártire, D.O.; Fabbri, D.; Boffa, V. Water Defluoridation: Nanofiltration vs Membrane Distillation. Ind. Eng. Chem. Res. 2018, 57, 14740–14748. [Google Scholar] [CrossRef]
- Hu, K.; Dickson, J.M. Nanofiltration membrane performance on fluoride removal from water. J. Membr. Sci. 2006, 279, 529–538. [Google Scholar] [CrossRef]
- Fadaei, A. Comparison of Water Defluoridation Using Different Techniques. Int. J. Chem. Eng. 2021, 2021, 2023895. [Google Scholar] [CrossRef]
- Peter-Varbanets, M.; Zurbrügg, C.; Swartz, C.; Pronk, W. Decentralized systems for potable water and the potential of membrane technology. Water Res. 2009, 43, 245–265. [Google Scholar] [CrossRef]
- Jadhav, S.V.; Bringas, E.; Yadav, G.D.; Rathod, V.K.; Ortiz, I.; Marathe, K.V. Arsenic and fluoride contaminated groundwaters: A review of current technologies for contaminants removal. J. Environ. Manag. 2015, 162, 306–325. [Google Scholar] [CrossRef]
- Singh, J.; Singh, P.; Singh, A. Fluoride ions vs removal technologies: A study. Arab. J. Chem. 2016, 9, 815–824. [Google Scholar] [CrossRef]
- Alkhudhiri, A.; Darwish, N.; Hilal, N. Membrane distillation: A comprehensive review. Desalination 2012, 287, 2–18. [Google Scholar] [CrossRef]
- Naidu, G.; Jeong, S.; Choi, Y.; Jang, E.; Hwang, T.-M.; Vigneswaran, S. Application of vacuum membrane distillation for small scale drinking water production. Desalination 2014, 354, 53–61. [Google Scholar] [CrossRef]
- Boubakri, A.; Bouchrit, R.; Hafiane, A.; Bouguecha, S.A. Fluoride removal from aqueous solution by direct contact membrane distillation: Theoretical and experimental studies. Environ. Sci. Pollut. Res. Int. 2014, 21, 10493–10501. [Google Scholar] [CrossRef] [PubMed]
- Ruiz Salmón, I.; Janssens, R.; Luis, P. Mass and heat transfer study in osmotic membrane distillation-crystallization for CO2 valorization as sodium carbonate. Sep. Purif. Technol. 2017, 176, 173–183. [Google Scholar] [CrossRef]
- Diawara, C.K.; Diop, S.N.; Diallo, M.A.; Farcy, M.; Deratani, A. Performance of nanofiltration (NF) and low pressure reverse osmosis (LPRO) membranes in the removal of fluorine and salinity from brackish drinking water. J. Water Resour. Prot. 2011, 3, 912. [Google Scholar] [CrossRef]
- Dolar, D.; Košutić, K.; Vučić, B. RO/NF treatment of wastewater from fertilizer factory—Removal of fluoride and phosphate. Desalination 2011, 265, 237–241. [Google Scholar] [CrossRef]
- Gedam, V.; Patil, J.; Kagne, S.; Sirsam, R.; Labhasetwar, P. Performance evaluation of polyamide reverse osmosis membrane for removal of contaminants in ground water collected from Chandrapur district. J. Memb. Sci. Technol. 2012, 2, 117. [Google Scholar] [CrossRef]
- Ezzeddine, A.; Meftah, N.; Hannachi, A. Removal of fluoride from an industrial wastewater by a hybrid process combining precipitation and reverse osmosis. Desalination Water Treat. 2015, 55, 2618–2625. [Google Scholar] [CrossRef]
- Owusu-Agyeman, I.; Jeihanipour, A.; Luxbacher, T.; Schäfer, A.I. Implications of humic acid, inorganic carbon and speciation on fluoride retention mechanisms in nanofiltration and reverse osmosis. J. Membr. Sci. 2017, 528, 82–94. [Google Scholar] [CrossRef]
