Abstract
Billions of litres of wastewater are produced daily from domestic and industrial areas, and whilst wastewater is often perceived as a problem, it has the potential to be viewed as a rich source for resources and energy. Wastewater contains between four and five times more energy than is required to treat it, and is a potential source of bio-hydrogen—a clean energy vector, a feedstock chemical and a fuel, widely recognised to have a role in the decarbonisation of the future energy system. This paper investigates sustainable, low-energy intensive routes for hydrogen production from wastewater, critically analysing five technologies, namely photo-fermentation, dark fermentation, photocatalysis, microbial photo electrochemical processes and microbial electrolysis cells (MECs). The paper compares key parameters influencing H2 production yield, such as pH, temperature and reactor design, summarises the state of the art in each area, and highlights the scale-up technical challenges. In addition to H2 production, these processes can be used for partial wastewater remediation, providing at least 45% reduction in chemical oxygen demand (COD), and are suitable for integration into existing wastewater treatment plants. Key advancements in lab-based research are included, highlighting the potential for each technology to contribute to the development of clean energy. Whilst there have been efforts to scale dark fermentation, electro and photo chemical technologies are still at the early stages of development (Technology Readiness Levels below 4); therefore, pilot plants and demonstrators sited at wastewater treatment facilities are needed to assess commercial viability. As such, a multidisciplinary approach is needed to overcome the current barriers to implementation, integrating expertise in engineering, chemistry and microbiology with the commercial experience of both water and energy sectors. The review concludes by highlighting MECs as a promising technology, due to excellent system modularity, good hydrogen yield (3.6–7.9 L/L/d from synthetic wastewater) and the potential to remove up to 80% COD from influent streams.
1. Introduction
The Intergovernmental Panel on Climate Change (IPCC) states that reducing CO2 emissions will play a critical role in addressing the challenges posed by climate change. Increasing energy production from renewable sources, and eliminating waste through the continual use of resources via a circular economy approach, are key aspects of the mitigation strategy [1]. The International Energy Agency (IEA) [2] and the UK Environment Agency (EA) [3] have emphasised the role the water industry can play to address the effects of climate change. Whilst the sector uses a significant amount of energy for water distribution and wastewater treatment (around 3–5% of the global energy use [4]), wastewater (WW) is widely recognised as a potential source of energy. Researchers have quantified the energy available in wastewater to be in the range of 17.8 to 28.7 kJ per gram of chemical oxygen demand (COD), with calculations demonstrating that wastewater contains between four and five times more energy than is required for treatment [4]. There is therefore potential to recover thermal, kinetic and chemical energy from wastewater, including energy-rich gases such as methane and hydrogen. Hydrogen is an energy vector, a feedstock chemical and a fuel, and it is widely recognised that hydrogen produced via low-carbon footprint technologies, commonly defined as bio-hydrogen, will play a significant role in the decarbonisation of the energy system [5]. To date, many processes have been developed for hydrogen production, but the majority are energy-intensive and based on fossil fuels, often termed “black” hydrogen [6], and as such contribute to CO2 emissions. Therefore, there is a clear need to identify both new sources and low-energy processes for hydrogen production, enabling decarbonisation of the energy system, coupled with improving the uptake of low-energy intensive wastewater treatment processes.
This paper reviews sustainable low-energy intensive processes that use domestic and/or industrial wastewater as a feedstock for bio-hydrogen production, describing the underpinning theory and state of the art relating to photo-fermentation (PF), photocatalysis, microbial photo electrochemical cells (MPEC), dark fermentation (DF) and microbial electrolysis cells (MEC). We examine the pros and cons of each technology, the key parameters influencing hydrogen yield, and highlight areas for technical development to drive commercial feasibility.
Previous reviews in this field have focused on a single technology or specific aspects of technologies producing bio-hydrogen from municipal wastewater. For example, Yasri et al. [7] reviewed MECs, focusing on the influence of the system design, electrolyte properties, and anode and cathode materials, while Preethi et al. [8] described the role and influence of various operating parameters on dark fermentative biohydrogen production from industrial wastewater. Banu et al. [9] discussed the recent developments, enhancement strategy, economical aspects and scale-up of dark fermentative hydrogen production from industrial wastewater. A recent review by Hay et al. [10] focused mainly on photo and dark fermentation, providing only a short introduction to microbial electrolysis cells. Pretreatment approaches to enhance hydrogen yields in microbial-based generation systems were recently reviewed by Sharmila et al. [11], and reduction in influent toxicity significantly increased bio-hydrogen production, thereby aiding economic viability. Hydrogen production through photocatalysis was reviewed by Rioja-Cabanillas et al., describing the underpinning materials involved in electrode/catalysts and the efficiencies of both photocatalysis and photochemical processes [12]. The need for researchers to move from synthetic to real wastewater substrates and an opportunity to couple treatments through simultaneous bioremediation was also highlighted. Capson-Tojo et al. [13] critically analysed the role of purple phototrophic bacteria (PPB) in recovering value-added products from wastewater, including bio-hydrogen, through artificial and solar irradiation (sometimes referred to as biological “batteries”, converting sewage to clean energy).
Some authors broaden the scope further to analyse bio-hydrogen production from organic waste, considering both organic waste and wastewater with the analysis of both fermentation-based processes and MECs [14], and indeed, the integration of low-energy intensive processes with existing technologies. Kadier et al. [15] reviewed MEC integration with MFC, anerobic digestion, dye-sensitised solar cells and thermoelectric microconverters to generate more sustainable hydrogen from wastewater, identifying strategies for scale-up. However, to the authors’ knowledge, there is no comprehensive review including light-dependent and -independent technologies to produce bio-hydrogen from domestic and industrial wastewater.
The objectives of the paper are to (1) develop a comparative analysis of both light-dependent and -independent H2 production technologies; (2) identify the key challenges facing low-energy processes; (3) discuss the current trends and future research pathways to improve H2 yield from wastewater.
2. Methodology
2.1. Web of Science-Based Trend Analysis
Clarivate’s Web of Science (WoS) advanced search tool was used to assess research activities related to “hydrogen production processes from wastewater”. The output from the tool (Table 1) identified 1030 documents from 2001 to July 2020 [16]. No acronyms were considered in this search operation. In addition, 13 query strings were selected based on five specific technologies presented in the review, and additional relevant variables/parameters were included with respect to low-energy intensive hydrogen production processes (Table 1). Although the number of publications returned in the search was comprehensive, some articles linked to the topic may not have been identified through the use of the specific strings. An analysis of the data in Table 1 shows that dark fermentation returned the highest number of related documents (125), followed by microbial electrolysis cell (64), photocatalysis (43), photo-fermentation (28) and microbial photo electrochemical cells (8). Among the results, “reactor” (set # 2) returned the highest number of publications (397), whereas “configuration” (set # 12) generated a low number of publications (38). Figure 1 shows the number of publications per year as a function of topic as well as other associated fields between 2001 and 2020. The data confirm the steady increase in research output within this field, with the number of publications across the technologies (set # 1) rising from 8 in 2001, to 144 in 2019 (an 18-fold increase). The refinement of technology and focus on increased efficiency were evident from the large number of papers from 2013 onwards focusing on reactor development, materials and/or fabrication techniques.

Table 1.
Publication results for the different search query strings used in this study.
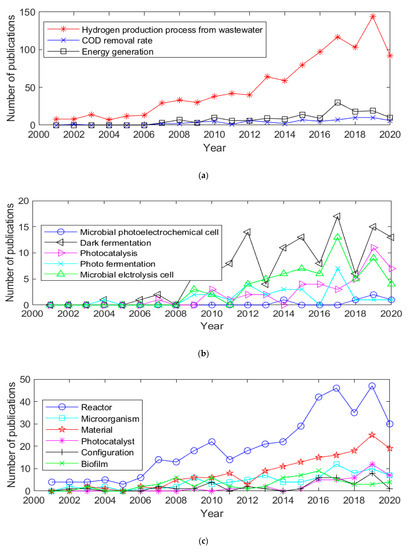
Figure 1.
Comparative publication output for selected search terms per year; (a) H2 production process, energy generation and COD reduction rate. (b) Microbial photoelectrochemical cell, dark fermentation, photocatalysis, photo fermentation and microbial electrolysis cell. (c) Reactor, microorganism, material, photocatalyst, configuration and biofilm.
Analysis of research publication trends not only permits the review and identification of “hot topics” and progress towards the production of clean renewable hydrogen, as it also permits the identification of research gaps. Figure 1a highlights the increase in research focused on hydrogen production; whilst this is coupled with wastewater remediation (primarily a reduction in COD), the analysis shows significant interest and growth specifically for hydrogen production within the sector. Figure 1b analyses trends in those low-energy intensive hydrogen production processes, demonstrating increased research activity in DF, photocatalysis and MEC. If areas such as photo-fermentation and PMEC are to make significant contributions to the area, additional focus is needed in these specific areas. Figure 1c details the specific research topics which underpin progress in the area; whilst there has been an increase over the past 10 years in articles related to reactor designs and materials, these laboratory-based advances have not yet transferred into industry application.
2.2. VOS Viewer-Based Strength Analysis
The 1030 publications selected by Clarivate’s WoS database were analysed by VOS viewer (version 1.6.15, April 2020) to establish and visualise bibliometric networks associated with the relative strength of the keywords. The clustering and co-occurrence networks demonstrate the synergy between the research areas, but highlight the use of differing terminology (bio-hydrogen relating to fermentation-based systems; anaerobic digestion (AD) favouring hydrogen linked with biogas). Of the 2595 keywords within the selected publications, 22 were identified based on the minimum occurrence threshold level of 15. Hydrogen exhibited the highest link strength (72) followed by biohydrogen (70) and wastewater (66). Among the process keywords, dark fermentation showed the highest strength (41) followed by MEC (26) and photocatalysis (10), whereas photo-fermentation and MPEC did not exhibit results. A concept map (Figure 2) was developed, visualising the cluster of keywords with their relative linked strengths. Four different clusters were identified; Cluster 1 (red) is the biggest cluster with 8 keywords, including MEC linked to wastewater treatment and bioenergy generation, whereas Cluster 4 (yellow) forms the smallest cluster with 4 keywords, including photocatalysis, linked to hydrogen production and olive mill wastewater. The growing interest in MEC was highlighted through association with the main cluster [17].
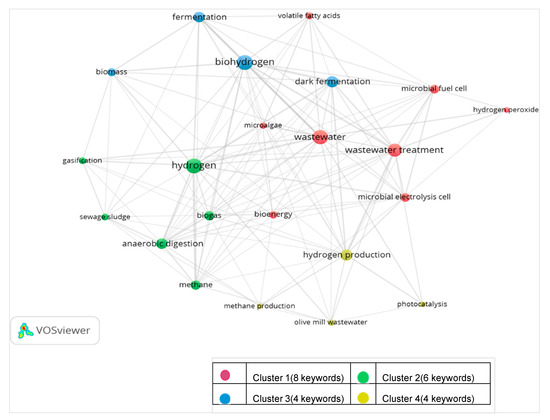
Figure 2.
