Experimental and Numerical Simulation Study of the Influence of Fe(C5H5)2-SiO2 Composite Dry Powders on Characteristics of Hydrogen/Methane/Air Explosion
Abstract
1. Introduction
2. Experimental and Numerical Methods
2.1. Materials and Preparation
2.2. Experimental Apparatus and Procedures
2.3. Numerical Simulation
3. Results and Discussion
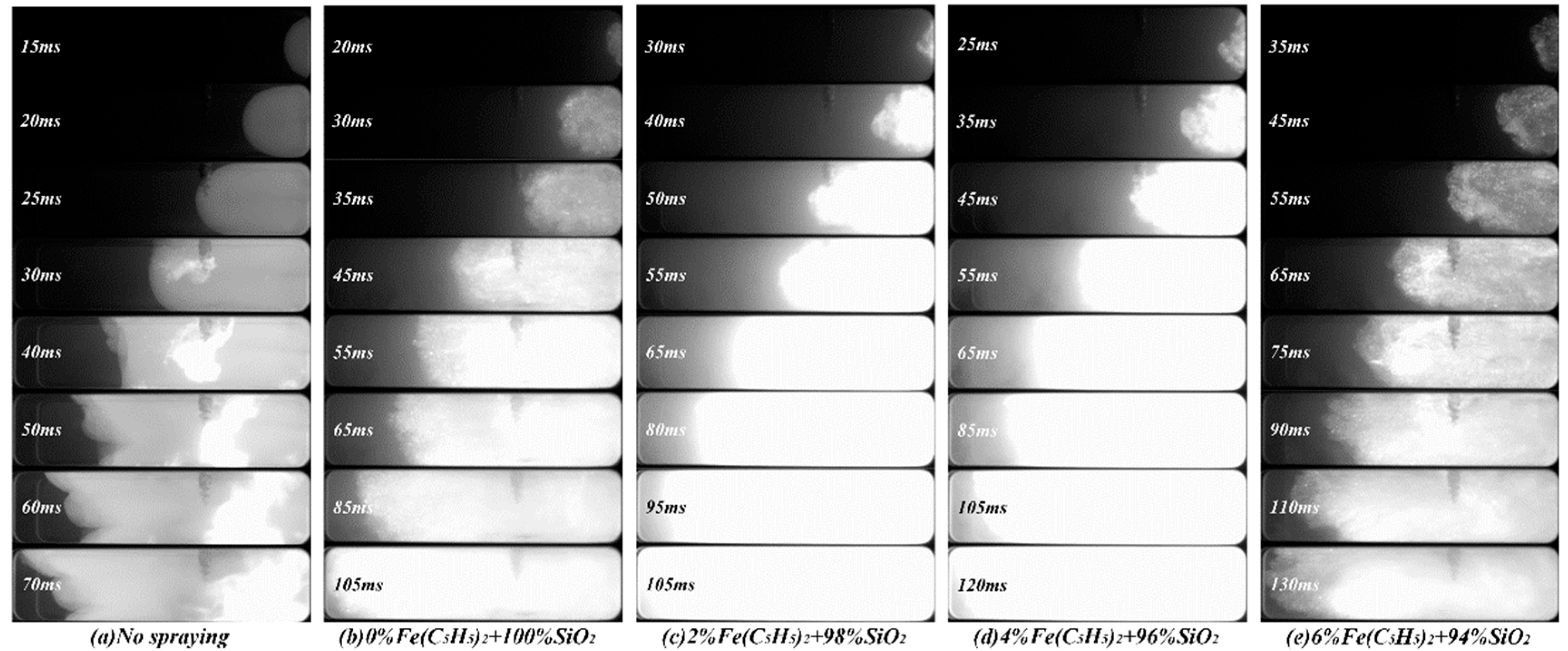
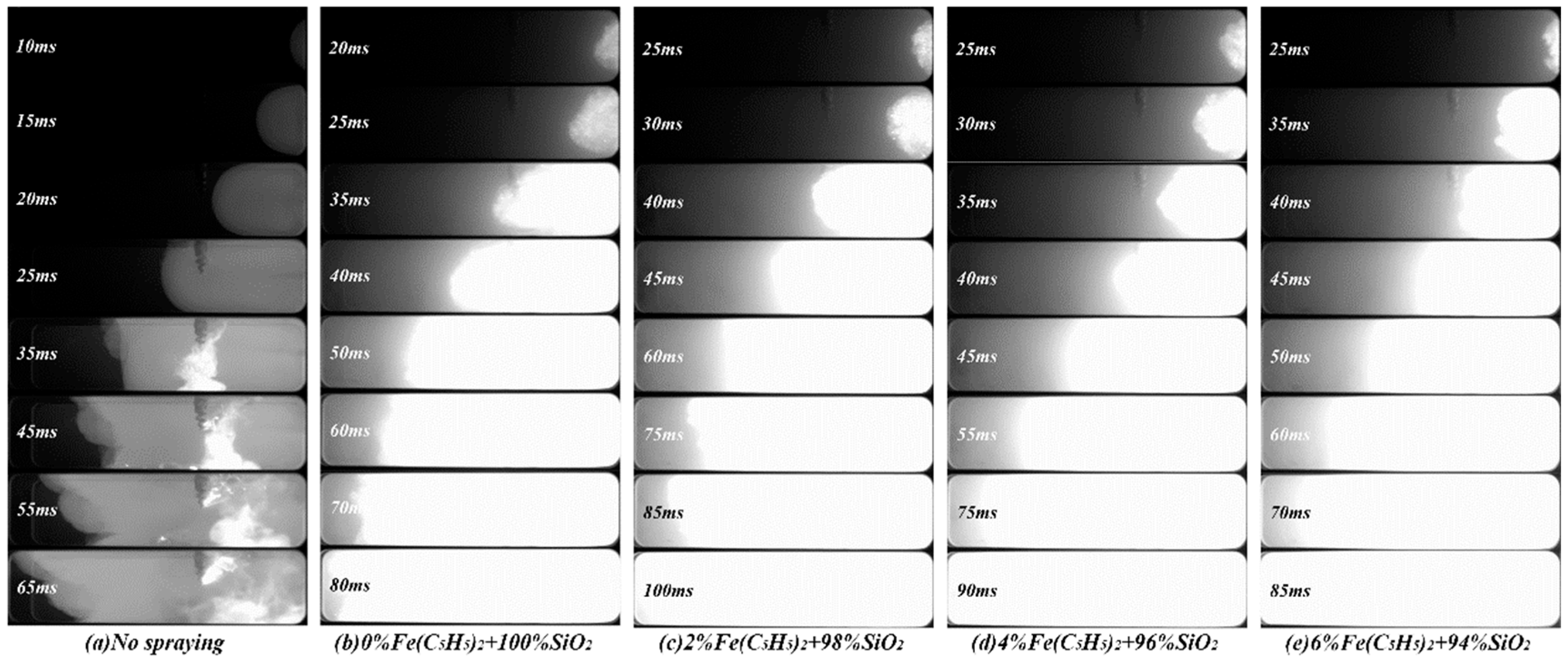
3.1. Effects on Flame Structure
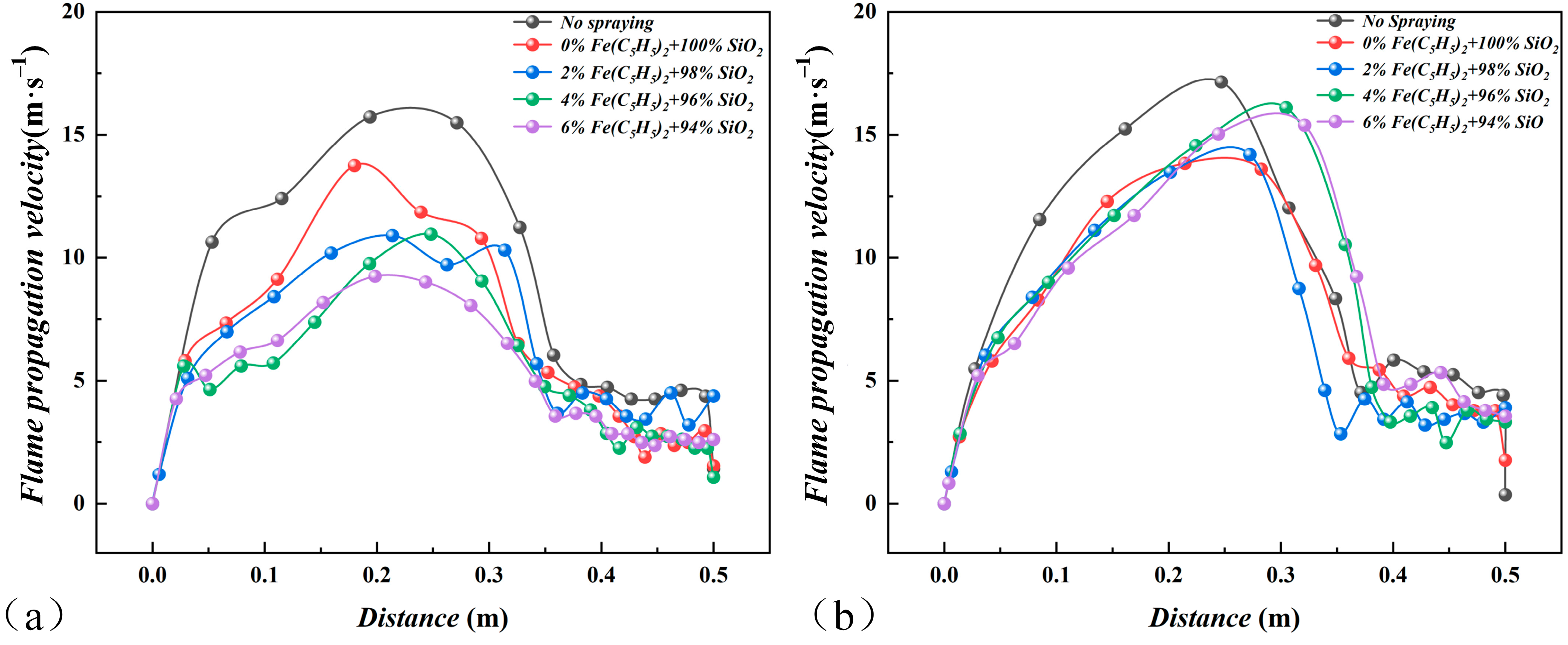
3.2. Effects on Flame Propagation Velocity
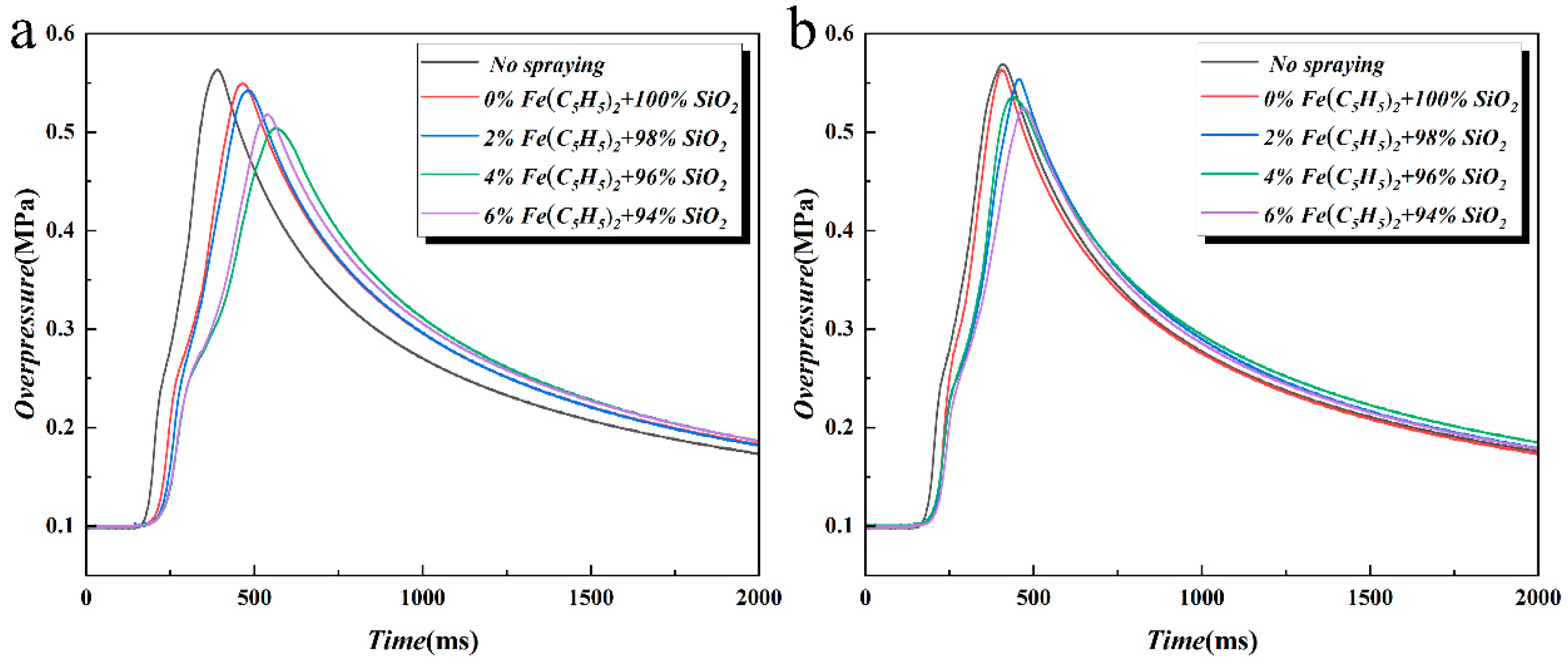
3.3. Effects on Pressure Propagation
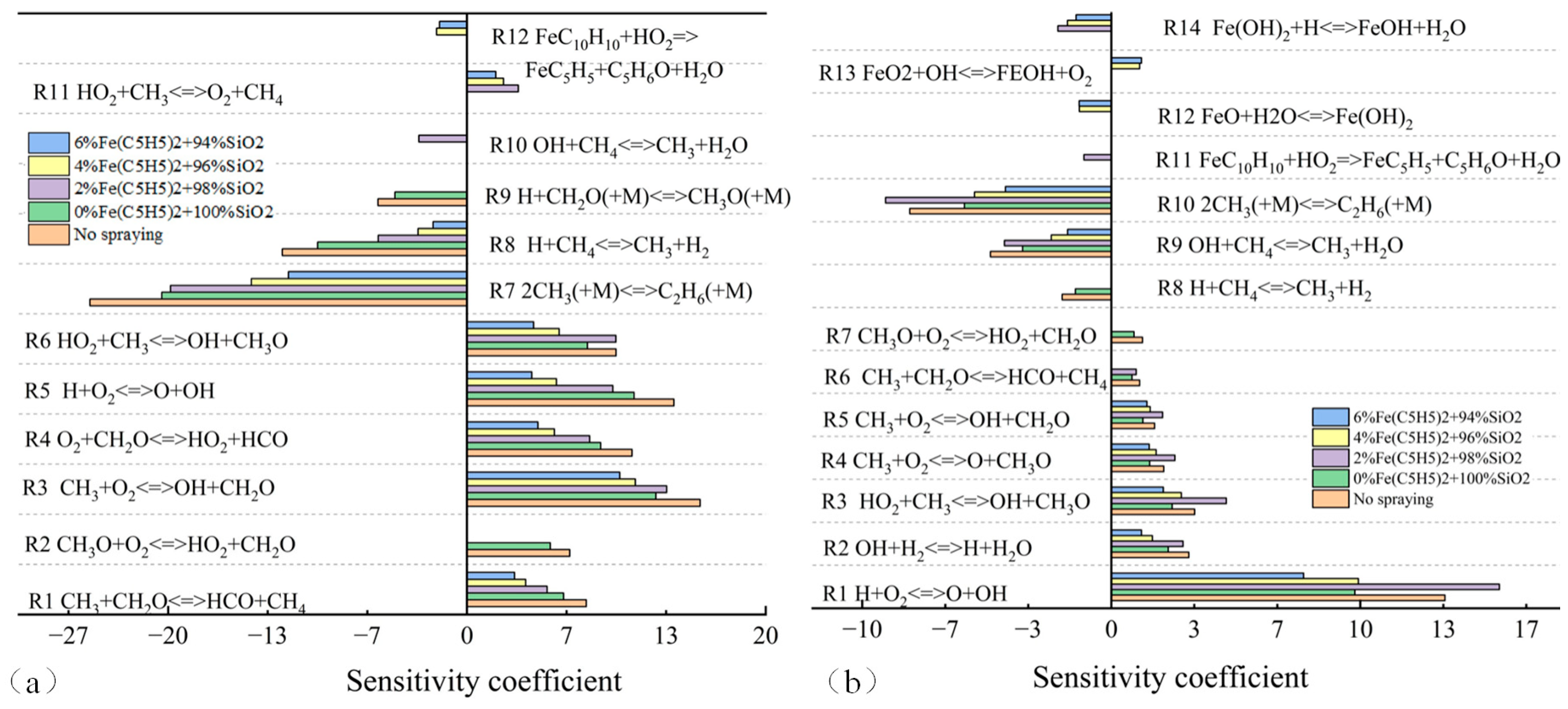
3.4. Temperature Sensitivity Analysis
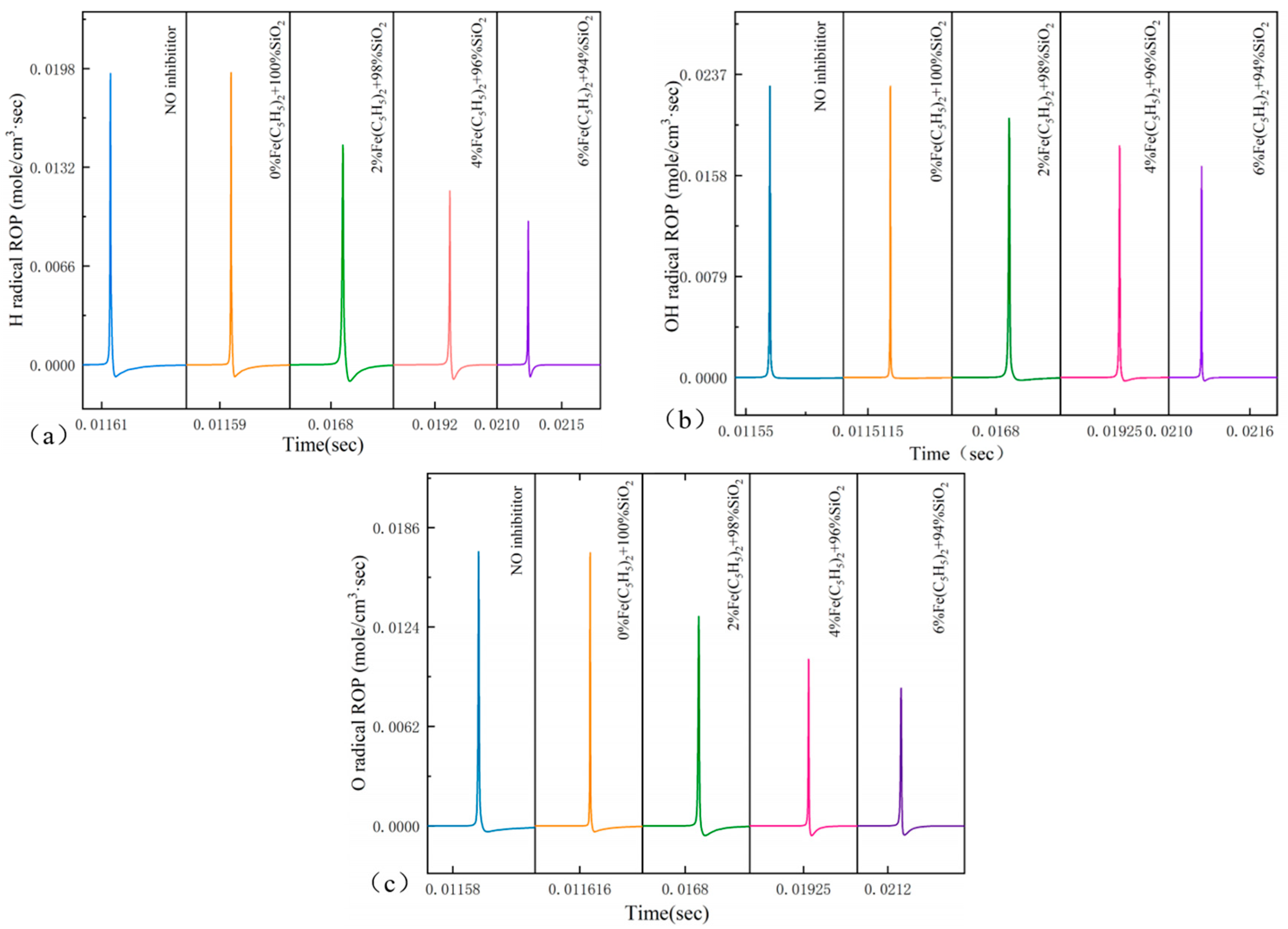
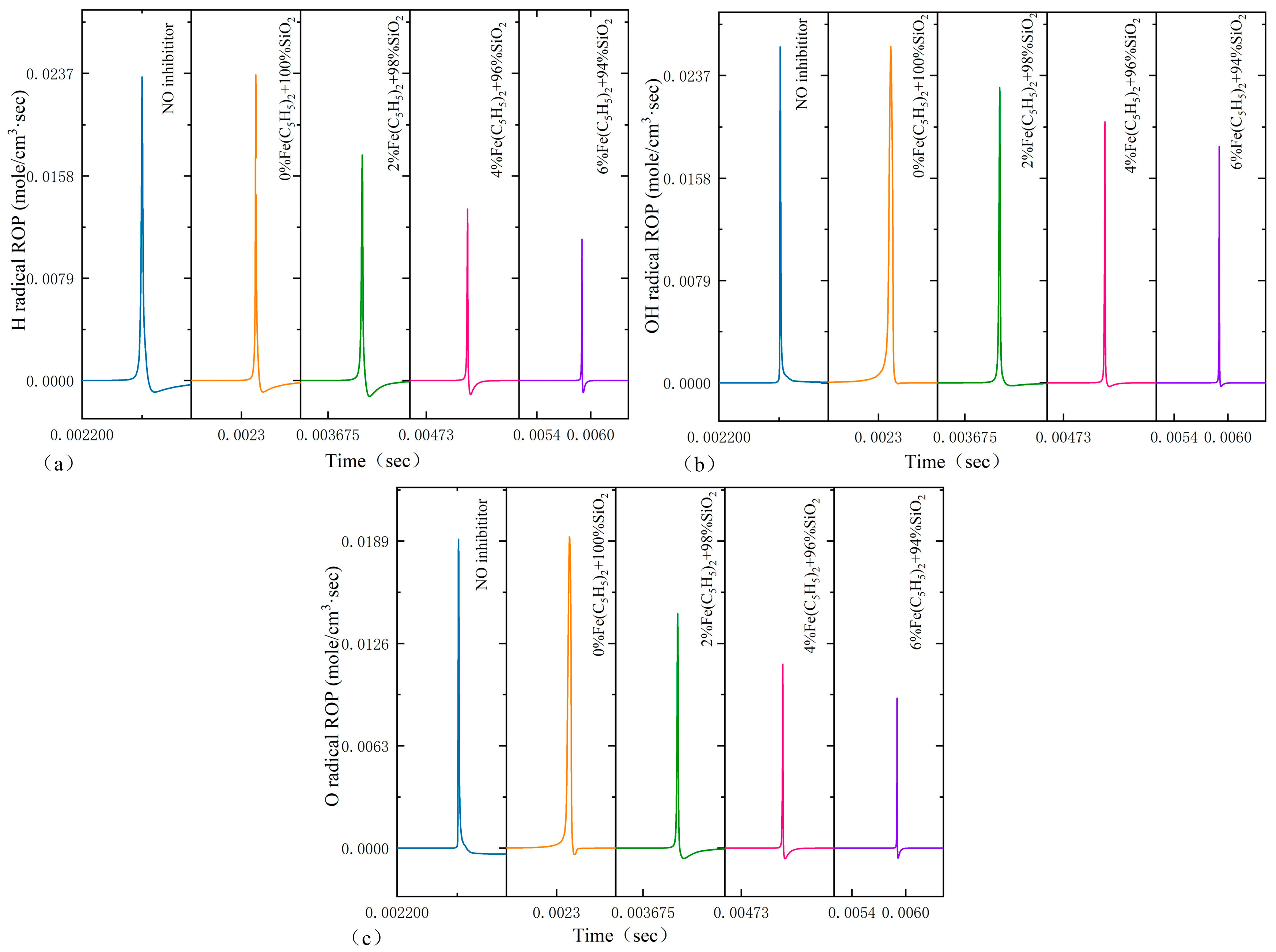
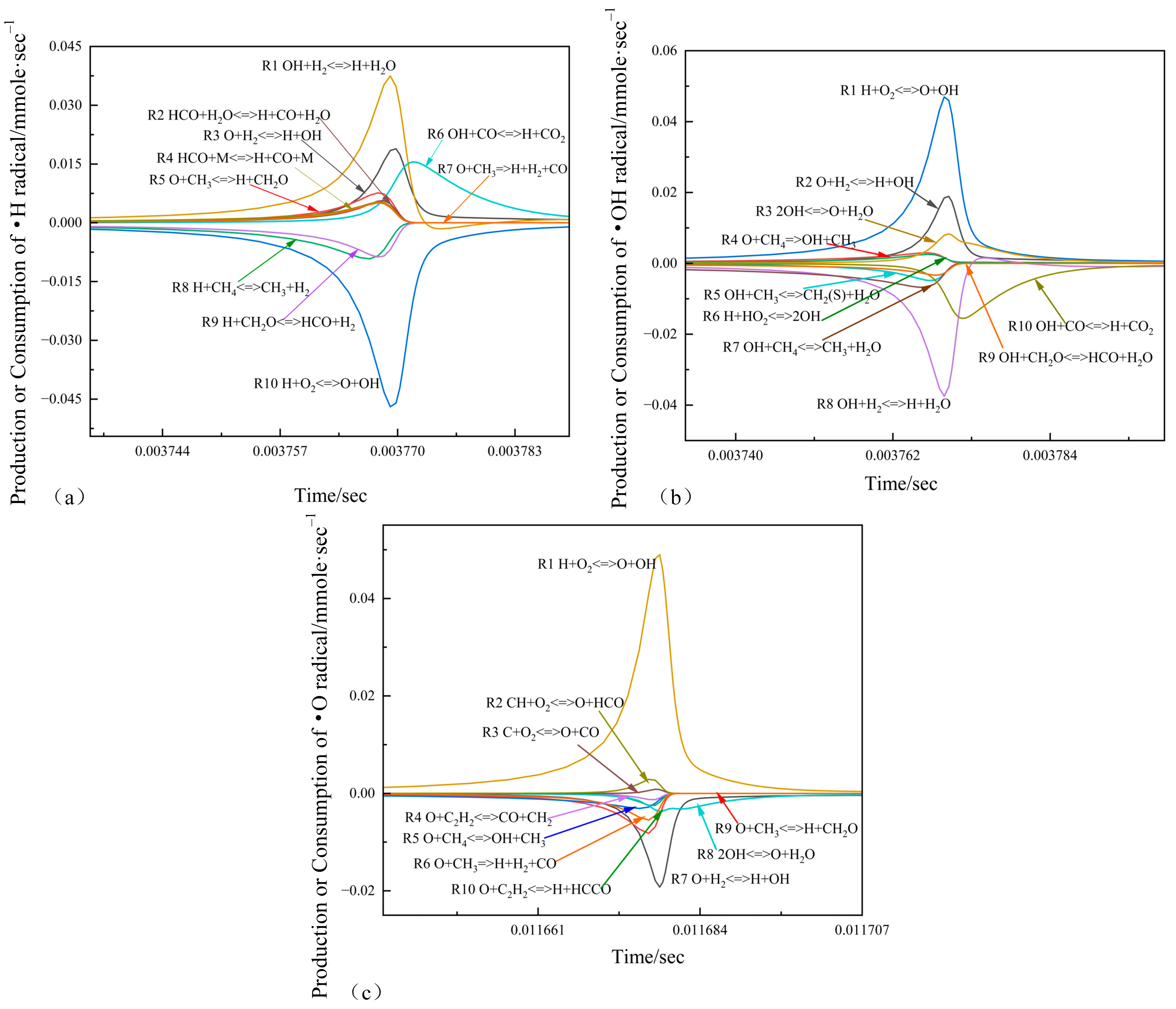
3.5. Rate of Production for Key Radicals
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Lee, J.E.; Jeon, K.J.; Show, P.L.; Lee, I.H.; Jung, S.C.; Choi, Y.J.; Rhee, G.H.; Lin, K.Y.; Park, Y.K. Mini review on H2 production from electrochemical water splitting according to special nanostructured morphology of electrocatalysts. Fuel 2022, 308, 122048. [Google Scholar] [CrossRef]
