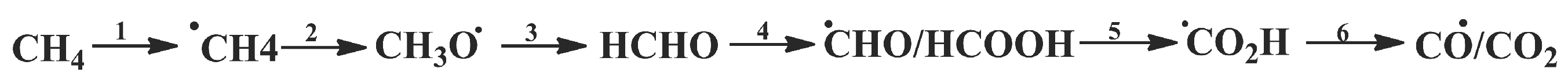1. Introduction
Energetic materials (EMs), whether they are explosives, propellants, or pyrotechnic materials, have found extensive civilian and military applications [
1,
2]. The ability of EMs to undergo highly vigorous decomposition, be it without combustion, through deflagration, or through detonation [
3,
4], is both an aspect of their utility and a significant safety concern. Although the safety parameters [
5] and energetic properties [
6] of materials belonging to the various groups of EMs can be monitored by a wide array of methods, shaping these parameters and properties requires a thorough understanding of the underlying physical and chemical processes.
In terms of safety parameters, EMs can undergo accidental initiation through a wide variety of stimuli, e.g., mechanical, thermal, electrical, electromagnetic. In many cases, the action of these stimuli causes internal heating of the EM, e.g., frictive heating of EM particles upon application of friction, absorption of electromagnetic radiation followed by non-radiant transitions. Simultaneously, the energetic properties of EMs are to a large extent shaped by the net heats of the underlying chemical reactions and the thermal features of the materials [
7]. Consequently, thermal analysis methods play an extremely important role in studying EMs [
8,
9] as they can provide insight into factors affecting both the safety and performance of these materials.
The results provided by thermal analysis, relevant as they are to the processes taking place in EMs, do not provide structural information. As such, in all but the most simple cases, they need to be supported with additional experimental evidence in order to provide well-grounded insights. A significant limitation, in comparison to the study of classical chemical reactions, is the fact that chemical reactions in EMs take place extremely rapidly, i.e., with complete conversion of the reagents taking on the order of milliseconds (deflagration) or even microseconds (detonation) [
10,
11]. Due to the rapid nature of the processes, few of the typical experimental methods can be applied directly and the mechanisms of the observed reactions often need to be pieced together based on fragmentary evidence. This fact, as well as the necessity to adapt the use of experimental methods to a significant degree can, however, lead to drawing extremely far-reaching conclusions without due experimental justification. This issue has already been noted in literature, as seen in the case of the criticism of kinetics studies that utilise a single heating rate in thermal analysis [
12].
In this work, we have reviewed a number of significant literature reports on the mechanisms of reactions taking place in EMs, examining them in term of the approaches used to study these mechanisms. Key experimental strategies, points of uncertainty, and approaches to adapting traditional instrumental methods are highlighted, so as to provide a critical perspective on the state of the art in this regard.
2. Combustion and Detonation of Energetic Materials
2.1. Case 1: Combustion of AN/C Mixtures
The combustion of mixtures of ammonium nitrate (AN) with various carbon materials (C) was examined in [
13]. Initially, the Authors worked on the assumption that the AN/C mixtures reacted according to Equation (
1). This assumption was deemed incorrect on the basis of stoichiometric mixtures being expected to show the highest combustion velocity, whereas experiments conducted for AN/C mixtures containing 7, 10, and 15 wt.% C showed that mixtures containing 15 wt.% C showed the highest combustion velocity. It should be noted, however, that no effort was made to determine whether mixtures containing even more carbon would have exhibited higher combustion velocities, and that a combustion velocity vs. carbon content dependence was posited based solely on these three data points. As such, the carbon content offering the highest combustion velocity, and, therefore, corresponding to a stoichiometric mixture, was not identified.
With Equation (
1) being proven incorrect, the authors conducted DSC measurements of the AN/C mixtures. Onset temperatures of the observed exothermic signals were taken as a criterion of different reaction mechanisms, i.e., of the oxidation of the carbon by either HNO
3 (peak onset at approx. 180
C) or NO
X (peak onset at approx. 230–240
C). No evidence was presented for this particular choice of oxidising agents and, according to the presented data, one sample, i.e., AN/carbon black (average particle diameter of 24 nm) underwent oxidation by either of the two agents when the experiment was repeated. In the case of oxidation by HNO
3, a summary reaction, given in Equation (
2), was proposed. This was posited without any experimental verification and without consideration of the boiling point of HNO
3 being approx. 85
C at ambient pressure [
14], i.e., about 100
C below the onset of the observed DSC peak.
