Development of a Biochar-Based Substrate Added with Nitrogen from a Mining Effluent for the Production of Picea mariana Seedlings
Abstract
:1. Introduction
2. Materials and Methods
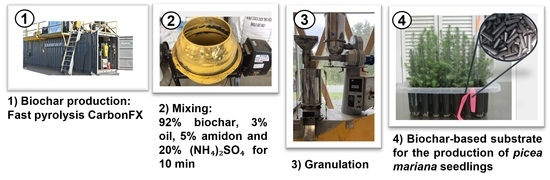
2.1. Biochar Production and Characterization
2.2. Pellet Manufacturing and Characterization
2.3. Seedling Production and Monitoring
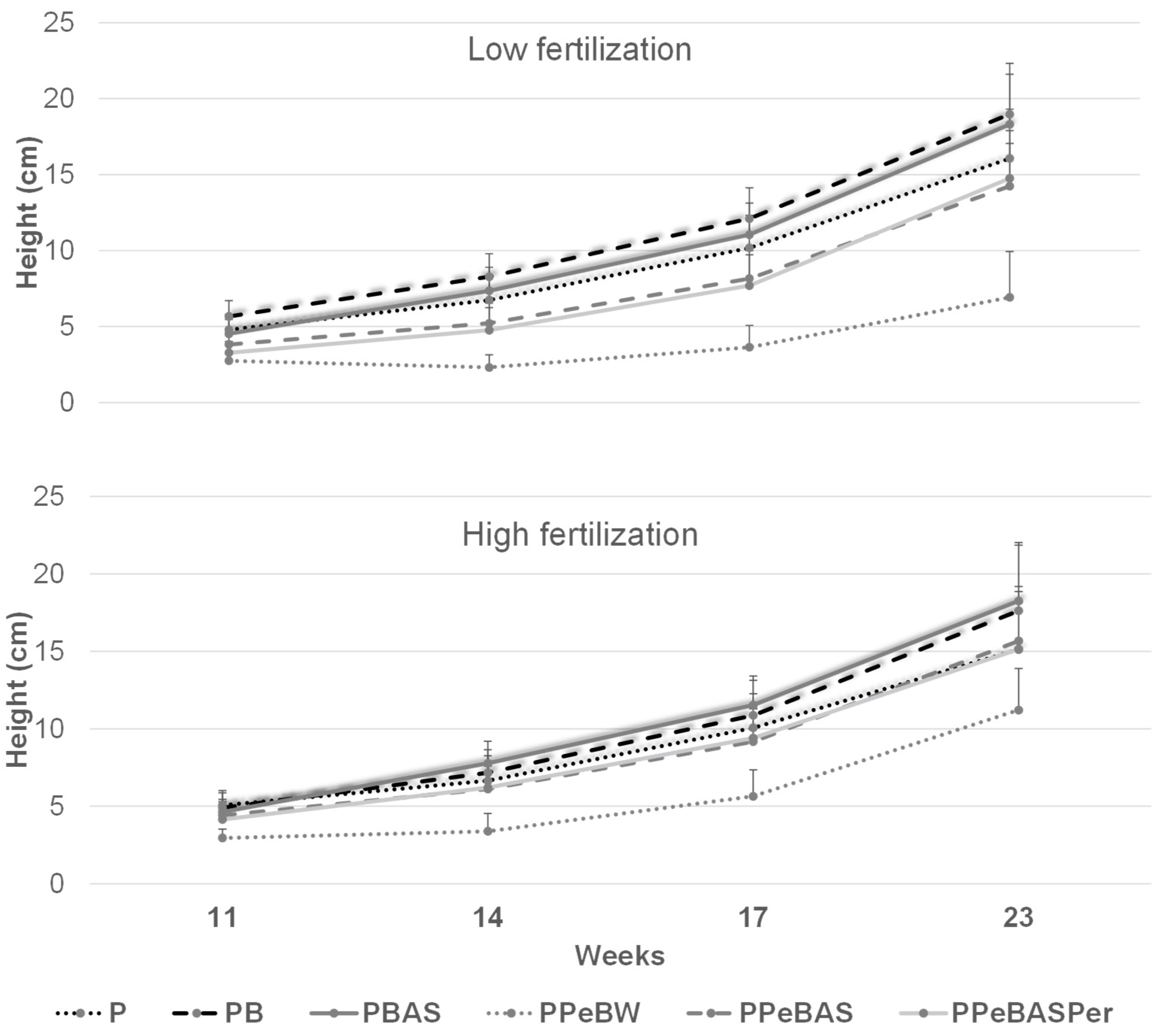
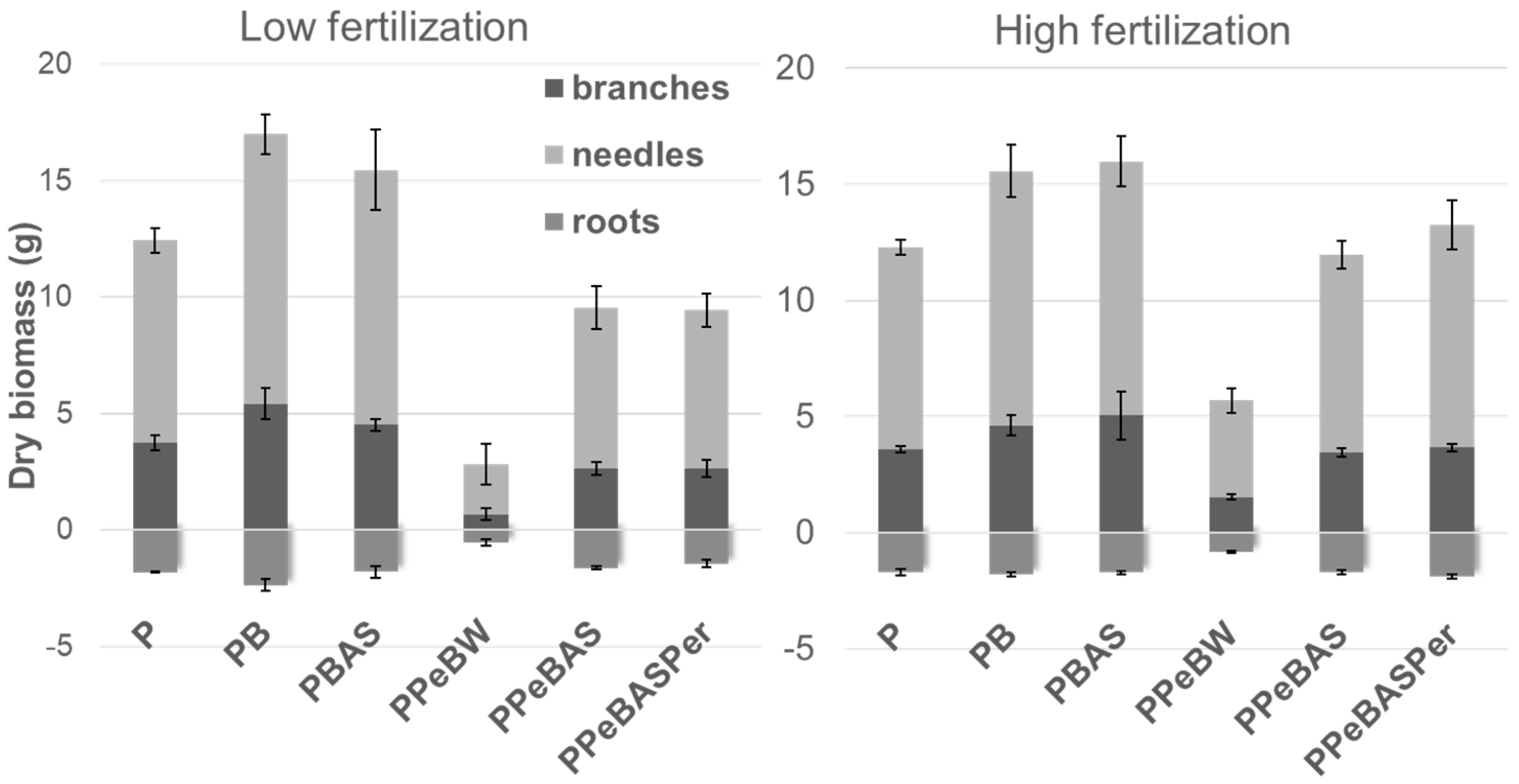
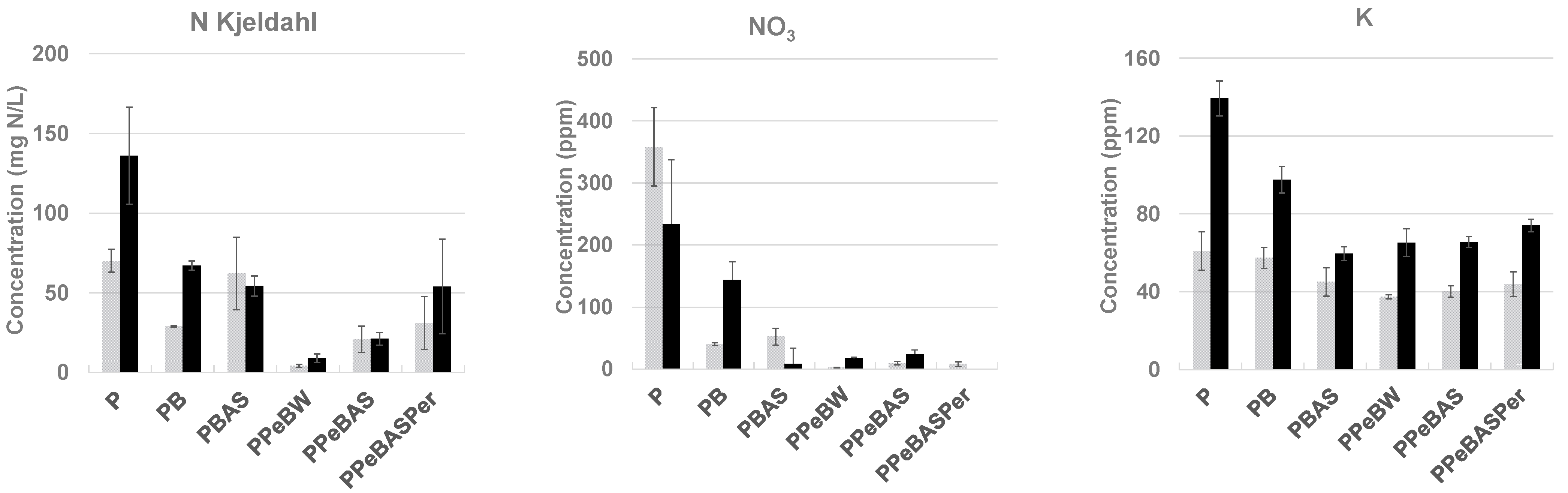
- 100% peat (P);
- 75% peat plus 25% bulk biochar (PB);
- 75% peat plus 25% bulk biochar impregnated with ammonium sulfate (PBAS);
- 75% peat plus 25% biochar pellets with water (PPeBW);