Cluster analysis of frequently used keywords based on associated strength.
3. Hydrogen Production Process Analysis
Low-energy intensive hydrogen production processes utilising domestic or industrial wastewater as a feedstock can be classified based on their dependence on the input of light (UV/Vis energy); however, many other routes are available (Figure 3) [18]. Light-dependent processes can be split into photo-fermentation, photocatalysis and microbial photoelectrochemical systems, whereas dark fermentation and microbial electrolysis cells do not depend on photonic based energy input. In addition to these low-energy intensive processes, several other processes have been used to generate hydrogen from wastewater, including electrolysis, reverse electrodialysis, microbial electrodialysis and super critical water gasification (energy-intensive H2 production processes, requiring significant electrical input and/or high temperature). It is noted that additional catalysts are required for photocatalysis [19], and small-voltage bias is often used for MEC and MPEC [20].
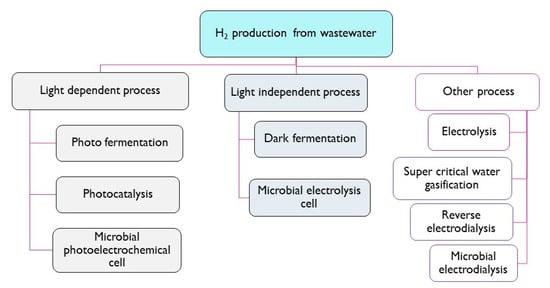
Figure 3.
Classification of hydrogen production processes from wastewater [18].
3.1. Photo-Fermentation (PF)
3.1.1. Description of the Process
Photo-fermentation has been well studied for the production of H2 from industrial wastewaters, as shown in Table 2. Batch-mode photoreactors ensure the absence of oxygen, permitting purple non-sulphur (PNS) bacteria [21] to anaerobically break down organic compounds producing hydrogen (Figure 4) [22]. The reactions shown in Equation (1) and (2) describe the energetics of the process for two model compounds (acetic acid and glucose), confirming the non-spontaneous reaction. As such, the input of photons is essential, typically limiting process efficiency. Photosynthetic bacteria convert the organic content to hydrogen, primarily via nitrogenase and hydrogenase enzyme systems. A greater molar yield is theoretically generated from glucose-based substrates.
2CH3COOH + 2H2O→ 4H2 + 2CO2, (ΔGo = +104 kJ)
C6H12O6 + 6H2O →12H2 + 6CO2 (∆Go = +3.2 kJ)

Table 2.
Batch photo fermentative hydrogen production.
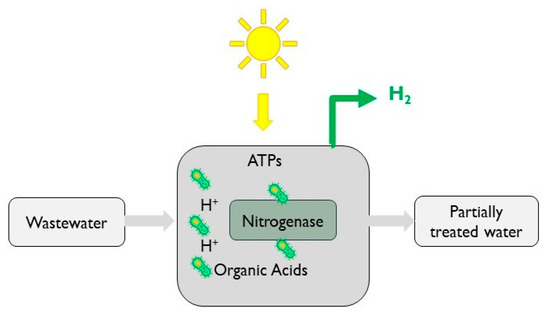
Figure 4.
Schematic diagram of photo-fermentation process.
In the presence of molecular nitrogen (N2), nitrogenase catalyses the formation of ammonium (NH4+) and H2 (Equation (3)), with the reaction also sustainable in the absence of N2 (Equation (4)).
where Pi signifies orthophosphate.
N2 + 8 e− +10 H+ + 16 MgATP → 2 NH4+ + H2 + 16 MgADP + 16 Pi
8 e− + 8 H+ + 16 MgATP → 4 H2 + 16 MgADP + 16 Pi
Table 2 describes hydrogen production by PF from different types of wastewater using both natural and artificial photon input. Whilst low-cost and carbon-free, using sunlight has drawbacks, including limited working time (daylight hours only) and intensity fluctuation due to seasonality and weather changes [23]. Generally, as a source of artificial light, mercury–tungsten lamps have been used with light intensities in the range of 4000 lux to 9000 lux. With dairy wastewater, the highest hydrogen production rate (0.057 L/L/h) was achieved using a 9000 lux lamp, suggesting that high photon flux can increase hydrogen production. A high hydrogen production rate (0.015 L/L/h) was reported from olive wastewater using a 150 W/m2 tungsten lamp [24]; conversely, a low H2 yield (0.009L/L/h) was reported from olive mill wastewater using a lamp intensity of 200 W/m2 [25]. Such variations provide an idea of the complexity of the process, the importance of reactor configuration and the need to optimise parameters to ensure efficient H2 production.
3.1.2. Important Process Parameters
Both the rate and yield of H2 production are influenced by light intensity, temperature, pH, hydraulic retention time (HRT) and substrate C/N ratio. The configuration of the photobioreactor also plays a key role relating to both efficient light capture and the growth of microbes inside the reactor. Table 3 summarises the parameters that influence hydrogen production, highlighting optimal ranges. Zhang et al. reported a temperature range from 30 to 40 °C to be optimal for hydrogen production, whereas the pH range depends on the influent type [28]. Baffle-based bioreactors were reported to show a high hydrogen production rate and cumulative hydrogen yield, this being effective for uninterrupted production of H2. Substrate C/N ratio plays a vital role, being inversely proportional to H2 production rate [29]. If C/N is higher than 2/3, then it is likely that H2 production will be hindered due to the accumulation of NH4+. In regard to HRT, no linear relationship with H2 production rate was reported; however, hydrogen yield may be decreased when HRT below 24 h [28].

Table 3.
Key parameters and optimal range influencing the H2 production in photo-fermentation.
3.1.3. Strengths, Weakness and H2 Production Enhancement
Photo-fermentative hydrogen production is not economically viable at a larger scale, but this is an active area of research with some promising recent progress. The main challenges include (1) the high cost and complexity of the photobioreactors [32], (2) the fact that microbes are highly sensitive to temperature and light intensity, (3) the low catalytic activity of nitrogenase [33], and (4) the bacterial contamination and synthesis of competing by-products, such as polyhydroxybutyrate. Although the process has several drawbacks, there is significant potential, as follows: (1) the possibility of using solar energy, a free, renewable and clean energy source [34]; (2) a variety of substrates can be utilised by the PNS bacteria, and (3) the process can be integrated with other technologies, such as dark fermentation (DF), enhancing hydrogen output [34]. Assawamongkholsiri & Reungsang [35] reported optimisation of the process by controlling key factors such as (1) microbial cell density, (2) initial pH, (3) light intensity and (4) molybdenum concentration, increasing the hydrogen production rate and yield 1.6–2.5-fold. Recent advancements in bio-nanotechnology, especially the application of nano-metal ions and oxides in photo-fermentative hydrogen processes, have shown promising results. The addition of TiO2 (300 mg/L), ZnO (100 mg/L) and SiC (200 mg/L) to the medium can enhance hydrogen production by up to 18.6% [36]. The use of genetically modified and mixed bacterial consortia have been reported to be effective when scaling-up the photo fermentative hydrogen production from dark fermented effluent (residue produced from anaerobic digester), with increased COD reduction also reported [37]. Improved photobioreactor kinetics, increasing light diffusion coupled with effective mixing, can also increase photoconversion efficiency [32]. Net hydrogen production can also be increased by improving the efficiency of key nitrogenase enzymes, with one study using genetic engineering to knock out specific PNS genes and reduce the suppression of nitrogenase by ammonium ions [38].
3.2. Photocatalysis
3.2.1. Description of the Process
Photocatalysis is defined as the acceleration of a photoreaction in the presence of a catalyst. Photocatalytic hydrogen generation can be achieved through the photo-splitting of water [39] or the photo-reforming/degradation of organic species [40]. Solar photocatalytic processes require the catalyst to absorb radiation in the UV and/or visible range to promote electrons from the valence band to the conduction band, thereby generating the required potential for surface-based redox reactions and, in this case, hydrogen formation (Figure 5) [41,42].
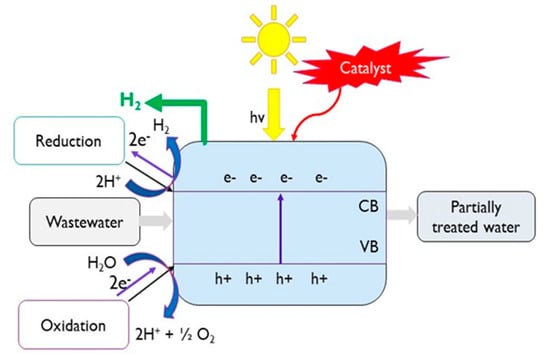
Figure 5.
Schematic diagram of photocatalytic H2 production.
Short-chain alcohols (e.g., methanol, ethanol and glycerol), carboxylic acids, (e.g., formic acid), and carbohydrates (e.g., glucose) present in wastewater are commonly oxidised in photo-reforming processes [43,44,45,46]. Photocatalytic treatment of industrial and domestic wastewater streams has been achieved, demonstrating potential for a combined process of wastewater treatment with simultaneous hydrogen generation [45]. For efficient hydrogen production, a high irradiated surface area to volume ratio is required [44].
Table 4 reports H2 production rate (micromole per gram of catalyst per hour) as a function of photocatalyst, wastewater source and light intensity. Much of the research being undertaken focuses on the implementation of new catalysts or the optimisation of process parameters and influent properties. The modification of the catalyst plays a vital role in hydrogen production; for example, the use of Au/TiO2 was reported to result in a greater rate of hydrogen production from municipal wastewater in comparison to Cu/TiO2 (22 and 0.1 μmol/g_cat/h, respectively), and achieved 115.2 and 10.27 μmol/g_cat/h, respectively, with a juice production wastewater as the substrate.

Table 4.