The abovementioned issues, as well as inconsistencies between the reported DSC and DTA data, cast significant doubt on the posited reaction mechanisms, suggesting the need for re-investigation of this subject.
2.2. Case 2: Combustion of Coloured Flame Pyrotechnics
An ambitious research effort was undertaken to elucidate the combustion mechanisms of a series of multi-component coloured flame pyrotechnic compositions [
15]. In this work, the Authors used the rate of heat emission, obtained via DSC measurements of the combustion of the samples, as a measure of the net reaction rate in the combusting systems. These data were used to attempt deconvolution of the thermograms, identification of on-going reactions, and matching them with known reaction pathways. Although no experimental verification of the identity of the on-going reactions was provided, the proposed model achieved a good fit to experimental data. Interestingly, the authors first concluded that the on-going processes were a superposition of reactions taking place for pure components, only to follow up with a conflicting conclusion that the changes in the apparent activation energy suggested a complex reaction mechanism.
As such, while the proposed approach appears to be highly useful for elucidating the number and kinetic features of on-going processes, its results need to be supported by additional data to confirm whether the correct chemical reaction equations were identified. Moreover, due to the fact that DSC offers information only about the net flow of heat through the sample, strongly exothermic processes, such as combustion, may serve to obscure the occurrence of endothermic processes, leading to incorrect conclusions.
2.3. Case 3: Deflagration to Detonation Transition of Loosely Packed Powdered Explosive (HMX)
In this work, the authors analyzed and calculated the mechanisms of deflagration and the deflagration to detonation transition in loosely packed powder explosive. For simulation, the authors used a two-phased flow model with the CE/SE method. The calculations allowed them to assume that detonations occurs more easily when there is a smaller difference in the porosity and a higher initial temperature. The authors also concluded that steady detonation occurs in areas where different physical quantities, like pressure, temperature, density, or velocity, reach equal peak values [
16]. In this paper, the authors did not provide any experimental study to confirm their computational studies. However, they confirmed that they results overlapped with high-temperature deflagration to detonation transition tube experiments [
17].
2.4. Case 4: The Combustion Process of Stoichiometric Aluminum/Copper(II) Oxide Thermite
Publication [
18] presented the results of investigation of a combustion process of Al/Cu
2O stoichiometric composition. The combustion process was experimentally investigated in an optical bomb and the reaction progress was monitored with a high-speed camera and a UV-VIS emission spectrometer. Unfortunately, the publication lacks information on the dimensions of the aluminum particles—this information is crucial to correctly characterize the burning process of thermites.
Despite this fact, the authors performed a reliable analysis of the burning process of this mixture. Determination of the condensed phase temperature was performed by calculating the grey body radiation of the background. The authors also provided an in-depth spectrum analysis and indicated the molecular bands—what is of interest is that the authors managed to model the diatomic band system of Cu2 and CuO.
2.5. Case 5: Investigation of the Burning Process and Determination of the Decomposition Reaction Mechanism of the Magnesium/Sodium Nitrate/Phenolic Resin Mixture
The aim of the study [
19] was the characterization of the combustion process of a Mg/NaNO
3 pyrotechnic mixture, as well as the determination of the mechanism of its decomposition reaction. The test was conducted under oxygen-free conditions and under pressure within the range of 1–101 kPa. The mass ratio of the components was chosen to achieve zero oxygen balance.
In the experimental part, the change in the flame structure depending on the pressure was shown. As the pressure decreased, the height of the mixture’s combustion flame also decreased, and at the top, a turbulent flame appeared. The authors rightly linked this to the decreasing influence of the buoyancy force, as a result of areas of lower pressure and thus lower air density. This, in turn, promoted the diffusion of gases produced by combustion. Additionally, the publication shows the results of the oxidation reaction zone of magnesium at different pressures and its dimensional parameters.
The publication also focuses on determining the dependence of light output on pressure. Using a transient light intensity meter, the dependence of the luminous intensity over time as a function of the pressure height (again, in the range of 1 kPa–101 kPa) was determined.The results showed that the luminescence period increased as the pressure decreased, but the intensity decreased. By using I-t curves, the burning rate of composition was also determined.
