The Influence of Black Soldier Fly Residue on Watermelon Growth and the Properties of a Coarse-Textured Ultisol
Abstract
1. Introduction
2. Materials and Methods
2.1. Laboratory Analysis
2.2. Percentage Changes in the Soil’s Chemical Properties Relative to the Baseline Soil
2.3. Statistical Analysis
3. Results and Discussion
3.1. Chemical Properties of BSF and Its Effect on the Physical Properties of the Soil
3.2. Effect of Black Soldier Fly Residue Application on the Chemical Properties of the Soil
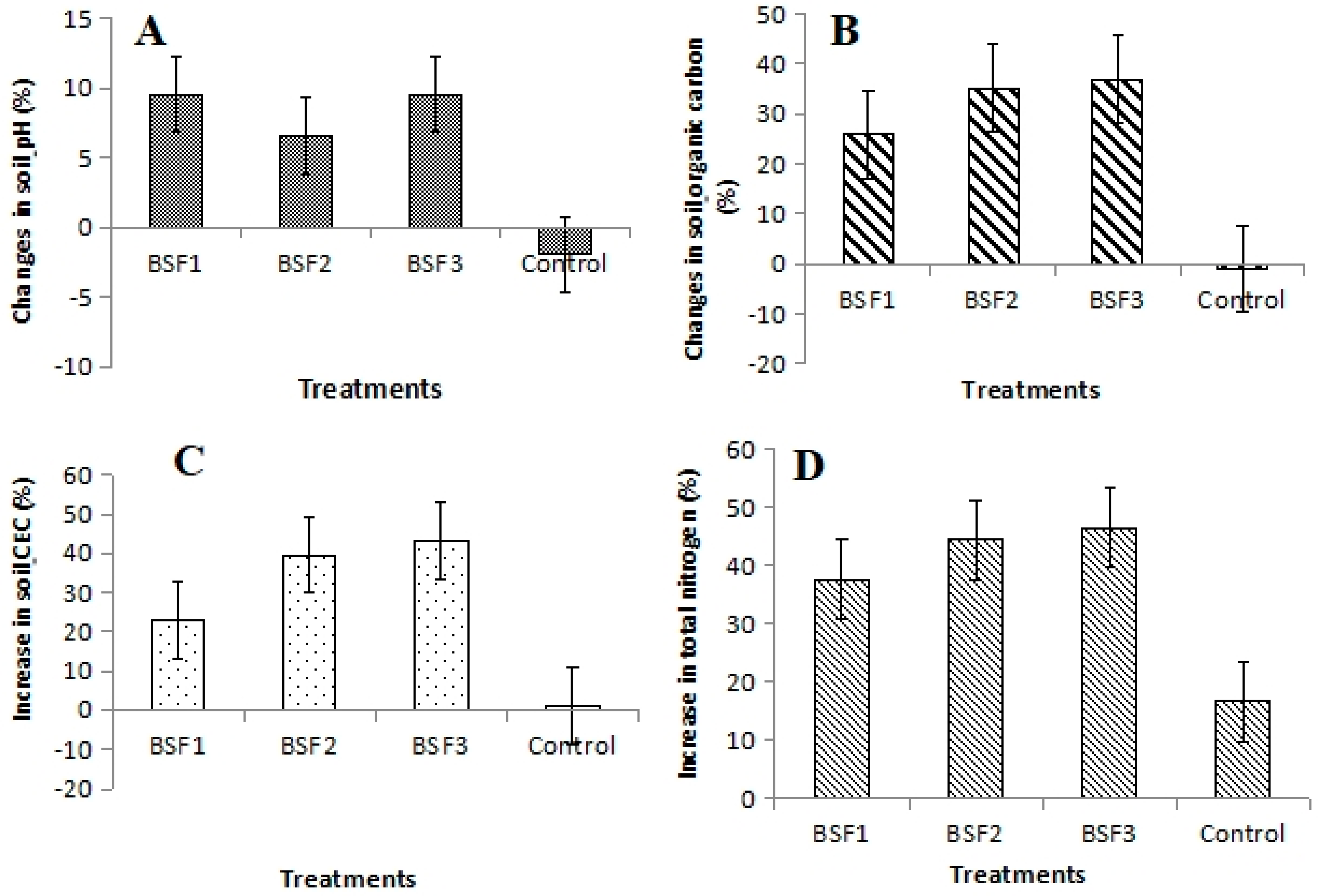
3.3. Changes in Soil pH, SOC, CEC, and TN Relative to the Baseline Soil Properties Following BSF Application
3.4. Changes in Available P and Exchangeable Nutrients Relative to the Baseline Soil Properties Following BSF Application
3.5. The Response of Watermelon Growth Indices to BSF Residue Amendment
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Unagwu, B.O.; Onah, I.; Chibuike, G. Residual effects of repeated animal manure application on coarse-textured Ultisol, nutrient uptake and cucumber yield. Zemdirbyste-Agriculture 2023, 110, 357–366. [Google Scholar] [CrossRef]
- Tajudeen, T.T.; Omotayo, A.; Ogundele, F.O.; Rathbun, L.C. The effect of climate change on food crop production in Lagos State. Foods 2022, 11, 3987. [Google Scholar] [CrossRef]
- United Nations. World Population Prospects 2022. Available online: https://www.un.org/development/desa/pd/sites/www.un.org.development.desa.pd/files/wpp2022_summary_of_results.pdf (accessed on 24 February 2025).