Photocatalytic hydrogen production from selected wastewater streams
3.2.2. Important Process Parameters
Experimental parameters which can be optimised include photocatalyst composition and dose, light intensity, reactor configuration, irradiated surface area to volume ratio, and energy input cost (Table 5). TiO2 is the most extensively utilised photocatalyst due to its commercial availability, low cost, stability in different pH conditions, and non-toxic properties. Temperature does not significantly influence photocatalytic kinetics, although pollutant desorption and gas solubility can de decreased at elevated temperatures. One study reports the use of a Pt/TiO2 photocatalyst to enhance H2 production with increasing the temperature from 45 °C to 55 °C Catalyst concentration significantly influences the efficiency of the photocatalytic process; upon reaching the optimal loading, H2 production is maximised; however, photon absorption can be limited due to scattering and blocking at high catalyst concentrations. Baniasadi et al. [48] reported additional hydrogen production (20% increase) with increasing light intensity (from 900 to 1000 W/m2) in the presence of a ZnS photocatalyst. Photocatalytic activity can also be enhanced through the introduction of biological material, which can play a hole-scavenging role, permitting increased change carrier separation efficiency. Photoreactor architecture plays a crucial role in efficient reaction kinetics. Compound parabolic concentrator reactors (CPC) are considered an effective option to capture both direct and diffuse solar radiation, leading to higher yields of hydrogen than flat plate systems [49]. Interestingly, Wei et al. [50] presented promising research investigating antibiotic degradation and simultaneous hydrogen production by photocatalysis. They demonstrated progress in the remediation of micropollutants with a reduction in effluent toxicity, and highlighted the significant role catalysts play in the generation of hydrogen production.

Table 5.
Key parameters influencing photocatalytic H2 production.
3.2.3. Strengths, Weaknesses and H2 Production Enhancement
Photocatalysis offers the following: (1) alternative low energy approach to water treatment compared to conventional treatment methods (activated carbon, ultrafiltration, reverse osmosis, coagulation, ion exchange) with the capacity to use solar energy [55]; (2) enhanced removal of a wide range of toxic recalcitrant compounds prior to conventional treatment, and full mineralisation of pollutants is theoretically possible [56]; (3) the reaction conditions for photocatalysis are moderate, the reaction time is limited, and the chemical by-products produced are of low toxicity [57].
Although widely researched within academia, this promising low-energy process is still in its infancy with respect to commercial-scale operation due to key drawbacks, including inefficient photon-absorption and, as such, energy utilisation [58]. A major challenge is the design of effective photocatalytic reactors [59], with parameters such as simple and low-cost construction and operational costs difficult to balance against photon efficiency and operation at large volume [60]. Photocatalytic hydrogen production could be improved by (1) doping catalysts with, for example, noble metallic nanoparticles, providing enhanced H2 generation via surface plasmon resonance (SPR) impacts [61], (2) designing and developing effective photoreactors [60], (3) greater academic–industry collaboration to drive commercial viability, and (4) the significant potential to use low-energy, high-output light-emitting diodes as photon sources, which can be tuned to catalyst absorption.
3.3. Microbial Photo Electrochemical Cells (MPEC)
3.3.1. Description of the Process
In MPEC systems, microbial activities and photochemical processes take place within the reactor generating hydrogen with or without a small external bias. The bioanode and photocathode are separated by a bipolar membrane (Figure 6). In this system, wastewater microbes, particularly electrochemically active bacteria (EABs), break down the organic substances within wastewater, releasing electrons and protons. Protons exchange through the membrane, with electrons passing through the external circuit to assist proton photoreduction to gaseous H2 [62]. A nanostructured cost-effective black silicon (b-Si) photocathode was reported to be efficient for the absorption of visible light energy and electron transport without the need of an external bias [62,63]. In this case, the H2 production rate was higher than in other classical MPEC systems, and greater than unassisted photo electrochemical cell (PEC) water splitting systems [64]. Table 6 shows the hydrogen production rate via MPEC from wastewater and synthetic wastewater. Unbiased hydrogen production using brewery wastewater, with black silicon (b-Si) catalyst and a light intensity ranging between 6.54 and 9.88 mW/cm2, showed normalised hydrogen production rates in the range of 0.31–0.43 L/L/d [62], which is higher than that observed with synthetic wastewater [64].
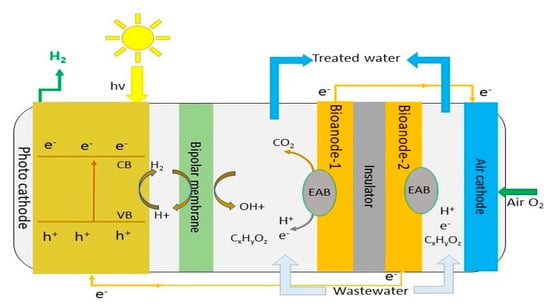
Figure 6.
Schematic diagram of microbial photo electrochemical cell (MPEC).

Table 6.
Hydrogen production via MPEC.
3.3.2. Important Process Parameters
Table 7 summaries key process variables, materials chosen for the photocathode and bioanode construction, coupled with light intensity play vital roles for the performance of MPEC. A sound reactor configuration is vital to ensure high surface area for both biological growth and chemical reactions, and therefore optimal efficiency [67].

Table 7.
Key parameters influencing H2 production via an MPEC.
3.3.3. Strengths, Weakness and H2 Enhancement
MPEC is a promising technology with strengths including the following: (1) a wide range of organic pollutants can be remediated without additional consumable chemicals; (2) the stability and good lifetime of common catalysts, and (3) the combination of microbial and chemical systems reduces energy input. The use of solar energy is a possibility [66]. However, MPEC technology is still at the laboratory stage of development, with several challenges, as follows: (1) low rates of H2 production in comparison to other processes; (2) the overall efficiency, and the quality of the produced effluent is low; (3) reliability and durability requires long-term study; (4) current fabrication and operational cost is high [68]. Additional opportunities for research include co-catalyst development to increase photocatalytic activity [69], and the fabrication of electrically conductive three-dimensional anode electrodes with large surface areas, which could enhance hydrogen productivity [70].
3.4. Dark Fermentation (DF)
3.4.1. Description of the Process
DF is the most widely studied fermentation process, whereby, in the absence of light and oxygen, hydrogen-producing microorganisms and microalgae can produce hydrogen from a wide variety of substrates, including the organic fraction of wastewater (Figure 7). Glucose is considered the ideal substance for fermentation, which, during glycolysis, is converted to pyruvate, and through various pathways, H2 can be produced (Equations (5)–(7)).
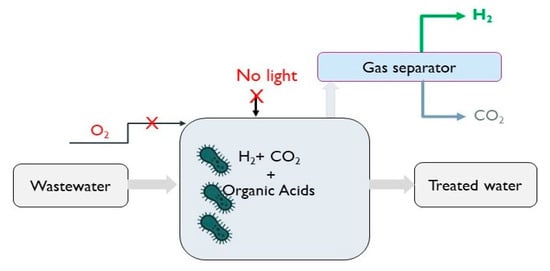
Figure 7.
Schematic diagram of dark fermentative H2 production process.
In the case of wastewater, the major soluble and bio-available organic products include organic acids (acetic, propionic, and butyric) and short-chain alcohols [71]. Theoretically, one mole of glucose can produce 12 mol of hydrogen (Equation (2)), but practically, the production rate is much lower (typically 3.47 mol H2/mol glucose via the acetic acid pathway [72]). A range of operational parameters can influence hydrogen production volume and rate of production in batch production, including the activity and growth rate of the anaerobic microorganisms [73], with continuous operation mode being more complex as the microbial activity is very sensitive to pH and toxic shock [74]. The content and bio-availability of organic and inorganic substrates also significantly influences process performance [75].
C6H12O6 + 2H2O → 2CH3COOH + 2CO2 + 4H2 (acetic acid pathway)
C6H12O6 → CH3CH2CH2COOH + 2CO2 + 2H2 (butyric acid pathway)
C6H12O6→CH3COOH + CH3CH2COOH + CO2 + H2 (propionic acid pathway)
Table 8 compares the dark fermentative hydrogen production rates from a range of industrial effluents by batch and continuous mode processes. DF operates in typically acidic conditions; the pH varies from 4.5 to 7.5, at low operational temperature (mesophilic conditions). The effects of the operational conditions, the COD of the influent wastewater, the HRT and the importance of the optimisation of the inoculum (the source of natural microorganisms) are all evident with respect to beverage wastewater treatment, with studies reporting both high H2 (1.75 L/L/d) and low H2 production rates (0.03 L/L/d). In continuous-mode cheese whey, wastewater was reported to yield higher rates of H2 production than studies using batch mode; however, close process control is required to maintain steady gas production in the continuous mode [76]. Although the substrate COD is a key element in the production of H2, DF is a multi-parametric process.

Table 8.
Hydrogen production by dark fermentation.
3.4.2. Important Process Parameters
The mode of operation, substrate and microorganism culture/inoculum source play a significant role in DF-mediated H2 production (Table 8). The control reactor parameters include pH, temperature and HRT, with organisms particularly sensitive to high-strength effluents. The key parameters investigated in the literature are described in Table 9.

Table 9.
Key parameters influencing dark fermentative H2 production.
3.4.3. Strengths, Weaknesses and H2 Enhancement Strategy
Dark fermentation is a relatively low-tech, low-cost process producing moderate rates of H2 and organic removal [87]. Additional advantages include the following: (1) complex forms of organic substrate can be utilised by anaerobic microorganisms or microalgae; (2) simple reactor construction; (3) the possibility of producing value-added by-products; (4) no need for external energy input (light or electrical bias); (5) continuous, all-day operation is possible. One challenge of anaerobic systems is managing the effluent gases to ensure that methane, hydrogen sulphide and carbon monoxide are separated from H2 [77]. The pre-treatment of feedstock, process optimisation, co-fermentation, the supplementation of additives such as metal ions, and improving inoculum specificity for H2 production are avenues for continued research [88]. The addition of nickel may also accelerate the action of hydrogenase, which ultimately contributes to the increased hydrogen evolution [88]. Recently, Rambabu et al. [89] demonstrated improved H2 yields as well as COD reduction rates with the introduction of nanoparticles (NiO and CoO) to the dark fermentation process using rice mill wastewater, confirming that research in the area is improving the outlook for this technology.
3.5. Microbial Electrolysis Cell (MEC)
3.5.1. Description of the Process
MECs have recently attracted significant attention as a means to produce sustainable hydrogen from wastewater streams [90]. MECs harness anaerobic microorganisms to convert organic waste matter into H2 in single-chamber or within double-chamber reactors; Figure 8 shows a schematic of a double-chamber MEC. The anode and cathode chambers are separated by a membrane, which can comprise anion exchange, cation exchange, bipolar, charge-mosaic and battery separator materials. Organic material entering the anode chamber is degraded by naturally occurring exo-electrogenic strains of anaerobic microorganisms, which form a biofilm on the anode’s electrode surface. Microbial degradation generates electrons, protons, and carbon dioxide (CO2), with the electrons flowing to the cathode through the exterior circuit. Protons migrate through the membrane, where cathodic reduction produces H2 gas. Methane (CH4) can also be produced in the cathode chamber, and careful control is required to optimise H2 production. A small external bias, ranging from 0.2 to 0.8 volt, is necessary to ensure electron migration and efficient proton reduction [20]. Model reactions occurring within the MEC using acetic acid as a model carbon source are shown in Equations (8)–(10).