- Gaikwad, M.S.; Balomajumder, C. Simultaneous rejection of fluoride and Cr (VI) from synthetic fluoride-Cr (VI) binary water system by polyamide flat sheet reverse osmosis membrane and prediction of membrane performance by CFSK and CFSD models. J. Mol. Liq. 2017, 234, 194–200. [Google Scholar] [CrossRef]
- Jeihanipour, A.; Shen, J.; Abbt-Braun, G.; Huber, S.A.; Mkongo, G.; Schäfer, A.I. Seasonal variation of organic matter characteristics and fluoride concentration in the Maji ya Chai River (Tanzania): Impact on treatability by nanofiltration/reverse osmosis. Sci. Total Environ. 2018, 637, 1209–1220. [Google Scholar] [CrossRef]
- Deswal, M.; Laura, J. Effect of change in trans-membrane pressure differences on the efficiency of a thin film reverse osmosis membrane. IOSR J. Eng. 2018, 8, 21–26. [Google Scholar]
- Owusu-Agyeman, I.; Reinwald, M.; Jeihanipour, A.; Schäfer, A.I. Removal of fluoride and natural organic matter from natural tropical brackish waters by nanofiltration/reverse osmosis with varying water chemistry. Chemosphere 2019, 217, 47–58. [Google Scholar] [CrossRef] [PubMed]
- Drioli, E.; Criscuoli, A.; Curcio, E. Membrane Contactors: Fundamentals, Applications and Potentialities; Elsevier: Amsterdam, The Netherlands, 2011. [Google Scholar]
- Salmón, I.R.; Luis, P. Membrane crystallization via membrane distillation. Chem. Eng. Process. Process Intensif. 2018, 123, 258–271. [Google Scholar] [CrossRef]
- Luis, P.; Van der Bruggen, B. The role of membranes in post-combustion CO2 capture. Greenh. Gases Sci. Technol. 2013, 3, 318–337. [Google Scholar] [CrossRef]
- Pramanik, B.K.; Thangavadivel, K.; Shu, L.; Jegatheesan, V. A critical review of membrane crystallization for the purification of water and recovery of minerals. Rev. Environ. Sci. Bio/Technol. 2016, 15, 411–439. [Google Scholar] [CrossRef]
- Curcio, E.; Drioli, E. Membrane distillation and related operations—A review. Sep. Purif. Rev. 2005, 34, 35–86. [Google Scholar] [CrossRef]
- Martínez, M.B.; Jullok, N.; Negrín, Z.R.; Van der Bruggen, B.; Luis, P. Membrane crystallization for the recovery of a pharmaceutical compound from waste streams. Chem. Eng. Res. Des. 2014, 92, 264–272. [Google Scholar] [CrossRef]
- Garcia Alvarez, M.; Sang Sefidi, V.; Beguin, M.; Collet, A.; Bahamonde Soria, R.; Luis, P. Osmotic Membrane Distillation Crystallization of NaHCO3. Energies 2022, 15, 2682. [Google Scholar] [CrossRef]
- Chen, Q.; Burhan, M.; Akhtar, F.H.; Ybyraiymkul, D.; Shahzad, M.W.; Li, Y.; Ng, K.C. A decentralized water/electricity cogeneration system integrating concentrated photovoltaic/thermal collectors and vacuum multi-effect membrane distillation. Energy 2021, 230, 120852. [Google Scholar] [CrossRef]
- Kurz, E.E.C.; Hellriegel, U.; Figoli, A.; Gabriele, B.; Bundschuh, J.; Hoinkis, J. Small-scale membrane-based arsenic removal for decentralized applications–Developing a conceptual approach for future utilization. Water Res. 2021, 196, 116978. [Google Scholar] [CrossRef]
- Yang, F.; Zhang, H.; Zhang, X.; Zhang, Y.; Li, J.; Jin, F.; Zhou, B. Performance analysis and evaluation of the 146 rural decentralized wastewater treatment facilities surrounding the Erhai Lake. J. Clean. Prod. 2021, 315, 128159. [Google Scholar] [CrossRef]
- Lenntech, Trisep X-20: The proven low fouling RO membrane. Available online: https://www.lenntech.com/Data-sheets/Trisep-X-20-Lit-L.pdf (accessed on 20 January 2023).
