Bacterial Community in Sugarcane Rhizosphere Under Bacillus subtilis Inoculation and Straw Return
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Site Characterization
2.2. Experimental Design and Treatments
2.3. Experimental Condition
2.4. DNA Extraction and Sequencing
2.5. Statistical Analysis
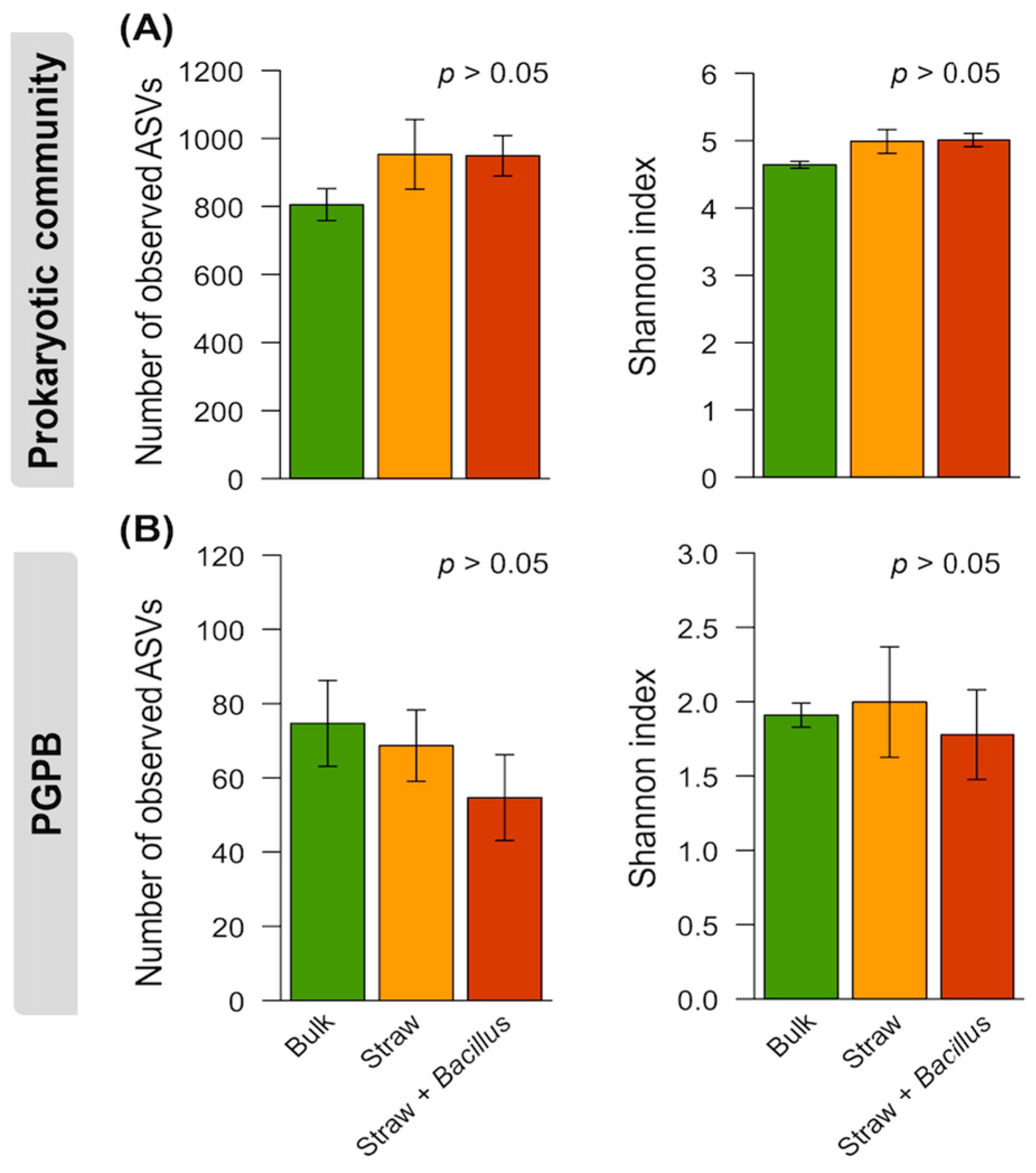
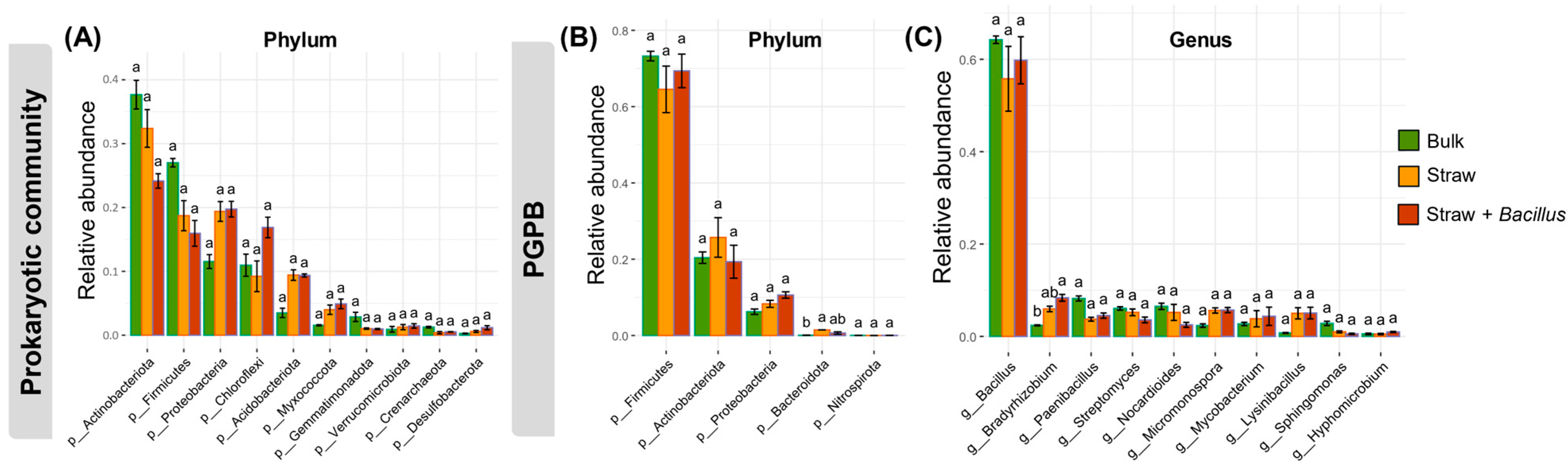
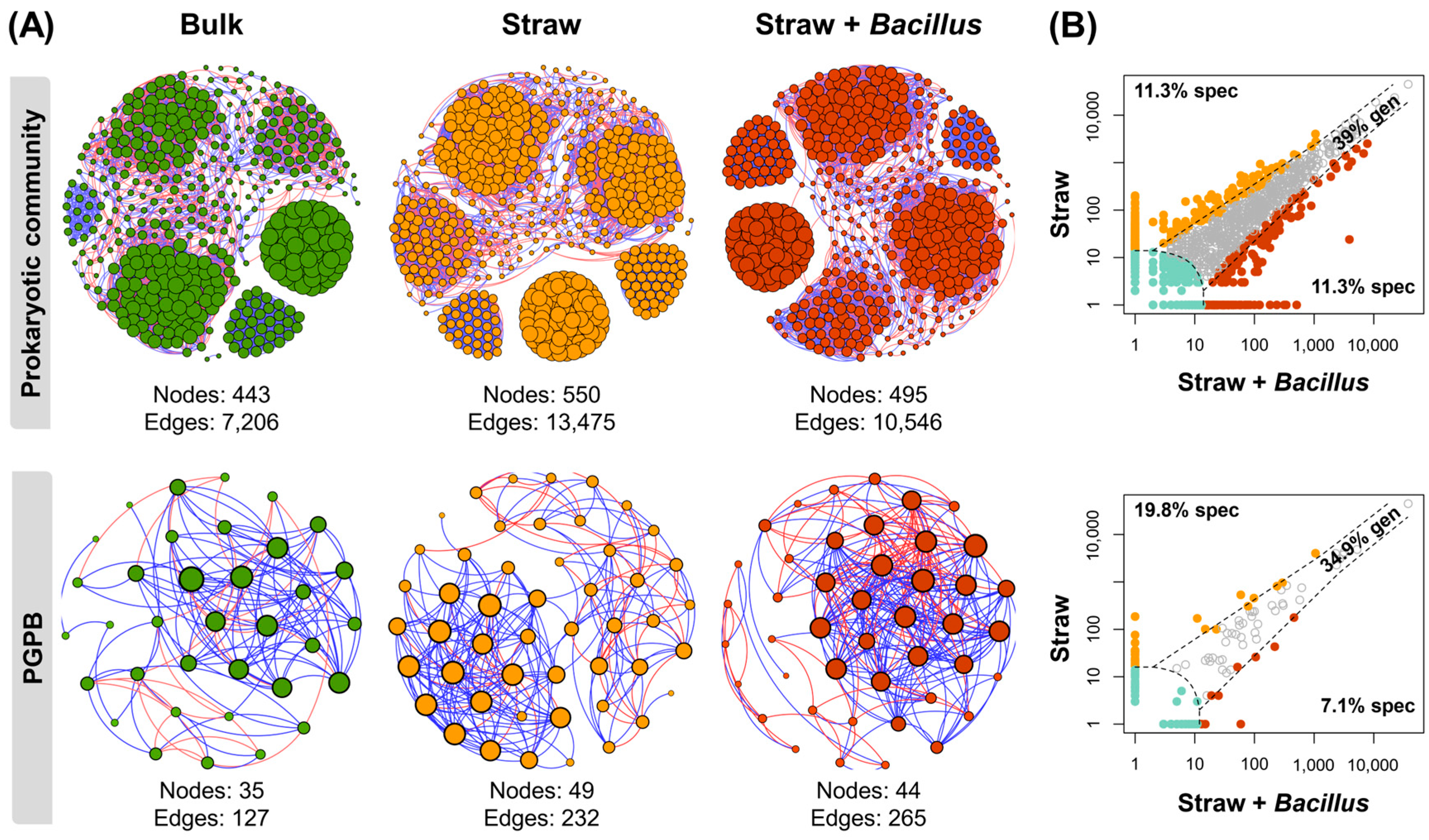
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Song, Y.; Gao, M.; Li, Z. Impacts of straw return methods on crop yield, soil organic matter, and salinity in saline-alkali land in North China. Field Crops Res. 2025, 322, 109752. [Google Scholar] [CrossRef]
- Ninkuu, V.; Liu, Z.; Qin, A.; Xie, Y.; Song, X.; Sun, X. Impact of straw returning on soil ecology and crop yield: A review. Heliyon 2025, 11, e41651. [Google Scholar] [CrossRef]
- Liu, R.Z.; Borjigin, Q.; Gao, J.L.; Yu, X.F.; Hu, S.P.; Li, R.P. Effects of different straw return methods on soil properties and yield potential of maize. Sci. Rep. 2024, 14, 28682. [Google Scholar] [CrossRef] [PubMed]
- Xia, L.; Lam, S.K.; Wolf, B.; Kiese, R.; Chen, D.; Butterbach-Bahl, K. Trade-offs between soil carbon sequestration and reactive nitrogen losses under straw return in global agroecosystems. Glob. Change Biol. 2018, 24, 5919–5932. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Dang, P.; Haegeman, B.; Han, X.; Wang, X.; Pu, X.; Qin, X.; Siddique, K.H.M. The effects of straw return on soil bacterial diversity and functional profiles: A meta-analysis. Soil. Biol. Biochem. 2024, 195, 109484. [Google Scholar] [CrossRef]
- Zhao, Y.H.; Wang, N.; Yu, M.K.; Yu, J.G.; Xue, L.H. Rhizosphere and straw return interactively shape rhizosphere bacterial community composition and nitrogen cycling in paddy soil. Front. Microbiol. 2022, 13, 945927. [Google Scholar] [CrossRef]
- Moreau, D.; Bardgett, R.D.; Finlay, R.D.; Jones, D.L.; Philippot, L.A. A plant perspective on nitrogen cycling in the rhizosphere. Funct. Ecol. 2019, 33, 540–552. [Google Scholar] [CrossRef]