- Zhang, J.; Zhu, X.; Guo, Y.; Teng, Y.; Liu, M.; Li, Q.; Wang, Q.; Wang, C. Numerical Study of Homogenous/Inhomogeneous Hydrogen–Air Explosion in a Long Closed Channel. Fire 2024, 7, 418. [Google Scholar] [CrossRef]
- Ban, J.; Zhu, L.; Shen, R.; Yang, W.; Hao, M.; Liu, G.; Wang, X. Research on hydrogen distribution characteristics in town hydrogen-doped methane pipeline. Sci. Rep. 2024, 14, 20347. [Google Scholar] [CrossRef]
- Li, R.; Luo, Z.; Wang, T.; Cheng, F.; Lin, H.; Zhu, X. Effect of initial temperature and H2 addition on explosion characteristics of H2-poor/CH4/air mixtures. Energy 2020, 213, 118979. [Google Scholar] [CrossRef]
- Su, B.; Luo, Z.; Wang, T.; Xie, C.; Cheng, F. Chemical kinetic behaviors at the chain initiation stage of CH4/H2/air mixture. J. Hazard. Mater. 2020, 403, 123680. [Google Scholar] [CrossRef]
- Mi, H.; Luo, N.; Shao, P.; Yi, H.; Wang, S.; Wang, W.; Niu, Y.; Yang, A.; Jiang, X.; Feng, Y.; et al. Interactive mechanisms of CF3CHFCF3 with H2-CH4-air mixture explosion: A synergistic study using chemical kinetic simulation and density functional theory. Fuel 2025, 381, 133603. [Google Scholar] [CrossRef]