- Mgbenka, R.N.; Onwubuya, E.A.; Ezeano, C.I. Organic farming in Nigeria: Need for popularization and policy. World J. Agric. Sci. 2015, 11, 346–355. [Google Scholar] [CrossRef]
- Unagwu, B.O.; Ene, J.; Azuka, C.V. Effects of Different Organic Amendment Sources on the Physico Chemical Properties of A Nutrient Depleted Vertisol. Niger. J. Soil Sci. 2020, 29, 68–73. [Google Scholar] [CrossRef]
- Choudhary, R.C.; Bairwa, H.L.; Mahawer, L.N.; Meena, V.K.; Tak, J.K. Effect of Organic Manures on Yield and Quality Characteristics of Pomegranate (Punica granatum L.) Cv. Bhagwa. Int. J. Plant Soil Sci. 2023, 35, 120–128. [Google Scholar] [CrossRef]
- Gelaye, Y. Effect of combined application of organic manure and nitrogen fertilizer rates on yield and yield components of potato: A review. Cogent Food Agric. 2023, 9, 2217603. [Google Scholar] [CrossRef]
- Unagwu, B.O.; Okpara, C.; Ebido, N.E. Suitability of Animal Manure for Melon Production and Soil Performance in an Utisol. Middle East J. Agric. Res. 2022, 11, 519–526. [Google Scholar] [CrossRef]
- Beesigamukama, D.; Mochoge, B.; Korir, N.K.K.M.; Fiaboe, K.; Nakimbugwe, D. Low-cost technology for recycling agro-industrial waste into nutrient-rich organic fertilizer using black soldier fly. Waste Manag. 2021, 119, 183–194. [Google Scholar] [CrossRef]
- Tan, J.K.N.; Lee, J.T.E.; Chiam, Z.; Song, S.; Arora, S.; Tong, Y.W.; Tan, H.T.W. Applications of food wastes-derived black soldier fly larval frass as incorporated compost, side-dress fertilizer and frass-tea drench for soilless cultivation of leafy vegetables in biochar-based growing media. Waste Manag. 2021, 130, 155–166. [Google Scholar] [CrossRef]
- Mannaa, M.; Mansour, A.; Park, I.; Lee, D.; Seo, Y. Insect-based agri-food waste valorization: Agricultural applications and roles of insect gut microbiota. Environ. Sci. Ecotechnol. 2024, 17, 100287. [Google Scholar] [CrossRef]
- Lalander, C.; Senecal, J.; Calvo, M.G.; Ahrens, L.; Josefsson, S.; Wiberg, K.; Vinnerås, B. Fate of pharmaceuticals and pesticides in fly larvae composting. Sci. Total Environ. 2016, 565, 279–286. [Google Scholar] [CrossRef] [PubMed]
- Chavez, M.Y.; Uchanski, M.; Tomberlin, J.K. Impacts of black soldier fly, Hermetia illucens, larval frass on lettuce and arugula production. Front. Sustain. Food Syst. 2024, 8, 1399932. [Google Scholar] [CrossRef]
- Vogel, H.; Müller, A.; Heckel, D.G.; Gutzeit, H.; Vilcinskas, A. Nutritional immunology: Diversification and diet-dependent expression of antimicrobial peptides in the black soldier fly Hermetia illucens. Develop. Comp. Immune 2018, 78, 141–148. [Google Scholar] [CrossRef]
- Beesigamukama, D.; Mochoge, B.; Korir, N.; Musyoka, M.W.; Fiaboe, K.K.M.; Nakimbugwe, D.; Khamis, F.M.; Subramanian, S.; Dubois, T.; Ekesi, S. Nitrogen Fertilizer Equivalence of Black Soldier Fly Frass Fertilizer and Synchrony of Nitrogen Mineralization for Maize Production. Agronomy 2020, 10, 1395. [Google Scholar] [CrossRef]