- Shen, J.; Schäfer, A. Removal of fluoride and uranium by nanofiltration and reverse osmosis: A review. Chemosphere 2014, 117, 679–691. [Google Scholar] [CrossRef] [PubMed]
- Bao, T.; Yu, Z.M.; Damtie, M.M.; Wu, K.; Jin, J.; Zhang, Y.; Wei, X.L.; Frost, R.L. Use of autoclaved aerated concrete particles for simultaneous removal of nitrogen and phosphorus as filter media from domestic wastewater. Environ. Technol. 2020, 41, 3032–3042. [Google Scholar] [CrossRef] [PubMed]
- Damtie, M.M.; Woo, Y.C.; Kim, B.; Hailemariam, R.H.; Park, K.-D.; Shon, H.K.; Park, C.; Choi, J.-S. Removal of fluoride in membrane-based water and wastewater treatment technologies: Performance review. J. Environ. Manag. 2019, 251, 109524. [Google Scholar] [CrossRef] [PubMed]
- Luis, P.; Van Aubel, D.; Van der Bruggen, B. Technical viability and exergy analysis of membrane crystallization: Closing the loop of CO2 sequestration. Int. J. Greenh. Gas Control 2013, 12, 450–459. [Google Scholar] [CrossRef]
- Faridi, J.; El Guendouzi, M. Study of Ion-Pairing and Thermodynamic Properties of Sodium Fluoride in Aqueous Solutions at Temperatures from 298.15 to 353.15 K. J. Solut. Chem. 2015, 44, 2194–2207. [Google Scholar] [CrossRef]