- Sun, Y.; Yuan, B.; Chen, X.; Li, K.; Wang, L.; Yun, Y.; Fan, A. Suppression of methane/air explosion by kaolinite-based multi-component inhibitor. Powder Technol. 2018, 343, 279–286. [Google Scholar] [CrossRef]
- Liang, X.; Zhou, X.; Lu, X.; Liu, A. Investigation on slag resource utilization: KHCO3/modified slag composite powder applied to methane/air explosion suppression. Powder Technol. 2024, 441, 119814. [Google Scholar] [CrossRef]
- Xiao, Q.; Meng, X.; Li, Z.; Ma, X.; Yan, K.; Wang, Z. Inhibition of aluminum-silicon alloy dust explosion and flame by KH2PO4/montmorillonite composite powder. Fire Mater. 2022, 46, 797–808. [Google Scholar] [CrossRef]
- Krasnyansky, M. Prevention and suppression of explosions in gas-air and dust-air mixtures using powder aerosol-inhibitor. J. Loss Prev. Process Ind. 2006, 19, 729–735. [Google Scholar] [CrossRef]
- Yu, M.; Wang, X.; Zheng, K.; Han, S. Experimental investigation of gas explosion suppression by catalytic composite powder inhibitor. J. China Coal Soc. 2021, 46, 3212–3220. [Google Scholar]
