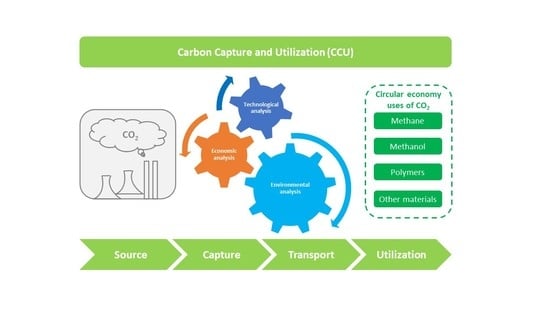
Critical Analysis and Evaluation of the Technology Pathways for Carbon Capture and Utilization
Abstract
1. Introduction
2. Literature Review
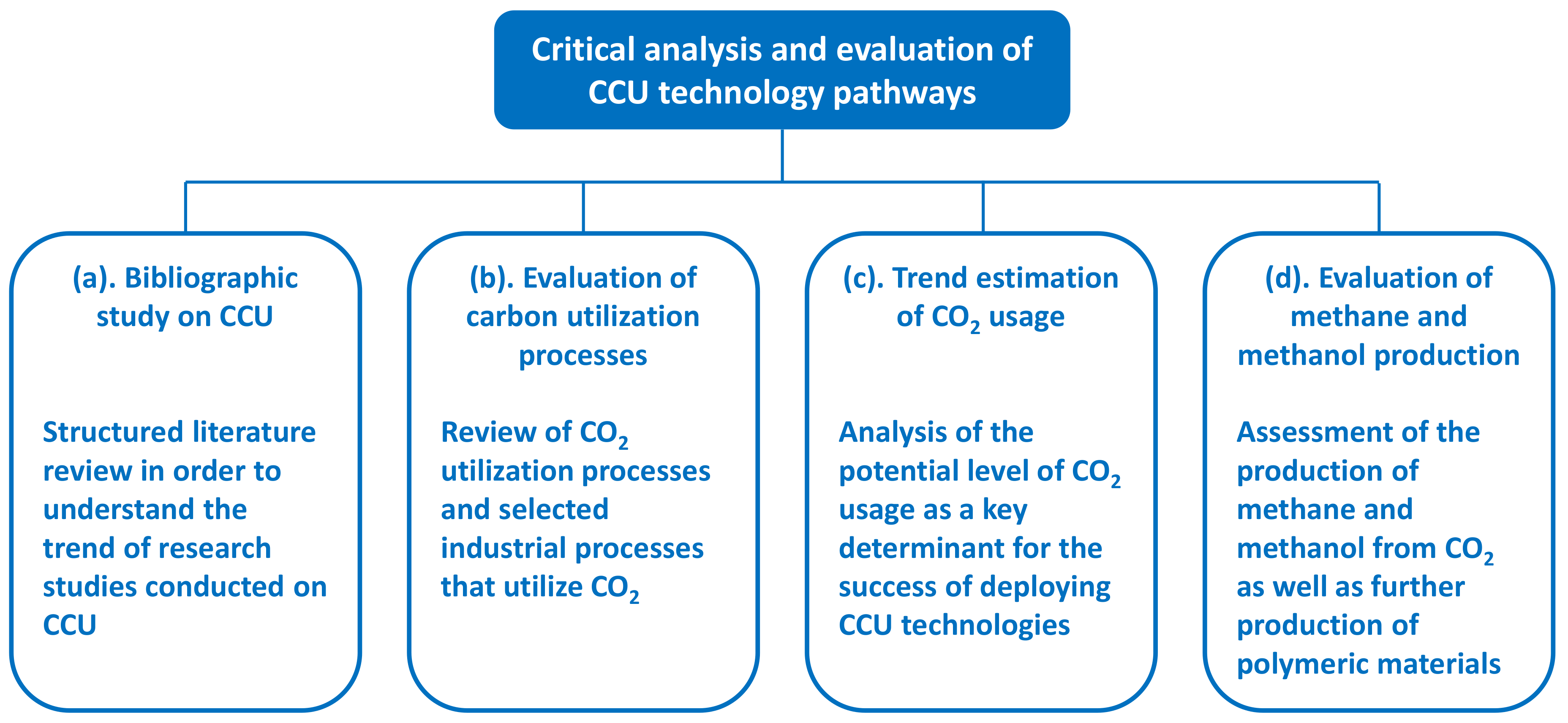
3. Method
4. Results
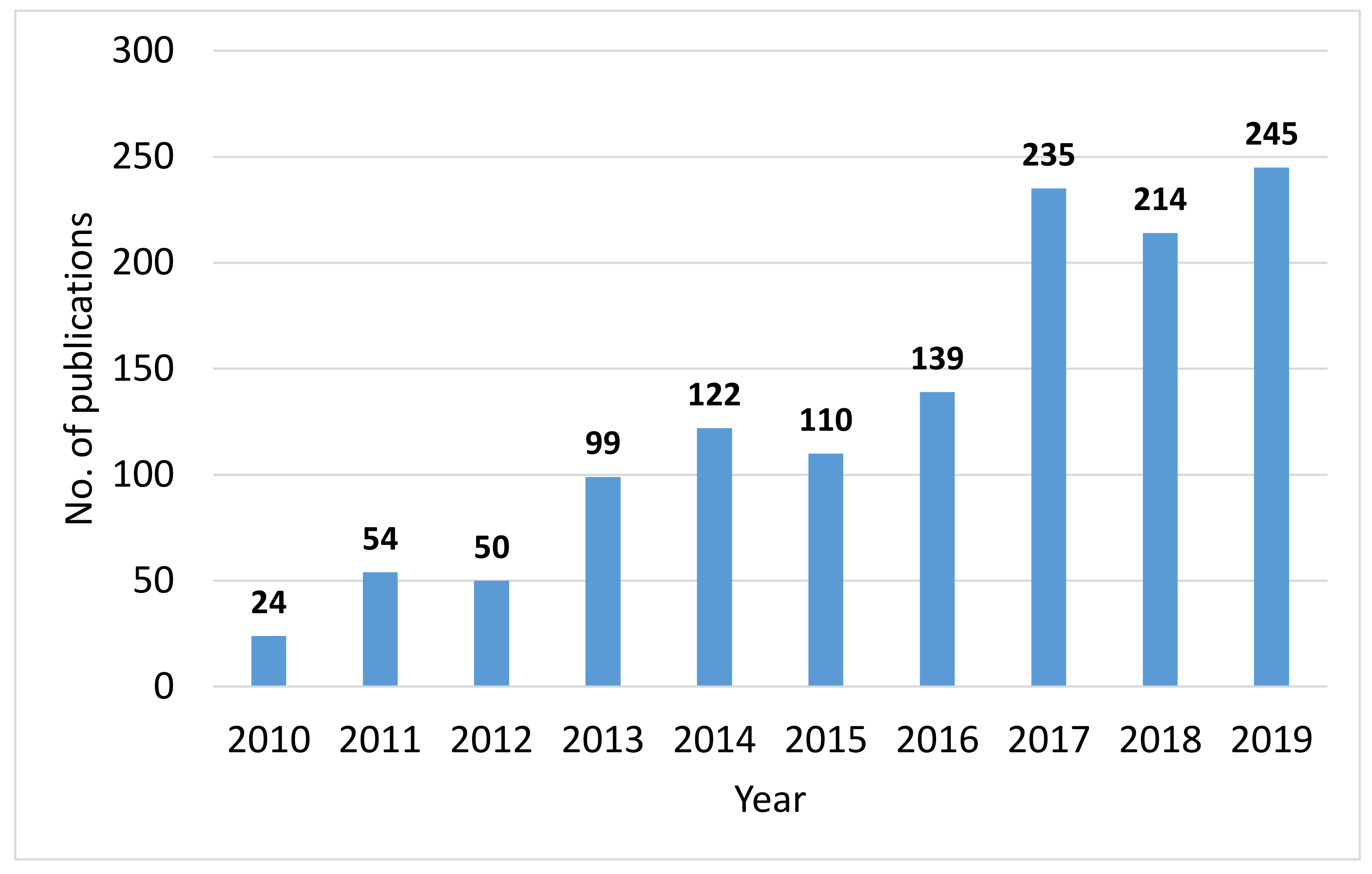
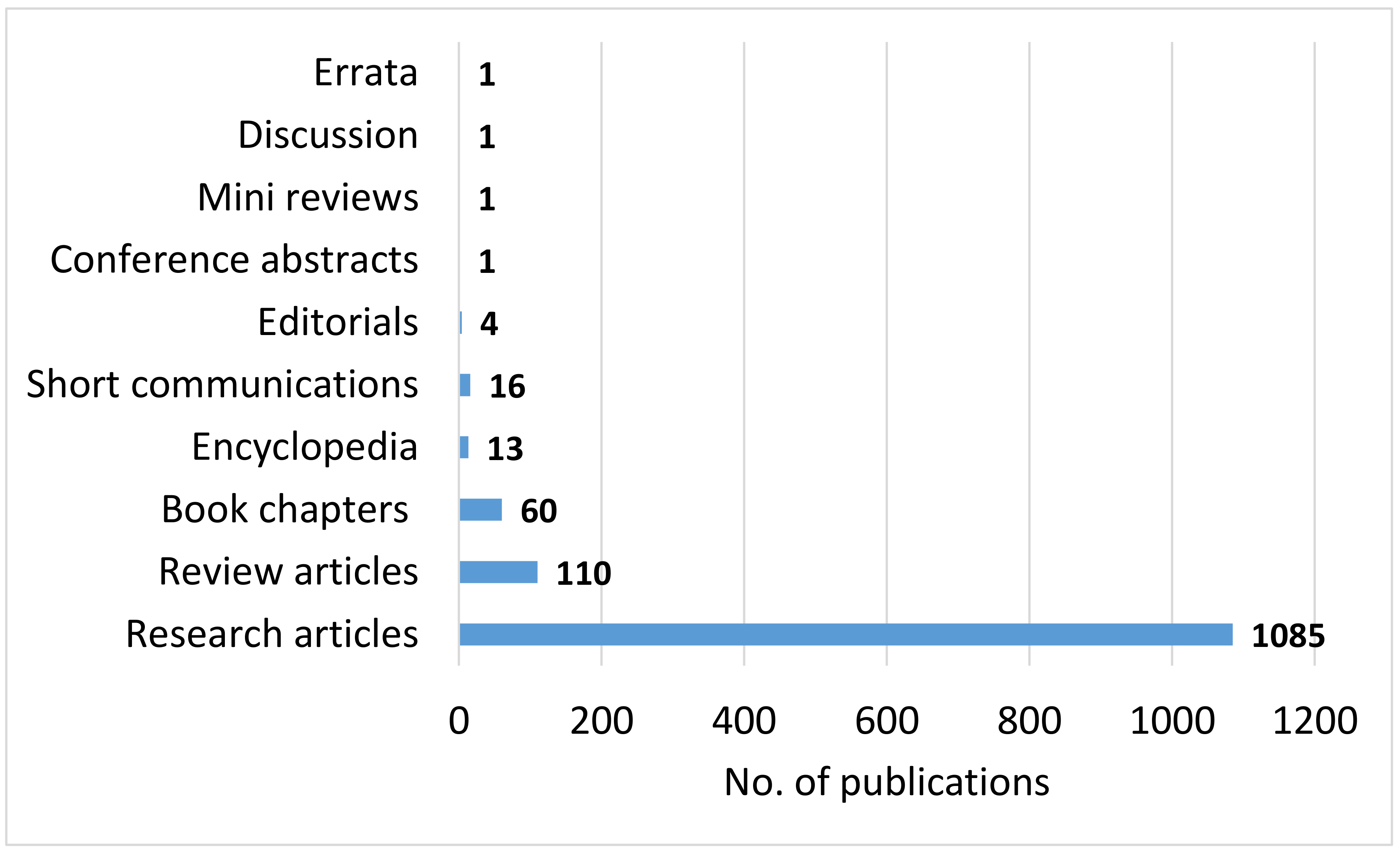
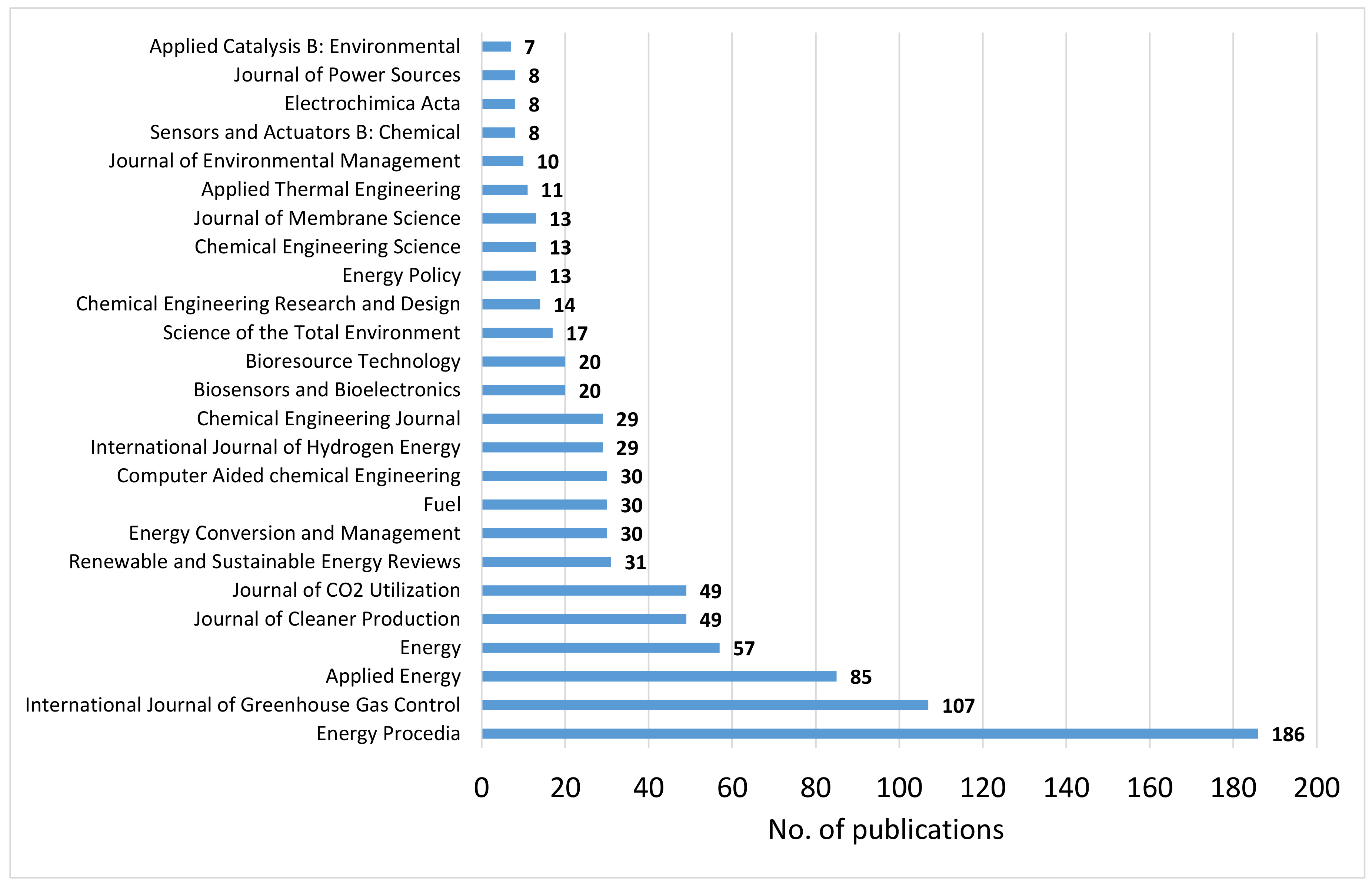
4.1. Bibliographic Study on CCU
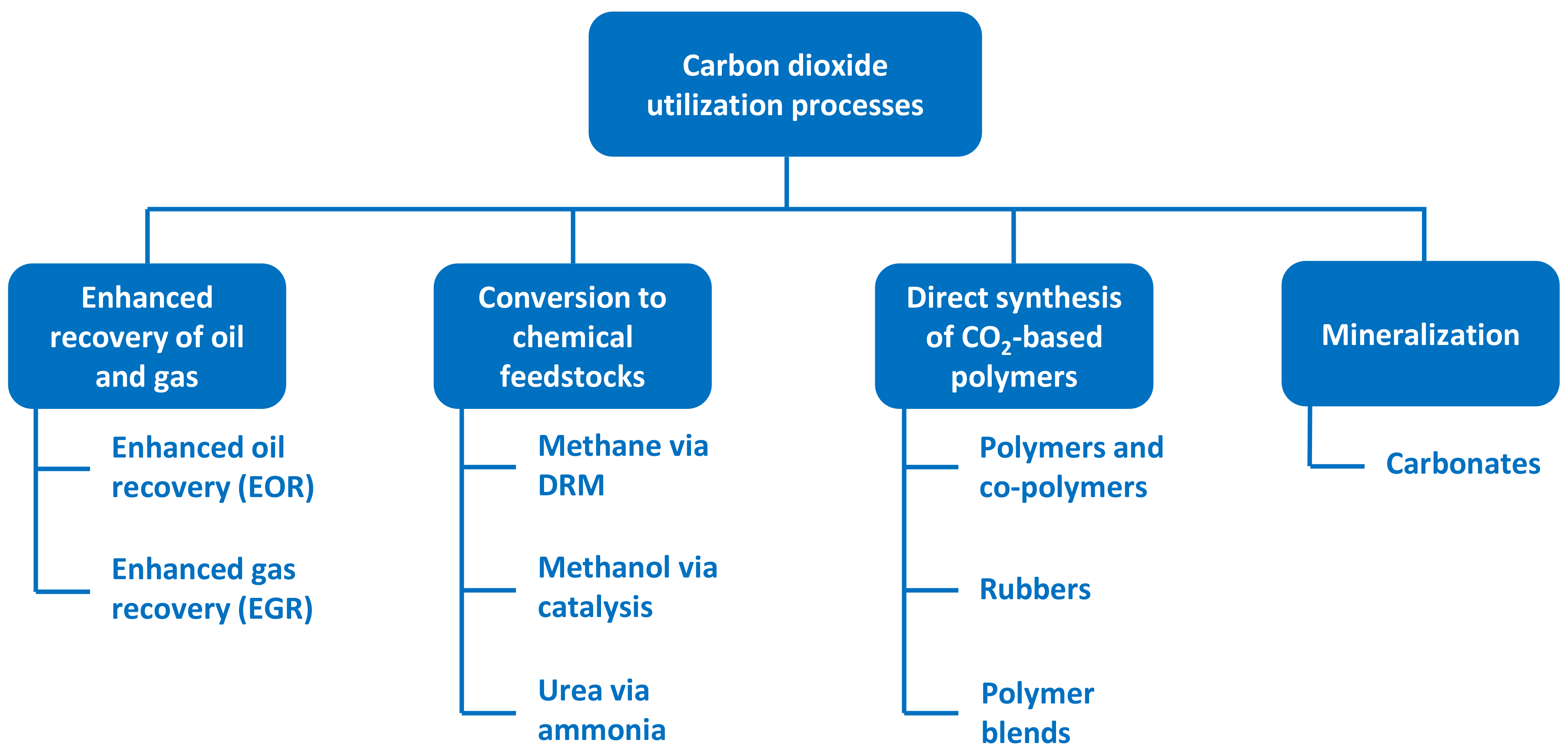
4.2. Evaluation of Carbon Utilization Processes
- Bauxite residue carbonation where CO2 is used to neutralize bauxite residues (TRL = 9).
- Horticulture production based on CO2 supplementation via plant growth (TRL = 9).
- Urea production from ammonia and CO2 (TRL = 9).
- Concrete curing of concrete blocks where CO2 used for precast concrete curing (TRL = 7–8).
- Mineral carbonation based on CO2 reacted with calcium or magnesium containing minerals (TRL = 7–8).
- Lignin production where CO2 is used in black liquor pH regulation (TRL = 7–8).
- Methanol production based on electrochemical reduction in CO2 (TRL = 7).
- Polyurethane production where CO2 is reacted with calcium or magnesium containing minerals (TRL = 7).
- Polycarbonate production where CO2 is used as raw material to produce plastics and fibres (TRL = 7).
- Carbon capture and sequestration from point sources (CCS): 15.2% and 32.2%.
- Carbon capture and sequestration from air (CCSA): 20.0% and 46.6%.
- Carbon capture and utilization as structural materials (CCUSM): 8.2% and 9.2%.
- Steam methane reforming with carbon capture and sequestration (SMR + CCS): 11.3% and 20.0%.