- 75% peat plus 25% biochar pellets with ammonium sulfate (PPeBAS);
- 62.5% peat plus 25% biochar pellets with ammonium sulfate and 12.5% perlite (PPeBASPer).
2.4. Leachate and Resultant Tissue Analysis
3. Results and Discussion
3.1. Biochar and Pellet Characterization
3.2. Seedling Production
3.2.1. Preliminary Test
3.2.2. Study Results
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zuttah, Y. Destruction de L’ammoniac dans les Effluents Miniers. Master’s Thesis, Département de mines et métaliurgie, Faculté des sciences et de génie, Université Laval, Québec, QC, Canada, 1999. [Google Scholar]
- Revey, G. Practical Methods to Control Explosives Losses and Reduce Ammonia and Nitrate Levels in Mine Water. Min. Eng. 1996, 48, 61–64. [Google Scholar]
- Bailey, B.L.; Smith, L.J.D.; Blowes, D.W.; Ptacek, C.J.; Smith, L.; Sego, D.C. The Diavik Waste Rock Project: Persistence of Contaminants from Blasting Agents in Waste Rock Effluent. Appl. Geochem. 2013, 36, 256–270. [Google Scholar] [CrossRef]
- Gherrou, A. L’azote Ammoniacal, Nouvelle Cible de La Réglementation Sur Les Rejets Industriels. In La revue de l’ordre des chimistes du Québec, Printemps 2012; 2012; Volume 27, pp. 15–20. Available online: https://www.ocq.qc.ca/wp-content/uploads/2015/03/chimistevol27no12012webl.pdf (accessed on 20 July 2021).