- Jia, J.; Tian, X. Experimental study on inhibiting methane-coal dust explosion by APP-diatomite composite explosion suppressant in the pipe network. Arab. J. Chem. 2024, 17, 105977. [Google Scholar] [CrossRef]
- Yan, C.; Pan, X.; Hua, M.; Li, S.; Guo, X.; Zhang, C. Study on the fire extinguishing efficiency and mechanism of composite superfine dry powder containing ferrocene. Fire Saf. J. 2022, 130, 103606. [Google Scholar] [CrossRef]
- Babushok, V.; Tsang, W. Inhibitor rankings for alkane combustion. Combust. Flame 2000, 123, 488–506. [Google Scholar] [CrossRef]
- Linteris, G.T.; Rumminger, M.D.; Babushok, V.; Tsang, W. Flame inhibition by ferrocene and blends of inert and catalytic agents. Proc. Combust. Inst. 2000, 28, 2965–2972. [Google Scholar] [CrossRef]
- Rumminger, M.D.; Reinelt, D.; Babushok, V.; Linteris, G.T. Inhibition of flames by iron pentacarbonyl. In Proceedings of the Halon Options Technical Working Conference, Albuquerque, NM, USA, 12–14 May 1998; pp. 145–156. [Google Scholar]
- Reinelt, D.; Linteris, G.T. Experimental study of the flame inhibition effect of iron pentacarbonyl. In Proceedings of the Halon Options Technical Working Conference, Albuquerque, NM, USA, 7–9 May 1996. [Google Scholar]
- Rausch, M.; Vogel, M.; Rosenberg, H. Ferrocene: A novel organometallic compound. J. Chem. Educ. 1957, 34, 268. [Google Scholar] [CrossRef]
- Howard, J.B.; Kausch, W.J., Jr. Soot control by fuel additives. Prog. Energy Combust. Sci. 1980, 6, 263–276. [Google Scholar] [CrossRef]
- Kasper, M.; Siegmann, K. The influence of ferrocene on PAH synthesis in acetylene and methane diffusion flames. Combust. Sci. Technol. 1998, 140, 333–350. [Google Scholar] [CrossRef]
- Kasper, M.; Sattler, K.; Siegmann, K.; Matter, U.; Siegmann, H. The influence of fuel additives on the formation of carbon during combustion. J. Aerosol. Sci. 1999, 30, 217–225. [Google Scholar] [CrossRef]
- Wang, Z.H.; Weng, W.B.; He, Y.; Li, Z.S.; Cen, K.F. Effect of H2/CO ratio and N2/CO2 dilution rate on laminar burning velocity of syngas investigated by direct measurement and simulation. Fuel 2015, 141, 285–292. [Google Scholar] [CrossRef]