2.6. Case 6: Dynamic Diffusion Combustion of the Metal Particles and Its Influence on Infrared Radiation Output Characterization
The influence of the metal particles, which undergo dynamic diffusion combustion in the flow field, on the characterization of the infrared radiation output was determined [
20]. Samples consisting of 55% Mg, 40% PTFE, and 5% Viton (fluorine rubber) by mass were tested. Additionally, samples were covered with an insulating layer made of heat-resistant silicone rubber to provide a quasi-one-dimensional regression of the combustion surface required to perform combustion characterization and determine the infrared radiation. Both experimental and numerical studies were undertaken.
In their calculations to mathematically describe the gaseous and solid phases, the authors used the coupled mode according to the Euler–Lagrangian method, the eddy dissipation concept (EDC), the realizable k- model, the finite volume method (FVM), and the discrete phase model (DPM). The authors also focused on the reaction kinetics model and on infrared radiation output characterization.
The authors as well as performing extensive calculations, also included a comparison of the results obtained from simulations with those obtained from experimental experiments. Possible errors and discrepancies were also taken into account, which indicates that the research was performed reliably.
2.7. Case 7: The Reaction Mechanism of Aluminum-Based Nanothermites
Nanoaluminum and the oxidising agents CuO, SnO2, and Fe2O3 were investigated to understand the reaction mechanism. The authors assumed that the most important element of the process was the decomposition of the oxidant. To support their hypothesis, they collected simultaneous pressure and optical signals during combustion. The optical results obtained for the mixture with CuO and SnO2 did not coincide with the pressure results. The increase in the pressure with these two mixtures occurred before the maximum of the optical signal. The authors assumed that the increase in pressure could not have occurred due to vaporization of the metal. To confirm their thesis, they performed calculations using the AC NASA CEA equilibrium code to check the decomposition behavior of metal oxides.
The obtained results indicate that the decomposition of CuO and SnO2 occurs fully in temperatures lower than adiabatic flame temperatures. However, in the case of Fe2O3, the oxidant is unable to efficiently decompose. In the second stage of the research, the authors added various WO3 contents to the mixtures in order to perturb the system gas release while keeping the temperature relatively constant. In the case of CuO and SnO2, the addition of WO3 significantly reduced the reactivity of the process.
The authors emphasized that they are not able to fully prove that the oxidants used decomposed completely at the tested pressure increase. However, they assumed that the reaction mechanism was limited by the decomposition of the oxidant. On the other hand, they did not provide any experimental temperature data, and the only indication of the temperature of the combustion was the optical signal. They provided an explanation of why they did not research the temperature in this paper. However, an attempt to investigate the temperatures occurring during the process could have provided additional confirmation of the obtained calculations [
6].
2.8. Case 8: Mechanism of Methane Oxidation under Explosion Condition
Methane explosion has been studied using reactive force field molecular dynamics (MD) simulations and density function theory (DFT) calculations [
21]. Both reactive force field molecular dynamics (MD) simulation and DFT calculations were used to investigate the detailed reaction process and key reaction steps. First, MD analysis was performed to investigate the entire explosion process and the main reaction pathways. The intrinsic kinetic properties of the key reaction steps were then checked using DFT calculations. Based on the calculations, it was found that the reaction of creating the HCHO molecule is the rate-limiting step in the oxidation of methane, which has the highest energy barrier of 27.12 kcal·mol
−1 for CH
3O→HCHO and 8.90 kcal·mol
−1 for CH
2OH→HCHO, respectively. It was shown that almost all the key methane combustion reactions involve the formation of the free radical OH, which is a critical factor in the oxidation of methane, especially in the case of the initial formation of radicals. It was proposed that the process of methane oxidation under explosive conditions consists of six main stages (
Figure 1).
The computational results show that the initiation stage of the gas explosion process is induced mainly by the OH free radical, and the chain transfer process takes place mainly through the conversion of the HO2 radical to the OH radical. The number of free radicals, such as CH3, HO2, and OH, has a huge impact on the reaction speed during the gas explosion process. However, the work is entirely theoretical and was not confirmed by experiment at any stage.
3. Thermally Induced Decomposition and Ageing of Energetic Materials
3.1. Case 1: Thermal Decomposition of Polymer-Bonded Explosives Using LLM-105 and Hydroxyl-Terminated Polybutadiene as an Example
An interesting approach to investigating the reaction mechanisms of energetic materials is to use computational methods to predict the strength of the relevant intra- and inter-molecular interactions [
22]. In this work, a polymer-bonded explosive (PBX), composed of LLM-105 and hydroxyl-terminated polybutadiene (HTPB), was modeled, allowing the authors to predict the total energy of the interactions, the amount of potential energy released at various temperatures, the kinetic rate constants for the relevant reactions, and even the composition of the gaseous reaction products. The only significant drawback of this approach was the omission of any comparison with actual experimental evidence. As such, as convincing as the proposed model may be, its accuracy remains unverified.