- Methane pyrolysis (MP): 45.0% and 45.0%
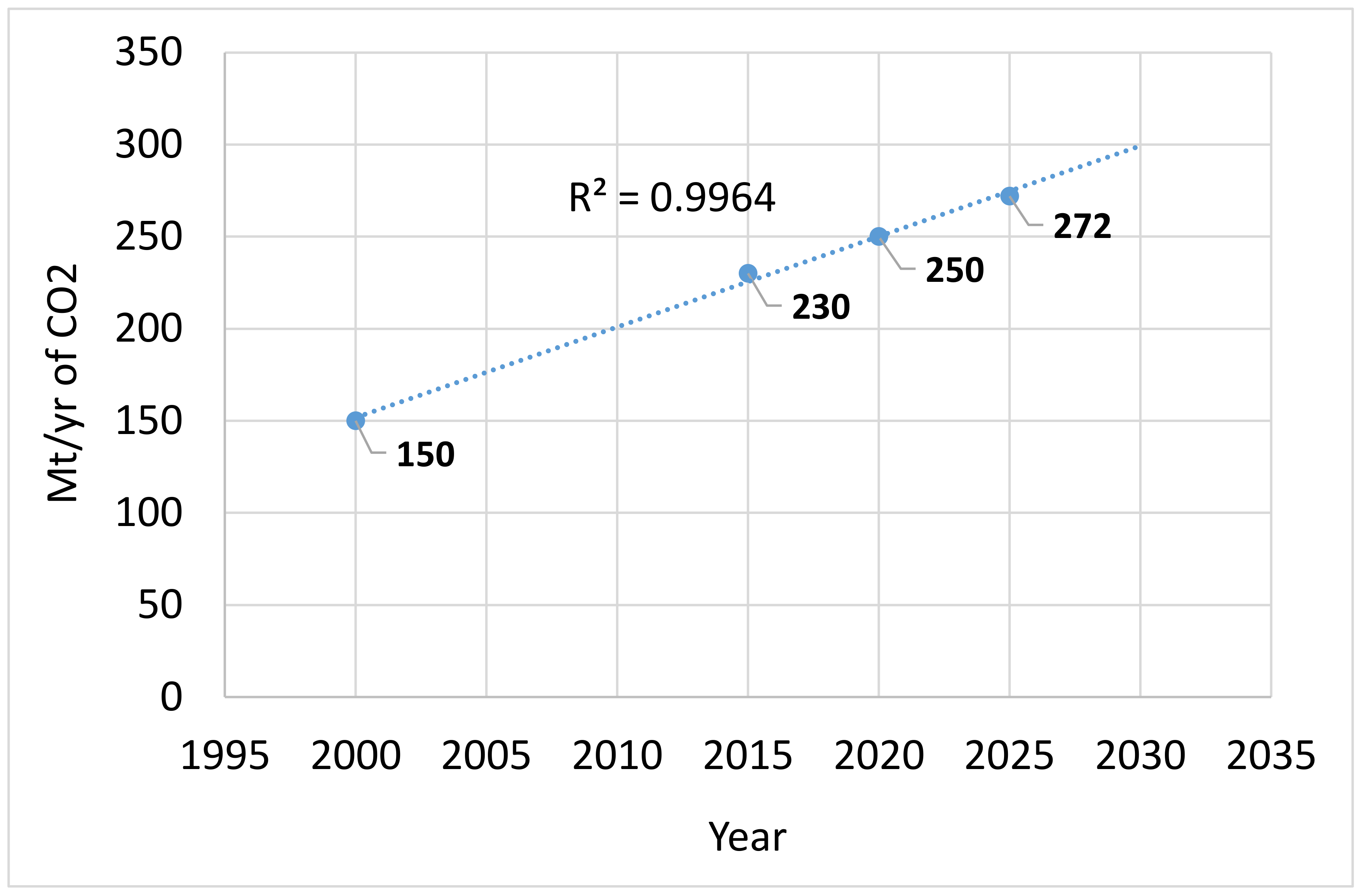
4.3. Trend Estimation of CO2 Usage
4.4. Evaluation of Methane and Methanol Production
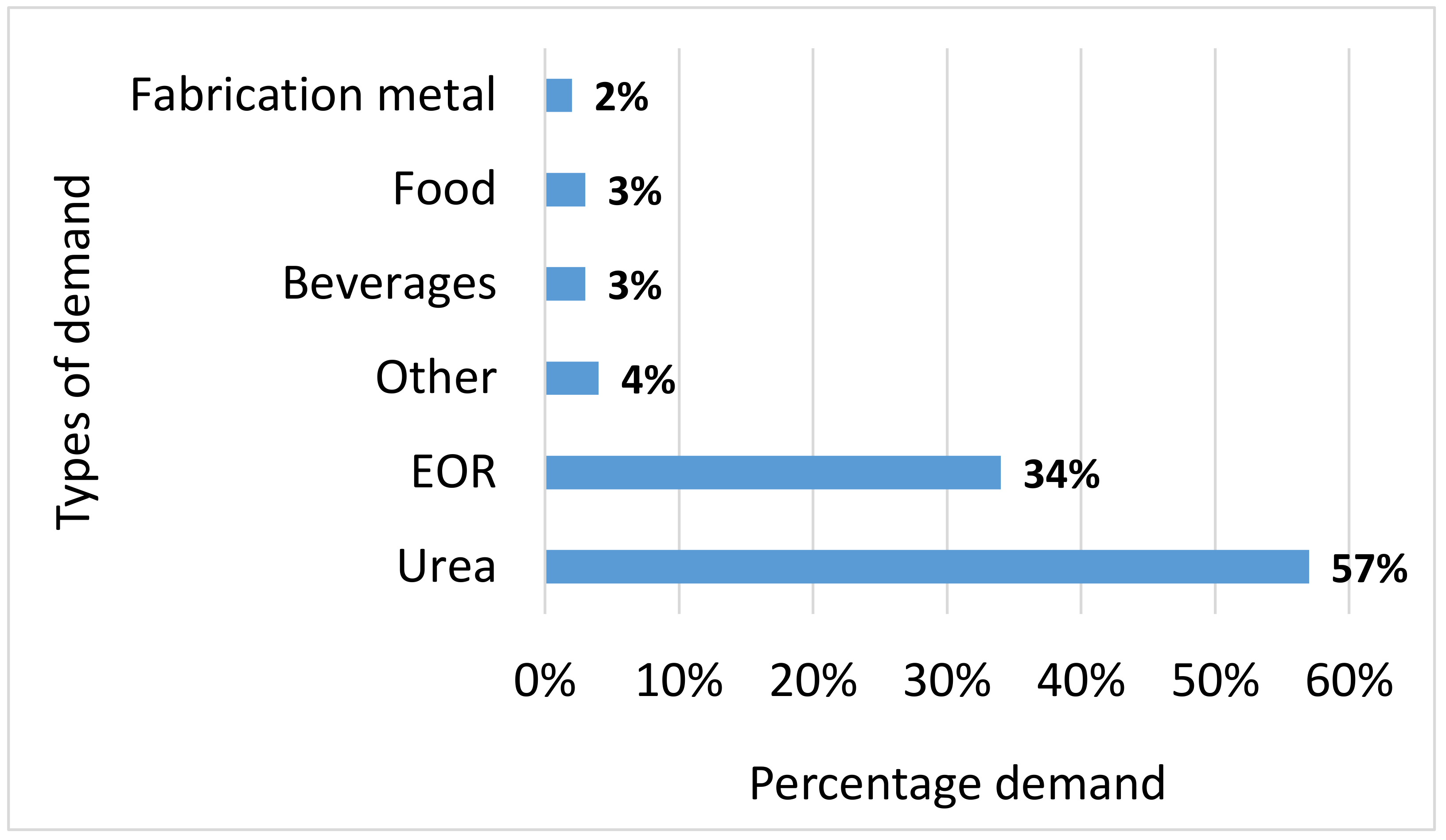
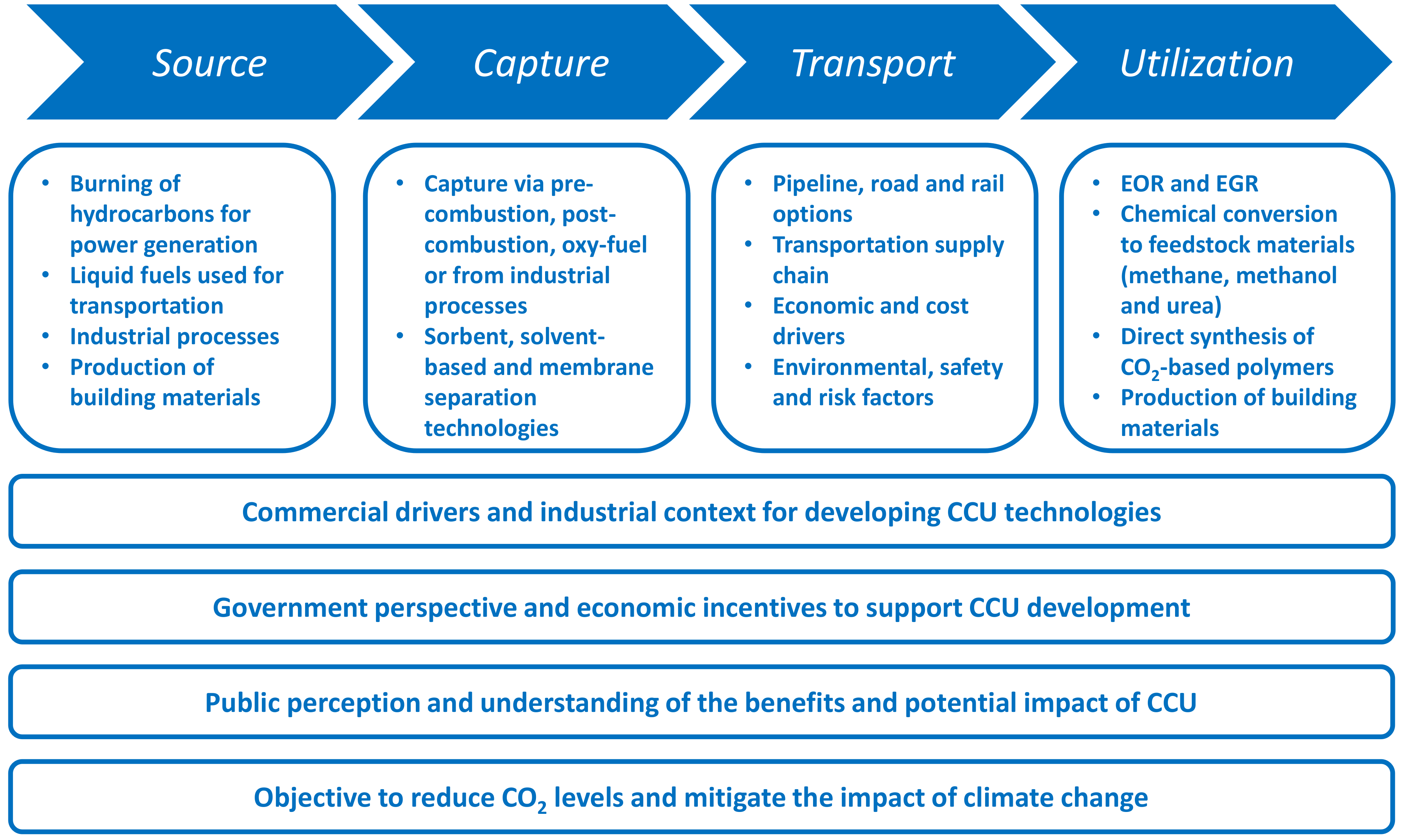
5. CCU Value Chain Analysis
6. Conclusions and Future Work
- (1)
- CCU is an emerging area of research as identified by the bibliographic study and the analysis suggests that despite the various challenges, CCU will continue to grow as an area of interest for researchers, industry and governmental organizations. The main subject areas where CCU has been investigated include energy, environmental science, materials science, chemical engineering and general engineering as well as several other areas to a lesser extent. Indeed, since there are many technological, economic and environmental challenges associated with implementing CCU, adoption of a multidisciplinary research perspective is encouraged and will be essential if the industrial development challenges identified in this article are to be surmounted.
- (2)
- There are a range of carbon dioxide utilization processes currently available, although not all are fully developed for industrial application. EOR is an existing practice (i.e., high TRL) available in certain cases to significantly increase the level of production from oil wells that are depleted via traditional extraction with the benefit of sequestering CO2 in geological rock formations on an effectively permanent basis, although there is the overall net effect on carbon emissions to be considered in regard to higher levels of petroleum products eventually arising from EOR. Chemical conversion to feedstock materials including methane and methanol have a relatively high TRL and are active areas of development alongside existing production of