CH3COOH + 2H2O → 2CO2 +8e− + 8H+ (Anode)
8e− + 8H+ → 4H2 (Cathode)
CH3COOH + 2H2O → 2CO2 +4H2 (Overall)
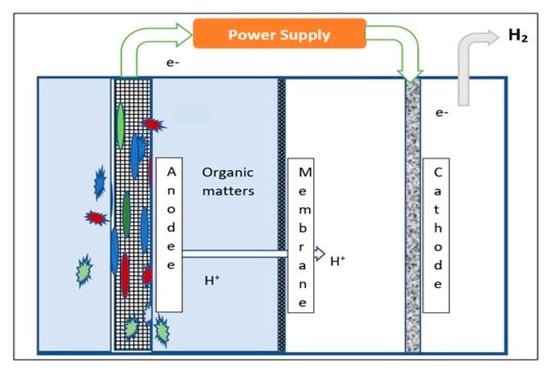
Figure 8.
Schematic representation of H2 production process through MEC.
In laboratory settings, MECs produce 1 to 4 L/L/d of hydrogen [91,92,93,94]; however, these rates of production have not been observed using real wastewater. Using a six-cassette-style double-chamber MEC, Heidrich et al. reported pure hydrogen production (0.015 L/L/d) using urban wastewater in a small-scale trial at a wastewater treatment plant, along with simultaneous COD reduction (44.5%) [95]. Trials by the same group increased H2 production to 0.6 L/L/d, using a cassette-type double-chamber MEC at ambient temperature, demonstrating stability for more than 12 months at the wastewater treatment plant with 33% COD reduction [96]. Researchers at the University of Leon reported around 90% COD reduction from both synthetic and municipal wastewater with a membrane-free MEC, but H2 production rates were relatively low [97,98]. Table 10 shows the application of MEC across a wide range of wastewater sources, highlighting the columbic efficiency, which ultimately drives the hydrogen production rate. COD reduction rates and H2 production rates vary significantly, depending on the effluent source and particular reactor configuration, demonstrating the challenge in developing technology to attain both hydrogen production and good-quality water treatment. However, with systems optimised specifically for H2 production, in which the external bias is carefully controlled, greater H2 production yields are reported.

Table 10.
H2 production from wastewater through MEC.
3.5.2. Important Process Parameters for MEC
A wide range of operational parameters require careful consideration, such as substrate flow rate, HRT, organic matter concentration (e.g., COD), pH, temperature and external bias. A range of reactor designs and material factors have been considered by researchers, including increasing the active area of the anode and cathode and the suitability of membrane materials [108]. Table 11 summarises the key parameters influencing the performance of this complex system.

Table 11.
Key parameters influencing H2 production via MEC.
3.5.3. Strengths, Weaknesses and H2 Performance Strategy
The primary advantage of MECs is the good H2 yield, with 67% to 91% conversion of the substrate reported from a wide range of effluents with significant COD reduction noted [113]. In addition, it has been reported that duel-chamber MECs can recover phosphorus, with accumulation reaching up to 95% and with simultaneous H2 production [114]. Although the maintenance costs for operation have been reported to be comparatively low [115], capex costs are often high. Technical challenges requiring attention include (1) voltage losses (electrode overpotentials and ohmic losses) that reduce efficiency, (2) high construction costs, (3) consistently meeting effluent discharge standards, and (4) the instability of MEC due to fouling and the blockage of membranes [112]. To enhance MEC performance, increasing the anode surface area, improving the anode microbiome, ensuring optimal temperature and adding catalysts to the electrode have all formed the basis of many studies. Bioaugmentation and -enrichment is reported to promote the growth of microbes, and as such leads to improved H2 yields. Wastewater pre-treatments have also been reported to impact MEC efficiency [116]. The inhibition of methanogens is an important strategy to aid in increased H2 yields; the strategies include using pure cultures or adding catalysts (e.g., 2-bromoethanesulfonate, 2-chloroethanesulfonate, etc.) to suppress methanogens [91].
4. Discussion
4.1. General Comparison
Although there is a significant body of research exploring H2 production using wastewater (WW) as a feedstock, no single technology is ready for large-scale application. Both photo- and dark fermentation are mature technologies, but in the case of photo-fermentation, efficiency is still too low and dependent on a small group of specific bacteria. Furthermore, there is a need to design expensive reactors with high-photon flux sources and high irradiation areas. For light-dependent technologies, the possibility of using solar energy is positive, but the most challenging element is the reduced working time and output intermittency. Photon losses, low conversion efficiencies and poor bio-catalyst stability reduce the yield of hydrogen in both photocatalysis and MPEC systems. In a recent study, Lu et al. reported a maximum MPEC stability of only 90 h [62]. Although most photocatalytic studies focus on pollutant degradation in wastewater, there is a growing body of research starting to focus on energy production coupled to wastewater treatment, but this is still at an early stage. Though dark fermentation is a mature technology [117] and the outcomes from several pilot trials show good yields and rates of hydrogen production, the process instability and inconsistency in COD reduction rate remain an issue. Conversely, studies with MECs demonstrate reasonable COD reduction rates from a range of wastewater streams, with inconsistency in hydrogen production rates. In theory, a H2 yield of 12 mol/mol hexose is possible with MEC, whereas only 4 mol/mol hexose can be generated in dark fermentation [90]. Pilot trials using domestic WW show 85% COD reduction within MECs, wherein the energy requirement was typically less than the energy required by aerobic WW treatment [96]. With respect to COD removal, studies comparing systems show MEC performance to be relatively high (80–95%) [98] compared to both fermentation processes (33%) [118] and water photocatalysis (65%) [119]. Although MEC technology has been shown to efficiently convert substrate energy to hydrogen, it is not widely utilised, due primarily to the cost of MEC construction and difficulties in scale-up. Table 12 summarises the main strengths and technical challenges of each H2 production process.

Table 12.
Process comparison, strengths and technical challenges.
Whilst it is difficult to draw direct comparisons, we consider the main parameters determining the application of processes via efficiency (expressed by mechanism-appropriate means), unit production cost of H2 and percentage COD reduction, and technology maturity (via technology readiness level (TRL)) in Table 13. Fermentative process efficiencies are articulated as the ratio of output energy based on the produced H2 to the total input energies, both from the substrate and the external sources (either light or electrical bias). For photocatalysis, the efficiency is expressed as apparent quantum efficiency (AQE), the ratio of two times the number of moles of H2 evolved to the number of incident photons, whereas the MPEC’s efficiency is expressed by the solar to hydrogen (STH) efficiency, which is the ratio of change in Gibbs free energy per mole of produced H2 to the total illuminated light energy on the reactor area. By this analysis, MEC shows the highest efficiency (80%); dark fermentation showed low efficiency (~25%) and photo-fermentation demonstrated a poor conversion efficiency (10%). With respect to COD reduction rate, MPEC is reported to remove the highest percentage (90%), followed by MEC (80%), with dark fermentation the lowest at 45%. Regarding the production cost and maturity of technologies of H2, dark fermentation is the most economical and the highest TRL, but efficiency and COD reduction are still great challenges. Photocatalysis shows an intermediate value of COD reduction with generally low H2 production efficiency. For MEC, Cheng and Logan [120] reported reasonable hydrogen production rates (8.55 mol H2/mol-glucose), whereas only 4 mol H2/mol-glucose can be produced by dark fermentation. Furthermore, if operational conditions are optimised, MECs can produce pure H2 gas without the requirement for clean-up. If the materials cost (ion exchange membranes, anode material in particular) could be reduced, MECs could be a very competitive technology; however, scale-up and pilot trials are needed to evaluate commercial applicability. Work in this area is accelerating, with Logan et al. having recently developed a low-cost MFC cathode using an activated carbon catalyst, reducing the cost to 15 USD/m−2 in comparison to the widely used platinum catalysts’ 1814 USD/m−2 [121].

Table 13.
Comparative assessment of H2-generating processes.
4.2. Strategy to Enhance H2 Production from Wastewater
To develop affordable technologies producing high yields of sustainable hydrogen from wastewater requires the optimisation of the key performance parameters, consideration of innovative ways to integrate and combine energy-producing systems into existing WWTP, and indeed the identification of viable opportunities for the use of produced hydrogen.
4.2.1. Optimising Process Parameters
Process parameters play a vital role for all systems. As shown above, the optimum production of hydrogen depends on several parameters, such as substrate type and concentration, pH, temperature and HRT. Temperature and pH play a key role in the high yield of hydrogen; low pH (below 5) and low temperature can prevent methanogenesis activity and increase pure H2 production. For light-dependent processes, the effective capture and utilisation of visible and UV photons is critical to efficient performance.
4.2.2. The Reactor Design
H2 production for all the processes analysed is significantly affected by the respective reactor architecture, configuration and design. Dark fermentation and MEC reactors are light-independent, but anaerobic conditions must be maintained for hydrogen production. Construction and configuration are generally simple for DF, but complex for MPEC and MEC, as they requires anode and cathode separation and an external power supply system. For photo-fermentation, photocatalysis, and in some instances MPEC, the photo reactor is designed in such a way as to capture maximum light energy from the reactor; however, this often negates efficient mixing. In these cases, the surface area for light collection needs to be increased, with CPC reactors starting to show promise under solar irradiation. MPEC further challenges reactor configuration with the complex interaction of the catalyst cathode with the bio-anode, and again progress is being made, but systems are at low TRL [62]. The design of dark fermentative H2 production systems is advanced, with a range of configurations examined. Continuous stirred tank reactors (CSTR) and anaerobic granular sludge beds (AnGSB) are popular, with the AnGSB producing higher hydrogen yields than traditional CSTR. In the case of photo-fermentation, up-flow anaerobic sludge blanket (UASB) bioreactors have been shown to maximise the hydrogen production [127]. For MEC, double-chamber cassette configuration as well as tubular-type reactors show increased performance, but their complex design results in greater capital costs. From a practical construction point of view, MPEC and MEC reactors are engineering-heavy, requiring careful consideration of the positions of the anode and cathode, the membrane, the influent flow pattern and the effluent exit route. It is also essential to have provision for the collection of debris, and to minimise membrane fouling.