3.2. Case 2: Ageing of Boron-Potassium Nitrate Pyrotechnic System
The issue of the significant loss in performance of the B/KNO
3 pyrotechnic system, which contained a polymer binder, upon aging was investigated using a range of experimental methods [
23]. The initial hypothesis of the decomposition of the polymer causing the decay of performance was rejected on the basis of IR spectroscopy and thermoanalysis data. Instead, transmission electron microscopy coupled with electron dispersive spectroscopy (TEM-EDS) was used to monitor the changes in the composition of the grains constituting the pyrotechnic system, revealing the formation of an inert oxide shell (B
2O
3) on the surface of the boron grains. This oxide shell was found to form due to reactions of boron with water, as evidenced by the lack of such a shell in accelerated aging experiments without the presence of moisture.
Although thermal analysis methods played only a supporting role in this investigation, it is noteworthy among the other cases due to its good scientific rigour, and it stands as a model of solid reasoning, with well-founded conclusions based on multi-aspect experimental evidence.
3.3. Case 3: Mechanism of Thermal Decomposition of 2,6-Diamino-3,5-dinitropyrazine-1-oxide
The mechanism for the two-step thermal decomposition of 2,6-diamino-3,5-dinitropyrazine-1-oxide (LLM-105) was determined by analyzing the gaseous products and solid samples at different decomposition stages [
24]. TG-DSC-FTIR-MS simultaneous analysis was used to study the evolved gaseous products of LLM-105 explosive. From the DSC and DTG curves, it can be seen that LLM-105 exhibited two obvious exothermic peaks and a two-step mass loss during the decomposition process. The thermal decomposition of LLM-105 is a two-stage process in which the overall reaction can be deconvoluted into two reaction steps. Comparing the two stages, it was found that the first decomposition stage had a higher heat release rate accompanied with a lower weight loss rate, while the second stage exhibited the opposite trend. Similarly to the DSC curves, the FTIR and MS spectra of the volatile products showed two stages during the entire decomposition process. The gaseous products of LLM-105 detected by FTIR were mainly CO
2 (2345 and 2360 cm
−1), NO (1845 and 1910 cm
−1), HCN (712 and 3336 cm
−1), C
2N
2 (2249 and 2290 cm
−1), and H
2O (3500–4000 cm
−1). The MS data indicated that N
2 and NO
2 were also produced during the decomposition process. Significant differences between the gases released in the two decomposition stages were clearly observed from the FTIR and MS results. NO and C
2N
2 were mainly generated in the second stage of thermal decomposition, while the other gases were produced throughout the entire decomposition process. The FTIR and MS signals of COC were strong from the initial reaction compared with the signals of other gases. As the element carbon exists in the pyrazine ring of LLM-105, this result indicates that breaking of the pyrazine ring occurred in the early stage of the reaction. The MS data also showed that the release of HCN and C
2N
2 ceased earlier than the other gases, and the external oxygen participated in the thermal decomposition when the second stage reaction was intense. However, the authors did not propose a detailed reaction mechanism.
3.4. Case 4: Determination of the Mechanism of Pyrolysis of 5-Amino-1H-tetrazoles(5AT)
In article [
25], the authors attempted to determine and compare the catalytic effects of selected oxidants (NH
4ClO
4 (AP), NaClO
4 and NH
4NO
3) and selected metal oxides (MnO
2 and Cr
2O
3) on the pyrolysis of 5AT. TGA/DSC was used to investigate the thermal decomposition of the samples. TG-FTIR and TG-MS were used to determine the gaseous products of decomposition. Additionally, the activation energy (E
a) was determined for each sample.
In the course of the study, the authors demonstrated the influence of individual components on the occurrence of pyrolysis and determined its mechanism, taking into account the products formed in each reaction and their possible interactions with each other. The experimental results indicated that 5AT-AN had the lowest activation energy, and the degradation process was simplified to a single step. The addition of AP, NaClO4, and MnO2 can promote the pyrolysis of 5AT as a result of redox reactions between melamine and the oxidant degradation products, while the catalytic effect of Cr2O3 mainly occurs above 400 °C without oxidation occurring. The catalytic mechanism of CrC2N2OC2N3 works towards polyaddition reactions and the ring opening of nitrogen-containing heterocycles.