- Kong, Z.; Liu, H. Modification of rhizosphere microbial communities: A possible mechanism of plant growth promoting rhizobacteria enhancing plant growth and fitness. Front. Plant Sci. 2022, 13, 920813. [Google Scholar] [CrossRef]
- Chen, S.; Xiang, X.; Ma, H.; Penttinen, P.; Zheng, T.; Huang, X.; Fan, G. Response of soil bacterial communities in wheat rhizosphere to straw mulching and N fertilization. Front. Microbiol. 2022, 13, 982109. [Google Scholar] [CrossRef]
- Wang, M.; Qi, X.; Shi, Y.; Zhao, J.; Ahmad, S.; Akhtar, K.; Chen, B.; Lian, T.; He, B.; Wen, R. Sugarcane straw returning is an approaching technique for the improvement of rhizosphere soil functionality, microbial community, and yield of different sugarcane cultivars. Front. Microbiol. 2023, 14, 1133973. [Google Scholar] [CrossRef]
- Xia, H.; Liu, H.; Gong, P.; Li, P.; Xu, Q.; Zhang, Q.; Sun, M.; Meng, Q.; Ye, F.; Yin, W. Study of the mechanism by which Bacillus subtilis improves the soil bacterial community environment in severely saline-alkali cotton fields. Sci. Total Environ. 2025, 958, 178000. [Google Scholar] [CrossRef] [PubMed]
- Sui, J.; Yu, Q.; Yang, K.; Yang, J.; Li, C.; Liu, X. Effects of Bacillus subtilis T6-1 on the Rhizosphere Microbial Community Structure of Continuous Cropping Poplar. Biology 2022, 11, 791. [Google Scholar] [CrossRef] [PubMed]
- Cantarella, H.; Quaggio, J.A.; van Raij, B.; Otto, R.; Penatti, C.P.; Rossetto, R.; Vitti, G.C.; Korndörfer, G.H.; Trivelin, P.C.O.; Mellis, E.V.; et al. Cana-de-açúcar. In Boletim 100: Recomendações de Adubação e Calagem para o Estado de São Paulo, 2nd ed.; Instituto Agronômico de Campinas (IAC): Campinas, Brazil, 2022; pp. 275–290. [Google Scholar]
- Caporaso, J.G.; Lauber, C.L.; Walters, W.A.; Berg-Lyons, D.; Lozupone, C.A.; Turnbaugh, P.J.; Knight, R. Global patterns of 16S rRNA diversity at a depth of millions of sequences per sample. Proc. Natl. Acad. Sci. USA 2011, 108, 4516–4522. [Google Scholar] [CrossRef]