4.2.3. Identification and Enrichment of Effective Microorganisms and Catalysts
Given the diversity of microorganisms, significant attention is being focused on the isolation and modification of efficient and tolerant hydrogen producers. Pure cultures optimised for hydrogen production are favourable when a single or defined substrate is employed for DF, MPEC or MEC, whereas a mixed culture was found to be more suitable for general wastewater, resulting in higher rates of hydrogen production. For photo-fermentation-specific strains of PNS, bacteria are widely used, but enrichment is often conducted for improved output. Bioaugmentation represents an opportunity to improve microbial performance, and is a hot area of research [128]. Combinations of heat-shock and chemical pre-treatment have been effectively employed to optimise cultures in terms of the temperature, energy consumption and elimination of hydrogen consumers [129]. Research into the development of novel catalysts has been ongoing for many years, and whilst reports demonstrate the absorption of wide-band energy via doping and the use of nanoparticles to increase the catalytic performance, stability and conversion efficiencies remain low. MPEC catalysts have predominately focused on modified silicon to enhance microbial interactions (e.g., b-Si), resulting in hydrogen production and organic degradation [62], and whilst there is scope for expansion, research is at the bench level.
4.2.4. Integrated Approaches Using Two or More Processes
The combinations of DF and PF, as well as DF and MEC, have been reported to show improved results in terms of hydrogen yield and substrate conversion [130]; integrating MEC with DF was reported to result in 41% more H2 production compared with fermentation alone [131]. Integrated DF and PF also showed a higher hydrogen yield than the individual processes, with close to the theoretical H2 yield with specific substrates [132]. The combination of MEC with microbial fuel cells (MFC) could potentially avoid the need for external power, leading to more energy efficient systems, but attaining the required voltage can be challenging. Triple combination of MEC–MFC–DF have been reported to produce more hydrogen than single processes, without the need for an external power supply [15]. This combination also permits the recovery of a range of resources in addition to bio-energy production. Moreover, integrating processes can also increase COD reduction rate, but does adds to operational complexity and costs. Recently, MECs have been incorporated with desalination units, defined as microbial electrodialysis cells (MEDCs), which resulted in increased desalination performance and energy recovery [133]. Enhanced bio-electrochemical systems, such as microbial reverse-electrodialysis electrolysis cell (MREC), microbial electrolysis desalination and chemical production (MEDCC) and microbial saline-waste water electrolysis cell (MSC), are in the early stages of development, with predicted performance and resource recovery from wastewater looking promising.
5. Conclusions
The present review demonstrates that wastewater has the potential and promise to be an exciting source of biohydrogen—initially aiding the decarbonisation of the sector and perhaps transforming an energy-consuming sector into an energy generator. Five low-energy intensive processes have been analysed and compared, namely, photo-fermentation, dark fermentation, photocatalysis, microbial photoelectrochemical systems and microbial electrolysis cells. Although none are ready for implementation at a large scale, each system offers positive aspects that could make hydrogen production from wastewater a reality if key technical challenges can be overcome. Whilst perhaps not the primary focus, the removal of COD (between 45% and 90%) is also possible, and therefore the systems could be introduced into selected areas of wastewater treatment plants to produce hydrogen.
The development of commercially available systems will require a multidisciplinary research approach with contributions from engineering, microbiology and chemistry. Optimising the bioreactor design, identifying the optimal working condition and selecting optimal microorganisms/catalysts are key challenges for the sector.
With four of the five technologies reported to be lower than TRL 4, there is a clear need to move from lab to pilot study and to large-scale field trials, for which collaboration with the wastewater sector is critical. The identification of the associated barriers within the wastewater sector requires significant attention, and in addition to technical factors, environmental sustainability, life cycle analysis and acceptance by internal and external stakeholders, policy and regulations become important commercial considerations.
As opposed to a technology push approach, building a case for user-pull in the form of the promotion of the diverse range of applications for H2 produced from wastewater treatment plants is important—if there is market demand, then the costs of technology can be addressed. H2 could be stored for off-site applications or used for on-site power generation, as fuel for vehicles and for heating. In the case of electricity generation, fuel cells could be used to produce electricity; however, fuel cells remain expensive with conversion efficiencies around 60 to 70%. For vehicles, H2 could be used to produce bio-hythane, a H2–CH4 blend, with H2 concentration between 10 and 30%. Another important use of H2 could be injection into natural gas pipelines, known as hydrogen injection. Around 15–20% H2 blend by volume could be added into the gas grid without any danger.
Informed Consent Statement Not applicable.
Author Contributions
Conceptualisation, P.S.M.D., N.J.H. and C.B.; methodology, P.S.M.D., C.B.; software, AKMK.I., validation, P.S.M.D., C.B. and AKMK.I.; formal analysis, C.B., AKMK.I.; investigation, P.S.M.D., R.L.; resources, AKMK.I., C.B., data curation, AKMK.I.; writing—original draft preparation, AKMK.I.; writing—P.S.M.D., C.B., R.L.; visualisation, C.B., N.J.H.; supervision, N.J.H.; project administration, C.B.; funding acquisition, C.B., P.S.M.D. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by EU Marie-Sklodowska-Curie EU H2020 project, ALICE “Accelerate innovation in wastewater management for climate change grant agreement”, No 734560. The authors would like to acknowledge financial support from the NI Department for Economy (DfE) and Ulster University in support of Khabirul Islam.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data is contained within the article.
Acknowledgments
The authors acknowledge the support of staff within CST and the Belfast School of Architecture and Built environment, Ulster University.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Intergovernmental Panel on Climate Change (IPCC) Special Report on Global Warming of 1.5 °C. Available online: https://www.ipcc.ch (accessed on 18 March 2018).
- International Energy Association (IEA) Key Energy World Energy Statistics. Available online: https://webstore.iea.org/key-world-energy-statistics-2018 (accessed on 20 March 2018).
- Environment Agency (EA) Report on Transforming wastewater treatment to reduce carbon emissions. Available online: https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachmentdata/file/291634/scho1209broaee.pdf (accessed on 18 March 2018).
- Dai, Z.; Heidrich, E.S.; Dolfing, J.; Jarvis, A.P. Determination of the Relationship between the Energy Content of Municipal Wastewater and Its Chemical Oxygen Demand. Environ. Sci. Technol. Lett. 2019, 6, 396–400. [Google Scholar] [CrossRef]
- Puyol, D.; Batstone, D.J.; Hülsen, T.; Astals, S.; Peces, M.; Krömer, J.O. Resource recovery from wastewater by biological technologies: Opportunities, challenges, and prospects. Front. Microbiol. 2017, 7, 2106. [Google Scholar] [CrossRef]
- Kalamaras, C.M.; Efstathiou, A.M. Hydrogen Production Technologies: Current State and Future Developments. Conf. Pap. Energy 2013, 2013, 1–9. [Google Scholar] [CrossRef]
- Yasri, N.; Roberts, E.P.L.; Gunasekaran, S. The electrochemical perspective of bioelectrocatalytic activities in microbial electrolysis and microbial fuel cells. Energy Rep. 2019, 5, 1116–1136. [Google Scholar] [CrossRef]
- Preethi; Mohamed Usman, T.M.; Rajesh Banu, J.; Gunasekaran, M.; Kumar, G. Biohydrogen production from industrial wastewater: An overview. Bioresour. Technol. Rep. 2019, 7, 100287. [Google Scholar] [CrossRef]
- Rajesh Banu, J.; Kavitha, S.; Yukesh Kannah, R.; Bhosale, R.R.; Kumar, G. Industrial wastewater to biohydrogen: Possibilities towards successful biorefinery route. Bioresour. Technol. 2020, 298, 122378. [Google Scholar] [CrossRef] [PubMed]
- Hay, J.X.W.; Wu, T.Y.; Juan, J.C.; Md. Jahim, J. Biohydrogen production through photo fermentation or dark fermentation using waste as a substrate: Overview, economics, and future prospects of hydrogen usage. Biofuels Bioprod. Biorefining 2013, 7, 334–352. [Google Scholar] [CrossRef]
- Sharmila, V.G.; Banu, J.R.; Kim, S.H.; Kumar, G. A review on evaluation of applied pretreatment methods of wastewater towards sustainable H2 generation: Energy efficiency analysis. Int. J. Hydrog. Energy 2020, 45, 8329–8345. [Google Scholar] [CrossRef]
- Rioja-Cabanillas, A.; Valdesueiro, D.; Fernández-Ibáñez, P.; Anthony Byrne, J. Hydrogen from wastewater by photocatalytic and photoelectrochemical treatment. J. Phys. Energy 2021, 3, 12006. [Google Scholar] [CrossRef]
- Capson-Tojo, G.; Batstone, D.J.; Grassino, M.; Vlaeminck, S.E.; Puyol, D.; Verstraete, W.; Kleerebezem, R.; Oehmen, A.; Ghimire, A.; Pikaar, I.; et al. Purple phototrophic bacteria for resource recovery: Challenges and opportunities. Biotechnol. Adv. 2020, 43. [Google Scholar] [CrossRef]
- Tian, H.; Li, J.; Yan, M.; Tong, Y.W.; Wang, C.H.; Wang, X. Organic waste to biohydrogen: A critical review from technological development and environmental impact analysis perspective. Appl. Energy 2019, 256, 113961. [Google Scholar] [CrossRef]
- Kadier, A.; Jain, P.; Lai, B.; Kalil, M.S.; Kondaveeti, S.; Alabbosh, K.F.S.; Abu-Reesh, I.M.; Mohanakrishna, G. Biorefinery perspectives of microbial electrolysis cells (MECs) for hydrogen and valuable chemicals production through wastewater treatment. Biofuel Res. J. 2020, 7, 1128–1142. [Google Scholar] [CrossRef]
- Web of Science Home Page. Hydrogen Production from Wastewtater. Available online: https://apps.webofknowledge.com/WOS_GeneralSearch_input.do?product=WOS&search_mode=GeneralSearch&SID=E36uOTQSu9KvjD4k7hg&preferencesSaved (accessed on 20 March 2020).