- Sandler, S.I. Chemical, Biochemical, and Engineering Thermodynamics; John Wiley & Sons: Hoboken, NJ, USA, 2017. [Google Scholar]
- Baek, Y.; Kang, J.; Theato, P.; Yoon, J. Measuring hydrophilicity of RO membranes by contact angles via sessile drop and captive bubble method: A comparative study. Desalination 2012, 303, 23–28. [Google Scholar] [CrossRef]
- Tang, C.Y.; Kwon, Y.-N.; Leckie, J.O. Effect of membrane chemistry and coating layer on physiochemical properties of thin film composite polyamide RO and NF membranes: I. FTIR and XPS characterization of polyamide and coating layer chemistry. Desalination 2009, 242, 149–167. [Google Scholar] [CrossRef]
- Liu, M.; Lü, Z.; Chen, Z.; Yu, S.; Gao, C. Comparison of reverse osmosis and nanofiltration membranes in the treatment of biologically treated textile effluent for water reuse. Desalination 2011, 281, 372–378. [Google Scholar] [CrossRef]
- Babu, B.R.; Rastogi, N.; Raghavarao, K. Mass transfer in osmotic membrane distillation of phycocyanin colorant and sweet-lime juice. J. Membr. Sci. 2006, 272, 58–69. [Google Scholar] [CrossRef]