- Fenard, Y.; Song, H.; Dauphin, R.; Vanhove, G. An engine-relevant kinetic investigation into the anti-knock effect of organometallics through the example of ferrocene. Proc. Combust. Inst. 2019, 37, 547–554. [Google Scholar] [CrossRef]
- Jensen, D.E.; Jones, G.A. Catalysis of radical recombination in flames by iron. J. Chem. Phys. 1974, 60, 3421–3425. [Google Scholar] [CrossRef]
- Rumminger, M.D.; Reinelt, D.; Babushok, V.; Linteris, G. Numerical study of the inhibition of premixed and diffusion flames by iron pentacarbonyl. Combust. Flame 1999, 116, 207–219. [Google Scholar] [CrossRef]
- Kellogg, C.B.; Irikura, K.K. Gas-Phase thermochemistry of iron oxides and hydroxides: Portrait of a super-efficient flame suppressant. J. Phys. Chem. A 1999, 103, 1150–1159. [Google Scholar] [CrossRef]
- Vanpee, M.; Shirodkar, P.P. A study of flame inhibition by metal compounds. Symp. (Int.) Combust. 1979, 17, 787–795. [Google Scholar] [CrossRef]
- Li, Z.; Zuo, Q. Experimental study on the inhibition characteristics of n-heptane pool fire by ferrocene. Fire Sci. Technol. 2010, 29, 752–754. [Google Scholar]