- Van Eck, N.J.; Waltman, L. Manual for VOSviewer Version 1.6.7. Univeristeit Leiden: Leiden, The Netherlands, 2018; pp. 1–50. [Google Scholar]
- Aydin, M.I.; Karaca, A.E.; Qureshy, A.M.M.I.; Dincer, I. A comparative review on clean hydrogen production from wastewaters. J. Environ. Manage. 2021, 279, 111793. [Google Scholar] [CrossRef]
- Fajrina, N.; Tahir, M. A critical review in strategies to improve photocatalytic water splitting towards hydrogen production. Int. J. Hydrog. Energy 2019, 44, 540–577. [Google Scholar] [CrossRef]
- Ditzig, J.; Liu, H.; Logan, B.E. Production of hydrogen from domestic wastewater using a bioelectrochemically assisted microbial reactor (BEAMR). Int. J. Hydrog. Energy 2007, 32, 2296–2304. [Google Scholar] [CrossRef]
- Abo-Hashesh, M.; Ghosh, D.; Tourigny, A.; Taous, A.; Hallenbeck, P.C. Single stage photofermentative hydrogen production from glucose: An attractive alternative to two stage photofermentation or co-culture approaches. Int. J. Hydrog. Energy 2011, 36, 13889–13895. [Google Scholar] [CrossRef]
- Li, R.Y.; Fang, H.H.P. Heterotrophic photo fermentative hydrogen production. Crit. Rev. Environ. Sci. Technol. 2009, 39, 1081–1108. [Google Scholar] [CrossRef]
- Seifert, K.; Waligorska, M.; Laniecki, M. Brewery wastewaters in photobiological hydrogen generation in presence of Rhodobacter sphaeroides O.U. 001. Int. J. Hydrog. Energy 2010, 35, 4085–4091. [Google Scholar] [CrossRef]
- Eroǧlu, E.; Eroǧlu, I.; Gündüz, U.; Yücel, M. Treatment of olive mill wastewater by different physicochemical methods and utilization of their liquid effluents for biological hydrogen production. Biomass Bioenergy 2009, 33, 701–705. [Google Scholar] [CrossRef]
- Eroǧlu, E.; Gündüz, U.; Yücel, M.; Türker, L.; Eroǧlu, I. Photobiological hydrogen production by using olive mill wastewater as a sole substrate source. Int. J. Hydrog. Energy 2004, 29, 163–171. [Google Scholar] [CrossRef]
- Seifert, K.; Waligorska, M.; Laniecki, M. Hydrogen generation in photobiological process from dairy wastewater. Int. J. Hydrogen Energy 2010, 35, 9624–9629. [Google Scholar] [CrossRef]
- Anam, K.; Habibi, M.S.; Harwati, T.U.; Susilaningsih, D. Photofermentative hydrogen production using Rhodobium marinum from bagasse and soy sauce wastewater. Int. J. Hydrog. Energy 2012, 37, 15436–15442. [Google Scholar] [CrossRef]
- Zhang, Z.; Wang, Y.; Hu, J.; Wu, Q.; Zhang, Q. Influence of mixing method and hydraulic retention time on hydrogen production through photo-fermentation with mixed strains. Int. J. Hydrog. Energy 2015, 40, 6521–6529. [Google Scholar] [CrossRef]
- Androga, D.D.; Özgür, E.; Eroglu, I.; Gündüz, U.; Yücel, M. Significance of carbon to nitrogen ratio on the long-term stability of continuous photofermentative hydrogen production. Int. J. Hydrog. Energy 2011, 36, 15583–15594. [Google Scholar] [CrossRef]
- Akkerman, I.; Janssen, M.; Rocha, J.; Wijffels, R.H. Photobiological hydrogen production: Photochemical efficiency and bioreactor design. Int. J. Hydrog. Energy 2002, 27, 1195–1208. [Google Scholar] [CrossRef]
- Fang, H.H.P.; Liu, H.; Zhang, T. Phototrophic hydrogen production from acetate and butyrate in wastewater. Int. J. Hydrog. Energy 2005, 30, 785–793. [Google Scholar] [CrossRef]
- Kumar Gupta, S.; Kumari, S.; Reddy, K.; Bux, F. Trends in biohydrogen production: Major challenges and state-of-the-art developments. Environ. Technol. 2013, 34, 1653–1670. [Google Scholar] [CrossRef] [PubMed]
- Oey, M.; Sawyer, A.L.; Ross, I.L.; Hankamer, B. Challenges and opportunities for hydrogen production from microalgae. Plant Biotechnol. J. 2016, 14, 1487–1499. [Google Scholar] [CrossRef]
- Nath, K.; Das, D. Biohydrogen production as a potential energy resource-Present state-of-art. J. Sci. Ind. Res. 2004, 63, 729–738. [Google Scholar]
- Assawamongkholsiri, T.; Reungsang, A. Photo-fermentational hydrogen production of Rhodobacter sp. KKU-PS1 isolated from an UASB reactor. Electron. J. Biotechnol. 2015, 18, 221–230. [Google Scholar] [CrossRef]
- Mishra, P.; Krishnan, S.; Rana, S.; Singh, L.; Sakinah, M.; Ab Wahid, Z. Outlook of fermentative hydrogen production techniques: An overview of dark, photo and integrated dark-photo fermentative approach to biomass. Energy Strateg. Rev. 2019, 24, 27–37. [Google Scholar] [CrossRef]
- Tiang, M.F.; Fitri Hanipa, M.A.; Abdul, P.M.; Jahim, J.M.; Mahmod, S.S.; Takriff, M.S.; Lay, C.H.; Reungsang, A.; Wu, S.Y. Recent advanced biotechnological strategies to enhance photo-fermentative biohydrogen production by purple non-sulphur bacteria: An overview. Int. J. Hydrog. Energy 2020, 45, 13211–13230. [Google Scholar] [CrossRef]
- Li, X.; Liu, T.; Wu, Y.; Zhao, G.; Zhou, Z. Derepressive effect of NH 4+ on hydrogen production by deleting the glnA1 gene in Rhodobacter sphaeroides. Biotechnol. Bioeng. 2010, 106, 564–572. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, H.; Kamarudin, S.K.; Minggu, L.J.; Kassim, M. Hydrogen from photo-catalytic water splitting process: A review. Renew. Sustain. Energy Rev. 2015, 43, 599–610. [Google Scholar] [CrossRef]
- Puga, A.V. Photocatalytic production of hydrogen from biomass-derived feedstocks. Coord. Chem. Rev. 2016, 315, 1–66. [Google Scholar] [CrossRef]
- Hisatomi, T.; Domen, K. Introductory lecture: Sunlight-driven water splitting and carbon dioxide reduction by heterogeneous semiconductor systems as key processes in artificial photosynthesis. Faraday Discuss. 2017, 198, 11–35. [Google Scholar] [CrossRef]
- Clarizia, L.; Russo, D.; Di Somma, I.; Andreozzi, R.; Marotta, R. Hydrogen Generation through Solar Photocatalytic Processes: A Review of the Configuration and the Properties of Effective Metal-Based Semiconductor Nanomaterials. Energies 2017, 10, 1624. [Google Scholar] [CrossRef]
- Clarizia, L. Hydrogen Production through Photoreforming of Oxygenated Organic Substrates over Cu/TiO 2 catalysts. Ph.D. Thesis, Industrial and Process Engineering, University of Naples “Federico II”, Naples, Italy, 2017. [Google Scholar]
- Chouhan, N.; Ameta, R.; Meena, R.K.; Mandawat, N.; Ghildiyal, R. Visible light harvesting Pt/CdS/Co-doped ZnO nanorods molecular device for hydrogen generation. Int. J. Hydrog. Energy 2016, 41, 2298–2306. [Google Scholar] [CrossRef]
- Imizcoz, M.; Puga, A.V. Assessment of Photocatalytic Hydrogen Production from Biomass or Wastewaters Depending on the Metal Co-Catalyst and Its Deposition Method on TiO2. Catalysts 2019, 9, 584. [Google Scholar] [CrossRef]
- Arzate Salgado, S.Y.; Ramírez Zamora, R.M.; Zanella, R.; Peral, J.; Malato, S.; Maldonado, M.I. Photocatalytic hydrogen production in a solar pilot plant using a Au/TiO2 photo catalyst. Int. J. Hydrog. Energy 2016, 41, 11933–11940. [Google Scholar] [CrossRef]
- Zhang, W.; Li, Y.; Wang, C.; Wang, P.; Wang, Q.; Wang, D. Mechanisms of simultaneous hydrogen production and estrogenic activity removal from secondary effluent though solar photocatalysis. Water Res. 2013, 47, 3173–3182. [Google Scholar] [CrossRef] [PubMed]
- Baniasadi, E.; Dincer, I.; Naterer, G.F. Measured effects of light intensity and catalyst concentration on photocatalytic hydrogen and oxygen production with zinc sulfide suspensions. Int. J. Hydrog. Energy 2013, 38, 9158–9168. [Google Scholar] [CrossRef]
- Corredor, J.; Rivero, M.J.; Rangel, C.M.; Gloaguen, F.; Ortiz, I. Comprehensive review and future perspectives on the photocatalytic hydrogen production. J. Chem. Technol. Biotechnol. 2019, 94, 3049–3063. [Google Scholar] [CrossRef]
- Wei, Z.; Liu, J.; Shangguan, W. A review on photocatalysis in antibiotic wastewater: Pollutant degradation and hydrogen production. Chinese J. Catal. 2020, 41, 1440–1450. [Google Scholar] [CrossRef]
- Badawy, M.I.; Ghaly, M.Y.; Ali, M.E.M. Photocatalytic hydrogen production over nanostructured mesoporous titania from olive mill wastewater. Desalination 2011, 267, 250–255. [Google Scholar] [CrossRef]
- Huaxu, L.; Fuqiang, W.; Ziming, C.; Shengpeng, H.; Bing, X.; Xiangtao, G.; Bo, L.; Jianyu, T.; Xiangzheng, L.; Ruiyang, C.; et al. Analyzing the effects of reaction temperature on photo-thermo chemical synergetic catalytic water splitting under full-spectrum solar irradiation: An experimental and thermodynamic investigation. Int. J. Hydrog. Energy 2017, 42, 12133–12142. [Google Scholar] [CrossRef]
- Konstantinou, I.K.; Albanis, T.A. TiO2-assisted photocatalytic degradation of azo dyes in aqueous solution: Kinetic and mechanistic investigations: A review. Appl. Catal. B Environ. 2004, 49, 1–14. [Google Scholar] [CrossRef]
- Police, A.K.R.; Basavaraju, S.; Valluri, D.K.; Muthukonda, V.S.; Machiraju, S.; Lee, J.S. CaFe2O4 sensitized hierarchical TiO2 photo composite for hydrogen production under solar light irradiation. Chem. Eng. J. 2014, 247, 152–160. [Google Scholar] [CrossRef]
- Nakata, K.; Fujishima, A. TiO 2 photocatalysis: Design and applications. J. Photochem. Photobiol. C Photochem. Rev. 2012, 13, 169–189. [Google Scholar] [CrossRef]
- Fujishima, A.; Rao, T.N.; Tryk, D.A. Titanium dioxide photocatalysis. J. Photochem. Photobiol. C Photochem. Rev. 2000, 1, 1–21. [Google Scholar] [CrossRef]