To determine the Ea of each decomposition reaction, the authors utilised Ozawa’s and Kissinger’s equation. Unfortunately, no calculations were provided to accurately trace the individual steps in the method used. It is also puzzling that the results obtained were based on only four different heating speeds. It is well-known that in order to obtain a statistically significant result at least five attempts should be made.
3.5. Case 5: Pyrolysis Mechanism of Octahydro-1,3,5,7-Tetranitro-1,3,5,7-tetrazocine (HMX)
A pyrolysis mechanism of HMX was determined using numerical methods [
26]. To determine the kinetics of the initial and the secondary stage of the decomposition reaction, the ab initio calculations and the RRKM/master equation simulation were used.
The publication presents the potential energy profiles of the reaction network and the transition states, with their simulated schematic structures, and accurately describes the pyrolysis reaction of HMX, including the factors that determine the processes taking place. The calculations performed were carried out very reliably, the entire description is consistent and the presented results of the calculations and simulations carried out seem accurate. However, the publication does not contain any information comparing the obtained results with experimental data.
3.6. Case 6: Zirconium-Based Pyrotechnic Igniters and Pyrotechnic Delays under Aging
The chemical changes taking place in pyrotechnic igniter (Zr/Fe
2O
3/SiO
2) and delay (Zr-Ni alloy/BaCrO
4/KClO
4) composition samples subjected to atmospheric and thermally accelerated ageing were investigated [
27]. XPS and SEM-EDS were used to monitor the composition of the ageing products. However, the work did not account for the fact that both methods were limited to investigating the surface of the samples only, yielding no information about the chemical composition of the interior of the sample grains. The reported XPS spectra showed significant overlap of numerous signals, making their deconvolution highly unreliable, casting any conclusions based on this deconvolution into doubt. In turn, SEM-EDS data were used to assess the oxygen content of the samples, which did not allow differentiating between oxygen adsorbed on the surface of the samples and oxygen present in the chemical structure of the proposed oxides. As such, for these data to be reliable, the adsorbed oxygen would have had to be thoroughly removed.
The research data presented in this work were used to support the claim that for atmospherically aged samples, the temperature caused by the oxidation of the metallic fuel and the humidity caused the decomposition of the oxidising agent. Jointly, this resulted in decreasing the amount of fuel available for combustion and lowering the net heat of the combustion reaction. It was also noted that the heat transfer coefficient of the metal oxide was lower than that of the corresponding metal, impairing the heat transfer across the pyrotechnic composition sample. Moreover, the thick layer of the metal oxide or hydroxide in the aged samples was noted to also limit diffusion of the reagents, lowering the rate of reactions leading to the ignition of samples. All these effects were concluded to lower the combustion velocity of the samples, impairing the performance of the pyrotechnic devices containing these compositions.
3.7. Case 7: Ageing-Induced Processes Effects on the Thermo-Kinetic and Combustion Characteristics of Tungsten Pyrotechnic Delay Composition
The ageing of a pyrotechnic delay composition (W/BaCrO
4/KClO
4/SiO
2) was investigated in an accelerated-ageing regime (71
C, 95% relative humidity) [
28]. Numerical simulation of the propagation of a reaction front in this composition, loaded into a pyrotechnic device, was conducted and compared with the reported experimental data. The composition of the combustion products of aged and unaged samples was investigated via XRD and SEM-EDS, revealing that BaWO
4 and Cr
2O
3 were the main combustion products regardless of whether ageing took place. In the case of aged samples, the presence of KCl and unreacted fuel was observed, which was linked to the decomposition of the oxidation agent. The presence of an oxide layer on the surface of the metallic fuel was linked to lower combustion velocities. In the case of ageing, two distinct processes were identified, differentially affecting the thermokinetic behavior and combustion of the samples.
The initial oxidation reaction caused an increase in the amount of metal oxides in the aged delay composition samples, which decreased the volumetric heat conductivity and the rate of diffusion of the reagents. The earlier decomposition reaction of the oxidising agent released oxygen that instead of undergoing reactions was lost to the environment, resulting in partial combustion taking place for the aged samples. This was further amplified by the presence of large agglomerates observed via SEM. All these aspects resulted in lowering the performance of the composition, i.e., the heat release, reactivity, temperature in the combustion zone, and the combustion velocity, through affecting the possible chemical reaction pathways. A two-dimensional simulation of a pyrotechnic delay device was used to predict the combustion time and to investigate the structure of the propagating combustion front inside a delay element, both for aged and unaged composition samples. The simulation was based on reaction heats and reaction kinetics obtained from DSC data and showed good agreement with the experimental data for the combustion of aged samples.