- Lehmann, J.; Joseph, S. (Eds.) Biochar for Environmental Management: Science, Technology and Implementation; Routledge: Abingdon, UK, 2015; ISBN 978-1-134-48953-4. [Google Scholar]
- Bonanomi, G.; Ippolito, F.; Scala, F. A “Black” Future for Plant Pathology? Biochar as a New Soil Amendment for Controlling Plant Diseases. J. Plant Pathol. 2015, 97, 223–234. [Google Scholar]
- Purakayastha, T.J.; Kumari, S.; Sasmal, S.; Pathak, H. Biochar Carbon Sequestration in Soil-A Myth or Reality? J. Bio-Resour. Stress Manag. 2015, 6, 623. [Google Scholar] [CrossRef]
- Selvarajh, G.; Ch’ng, H.Y.; Md Zain, N.; Sannasi, P.; Mohammad Azmin, S.N.H. Improving Soil Nitrogen Availability and Rice Growth Performance on a Tropical Acid Soil via Mixture of Rice Husk and Rice Straw Biochars. Appl. Sci. 2020, 11, 108. [Google Scholar] [CrossRef]
- Ding, Y.; Liu, Y.; Liu, S.; Li, Z.; Tan, X.; Huang, X.; Zeng, G.; Zhou, L.; Zheng, B. Biochar to Improve Soil Fertility. A Review. Agron. Sustain. Dev. 2016, 36, 36. [Google Scholar] [CrossRef] [Green Version]
- Rawat, J.; Saxena, J.; Sanwal, P. Biochar: A Sustainable Approach for Improving Plant Growth and Soil Properties. In Biochar-An Imperative Amendment for Soil and the Environment; IntechOpen: London, UK, 2019. [Google Scholar]
- Wardle, D.A.; Zackrisson, O.; Nilsson, M.-C. The Charcoal Effect in Boreal Forests: Mechanisms and Ecological Consequences. Oecologia 1998, 115, 419–426. [Google Scholar] [CrossRef]
- Thomas, S.C.; Gale, N. Biochar and Forest Restoration: A Review and Meta-Analysis of Tree Growth Responses. New For. 2015, 46, 931–946. [Google Scholar] [CrossRef]
- Jeffery, S.; Verheijen, F.G.A.; van der Velde, M.; Bastos, A.C. A Quantitative Review of the Effects of Biochar Application to Soils on Crop Productivity Using Meta-Analysis. Agric. Ecosyst. Environ. 2011, 144, 175–187. [Google Scholar] [CrossRef]
- Liang, B.; Lehmann, J.; Solomon, D.; Kinyangi, J.; Grossman, J.; O’Neill, B.; Skjemstad, J.O.; Thies, J.; Luizão, F.J.; Petersen, J.; et al. Black Carbon Increases Cation Exchange Capacity in Soils. Soil Sci. Soc. Am. J. 2006, 70, 1719–1730. [Google Scholar] [CrossRef] [Green Version]
- DeLuca, T.H.; MacKenzie, M.D.; Gundale, M.J.; Holben, W.E. Wildfire-Produced Charcoal Rirectly Influences Nitrogen Cycling in Ponderosa Pine Forests. Soil Sci. Soc. Am. J. 2006, 70, 448–453. [Google Scholar] [CrossRef] [Green Version]
- Clough, T.J.; Condron, L.M. Biochar and the Nitrogen Cycle: Introduction. J. Environ. Qual. 2010, 39, 1218–1223. [Google Scholar] [CrossRef] [PubMed]
- Aghoghovwia, M.P.; Hardie, A.G.; Rozanov, A.B. Characterisation, Adsorption and Desorption of Ammonium and Nitrate of Biochar Derived from Different Feedstocks. Environ. Technol. 2022, 43, 774–787. [Google Scholar] [CrossRef] [PubMed]
- Amoriello, T.; Fiorentino, S.; Vecchiarelli, V.; Pagano, M. Evaluation of Spent Grain Biochar Impact on Hop (Humulus Lupulus L.) Growth by Multivariate Image Analysis. Appl. Sci. 2020, 10, 533. [Google Scholar] [CrossRef] [Green Version]
- Taghizadeh-Toosi, A.; Clough, T.J.; Sherlock, R.R.; Condron, L.M. Biochar Adsorbed Ammonia Is Bioavailable. Plant Soil 2012, 350, 57–69. [Google Scholar] [CrossRef]
- Spokas, K.A.; Novak, J.M.; Venterea, R.T. Biochar’s Role as an Alternative N-Fertilizer: Ammonia Capture. Plant Soil 2012, 350, 35–42. [Google Scholar] [CrossRef]