- Otunaiya, A.O.; Adedeji, I.A. Technical efficiency of watermelon (Citrullus lanatus) production in Ogun State, Nigeria. Int. J. Appl. Agric. Apic. Res. 2014, 10, 44–53. [Google Scholar]
- Swamy, K.R.M. Origin, Distribution, Taxonomy, Botanical Description, Genetics and Crop Improvement of Watermelon {Citrullus lanatus (Thunb.) Matsum and Nakai}. Int. J. Curr. Res. 2022, 14, 22564–22582. [Google Scholar] [CrossRef]
- Enujeke, E.C. An assessment of some growth and yield indices of six varieties of watermelon (Citrulus lanatus thumb) in Asaba area of Delta state, Nigeria. Trop. Agric. Res. Ext. 2015, 16, 123–129. [Google Scholar] [CrossRef]
- Adojutelegan, O.T.; Adereti, F.O.; Makanju, T.S.; Olorunfemi, O.D. Analysis of Factors Affecting Watermelon Production in Ekiti State Nigeria. Sci. Technol. Arts Res. J. 2015, 4, 324–329. [Google Scholar] [CrossRef]
- Kang, F.; Wang, Z.; Xiong, H.; Li, Y.; Wang, Y.; Fan, Z.; Zhao, H.; Kuang, D.; Chen, Z.; Wang, J.; et al. Estimation of watermelon nutrient requirements based on the QUEFTS model. Agronomy 2020, 10, 1776. [Google Scholar] [CrossRef]
- Blake, G.R.; Hartge, K.H. Bulk density. In Methods of Soil Analysis. Part 1. Physical and Mineralogical Methods ASA No. 9; Klute, A., Ed.; Soil Science Society of America (SSSA): Madison, WI, USA, 1986; pp. 162–167. [Google Scholar]
- Kemper, W.D.; Rosenau, R.C. Aggregate stability and size distribution. In Method of Soil Analysis. Part 1. Physical and Mineralogical Methods, Soil Science Society of American Agronomy Monograph No. 9, 2nd ed.; Soil Science Society of America (SSSA): Madison, WI, USA, 1986; pp. 425–440. [Google Scholar]
- Klute, A.; Dirksen, C. Hydraulic conductivity and diffusivity: Laboratory methods. In Methods of Soil Analysis. Part 1. Soil Science Society of America; Klute, A., Ed.; Soil Science Society of America (SSSA): Madison, WI, USA, 1986; pp. 687–732. [Google Scholar]
- Nelson, D.W.; Sommers, L.S. Total carbon, organic carbon, and organic matter. In Methods of Soil Analysis. Part II. Chemical and Microbiological Properties. Agronomy Monographs; Soil Science Society of America (SSSA): Madison, WI, USA, 1982; pp. 539–579. [Google Scholar]
- Thomas, G.W. Exchangeable cations. In Methods of Soil Analysis, 2nd Edition, Part 2. American Society of Agronomy; Page, A.L., Miller, R.H., Keeney, D.R., Eds.; Soil Science Society of America (SSSA): Madison, WI, USA, 1982; pp. 159–165. [Google Scholar]
- Rhoades, J.D. Cation exchange capacity. In Methods of Soil Analysis. Part II. Chemical and Microbiological Properties. Agronomy Monographs; Page, A.L., Ed.; Soil Science Society of America (SSSA): Madison, WI, USA, 1982; pp. 149–157. [Google Scholar]