urea (although this will still likely lead to carbon emissions). Additional synthetic pathways to polymers and rubbers represent an emerging area of CCU, which is currently at a low TRL but has significant potential for the future. Other CCU processes (such as mineralization as well as application to the production of building materials, e.g., concrete) are less developed and have a lower TRL.
- (3)
- The global level of demand for CO2 is expected to continue to grow in a steady and linear fashion, with much of this current demand being driven by EOR and urea production, although there is scope for growth in chemical conversion of CO2 to other materials, as stated above. There is the potential for the level of EOR to grow at an exponential rate, although this will be impacted by the demand for oil and gas as well as the parallel development, maturity and lowering of costs for renewable forms of energy. However, the long term environmental impact of EOR needs to be properly evaluated due to the higher levels of oil and gas production and resulting carbon emissions that eventually arise from adoption of this process.
- (4)
- The production of methane and methanol as well as further derivative materials represents an area of active development where there is scope to deploy new technologies to facilitate the transition to greater levels of CCU. The development of new CCU-enabled economies and business models for CO2-based products, including methane, methanol as well as polymers such as polyoxymethylene (via syngas), is an area subject for further investigation and technological commercialization. In cases where there are favorable changes to the regulatory environment and a supporting economic case, such production routes are likely to become more viable and subject to accelerated industrial development.