- Kim, H.-J.; Kim, W.-S. Configuration of sodium fluoride crystal characterized by surface crystals on mother crystal. J. Cryst. Growth 2001, 233, 326–335. [Google Scholar] [CrossRef]

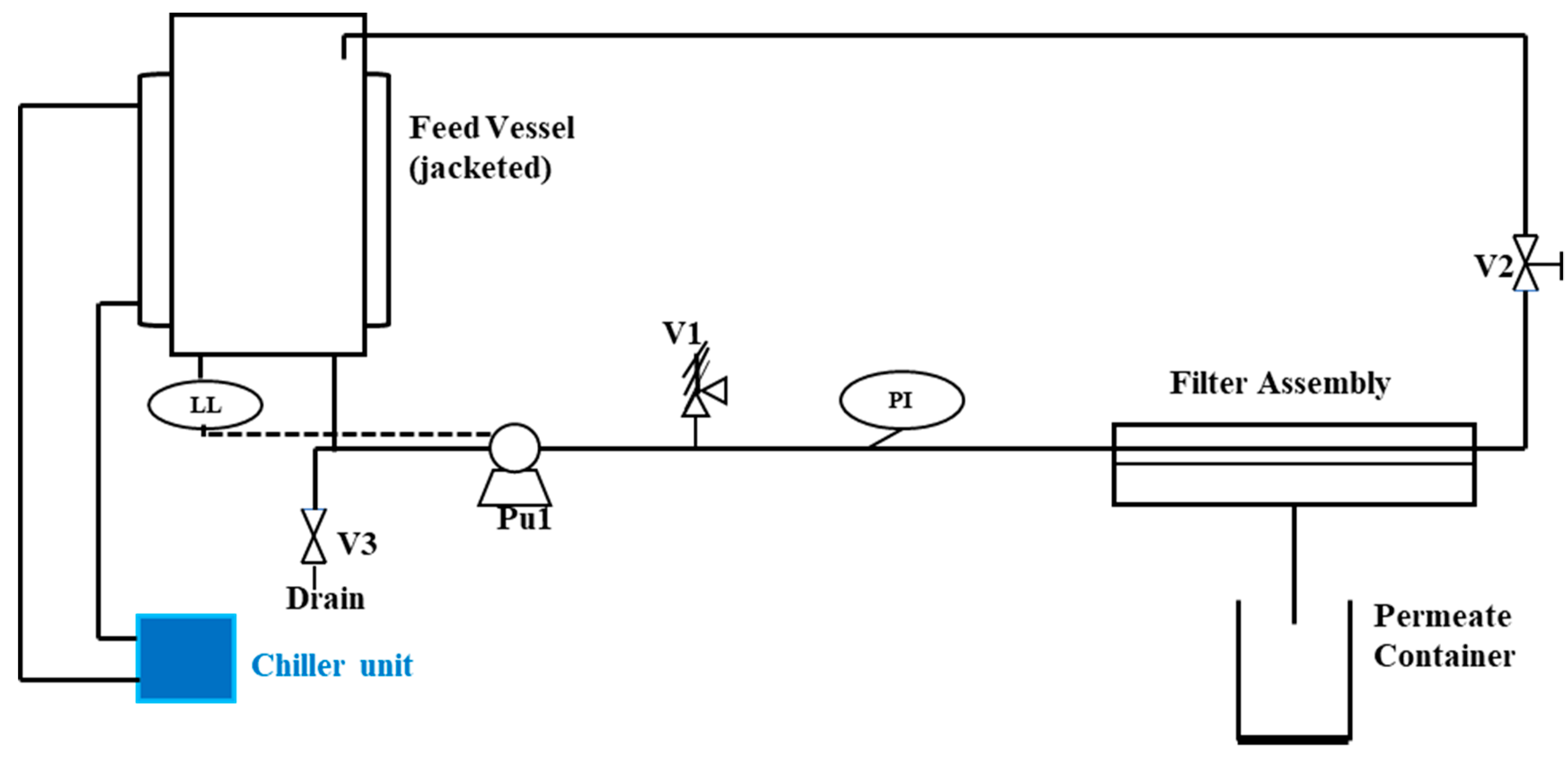
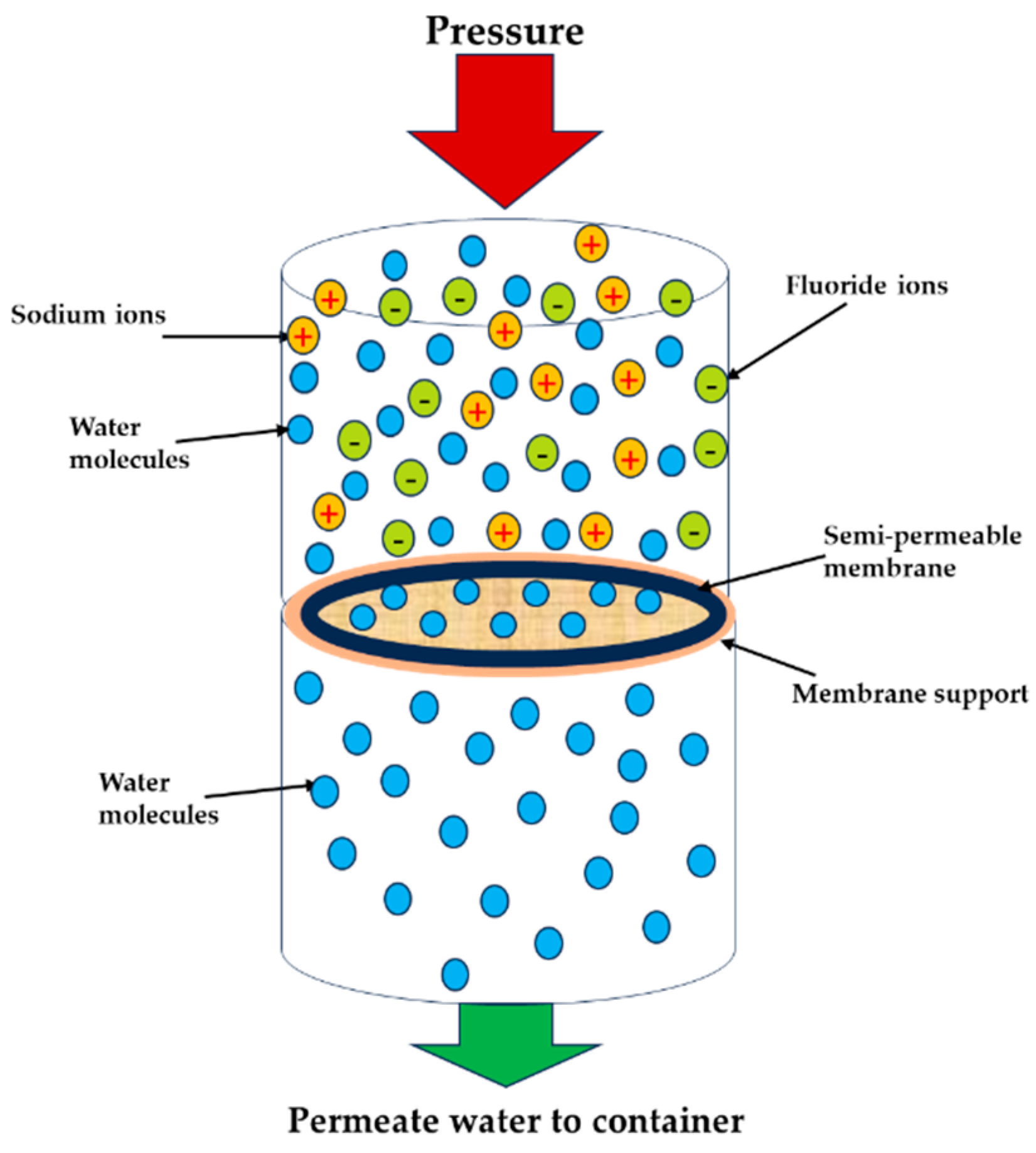
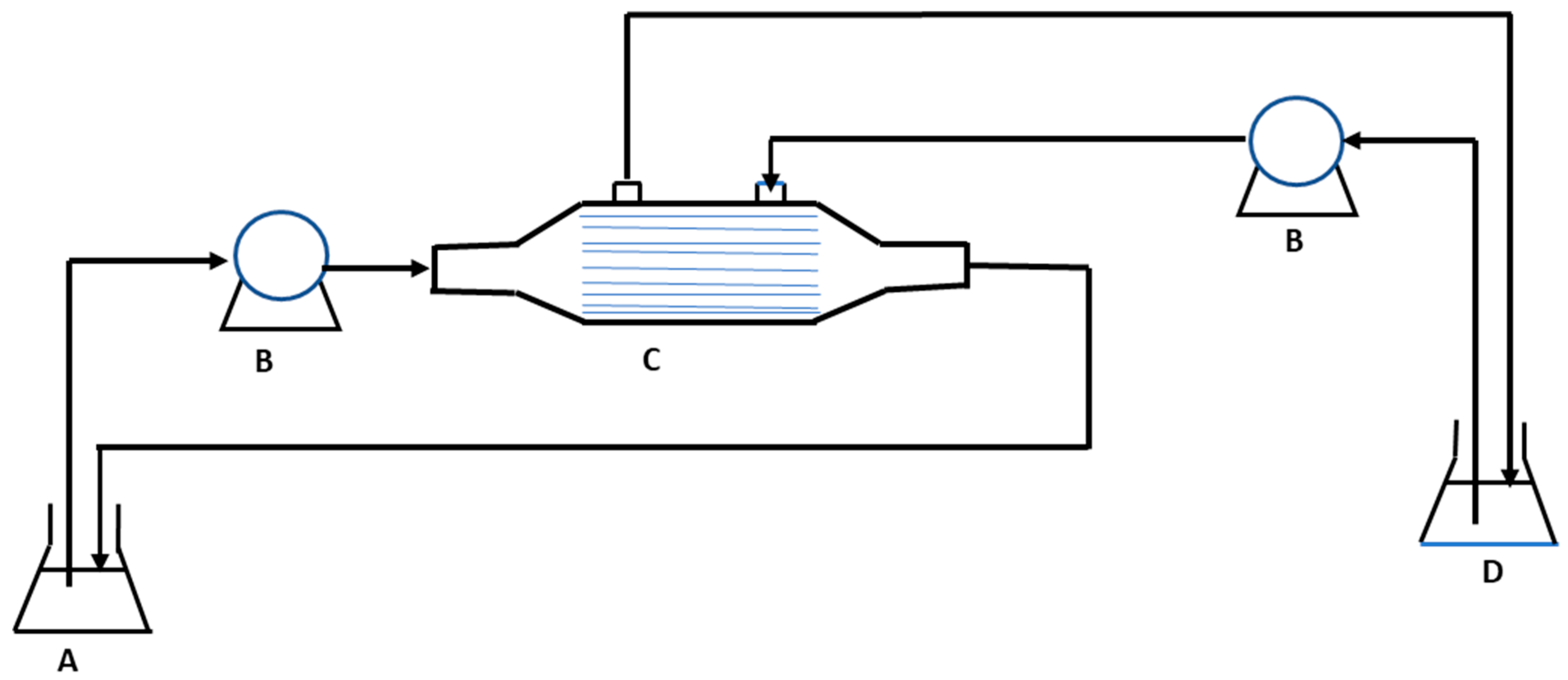
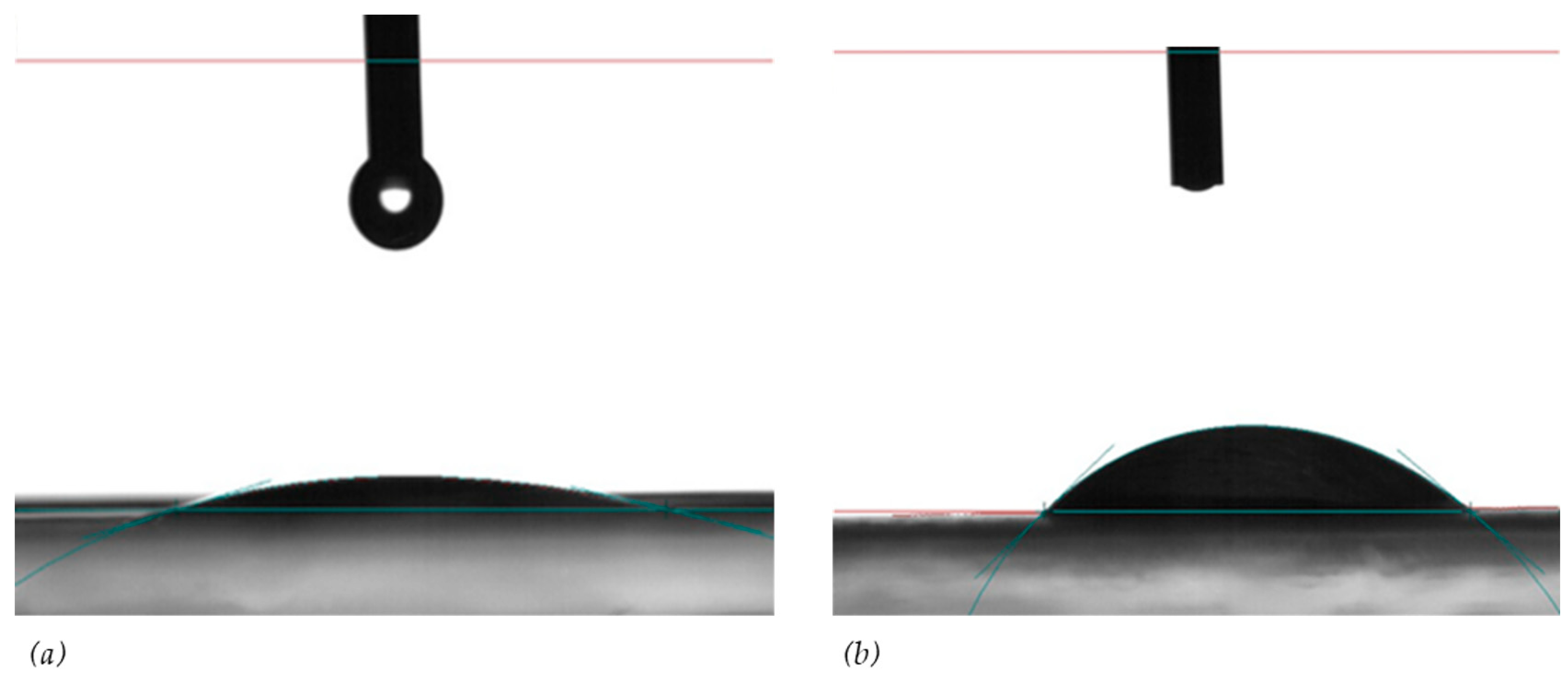






| References | Source of Feed | Membrane Type | Initial Feed Concentration | Removal Efficiency (%) |
|---|---|---|---|---|
| [26] | Bore hole | Low- and high-pressure RO membrane | 10 to 17 mgL−1 | 98 |
| [27] | Brackish drinking water | Low pressure RO, Polyamide | - | 97–98.9 |
| [28] | Artificial model waters | RO membranes (LFC, ULP, XLE, and TFC) | 2–20 mgL−1 | >80 |
| [29] | Groundwater | Polyamide (TW30-1812-75) RO membrane | 2.13 mgL−1 | 95–98 |
| [3] | Modeled water | RO-SG-2514 supplied by Osmonics | 8.4 mgL−1 | 96 |
| [3] | Metal packaging industrial effluent | RO-SG-2514 supplied by Osmonics | 34.96 mgL−1 | 96.1 |
| [30] | Real industrial wastewater | RO membrane | 8–60.1 mgL−1 | 98.1 |