- Brady, C.N.; Weil, R.R. The Nature and Properties of Soils, 14th ed.; Pearson Prentice Hall: Upper Saddle River, NJ, USA, 2008; pp. 972–975. [Google Scholar]
- Bremner, J.M. Nitrogen-total. In Methods of Soil Analysis, Part III. Chemical Methods; Sparks, D.L., Ed.; SSSA Book Series No. 5. American Society of Agronomy; Soil Science Society of America (SSSA): Madison, WI, USA, 1996; pp. 1085–1121. [Google Scholar]
- Sushma, B.; Srinivas, J.; Rajasekhar, M.; Saidaiah, P.; Sheker, B.C. Effect of black soldier fly digested organic manures on physico-chemical properties and nutrient status of soil in okra (Abelmoschus esculentus L.). Int. J. Res. Agron. 2024, 7, 23–27. [Google Scholar] [CrossRef]
- Unagwu, B.O. Organic amendments applied to a degraded soil: Short term effects on soil quality indicators. Afr. J. Agric. Res. 2019, 14, 218–225. [Google Scholar] [CrossRef]
- Dzepe, D.; Mbenda, T.K.; Ngassa, G.; Mube, H.; Chia, S.Y.; Aoudou, Y.; Djouaka, R. Application of black soldier fly frass, Hermetia illucens (Diptera: Stratiomyidae) as sustainable organic fertilizer for lettuce, Lactuca sativa production. Open J. Appl. Sci. 2022, 12, 1632–1648. [Google Scholar] [CrossRef]
- Wang, Q.; Ren, X.; Sun, Y.; Zhao, J.; Awasthi, M.K.; Liu, T.; Li, R.; Zhang, Z. Improvement of the composition and humification of different animal manures by black soldier fly bioconversion. J. Clean. Prod. 2022, 278, 123397. [Google Scholar] [CrossRef]
- Beesigamukama, D.; Subramanian, S.; Tanga, C.M. Nutrient quality and maturity status of frass fertilizer from nine edible insects. Sci. Rep. 2022, 12, 7182. [Google Scholar] [CrossRef]
- Klammsteiner, T.; Turan, V.; Juárez, M.F.; Oberegger, S.; Insam, H. Suitability of Black Soldier Fly Frass as Soil Amendment and Implication for Organic Waste Hygienization. Agronomy 2020, 10, 1578. [Google Scholar] [CrossRef]
- Anyega, A.O.; Korir, N.K.; Beesigamukama, D.; Changeh, G.J.; Nkoba, K.; Subramanian, S.; van Loon, J.J.A.; Dicke, M.; Tanga, C.M. Black Soldier Fly-Composted Organic Fertilizer Enhances Growth, Yield, and Nutrient Quality of Three Key Vegetable Crops in Sub-Saharan Africa. Front. Plant Sci. 2021, 12, 680312. [Google Scholar] [CrossRef]
- Tittonell, P.; Corbeels, M.; van Wijk, M.T.; Vanlauwe, B.; Giller, K.E. Combining Organic and Mineral Fertilizers for Integrated Soil Fertility Management in Smallholder Farming Systems of Kenya: Explorations Using the Crop-Soil Model Field. Agron. J. 2018, 100, 1511–1526. [Google Scholar] [CrossRef]
- Wang, M.; Zheng, Q.; Shen, Q.; Guo, S. The critical role of potassium in plant stress response. Int. J. Mol. Sci. 2013, 14, 7370–7390. [Google Scholar] [CrossRef]
- Fageria, N.K. The Use of Nutrients in Crop Plants; CRC Press: Boca Raton, FL, USA, 2016; pp. 67–74. [Google Scholar]