- Wang, Q.; Sun, Y.; Jiang, J.; Deng, J.; Shu, C.-M.; Luo, Z.; Wang, Q. Inhibiting effects of gas–particle mixtures containing CO2, Mg(OH)2 particles, and NH4H2PO4 particles on methane explosion in a 20-L closed vessel. J. Loss Prev. Process Ind. 2020, 64, 104082. [Google Scholar] [CrossRef]
- Wei, C.; Li, H.; Luo, Z.; Wang, T.; Yu, Y.; Wu, M.; Qi, B.; Yu, M. Quantitative analysis of flame luminance and explosion pressure in liquefied petroleum gas explosion and inerting: Grey relation analysis and kinetic mechanisms. Energy 2024, 304, 132046. [Google Scholar] [CrossRef]
- Bhattacharjee, A.; Rooj, A.; Roy, D.; Roy, M. Thermal Decomposition study of ferrocene [(C5H5)2Fe]. J. Exp. Phys. 2014, 2014, 513268. [Google Scholar] [CrossRef]
- Xiao, H.; Duan, Q.; Sun, J. Premixed flame propagation in hydrogen explosions. Renew. Sustain. Energy Rev. 2018, 81, 1988–2001. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zheng, Z.; Liao, H.; Mi, H.; Liao, K.; Zhang, H.; Li, Y.; Ren, Y.; Li, Z.; Li, N.; Xia, W. Experimental and Numerical Simulation Study of the Influence of Fe(C5H5)2-SiO2 Composite Dry Powders on Characteristics of Hydrogen/Methane/Air Explosion. Fire 2025, 8, 198. https://doi.org/10.3390/fire8050198