Funding
Acknowledgments
Conflicts of Interest
References
- Mac Dowell, N.; Fennell, P.S.; Shah, N.; Maitland, G. The role of CO2 capture and utilization in mitigating climate change. Nat. Clim. Chang. 2017, 7, 243–249. [Google Scholar] [CrossRef]
- Zimmermann, A.W.; Wunderlich, J.; Müller, L.; Buchner, G.A.; Marxen, A.; Michailos, S.; Armstrong, K.; Naims, H.; McCord, S.; Styring, P.; et al. Techno-Economic Assessment Guidelines for CO2 Utilization. In Fundamentals Carbon Dioxide Utilization; North, M., Styring, P., Eds.; De Gruyter: Berlin, Germany, 2020; pp. 63–78. [Google Scholar] [CrossRef]
- Gibbins, J.; Chalmers, H. Carbon capture and storage. Energy Policy 2008, 36, 4317–4322. [Google Scholar] [CrossRef]
- IPCC. Intergovernmental Panel on Climate Change (IPCC) Special Report on Global Warming of 1.5 °C. 2018. Available online: https://www.ipcc.ch/sr15/ (accessed on 8 August 2020).
- Cornwall, W. Inside the Paris climate deal. Science 2015, 350, 1451. [Google Scholar] [CrossRef] [PubMed]
- IPCC. Intergovernmental Panel on Climate Change (IPCC) Climate Change 2013: The Physical Science Basis. 2013. Available online: https://www.ipcc.ch/report/ar5/wg1/ (accessed on 8 August 2020).
- Gao, Y.; Gao, X.; Zhang, X. The 2 °C Global Temperature Target and the Evolution of the Long-Term Goal of Addressing Climate Change—From the United Nations Framework Convention on Climate Change to the Paris Agreement. Engineering 2017, 3, 272–278. [Google Scholar] [CrossRef]
- Cuéllar-Franca, R.M.; Azapagic, A. Carbon capture, storage and utilisation technologies: A critical analysis and comparison of their life cycle environmental impacts. J. CO2 Util. 2015, 9, 82–102. [Google Scholar] [CrossRef]
- Gielen, D.; Boshell, F.; Saygin, D.; Bazilian, M.D.; Wagner, N.; Gorini, R. The role of renewable energy in the global energy transformation. Energy Strat. Rev. 2019, 24, 38–50. [Google Scholar] [CrossRef]
- Nykvist, B.; Sprei, F.; Nilsson, M. Assessing the progress toward lower priced long range battery electric vehicles. Energy Policy 2019, 124, 144–155. [Google Scholar] [CrossRef]
- Kumar, A.; Madden, D.G.; Lusi, M.; Chen, K.J.; Daniels, E.A.; Curtin, T.; Perry, I.V.; Zaworotko, M.J. Direct air capture of CO2 by physisorbent materials. Angew. Chem. Int. Ed. 2015, 54, 14372–14377. [Google Scholar] [CrossRef]
- Zeman, F. Reducing the cost of Ca-based direct air capture of CO2. Environ. Sci. Technol. 2014, 48, 11730–11735. [Google Scholar] [CrossRef]
- Kätelhön, A.; Meys, R.; Deutz, S.; Suh, S.; Bardow, A. Climate change mitigation potential of carbon capture and utilization in the chemical industry. Proc. Natl. Acad. Sci. USA 2019, 116, 11187–11194. [Google Scholar] [CrossRef]
- Smit, B.; Park, A.-H.A.; Gadikota, G. The Grand Challenges in Carbon Capture, Utilization, and Storage. Front. Energy Res. 2014, 2, 55. [Google Scholar] [CrossRef]
- Tsvetkov, P.; Cherepovitsyn, A.; Fedoseev, S. The Changing Role of CO2 in the Transition to a Circular Economy: Review of Carbon Sequestration Projects. Sustainability 2019, 11, 5834. [Google Scholar] [CrossRef]
- Monkman, S.; Macdonald, M. On carbon dioxide utilization as a means to improve the sustainability of ready-mixed concrete. J. Clean. Prod. 2017, 167, 365–375. [Google Scholar] [CrossRef]
- Liang, L.; Liu, C.; Jiang, F.; Chen, Q.; Zhang, L.; Xue, H.; Jiang, H.-L.; Qian, J.; Yuan, D.; Hong, M. Carbon dioxide capture and conversion by an acid-base resistant metal-organic framework. Nat. Commun. 2017, 8, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Kaiser, S.; Bringezu, S. Use of carbon dioxide as raw material to close the carbon cycle for the German chemical and polymer industries. J. Clean. Prod. 2020, 271, 122775. [Google Scholar] [CrossRef]
- Kang, D.; Lee, M.-G.; Jo, H.; Yoo, Y.; Lee, S.Y.; Park, J.-W. Carbon capture and utilization using industrial wastewater under ambient conditions. Chem. Eng. J. 2017, 308, 1073–1080. [Google Scholar] [CrossRef]
- Choi, Y.H.; Jang, Y.J.; Park, H.; Kim, W.Y.; Lee, Y.H.; Choi, S.H.; Lee, J.S. Carbon dioxide Fischer-Tropsch synthesis: A new path to carbon-neutral fuels. Appl. Catal. B Environ. 2017, 202, 605–610. [Google Scholar] [CrossRef]
- Miguel, C.V.; Soria, M.; Mendes, A.; Madeira, L.M. A sorptive reactor for CO2 capture and conversion to renewable methane. Chem. Eng. J. 2017, 322, 590–602. [Google Scholar] [CrossRef]
- Arning, K.; Van Heek, J.; Ziefle, M. Acceptance profiles for a carbon-derived foam mattress. Exploring and segmenting consumer perceptions of a carbon capture and utilization product. J. Clean. Prod. 2018, 188, 171–184. [Google Scholar] [CrossRef]
- Khoo, H.H.; Bu, J.; Wong, R.L.; Kuan, S.; Sharratt, P. Carbon capture and utilization: Preliminary life cycle CO2, energy, and cost results of potential mineral carbonation. Energy Procedia 2011, 4, 2494–2501. [Google Scholar] [CrossRef]
- De Ras, K.; Van De Vijver, R.; Galvita, V.V.; Marin, G.B.; Van Geem, K.M. Carbon capture and utilization in the steel industry: Challenges and opportunities for chemical engineering. Curr. Opin. Chem. Eng. 2019, 26, 81–87. [Google Scholar] [CrossRef]
- Bonaventura, D.; Friedrich, D.; Valverde, J.; Becerra, J.; Verda, V. Carbon capture and utilization for sodium bicarbonate production assisted by solar thermal power. Energy Convers. Manag. 2017, 149, 860–874. [Google Scholar] [CrossRef]
- Lu, L.; Guest, J.S.; Peters, C.A.; Zhu, X.; Rau, G.H.; Ren, Z.J. Wastewater treatment for carbon capture and utilization. Nat. Sustain. 2018, 1, 750–758. [Google Scholar] [CrossRef]
- Zhang, Q.; Nurhayati; Cheng, C.-L.; Nagarajan, D.; Chang, J.; Hu, J.; Lee, D.-J. Carbon capture and utilization of fermentation CO2: Integrated ethanol fermentation and succinic acid production as an efficient platform. Appl. Energy 2017, 206, 364–371. [Google Scholar] [CrossRef]
- Wiesberg, I.L.; Brigagão, G.V.; De Medeiros, J.L.; Araújo, O.D.Q.F. Carbon dioxide utilization in a microalga-based biorefinery: Efficiency of carbon removal and economic performance under carbon taxation. J. Environ. Manag. 2017, 203, 988–998. [Google Scholar] [CrossRef]
- Leung, D.Y.C.; Caramanna, G.; Maroto–Valer, M.M. An overview of current status of carbon dioxide capture and storage technologies. Renew. Sustain. Energy Rev. 2014, 39, 426–443. [Google Scholar] [CrossRef]
- Kanniche, M.; Gros-Bonnivard, R.; Jaud, P.; Valle-Marcos, J.; Amann, J.M.; Bouallou, C. Pre-combustion, post-combustion and oxy-combustion in thermal power plant for CO2 capture. Appl. Therm. Eng. 2010, 30, 53–62. [Google Scholar] [CrossRef]