- Baiyeri, K.P.; Chukwudi, U.P.; Chizaram, C.A.; Aneke, N. Maximizing rice husk waste for Daucus carota production. Int. J. Recycl. Org. Waste Agric. 2019, 8, 399–406. [Google Scholar] [CrossRef]
- Agustiyani, D.; Agandi, R.; Arinafril; Nugroho, A.A.; Antonius, S. The effect of application of compost and frass from Black Soldier Fly Larvae (Hermetia illucens L.) on growth of Pakchoi (Brassica rapa L.). IOP Conf. Ser. Earth Environ. Sci. 2021, 762, 012036. [Google Scholar] [CrossRef]
- Fuhrmann, A.; Wilde, B.; Conz, R.F.; Kantengwa, S.; Konlambigue, M.; Masengesho, B.; Kintche, K.; Kassa, K.; Musazura, W.; Späth, L.; et al. Residues from black soldier fly (Hermetia illucens) larvae rearing influence the plant-associated soil microbiome in the short term. Front. Microbiol. 2022, 13, 994091. [Google Scholar] [CrossRef] [PubMed]
- Risman, R.D.S.; Nonkhonman, C.; Sungthongwises, K. Integrating vermicompost, black soldier fly, and inorganic fertilizers enhances corn growth and yield. Heliyon 2025, 11, e41314. [Google Scholar] [CrossRef] [PubMed]
- Chukwudi, U.P. Ginger germplasm classification and identification of morphological markers related to rhizome yield. Crop Sci. 2023, 63, 248–254. [Google Scholar] [CrossRef]
- Silva Junior, F.; Sousa, G.; Sousa, J.T.; Nojosa Lessa, C.; Silva, F. Salt Stress and Ambience on the Production of Watermelon Seedlings. Rev. Caatinga 2020, 33, 518–528. [Google Scholar] [CrossRef]

| Amendments | pH (H2O) | pH (KCl) | Total N (g kg−1) | Total P (g kg−1) | Total K (g kg−1) | Total Ca (g kg−1) | Total Mg (g kg−1) | OM (g kg−1) |
|---|---|---|---|---|---|---|---|---|
| BSF residue | 6.6 | 5.3 | 3.80 | 1.86 | 4.60 | 42.7 | 48.8 | 298 |
| Treatment | Bulk Density (g cm−3) | Aggregate Stability (%) | Hydraulic Conductivity (cm hr−1) |
|---|---|---|---|
| BSF1 | 1.68 (0.098) | 12.4 (1.09) | 0.15 (0.023) |
| BSF2 | 1.56 (0.087) | 14.0 (1.18) | 0.25 (0.051) |
| BSF3 | 1.66 (0.086) | 15.4 (1.06) | 0.26 (0.090) |
| Control | 1.58 (0.069) | 10.3 (1.11) | 0.10 (0.008) |
| F-LSD | NS | 1.02 | 0.03 |
| Treatment | pH (H2O) | pH (KCl) | Total N (g/kg) | Available P (mg/kg) | Ex. K (mg/kg) | Ex. Ca (c mol/kg) | Ex. Mg (cmolc/kg) | CEC (cmolc/kg) | OC (g/kg) |
|---|---|---|---|---|---|---|---|---|---|
| BSF1 | 6.30 (0.058) | 5.25 (0.029) | 0.24 | 3.26 (0.081) | 0.93 (0.013) | 1.20 (0.115) | 1.90 (0.058) | 12.40 (0.922) | 1.39 (0.109) |
| BSF2 | 6.10 (0.058) | 5.05 (0.144) | 0.27 | 7.00 (0.018) | 1.01 (0.017) | 2.30 (0.172) | 2.10 (0.520) | 15.80 (0.808) | 1.59 (0.111) |
| BSF3 | 6.30 (0.058) | 5.45 (0.087) | 0.28 | 10.05 (0.025) | 1.27 (0.105) | 2.40 (0.188) | 1.90 (0.758) | 16.80 (0.924) | 1.63 (0.102) |
| Control | 5.59 (0.062) | 4.95 (0.059) | 0.18 | 2.33 (0.068) | 0.80 (0.006) | 1.00 (0.005) | 1.40 (0.061) | 9.60 (0.428) | 1.02 (0.098) |