- López-Valdez, F.; Fernández-Luqueno, F. (Eds.) Fertilizers: Components, Uses in Agriculture and Environmental Impacts (Biotechnology in Agriculture, Industry and Medicine); Nova Publishers: New York, NY, USA, 2014; ISBN 978-1-63321-051-6. [Google Scholar]
- Kim, P.; Hensley, D.; Labbé, N. Nutrient Release from Switchgrass-Derived Biochar Pellets Embedded with Fertilizers. Geoderma 2014, 232–234, 341–351. [Google Scholar] [CrossRef]
- Allaire, S.E.; Lange, S.F. Substrats Horticoles à Base de Biochars: Performance et Économie; Centre de Recherche sur les Matériaux, Université Laval Renouvelables: Québec, QC, Canada, 2017; p. 40. [Google Scholar]
- Landis, T.D.; Tinus, R.W.; McDonald, S.E.; Barnett, J.P. Seedling Nutrition and Irrigation, Volume 4, The Container Tree Nursery Manual. In Manual Agriculture Handbook; U.S. Department of Agriculture, Forest Service: Washington, DC, USA, 1989; Volume 674. [Google Scholar]
- Gauthier, S.; Vaillancourt, M.-A.; Leduc, A.; De Grandpré, L.; Kneeshaw, D.; Morin, H.; Drapeau, P.; Bergeron, Y. (Eds.) Aménagement Écosystémique En Forêt Boréale, 1st ed.; Presses de l’Université du Québec: Quebec, QC, Canada, 2008; ISBN 978-2-7605-1959-6. [Google Scholar]
- Arous, S.; Koubaa, A.; Bouafif, H.; Bouslimi, B.; Braghiroli, F.L.; Bradai, C. Effect of Pyrolysis Temperature and Wood Species on the Properties of Biochar Pellets. Energies 2021, 14, 6529. [Google Scholar] [CrossRef]
- García, R.; Gil, M.V.; Fanjul, A.; González, A.; Majada, J.; Rubiera, F.; Pevida, C. Residual Pyrolysis Biochar as Additive to Enhance Wood Pellets Quality. Renew. Energy 2021, 180, 850–859. [Google Scholar] [CrossRef]
- Mohammadi, A. Overview of the Benefits and Challenges Associated with Pelletizing Biochar. Processes 2021, 9, 1591. [Google Scholar] [CrossRef]
- García, R.; González-Vázquez, M.P.; Pevida, C.; Rubiera, F. Pelletization Properties of Raw and Torrefied Pine Sawdust: Effect of Co-Pelletization, Temperature, Moisture Content and Glycerol Addition. Fuel 2018, 215, 290–297. [Google Scholar] [CrossRef]
- Bergman, R.; Sahoo, K.; Englund, K.; Mousavi-Avval, S.H. Lifecycle Assessment and Techno-Economic Analysis of Biochar Pellet Production from Forest Residues and Field Application. Energies 2022, 15, 1559. [Google Scholar] [CrossRef]
- Dumroese, R.K.; Heiskanen, J.; Englund, K.; Tervahauta, A. Pelleted Biochar: Chemical and Physical Properties Show Potential Use as a Substrate in Container Nurseries. Biomass Bioenergy 2011, 35, 2018–2027. [Google Scholar] [CrossRef]
- Dumroese, R.; Pinto, J.; Heiskanen, J.; Tervahauta, A.; McBurney, K.; Page-Dumroese, D.; Englund, K. Biochar Can Be a Suitable Replacement for Sphagnum Peat in Nursery Production of Pinus Ponderosa Seedlings. Forests 2018, 9, 232. [Google Scholar] [CrossRef] [Green Version]
- Köster, E.; Pumpanen, J.; Palviainen, M.; Zhou, X.; Köster, K. Effect of Biochar Amendment on the Properties of Growing Media and Growth of Containerized Norway Spruce, Scots Pine, and Silver Birch Seedlings. Can. J. For. Res. 2021, 51, 31–40. [Google Scholar] [CrossRef]
- Brunauer, S.; Emmett, P.H.; Teller, E. Adsorption of Gases in Multimolecular Layers. J. Am. Chem. Soc. 1938, 60, 309–319. [Google Scholar] [CrossRef]
- CEN/TS 15210-1 2006 Solid Biofuels-Methods for the Determination of Mechanical Durability of PELLETS and briquettes-Part 1: Pellets. Available online: https://standards.iteh.ai/catalog/standards/sist/13971322-aa71-4d22-b678-c5469189521c/sist-ts-cen-ts-15210-1-2006 (accessed on 1 March 2018).