- Philbin, S.P.; Wang, S.H.-M. Perspectives on the Techno-Economic Analysis of Carbon Capture and Storage. J. Technol. Manag. Innov. 2019, 14, 3–17. [Google Scholar] [CrossRef]
- Dehjalali, F.R.; Avami, A. A design procedure for the assessment of carbon capturing and utilization of flue gas from power plant using experimental data. Chem. Eng. Res. Des. 2018, 131, 393–405. [Google Scholar] [CrossRef]
- Karjunen, H.; Tynjälä, T.; Hyppänen, T. A method for assessing infrastructure for CO2 utilization: A case study of Finland. Appl. Energy 2017, 205, 33–43. [Google Scholar] [CrossRef]
- Ros, M.; Read, A.; Uilenreef, J.; Limbeek, J. Start of a CO2 Hub in Rotterdam: Connecting CCS and CCU. Energy Procedia 2014, 63, 2691–2701. [Google Scholar] [CrossRef]
- McCoy, S.T.; Rubin, E.S. An engineering-economic model of pipeline transport of CO2 with application to carbon capture and storage. Int. J. Greenh. Gas Control. 2008, 2, 219–229. [Google Scholar] [CrossRef]
- Arning, K.; Heek, J.O.-V.; Linzenich, A.; Kätelhön, A.; Sternberg, A.D.; Bardow, A.; Ziefle, M. Same or different? Insights on public perception and acceptance of carbon capture and storage or utilization in Germany. Energy Policy 2019, 125, 235–249. [Google Scholar] [CrossRef]
- Huh, T.; Kim, H.-J. Korean Experimentation of Knowledge and Technology Transfer to Address Climate Change in Developing Countries. Sustainability 2018, 10, 1263. [Google Scholar] [CrossRef]
- Von Der Assen, N.; Voll, P.; Peters, M.; Bardow, A. Life cycle assessment of CO2capture and utilization: A tutorial review. Chem. Soc. Rev. 2014, 43, 7982–7994. [Google Scholar] [CrossRef]
- Mora, M.M.; Vergara, C.P.; Leiva, M.; Delgadillo, S.M.; Domínguez, R. Life cycle assessment of carbon capture and utilization from ammonia process in Mexico. J. Environ. Manag. 2016, 183, 998–1008. [Google Scholar] [CrossRef]
- Jens, C.M.; Muüller, L.; Leonhard, K.; Bardow, A. To Integrate or Not to Integrate—Techno-Economic and Life Cycle Assessment of CO2 Capture and Conversion to Methyl Formate Using Methanol. ACS Sustain. Chem. Eng. 2019, 7, 12270–12280. [Google Scholar]
- Sick, V.; Armstrong, K.; Cooney, G.; Cremonese, L.; Eggleston, A.; Faber, G.; Hackett, G.; Kätelhön, A.; Keoleian, G.; Marano, J.; et al. The Need for and Path to Harmonized Life Cycle Assessment and Techno-Economic Assessment for Carbon Dioxide Capture and Utilization. Energy Technol. 2020, 8, 1901034. [Google Scholar] [CrossRef]
- Thonemann, N.; Pizzol, M. Consequential life cycle assessment of carbon capture and utilization technologies within the chemical industry. Energy Environ. Sci. 2019, 12, 2253–2263. [Google Scholar] [CrossRef]
- Von Der Assen, N.; Jung, J.; Bardow, A. Life-cycle assessment of carbon dioxide capture and utilization: Avoiding the pitfalls. Energy Environ. Sci. 2013, 6, 2721–2734. [Google Scholar] [CrossRef]
- IEA. Putting CO2 to Use—Creating Value from Emissions. International Energy Agency (IEA). 2019. Available online: https://webstore.iea.org/putting-co2-to-use (accessed on 8 August 2020).
- Koytsoumpa, E.I.; Bergins, C.; Kakaras, E. The CO2 economy: Review of CO2 capture and reuse technologies. J. Supercrit. Fluids 2018, 132, 3–16. [Google Scholar] [CrossRef]
- Elia, V.; Gnoni, M.G.; Tornese, F. Measuring circular economy strategies through index methods: A critical analysis. J. Clean. Prod. 2017, 142, 2741–2751. [Google Scholar] [CrossRef]
- Darko, A.; Chan, A.P. Critical analysis of green building research trend in construction journals. Habitat Int. 2016, 57, 53–63. [Google Scholar] [CrossRef]
- Almeida, A.; Mulero, R.; Rametta, P.; Urošević, V.; Andrić, M.; Patrono, L. A critical analysis of an IoT—aware AAL system for elderly monitoring. Futur. Gener. Comput. Syst. 2019, 97, 598–619. [Google Scholar] [CrossRef]
- Al-Mamoori, A.; Krishnamurthy, A.; Rownaghi, A.A.; Rezaei, F. Carbon Capture and Utilization Update. Energy Technol. 2017, 5, 834–849. [Google Scholar] [CrossRef]
- Alvarado, V.; Manrique, E. Enhanced Oil Recovery: An Update Review. Energies 2010, 3, 1529–1575. [Google Scholar] [CrossRef]
- Zakaria, Z.; Kamarudin, S.K. Direct conversion technologies of methane to methanol: An overview. Renew. Sustain. Energy Rev. 2016, 65, 250–261. [Google Scholar] [CrossRef]
- Zhou, W.; Cheng, K.; Kang, J.; Zhou, C.; Subramanian, V.; Zhang, Q.; Wang, Y. New horizon in C1 chemistry: Breaking the selectivity limitation in transformation of syngas and hydrogenation of CO2 into hydrocarbon chemicals and fuels. Chem. Soc. Rev. 2019, 48, 3193–3228. [Google Scholar] [CrossRef]
- Yang, H.; Zhang, C.; Gao, P.; Wang, H.; Li, X.; Zhong, L.; Wei, W.; Sun, Y. A review of the catalytic hydrogenation of carbon dioxide into value-added hydrocarbons. Catal. Sci. Technol. 2017, 7, 4580–4598. [Google Scholar] [CrossRef]
- Aramouni, N.A.K.; Touma, J.G.; Abu Tarboush, B.; Zeaiter, J.; Ahmad, M. Catalyst design for dry reforming of methane: Analysis review. Renew. Sustain. Energy Rev. 2018, 82, 2570–2585. [Google Scholar] [CrossRef]
- Meys, R.; Kätelhön, A.; Bardow, A. Towards sustainable elastomers from CO2: Life cycle assessment of carbon capture and utilization for rubbers. Green Chem. 2019, 21, 3334–3342. [Google Scholar] [CrossRef]
- Valderrama, M.A.M.; Van Putten, R.-J.; Gruter, G.-J.M. The potential of oxalic—And glycolic acid based polyesters (review). Towards CO2 as a feedstock (Carbon Capture and Utilization—CCU). Eur. Polym. J. 2019, 119, 445–468. [Google Scholar] [CrossRef]
- Muthuraj, R.; Mekonnen, T.H. Recent progress in carbon dioxide (CO2) as feedstock for sustainable materials development: Co-polymers and polymer blends. Polymer 2018, 145, 348–373. [Google Scholar] [CrossRef]
- Patricio, J.; Angelis-Dimakis, A.; Castillo-Castillo, A.; Kalmykova, Y.; Rosado, L. Region prioritization for the development of carbon capture and utilization technologies. J. CO2 Util. 2017, 17, 50–59. [Google Scholar] [CrossRef]
- Farmer, T.C.; Doherty, M.F. Thermodynamic assessment of carbon dioxide emission reduction during fossil fuel derived energy production. Energy 2019, 177, 565–573. [Google Scholar] [CrossRef]
- He, Y.; Zhu, L.; Li, L.; Sun, L. Zero-energy penalty carbon capture and utilization for liquid fuel and power cogeneration with chemical looping combustion. J. Clean. Prod. 2019, 235, 34–43. [Google Scholar] [CrossRef]
- Aresta, M.; DiBenedetto, A.; Angelini, A. The changing paradigm in CO2 utilization. J. CO2 Util. 2013, 3, 65–73. [Google Scholar] [CrossRef]
- Statista. Production Capacity of Urea Worldwide in 2018 and 2030 (in Million Metric Tons). 2020. Available online: https://www.statista.com/statistics/1063689/global-urea-production-capacity/ (accessed on 8 August 2020).