| [7] | Surface water, groundwater, and soda lakes | RO membranes (BW30LE and BW30) | 42.4 mgL−1 | 99 and 98 |
| [31] | Natural water | Flat sheet TFC BW30, Dow FilmTech™ | 59.7 mgL−1 | 99 |
| [32] | Synthetic water | Polyamide | 400 mgL−1 | 95 |
| [33] | Natural water | Flat sheet TFC BW30, Dow FilmTech™ membranes | 40 mgL−1 | 99 |
| [34] | Synthetic solution | RO Spiral-wound TFC (Vontron) | 10 mgL−1 | 89.8 |
| [35] | Natural tropical brackish water | Flat sheet TFC BW30, Dow FilmTech™ | 50 mgL−1 | 95 |
| Trisep X—20TM | Specification |
|---|---|
| Membrane type | Polyamide–urea thin film |
| Maximum operating T° | 50 °C |
| Minimum salt rejection (%) | 98.5 |
| Maximum operating pressure | 41 bar |
| Continuous operating pH range | 4–11 |
| Maximum Silt Density Index (SDI) (15 min) | 5.0 |
| Maximum turbidity | 2 NTU |
| Parameters | Data from Manufacturer |
|---|---|
| Module configuration | Hollow fibers |
| Membrane/potting material | Polypropylene/polyethylene |
| Fiber inner/outer diameter (μm) | 240/300 |
| Wall thickness (μm) | 40 |
| Effective pore size (μm) | 0.04 |
| Porosity (%) | 40 |
| Effective fiber length (m) | 0.16 |