- Ministère des Forêts, de la Faune et des Parcs Production de Résineux En Récipients. Available online: https://mffp.gouv.qc.ca/les-forets/production-semences-plants-forestiers/plants/techniques/resineuses/recipients/ (accessed on 25 July 2022).
- Kjeldahl, J. New Method for the Determination of Nitrogen. Chem. News 1883, 48, 101–102. [Google Scholar] [CrossRef]
- Braghiroli, F.L.; Bouafif, H.; Neculita, C.M.; Koubaa, A. Performance of Physically and Chemically Activated Biochars in Copper Removal from Contaminated Mine Effluents. Water Air Soil Pollut. 2019, 230, 178. [Google Scholar] [CrossRef]



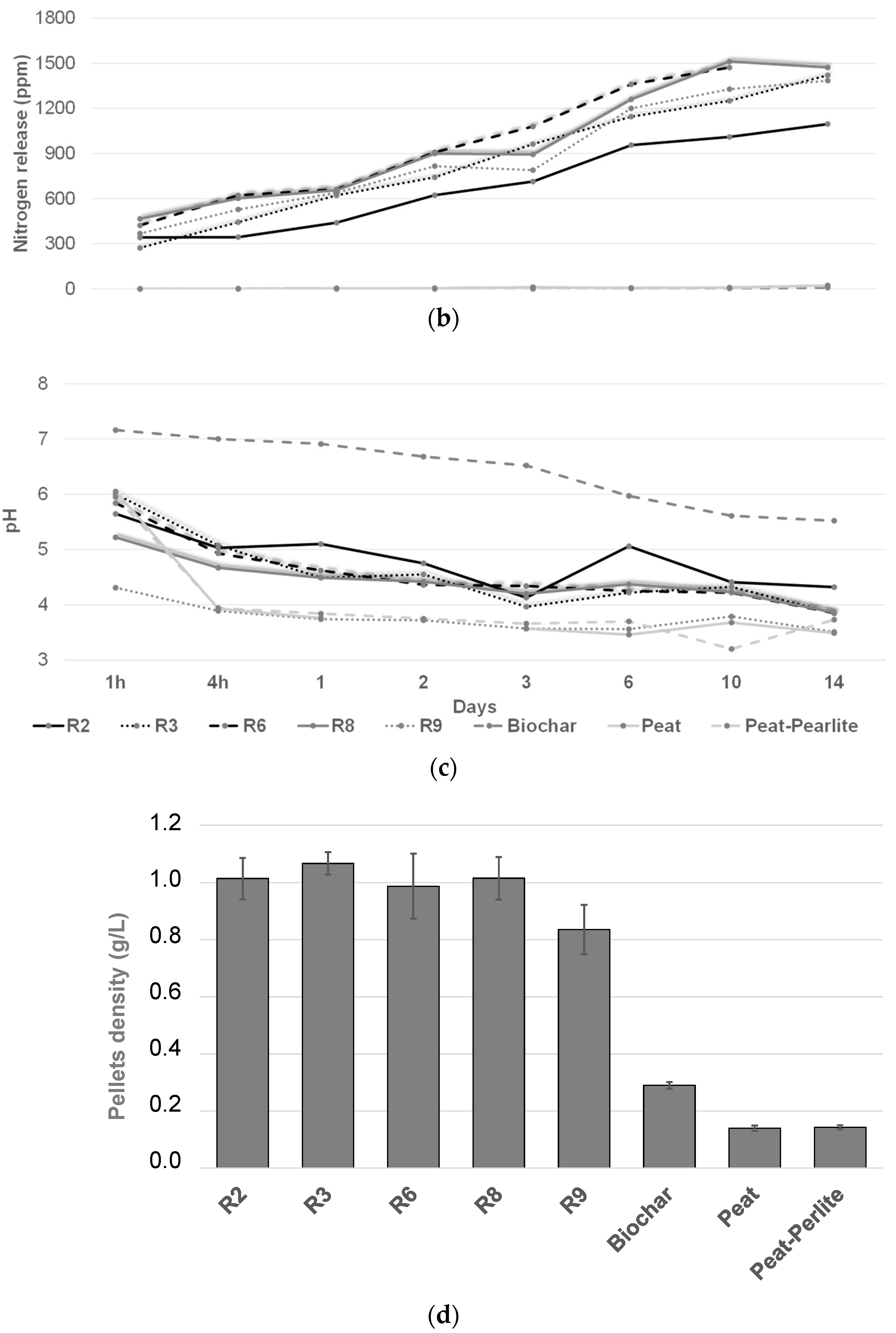



| Recipes | Biochar (%) | Wood Sawdust (%) | Oil (%) | Starch (%) | Moisture (%) | Pellets Efficacy | DI (%) |
|---|---|---|---|---|---|---|---|
| R1 | 80 | 20 | 0 | 0 | 20 | - | - |
| R2 | 77.6 | 19.4 | 3 | 0 | 20 | ✓ | 94 |
| R3 | 73.6 | 18.4 | 3 | 5 | 20 | ✓ | 99 |
| R4 | 100 | 0 | 0 | 0 | 20 | - | - |
| R5 | 97 | 0 | 3 | 0 | 20 | - | - |
| R6 | 92 | 0 | 3 | 5 | 20 | ✓ | 99 |
| R7 | 90 | 10 | 0 | 0 | 20 | - | - |
| R8 | 87.3 | 9.7 | 3 | 0 | 20 | ✓ | 94 |
| R9 | 82.8 | 9.2 | 3 | 5 | 20 | ✓ | 95 |
| C (%) | H (%) | N (%) | S (%) | O (%) | SBET (m2/g) | |