- Pérez-Fortes, M.; Bocin-Dumitriu, A.; Tzimas, E. CO2 Utilization Pathways: Techno-Economic Assessment and Market Opportunities. Energy Procedia 2014, 63, 7968–7975. [Google Scholar] [CrossRef]
- Xiang, X.; Guo, L.; Wu, X.; Ma, X.; Xia, Y. Urea formation from carbon dioxide and ammonia at atmospheric pressure. Environ. Chem. Lett. 2012, 10, 295–300. [Google Scholar] [CrossRef]
- Koohestanian, E.; Sadeghi, J.; Mohebbi-Kalhori, D.; Shahraki, F.; Samimi, A. A novel process for CO2 capture from the flue gases to produce urea and ammonia. Energy 2018, 144, 279–285. [Google Scholar] [CrossRef]
- McGlade, C.; Sondak, G.; Han, M. Commentary: Whatever Happened to Enhanced Oil Recovery? International Energy Agency (IEA). 2018. Available online: https://www.iea.org/commentaries/whatever-happened-to-enhanced-oil-recovery (accessed on 8 August 2020).
- Jaramillo, P.; Griffin, W.M.; McCoy, S.T. Life Cycle Inventory of CO2 in an Enhanced Oil Recovery System. Environ. Sci. Technol. 2009, 43, 8027–8032. [Google Scholar] [CrossRef] [PubMed]
- Schakel, W.; Oreggioni, G.; Singh, B.; Strømman, A.; Ramirez, A. Assessing the techno-environmental performance of CO2 utilization via dry reforming of methane for the production of dimethyl ether. J. CO2 Util. 2016, 16, 138–149. [Google Scholar] [CrossRef]
- Schildhauer, T.J.; Calbry-Muzyka, A.; Witte, J.; Biollaz, S.; Jansohn, P. Producing Renewable Methane—Demonstration of CCU from Biomass. SSRN Electron. J. 2019, 21–26. [Google Scholar] [CrossRef]
- Fan, M.-S.; Abdullah, A.Z.; Bhatia, S. Catalytic Technology for Carbon Dioxide Reforming of Methane to Synthesis Gas. ChemCatChem 2009, 1, 192–208. [Google Scholar] [CrossRef]
- Hoppe, W.; Bringezu, S.; Wachter, N. Economic assessment of CO2-based methane, methanol and polyoxymethylene production. J. CO2 Util. 2018, 27, 170–178. [Google Scholar] [CrossRef]
- Milani, D.; Khalilpour, R.; Zahedi, G.; E Abbas, A. A model-based analysis of CO2 utilization in methanol synthesis plant. J. CO2 Util. 2015, 10, 12–22. [Google Scholar] [CrossRef]
- Cheng, W.H. (Ed.) Methanol Production and Use; CRC Press: Boca Raton, FL, USA, 1994. [Google Scholar]
- Luu, M.T.; Milani, D.; Bahadori, A.; Abbas, A. A comparative study of CO2 utilization in methanol synthesis with various syngas production technologies. J. CO2 Util. 2015, 12, 62–76. [Google Scholar] [CrossRef]
- Huo, Z.; Hu, M.; Zeng, X.; Yun, J.; Jin, F. Catalytic reduction of carbon dioxide into methanol over copper under hydrothermal conditions. Catal. Today 2012, 194, 25–29. [Google Scholar] [CrossRef]
- Collodi, G.; Azzaro, G.; Ferrari, N.; Santos, S. Demonstrating Large Scale Industrial CCS through CCU—A Case Study for Methanol Production. Energy Procedia 2017, 114, 122–138. [Google Scholar] [CrossRef]
- Kaplinsky, R. Globalisation and Unequalisation: What Can Be Learned from Value Chain Analysis? J. Dev. Stud. 2000, 37, 117–146. [Google Scholar] [CrossRef]
- Vonsée, B.; Crijns-Graus, W.; Liu, W. Energy technology dependence—A value chain analysis of geothermal power in the EU. Energy 2019, 178, 419–435. [Google Scholar] [CrossRef]
- Baral, N.R.; Davis, R.; Bradley, T.H. Supply and value chain analysis of mixed biomass feedstock supply system for lignocellulosic sugar production. Biofuels Bioprod. Biorefin. 2019, 13, 635–659. [Google Scholar] [CrossRef]
- Hasan, M.F.; First, E.L.; Boukouvala, F.; Floudas, C.A. A multi-scale framework for CO2 capture, utilization, and sequestration: CCUS and CCU. Comput. Chem. Eng. 2015, 81, 2–21. [Google Scholar] [CrossRef]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Philbin, S.P. Critical Analysis and Evaluation of the Technology Pathways for Carbon Capture and Utilization. Clean Technol. 2020, 2, 492-512. https://doi.org/10.3390/cleantechnol2040031
Philbin SP. Critical Analysis and Evaluation of the Technology Pathways for Carbon Capture and Utilization. Clean Technologies. 2020; 2(4):492-512. https://doi.org/10.3390/cleantechnol2040031
Chicago/Turabian StylePhilbin, Simon P. 2020. "Critical Analysis and Evaluation of the Technology Pathways for Carbon Capture and Utilization" Clean Technologies 2, no. 4: 492-512. https://doi.org/10.3390/cleantechnol2040031
APA StylePhilbin, S. P. (2020). Critical Analysis and Evaluation of the Technology Pathways for Carbon Capture and Utilization. Clean Technologies, 2(4), 492-512. https://doi.org/10.3390/cleantechnol2040031