| Effective membrane surface area (m2) | 1.4 |
| Number of fibers | 10,200 |
| Burst strength (bar) | 27 |
| Contact angle (°) | 112 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ousman, W.Z.; Alemayehu, E.; Luis, P. Fluoride Removal and Recovery from Water Using Reverse Osmosis and Osmotic Membrane Crystallization. Clean Technol. 2023, 5, 973-996. https://doi.org/10.3390/cleantechnol5030049
Ousman WZ, Alemayehu E, Luis P. Fluoride Removal and Recovery from Water Using Reverse Osmosis and Osmotic Membrane Crystallization. Clean Technologies. 2023; 5(3):973-996. https://doi.org/10.3390/cleantechnol5030049
Chicago/Turabian StyleOusman, Wuhib Zeine, Esayas Alemayehu, and Patricia Luis. 2023. "Fluoride Removal and Recovery from Water Using Reverse Osmosis and Osmotic Membrane Crystallization" Clean Technologies 5, no. 3: 973-996. https://doi.org/10.3390/cleantechnol5030049
APA StyleOusman, W. Z., Alemayehu, E., & Luis, P. (2023). Fluoride Removal and Recovery from Water Using Reverse Osmosis and Osmotic Membrane Crystallization. Clean Technologies, 5(3), 973-996. https://doi.org/10.3390/cleantechnol5030049