- Callahan, B.J.; McMurdie, P.J.; Holmes, S.P. Exact sequence variants should replace operational taxonomic units in marker-gene data analysis. ISME J. 2017, 11, 2639–2643. [Google Scholar] [CrossRef]
- Quast, C.; Pruesse, E.; Yilmaz, P.; Gerken, J.; Schweer, T.; Yarza, P.; Peplies, J.; Glöckner, F.O. The SILVA ribosomal RNA gene database project: Improved data processing and web-based tools. Nucleic Acids Res. 2013, 41, D590–D596. [Google Scholar] [CrossRef] [PubMed]
- Bastian, M.; Heymann, S.; Jacomy, M. Gephi: An Open Source Software for Exploring and Manipulating Networks. In Proceedings of the Third International AAAI Conference on Weblogs and Social Media, San Jose, CA, USA, 17–20 May 2009. [Google Scholar]
- Chazdon, R.L.; Chao, A.; Colwell, R.K.; Lin, S.Y.; Norden, N.; Letcher, S.G.; Clark, D.B.; Finegan, B.; Arroyo, J.P. A Novel Statistical Method for Classifying Habitat Generalists and Specialists. Ecology 2011, 92, 1332–1343. [Google Scholar] [CrossRef]
- Schwab, S.; Pires, A.S.; Candido, G.Z.; Saggin Júnior, O.J.; Reis, V.M.; Cruz, L.M. Analysis of the endophytic microbiota of roots and culms of two commercial sugarcane cultivars inoculated with a synthetic microbial community. Appl. Soil Ecol. 2024, 195, 105235. [Google Scholar] [CrossRef]
- Chen, J.; Li, N.; Chang, J.; Ren, K.; Zhou, J.; Yang, G. Taxonomic structure of rhizosphere bacterial communities and its association with the accumulation of alkaloidal metabolites in Sophora flavescens. Front. Microbiol. 2021, 12, 781316. [Google Scholar] [CrossRef]
- Bak, G.R.; Lee, K.K.; Clark, I.M.; Mauchline, T.H.; Kavamura, V.N.; Jee, S.; Lee, J.T.; Kim, H.; Lee, Y.H. Changes in the potato rhizosphere microbiota richness and diversity occur in a growth stage-dependent manner. Sci. Rep. 2025, 15, 2284. [Google Scholar] [CrossRef]