Zheng Z, Liao H, Mi H, Liao K, Zhang H, Li Y, Ren Y, Li Z, Li N, Xia W. Experimental and Numerical Simulation Study of the Influence of Fe(C5H5)2-SiO2 Composite Dry Powders on Characteristics of Hydrogen/Methane/Air Explosion. Fire. 2025; 8(5):198. https://doi.org/10.3390/fire8050198
Chicago/Turabian StyleZheng, Zhiqian, Huiqian Liao, Hongfu Mi, Kaixuan Liao, Haoliang Zhang, Yi Li, Yanhui Ren, Zhijun Li, Nanfang Li, and Wei Xia. 2025. "Experimental and Numerical Simulation Study of the Influence of Fe(C5H5)2-SiO2 Composite Dry Powders on Characteristics of Hydrogen/Methane/Air Explosion" Fire 8, no. 5: 198. https://doi.org/10.3390/fire8050198
APA StyleZheng, Z., Liao, H., Mi, H., Liao, K., Zhang, H., Li, Y., Ren, Y., Li, Z., Li, N., & Xia, W. (2025). Experimental and Numerical Simulation Study of the Influence of Fe(C5H5)2-SiO2 Composite Dry Powders on Characteristics of Hydrogen/Methane/Air Explosion. Fire, 8(5), 198. https://doi.org/10.3390/fire8050198