|---|---|---|---|---|---|---|
| Spruce wood residues | 48.4 | 6.6 | 0.1 | 1.0 | 43.9 | 0.5 |
| Biochar | 53.0 | 5.7 | 0.7 | 0.8 | 39.8 | 42 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Robert, É.; Braghiroli, F.L. Development of a Biochar-Based Substrate Added with Nitrogen from a Mining Effluent for the Production of Picea mariana Seedlings. Clean Technol. 2022, 4, 770-784. https://doi.org/10.3390/cleantechnol4030047
Robert É, Braghiroli FL. Development of a Biochar-Based Substrate Added with Nitrogen from a Mining Effluent for the Production of Picea mariana Seedlings. Clean Technologies. 2022; 4(3):770-784. https://doi.org/10.3390/cleantechnol4030047
Chicago/Turabian StyleRobert, Émilie, and Flavia Lega Braghiroli. 2022. "Development of a Biochar-Based Substrate Added with Nitrogen from a Mining Effluent for the Production of Picea mariana Seedlings" Clean Technologies 4, no. 3: 770-784. https://doi.org/10.3390/cleantechnol4030047
APA StyleRobert, É., & Braghiroli, F. L. (2022). Development of a Biochar-Based Substrate Added with Nitrogen from a Mining Effluent for the Production of Picea mariana Seedlings. Clean Technologies, 4(3), 770-784. https://doi.org/10.3390/cleantechnol4030047