- Paes da Costa, D.; das Graças Espíndola da Silva, T.; Sérgio Ferreira Araujo, A.; Prudêncio de Araujo Pereira, A.; William Mendes, L.; dos Santos Borges, W.; Felix da França, R.; Alberto Fragoso de Souza, C.; Alves da Silva, B.; Oliveira Silva, R.; et al. Soil fertility impact on recruitment and diversity of the soil microbiome in sub-humid tropical pastures in Northeastern Brazil. Sci. Rep. 2024, 14, 3919. [Google Scholar] [CrossRef]
- Primo, A.C.A.; Lustosa Filho, J.F.; Marota, H.B.; Tonucci, R.G.; da Silva, I.R.; de Oliveira, T.S. Different composition of plant residues as a driver of microbial community structure and soil organic matter composition: A microcosm study. Pedobiologia 2024, 106, 150985. [Google Scholar] [CrossRef]
- Romero, F.; Hilfiker, S.; Edlinger, A.; Held, A.; Hartman, K.; Labouyrie, M.; van der Heijden, M.G.A. Soil microbial biodiversity promotes crop productivity and agro-ecosystem functioning in experimental microcosms. Sci. Total Environ. 2023, 885, 163683. [Google Scholar] [CrossRef] [PubMed]
- Di Benedetto, N.A.; Corbo, M.R.; Campaniello, D.; Cataldi, M.P.; Bevilacqua, A.; Sinigaglia, M.; Flagella, Z. The role of Plant Growth Promoting Bacteria in improving nitrogen use efficiency for sustainable crop production: A focus on wheat. AIMS Microbiol. 2017, 3, 413–434. [Google Scholar] [CrossRef] [PubMed]
- Fanai, A.; Bohia, B.; Lalremruati, F.; Lalhriatpuii, N.; Lalrokimi; Lalmuanpuii, R.; Singh, P.K.; Zothanpuia. Plant growth promoting bacteria (PGPB)-induced plant adaptations to stresses: An updated review. PeerJ 2024, 12, e17882. [Google Scholar] [CrossRef]
- Nishisaka, C.S.; Ventura, J.P.; Bais, H.P.; Mendes, R. Role of Bacillus subtilis exopolymeric genes in modulating rhizosphere microbiome assembly. Environ. Microbiome 2024, 19, 33. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Zhang, W.; Liu, Y.; Chen, L.; Li, Y.; Wang, J.; Zhang, X.; Shen, G. Long-term straw return enhances soil microbial co-occurrence patterns and network stability in a rice-wheat cropping system. Front. Microbiol. 2025, 16, 1533839. [Google Scholar] [CrossRef]