- Rajeshwar, K.; Osugi, M.E.; Chanmanee, W.; Chenthamarakshan, C.R.; Zanoni, M.V.B.; Kajitvichyanukul, P.; Krishnan-Ayer, R. Heterogeneous photocatalytic treatment of organic dyes in air and aqueous media. J. Photochem. Photobiol. C Photochem. Rev. 2008, 9, 171–192. [Google Scholar] [CrossRef]
- Daskalaki, V.M.; Antoniadou, M.; Li Puma, G.; Kondarides, D.I.; Lianos, P. Solar light-responsive Pt/CdS/TiO2 photocatalysts for hydrogen production and simultaneous degradation of inorganic or organic sacrificial agents in wastewater. Environ. Sci. Technol. 2010, 44, 7200–7205. [Google Scholar] [CrossRef]
- Mukherjee, P.S.; Ray, A.K. Major challenges in the design of a large-scale photocatalytic reactor for water treatment. Chem. Eng. Technol. 1999, 22, 253–260. [Google Scholar] [CrossRef]
- Lazar, M.; Varghese, S.; Nair, S. Photocatalytic Water Treatment by Titanium Dioxide: Recent Updates. Catalysts 2012, 2, 572–601. [Google Scholar] [CrossRef]
- Tahir, M.; Amin, N.S. Advances in visible light responsive titanium oxide-based photocatalysts for CO2 conversion to hydrocarbon fuels. Energy Convers. Manag. 2013, 76, 194–214. [Google Scholar] [CrossRef]
- Lu, L.; Vakki, W.; Aguiar, J.A.; Xiao, C.; Hurst, K.; Fairchild, M.; Chen, X.; Yang, F.; Gu, J.; Ren, Z.J. Unbiased solar H2 production with current density up to 23 mA cm-2 by Swiss-cheese black Si coupled with wastewater bioanode. Energy Environ. Sci. 2019, 12, 1088–1099. [Google Scholar] [CrossRef]
- Aguiar, J.A.; Anderson, N.C.; Neale, N.R. Revealing the semiconductor-catalyst interface in buried platinum black silicon photocathodes. J. Mater. Chem. A 2016, 4, 8123–8129. [Google Scholar] [CrossRef]
- Peerakiatkhajohn, P.; Yun, J.-H.; Wang, S.; Wang, L. Review of recent progress in unassisted photoelectrochemical water splitting: From material modification to configuration design. J. Photonics Energy 2016, 7, 012006. [Google Scholar] [CrossRef]
- Singh, R.; Dutta, S. A review on H2 production through photocatalytic reactions using TiO2/TiO2-assisted catalysts. Fuel 2018, 220, 607–620. [Google Scholar] [CrossRef]
- Liang, D.; Han, G.; Zhang, Y.; Rao, S.; Lu, S.; Wang, H.; Xiang, Y. Efficient H2 production in a microbial photoelectrochemical cell with a composite Cu2O/NiOx photocathode under visible light. Appl. Energy 2016, 168, 544–549. [Google Scholar] [CrossRef]
- Lianos, P. Production of electricity and hydrogen by photocatalytic degradation of organic wastes in a photoelectrochemical cell. The concept of the Photofuelcell: A review of a re-emerging research field. J. Hazard. Mater. 2011, 185, 575–590. [Google Scholar] [CrossRef]
- Chae, K.J.; Choi, M.J.; Kim, K.Y.; Ajayi, F.F.; Chang, I.S.; Kim, I.S. A solar-powered microbial electrolysis cell with a platinum catalyst-free cathode to produce hydrogen. Environ. Sci. Technol. 2009, 43, 9525–9530. [Google Scholar] [CrossRef] [PubMed]
- Han, H.; Huang, Z.; Lee, W. Metal-assisted chemical etching of silicon and nanotechnology applications. Nano Today 2014, 9, 271–304. [Google Scholar] [CrossRef]
- Lee, K.M.; Chen, P.Y.; Hsu, C.Y.; Huang, J.H.; Ho, W.H.; Chen, H.C.; Ho, K.C. A high-performance counter electrode based on poly(3,4-alkylenedioxythiophene) for dye-sensitized solar cells. J. Power Sources 2009, 188, 313–318. [Google Scholar] [CrossRef]
- Van Ginkel, S.; Logan, B.E. Inhibition of Biohydrogen Production by Undissociated Acetic and Butyric Acids. Environ. Sci. Technol. 2005, 39, 9351–9356. [Google Scholar] [CrossRef]
- Ortigueira, J.; Alves, L.; Gouveia, L.; Moura, P. Third generation biohydrogen production by Clostridium butyricum and adapted mixed cultures from Scenedesmus obliquus microalga biomass. Fuel 2015, 153, 128–134. [Google Scholar] [CrossRef]
- Won, S.G.; Lau, A.K. Effects of key operational parameters on biohydrogen production via anaerobic fermentation in a sequencing batch reactor. Bioresour. Technol. 2011, 102, 6876–6883. [Google Scholar] [CrossRef] [PubMed]
- Kumar, G.; Sivagurunathan, P.; Park, J.H.; Park, J.H.; Park, H.D.; Yoon, J.J.; Kim, S.H. HRT dependent performance and bacterial community population of granular hydrogen-producing mixed cultures fed with galactose. Bioresour. Technol. 2016, 206, 188–194. [Google Scholar] [CrossRef]
- Venkata Mohan, S.; Lalit Babu, V.; Sarma, P.N. Anaerobic biohydrogen production from dairy wastewater treatment in sequencing batch reactor (AnSBR): Effect of organic loading rate. Enzym. Microb. Technol. 2007, 41, 506–515. [Google Scholar] [CrossRef]
- Azbar, N.; Çetinkaya Dokgöz, F.T.; Keskin, T.; Korkmaz, K.S.; Syed, H.M. Continuous fermentative hydrogen production from cheese whey wastewater under thermophilic anaerobic conditions. Int. J. Hydrog. Energy 2009, 34, 7441–7447. [Google Scholar] [CrossRef]
- Kumar, G.; Sivagurunathan, P.; Pugazhendhi, A.; Thi, N.B.D.; Zhen, G.; Chandrasekhar, K.; Kadier, A. A comprehensive overview on light independent fermentative hydrogen production from wastewater feedstock and possible integrative options. Energy Convers. Manag. 2017, 141, 390–402. [Google Scholar] [CrossRef]
- Kumar, G.; Bakonyi, P.; Sivagurunathan, P.; Kim, S.H.; Nemestóthy, N.; Bélafi-Bakó, K.; Lin, C.Y. Enhanced biohydrogen production from beverage industrial wastewater using external nitrogen sources and bioaugmentation with facultative anaerobic strains. J. Biosci. Bioeng. 2015, 120, 155–160. [Google Scholar] [CrossRef]
- Yang, P.; Zhang, R.; McGarvey, J.A.; Benemann, J.R. Biohydrogen production from cheese processing wastewater by anaerobic fermentation using mixed microbial communities. Int. J. Hydrog. Energy 2007, 32, 4761–4771. [Google Scholar] [CrossRef]
- Wicher, E.; Seifert, K.; Zagrodnik, R.; Pietrzyk, B.; Laniecki, M. Hydrogen gas production from distillery wastewater by dark fermentation. Int. J. Hydrog. Energy 2013, 38, 7767–7773. [Google Scholar] [CrossRef]
- Moreno-Andrade, I.; Moreno, G.; Kumar, G.; Buitrón, G. Biohydrogen production from industrial wastewaters. Water Sci. Technol. 2015, 71, 105–110. [Google Scholar] [CrossRef] [PubMed]
- Gonçalves, M.R.; Costa, J.C.; Pereira, M.A.; Abreu, A.A.; Alves, M.M. On the independence of hydrogen production from methanogenic suppressor in olive mill wastewater. Int. J. Hydrog. Energy 2014, 39, 6402–6406. [Google Scholar] [CrossRef]
- Li, Y.C.; Chu, C.Y.; Wu, S.Y.; Tsai, C.Y.; Wang, C.C.; Hung, C.H.; Lin, C.Y. Feasible pretreatment of textile wastewater for dark fermentative hydrogen production. Int. J. Hydrog. Energy 2012, 37, 15511–15517. [Google Scholar] [CrossRef]
- Wang, B.; Li, Y.; Ren, N. Biohydrogen from molasses with ethanol-type fermentation: Effect of hydraulic retention time. Int. J. Hydrog. Energy 2013, 38, 4361–4367. [Google Scholar] [CrossRef]
- Wang, J.; Wan, W. Factors influencing fermentative hydrogen production: A review. Int. J. Hydrog. Energy 2009, 34, 799–811. [Google Scholar] [CrossRef]
- Leaño, E.P.; Babel, S. Effects of pretreatment methods on cassava wastewater for biohydrogen production optimization. Renew. Energy 2012, 39, 339–346. [Google Scholar] [CrossRef]
- Nikolaidis, P.; Poullikkas, A. A comparative overview of hydrogen production processes. Renew. Sustain. Energy Rev. 2017, 67, 597–611. [Google Scholar] [CrossRef]
- Sun, Y.; He, J.; Yang, G.; Sun, G.; Sage, V. A review of the enhancement of bio-hydrogen generation by chemicals addition. Catalysts 2019, 9, 353. [Google Scholar] [CrossRef]
- Rambabu, K.; Bharath, G.; Thanigaivelan, A.; Das, D.B.; Show, P.L.; Banat, F. Augmented biohydrogen production from rice mill wastewater through nano-metal oxides assisted dark fermentation. Bioresour. Technol. 2021, 319, 124243. [Google Scholar] [CrossRef]
- Logan, B.E.; Call, D.; Cheng, S.; Hamelers, H.V.M.; Sleutels, T.H.J.A.; Jeremiasse, A.W.; Rozendal, R.A. Microbial electrolysis cells for high yield hydrogen gas production from organic matter. Environ. Sci. Technol. 2008, 42, 8630–8640. [Google Scholar] [CrossRef] [PubMed]
- Rago, L.; Baeza, J.A.; Guisasola, A. Increased performance of hydrogen production in microbial electrolysis cells under alkaline conditions. Bioelectrochemistry 2016, 109, 57–62. [Google Scholar] [CrossRef]
- Montpart, N.; Rago, L.; Baeza, J.A.; Guisasola, A. Hydrogen production in single chamber microbial electrolysis cells with different complex substrates. Water Res. 2015, 68, 601–615. [Google Scholar] [CrossRef]
- Lu, L.; Ren, Z.J. Microbial electrolysis cells for waste biorefinery: A state of the art review. Bioresour. Technol. 2016, 215. [Google Scholar] [CrossRef]
- Kadier, A.; Kalil, M.S.; Abdeshahian, P.; Chandrasekhar, K.; Mohamed, A.; Azman, N.F.; Logroño, W.; Simayi, Y.; Hamid, A.A. Recent advances and emerging challenges in microbial electrolysis cells (MECs) for microbial production of hydrogen and value-added chemicals. Renew. Sustain. Energy Rev. 2016, 61, 501–525. [Google Scholar] [CrossRef]
- Heidrich, E.S.; Dolfing, J.; Scott, K.; Edwards, S.R.; Jones, C.; Curtis, T.P. Production of hydrogen from domestic wastewater in a pilot-scale microbial electrolysis cell. Appl. Microbiol. Biotechnol. 2013, 97, 6979–6989. [Google Scholar] [CrossRef]