| F-LSD | 0.212 | 0.241 | 0.022 | 4.831 | 0.224 | 0.371 | 0.341 | 2.420 | 0.131 |
| Treatment | WAS 2 | WAS 4 | WAS 6 | WAS 8 | WAS 10 |
|---|---|---|---|---|---|
| Leaf length (cm) | |||||
| BSF1 | 4.67 | 4.17 | 7.37 | 12.7 | 12.3 |
| BSF2 | 3.67 | 4.83 | 6.00 | 11.3 | 12.7 |
| BSF3 | 2.17 | 3.47 | 7.67 | 10.0 | 12.0 |
| Control | 2.10 | 4.33 | 5.00 | 9.00 | 9.33 |
| F-LSD | 0.99 | NS | 1.05 | 1.73 | 1.57 |
| Leaf number | |||||
| BSF1 | 5.67 | 7.67 | 9.33 | 15.7 | 13.0 |
| BSF2 | 5.00 | 7.67 | 9.67 | 18.7 | 23.0 |
| BSF3 | 4.33 | 6.67 | 10.7 | 20.3 | 25.0 |
| Control | 4.33 | 7.67 | 8.67 | 12.0 | 11.0 |
| F-LSD | 0.761 | NS | 1.655 | 1.961 | 3.234 |
| Leaf width (cm) | |||||
| BSF1 | 4.00 | 4.83 | 5.33 | 8.80 | 9.67 |
| BSF2 | 3.13 | 4.33 | 5.33 | 10.0 | 10.7 |
| BSF3 | 1.67 | 2.50 | 8.00 | 9.00 | 11.0 |
| Control | 1.60 | 3.97 | 5.30 | 7.00 | 7.67 |
| F-LSD | 0.98 | 0.72 | 1.22 | 1.10 | 1.05 |
| Stem girth (cm) | |||||
| BSF1 | 1.27 | 1.30 | 1.43 | 2.17 | 2.33 |
| BSF2 | 1.27 | 1.43 | 1.70 | 2.50 | 2.57 |
| BSF3 | 1.20 | 1.30 | 1.67 | 2.57 | 2.47 |
| Control | 1.30 | 1.37 | 1.70 | 2.63 | 2.73 |
| F-LSD | 0.19 | 0.16 | Ns | Ns | 0.23 |
| Vine Length (cm) | |||||
| BSF 1 | 8.67 | 17.0 | 30.0 | 54.7 | 70.0 |
| BSF2 | 10.0 | 27.3 | 40.0 | 92.3 | 96.3 |
| BSF3 | 7.00 | 16.3 | 44.3 | 83.7 | 113 |
| Control | 9.00 | 16.7 | 21.7 | 42.3 | 55. 7 |
| F-LSD | 3.709 | 5.97 | 12.02 | 9.59 | 12.59 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Unagwu, B.O.; Odu, C.F.; Amuji, C.F.; Eze, M.O.; Ebido, N.E.; Abara, C.U.; Igboka, C.R.; Chukwudi, U.P. The Influence of Black Soldier Fly Residue on Watermelon Growth and the Properties of a Coarse-Textured Ultisol. Soil Syst. 2025, 9, 43. https://doi.org/10.3390/soilsystems9020043
Unagwu BO, Odu CF, Amuji CF, Eze MO, Ebido NE, Abara CU, Igboka CR, Chukwudi UP. The Influence of Black Soldier Fly Residue on Watermelon Growth and the Properties of a Coarse-Textured Ultisol. Soil Systems. 2025; 9(2):43. https://doi.org/10.3390/soilsystems9020043
Chicago/Turabian StyleUnagwu, Benedict Onyebuchi, Chidiebere Fransica Odu, Chinedu Felix Amuji, Michael Onyedika Eze, Nancy Ekene Ebido, Chidike Ude Abara, Chioma Rosita Igboka, and Uchechukwu Paschal Chukwudi. 2025. "The Influence of Black Soldier Fly Residue on Watermelon Growth and the Properties of a Coarse-Textured Ultisol" Soil Systems 9, no. 2: 43. https://doi.org/10.3390/soilsystems9020043
APA StyleUnagwu, B. O., Odu, C. F., Amuji, C. F., Eze, M. O., Ebido, N. E., Abara, C. U., Igboka, C. R., & Chukwudi, U. P. (2025). The Influence of Black Soldier Fly Residue on Watermelon Growth and the Properties of a Coarse-Textured Ultisol. Soil Systems, 9(2), 43. https://doi.org/10.3390/soilsystems9020043