- Zhang, S.; Deng, Q.; Wang, Y.P.; Chen, J.; Yu, M.; Fang, X.; He, H.; Chen, J.; Xu, P.; Wang, S.; et al. Linkage of microbial living communities and residues to soil organic carbon accumulation along a forest restoration gradient in southern China. For. Ecosyst. 2021, 8, 57. [Google Scholar] [CrossRef]
- Akinsemolu, A.A.; Onyeaka, H.; Odion, S.; Adebanjo, I. Exploring Bacillus subtilis: Ecology, biotechnological applications, and future prospects. J. Basic Microbiol. 2024, 64, 2300614. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
de Alcântara Neto, F.; Pinheiro, D.A.; Rocha, S.M.B.; Leite, M.R.L.; Costa, R.M.; da Silva, J.M.; Ventura, S.H.; Costa, M.K.L.; Sousa, T.K.d.S.A.; Miranda, R.d.S.; et al. Bacterial Community in Sugarcane Rhizosphere Under Bacillus subtilis Inoculation and Straw Return. Soil Syst. 2025, 9, 44. https://doi.org/10.3390/soilsystems9020044
de Alcântara Neto F, Pinheiro DA, Rocha SMB, Leite MRL, Costa RM, da Silva JM, Ventura SH, Costa MKL, Sousa TKdSA, Miranda RdS, et al. Bacterial Community in Sugarcane Rhizosphere Under Bacillus subtilis Inoculation and Straw Return. Soil Systems. 2025; 9(2):44. https://doi.org/10.3390/soilsystems9020044
Chicago/Turabian Stylede Alcântara Neto, Francisco, Danielly Araújo Pinheiro, Sandra Mara Barbosa Rocha, Marcos Renan Lima Leite, Romário Martins Costa, Janderson Moura da Silva, Sabrina Hermelindo Ventura, Mayanna Karlla Lima Costa, Thâmara Kelly dos Santos Apollo Sousa, Rafael de Souza Miranda, and et al. 2025. "Bacterial Community in Sugarcane Rhizosphere Under Bacillus subtilis Inoculation and Straw Return" Soil Systems 9, no. 2: 44. https://doi.org/10.3390/soilsystems9020044
APA Stylede Alcântara Neto, F., Pinheiro, D. A., Rocha, S. M. B., Leite, M. R. L., Costa, R. M., da Silva, J. M., Ventura, S. H., Costa, M. K. L., Sousa, T. K. d. S. A., Miranda, R. d. S., Caetano, K. R., Medeiros, E. V. d., Pereira, A. P. d. A., Mendes, L. W., & Araujo, A. S. F. (2025). Bacterial Community in Sugarcane Rhizosphere Under Bacillus subtilis Inoculation and Straw Return. Soil Systems, 9(2), 44. https://doi.org/10.3390/soilsystems9020044