- Heidrich, E.S.; Edwards, S.R.; Dolfing, J.; Cotterill, S.E.; Curtis, T.P. Performance of a pilot scale microbial electrolysis cell fed on domestic wastewater at ambient temperatures for a 12 month period. Bioresour. Technol. 2014, 173, 87–95. [Google Scholar] [CrossRef] [PubMed]
- Escapa, A.; San Martin, M.I.; Moran, A. Potential Use of Microbial Electrolysis Cells in Domestic Wastewater Treatment Plants for Energy Recovery. Front. Energy Res. 2014, 2, 19. [Google Scholar] [CrossRef]
- Escapa, A.; San-Martín, M.I.; Mateos, R.; Morán, A. Scaling-up of membraneless microbial electrolysis cells (MECs) for domestic wastewater treatment: Bottlenecks and limitations. Bioresour. Technol. 2015, 180, 72–78. [Google Scholar] [CrossRef]
- Gil-Carrera, L.; Escapa, A.; Moreno, R.; Morán, A. Reduced energy consumption during low strength domestic wastewater treatment in a semi-pilot tubular microbial electrolysis cell. J. Environ. Manage. 2013, 122, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Escapa, A.; Gil-Carrera, L.; García, V.; Morán, A. Performance of a continuous flow microbial electrolysis cell (MEC) fed with domestic wastewater. Bioresour. Technol. 2012, 117, 55–62. [Google Scholar] [CrossRef]
- Cotterill, S.E.; Dolfing, J.; Jones, C.; Curtis, T.P.; Heidrich, E.S. Low Temperature Domestic Wastewater Treatment in a Microbial Electrolysis Cell with 1 m 2 Anodes: Towards System Scale-Up. Fuel Cells 2017, 17, 584–592. [Google Scholar] [CrossRef]
- Baeza, J.A.; Martínez-Miró, À.; Guerrero, J.; Ruiz, Y.; Guisasola, A. Bioelectrochemical hydrogen production from urban wastewater on a pilot scale. J. Power Sources 2017, 356, 500–509. [Google Scholar] [CrossRef]
- Ullery, M.L.; Logan, B.E. Comparison of complex effluent treatability in different bench scale microbial electrolysis cells. Bioresour. Technol. 2014, 170, 530–537. [Google Scholar] [CrossRef]
- Tenca, A.; Cusick, R.D.; Schievano, A.; Oberti, R.; Logan, B.E. Evaluation of low cost cathode materials for treatment of industrial and food processing wastewater using microbial electrolysis cells. Int. J. Hydrog. Energy 2013, 38, 1859–1865. [Google Scholar] [CrossRef]
- Wang, Y.; Guo, W.Q.; Xing, D.F.; Chang, J.S.; Ren, N.Q. Hydrogen production using biocathode single-chamber microbial electrolysis cells fed by molasses wastewater at low temperature. Int. J. Hydrogen Energy 2014, 39, 19369–19375. [Google Scholar] [CrossRef]
- Jia, Y.H.; Choi, J.Y.; Ryu, J.H.; Kim, C.H.; Lee, W.K.; Tran, H.T.; Zhang, R.H.; Ahn, D.H. Hydrogen production from wastewater using a microbial electrolysis cell. Korean J. Chem. Eng. 2010, 27, 1854–1859. [Google Scholar] [CrossRef]
- Wagner, R.C.; Regan, J.M.; Oh, S.E.; Zuo, Y.; Logan, B.E. Hydrogen and methane production from swine wastewater using microbial electrolysis cells. Water Res. 2009, 43, 1480–1488. [Google Scholar] [CrossRef]
- Kadier, A.; Simayi, Y.; Abdeshahian, P.; Azman, N.F.; Chandrasekhar, K.; Kalil, M.S. A comprehensive review of microbial electrolysis cells (MEC) reactor designs and configurations for sustainable hydrogen gas production. Alex. Eng. J. 2016, 55, 427–443. [Google Scholar] [CrossRef]
- Kokko, M.; Epple, S.; Gescher, J.; Kerzenmacher, S. Effects of wastewater constituents and operational conditions on the composition and dynamics of anodic microbial communities in bioelectrochemical systems. Bioresour. Technol. 2018, 258, 376–389. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Chen, G. Factors affecting the effectiveness of bioelectrochemical system applications: Data synthesis and meta-analysis. Batteries 2018, 4, 34. [Google Scholar] [CrossRef]
- Gil-Carrera, L.; Escapa, A.; Carracedo, B.; Morán, A.; Gómez, X. Performance of a semi-pilot tubular microbial electrolysis cell (MEC) under several hydraulic retention times and applied voltages. Bioresour. Technol. 2013, 146, 63–69. [Google Scholar] [CrossRef]
- Saravanan, A.; Karishma, S.; Kumar, P.S.; Yaashikaa, P.R.; Jeevanantham, S.; Gayathri, B. Microbial electrolysis cells and microbial fuel cells for biohydrogen production: Current advances and emerging challenges. Biomass Convers. Biorefinery 2020. [Google Scholar] [CrossRef]
- Escapa, A.; Lobato, A.; García, D.M.; Morán, A. Hydrogen production and COD elimination rate in a continuous microbial electrolysis cell: The influence of hydraulic retention time and applied voltage. Environ. Prog. Sustain. Energy 2013. [Google Scholar] [CrossRef]
- Pant, D.; Singh, A.; Van Bogaert, G.; Gallego, Y.A.; Diels, L.; Vanbroekhoven, K. An introduction to the life cycle assessment (LCA) of bioelectrochemical systems (BES) for sustainable energy and product generation: Relevance and key aspects. Renew. Sustain. Energy Rev. 2011, 15, 1305–1313. [Google Scholar] [CrossRef]
- Aiken, D.C.; Curtis, T.P.; Heidrich, E.S. Avenues to the financial viability of microbial electrolysis cells [MEC] for domestic wastewater treatment and hydrogen production. Int. J. Hydrog. Energy 2019, 44, 2426–2434. [Google Scholar] [CrossRef]
- Zhang, X.; Li, R. Electrodes bioaugmentation promotes the removal of antibiotics from concentrated sludge in microbial electrolysis cells. Sci. Total Environ. 2020, 715, 136997. [Google Scholar] [CrossRef]
- Rozendal, R.A.; Hamelers, H.V.M.; Rabaey, K.; Keller, J.; Buisman, C.J.N. Towards practical implementation of bioelectrochemical wastewater treatment. Trends Biotechnol. 2008, 26, 450–459. [Google Scholar] [CrossRef]
- Mohan, S.V.; Chandrasekhar, K.; Chiranjeevi, P.; Babu, P.S. Biohydrogen Production from Wastewater. Biohydrogen 2013, 223–257. [Google Scholar] [CrossRef]
- Sujatha, G.; Shanthakumar, S.; Chiampo, F. UV Light-Irradiated Photocatalytic Degradation of Coffee Processing Wastewater Using TiO2 as a Catalyst. Environments 2020, 7, 47. [Google Scholar] [CrossRef]
- Cheng, S.; Logan, B.E. Sustainable and efficient biohydrogen production via electrohydrogenesis. PNAS 2007, 104, 18871–18873. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.; He, W.; Zhang, F.; Hickner, M.A.; Logan, B.E. Single-Step Fabrication Using a Phase Inversion Method of Poly(vinylidene fluoride) (PVDF) Activated Carbon Air Cathodes for Microbial Fuel Cells. Environ. Sci. Technol. Lett. 2014, 1, 416–420. [Google Scholar] [CrossRef]
- Miller, E.; Studer, S. H2 Production Status & Threshold Costs Plot. Available online: https://www.hydrogen.energy.gov/pdfs/12002_h2_prod_status_cost_plots.pdf (accessed on 10 January 2019).
- James, B.D.; DeSantis, D.A.; Saur, G. Final Report: Hydrogen Production Pathways Cost Analysis (2013–2016). 2016; 1–55. [Google Scholar]
- IEA (International Energy Agency) H2 Implementing Agreement (HIA) 2013 Anual Report Background TASK 21. 2013; 1–13.
- Pinaud, B.A.; Benck, J.D.; Seitz, L.C.; Forman, A.J.; Chen, Z.; Deutsch, T.G.; James, B.D.; Baum, K.N.; Baum, G.N.; Ardo, S.; et al. Technical and economic feasibility of centralized facilities for solar hydrogen production via photocatalysis and photoelectrochemistry. Energy Environ. Sci. 2013, 6, 1983–2002. [Google Scholar] [CrossRef]
- Cusick, R.D.; Kiely, P.D.; Logan, B.E. A monetary comparison of energy recovered from microbial fuel cells and microbial electrolysis cells fed winery or domestic wastewaters. Int. J. Hydrog. Energy 2010, 35, 8855–8861. [Google Scholar] [CrossRef]
- Jung, K.W.; Kim, D.H.; Kim, S.H.; Shin, H.S. Bioreactor design for continuous dark fermentative hydrogen production. Bioresour. Technol. 2011, 102, 8612–8620. [Google Scholar] [CrossRef] [PubMed]
- Ma, F.; Guo, J.-B.; Zhao, L.-J.; Chang, C.-C.; Cui, D. Application of bioaugmentation to improve the activated sludge system into the contact oxidation system treating petrochemical wastewater. Bioresour. Technol. 2009, 100, 597–602. [Google Scholar] [CrossRef]
- Wang, J.; Yin, Y. Principle and application of different pretreatment methods for enriching hydrogen-producing bacteria from mixed cultures. Int. J. Hydrog. Energy 2017, 42, 4804–4823. [Google Scholar] [CrossRef]
- Kuppam, C.; Pandit, S.; Kadier, A.; Dasagrandhi, C.; Velpuri, J. Biohydrogen production: Integrated approaches to improve the process efficiency. In Microbial Applications; Springer: Berlin, Germany, 2017; Volume 1, pp. 189–210. [Google Scholar]
- Wang, A.; Sun, D.; Cao, G.; Wang, H.; Ren, N.; Wu, W.M.; Logan, B.E. Integrated hydrogen production process from cellulose by combining dark fermentation, microbial fuel cells, and a microbial electrolysis cell. Bioresour. Technol. 2011, 102, 4137–4143. [Google Scholar] [CrossRef]
- Gude, V.G. Integrating bioelectrochemical systems for sustainable wastewater treatment. Clean Technol. Env. Policy 2018, 20, 911–924. [Google Scholar] [CrossRef]
- Chen, S.; Liu, G.; Zhang, R.; Qin, B.; Luo, Y. Development of the microbial electrolysis desalination and chemical-production cell for desalination as well as acid and alkali productions. Environ. Sci. Technol. 2012, 46, 2467–2472. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).