3.8. Case 8: Thermal Behavior and Decomposition Kinetics of CL-20-Based Plastic-Bonded Explosives
The article [
29] investigated formulas composed of 2,4,6,8,10,12-hexanitro-2,4,6,8,10,12-hexaaza-tetracyclo-[5.5.0.05,9.03,11]-dodecane [CL-20] and various PBXs, in varying mass ratios.
The authors also sought to determine the effect of additives, such as BDNPF/A plasticizer and desensitizer, on the prepared formulas.The authors used thermal methods, such as DSC, TG, and accelerating rate calorimetry (ARC) in their study. Unfortunately, despite the thorough presentation and description of the methods used, as well as the results obtained, the calculated values of the activation energy of the high-energy distribution of the compositions studied cannot be considered reliable. Both the Ozawa and Kissinger methods were used to determine the aforementioned activation energy. Although the calculations were performed correctly, thermograms were prepared using only four different heating rates. In order to obtain a statistically significant result, there should be a minimum of five of these repetitions.
3.9. Case 9: Thermal Decomposition Performance of HNIW-Based PBXs
In [
30], the authors attempted to determine the long-term storage performance of HNIW-based PBXs, using an accelerated aging method.
The authors prepared two different types of HNIW-based PBXs: HNIW/CAB and HNIW/F2311 (fluorine rubber). The formulations prepared in this way were transformed into columns and pressurized at 400 MPa enabling achievement of 97% of the theoretical maximum density. The prepared columns were used to determine the mass change, the mechanical strength, and the detonation velocity. The powders were then used to analyze the surface microtopography, the thermal decomposition efficiency, and the mechanical sensitivity.
As in many of the previously described publications, the authors determined the activation energy and compared its value depending on the length of storage (0, 15, 30, 45, and 60 days). As with other publications, they used thermograms for only four different heating rates to determine the Ea values. Obtaining a final result from less than five measurements (in case of Ea determination-heating rates), can lead to some irregularities.
Changes in the surface topography of the samples after different storage times were observed using a scanning electron microscope. Unfortunately, the method description and the presented images cannot be considered sufficient. The description lacks information about the resolution capacity and the magnification range.
3.10. Case 10: Thermal Behavior and Decomposition Kinetics of Formex-Bonded Explosives Containing Different Cyclic Nitramines
In [
31], the effect of the polymer matrix Formex P1 (consisting of 25% styrene-butadiene rubber (SBR), which was plasticized by 75% oily material) on the thermal decomposition of some typical cyclic nitramines, such as 1,3,5-trinitro-1,3,5-triazinane (RDX), 1,3,5,7-tetranitro-1,3,5,7-tetrazocane (HMX), cis-1,3,4,6-tetranitrooctahy droimidazo-[4,5-d]imidazole (BCHMX), and 2,4,6,8,10,12-hexanitro-2,4,6,8,10, 12-hexaazaisowurtzitane (CL-20), was examined.
Using thermal methods (TG/DTG and DSC), the authors determined the activation energy of the PBX samples studied. The TG/DTG method was used to investigate the modified samples and the DSC method was used to investigate both modified and non-modified nitramines. The Kissinger and the modified Kissinger–Akahira–Sunose equations were used to calculate the correct kinetic parameters of the abovementioned PBXs. As in many cases referred to in this article, the authors prepared thermograms for only four different heating rates. Despite the detailed calculations performed, the factual description provided, and the thorough discussion of the results, the results presented by the authors cannot be considered to be clearly correct.
3.11. Case 11: Combustion Performance of B/PVDF/Al Composite
The thermal properties of B NPs (boron nanoparticles), B/PVDF, and B/PVDF/Al were examined. Based on the results obtained from TG–DSC, it was found that the B NPs had two exothermic peaks and the B/PVDF composites showed four exothermic peaks. Based on these results, the authors proposed a 4-step low-temperature thermal reaction mechanism. In the first stage, occurring in the temperature range 200–400 C, the C–H and C–F bonds in PVDF are split, releasing large amounts of HF, which react with Al2O3 and B2O3 by creating AlF3 and BF3. In the second stage, in the range of 400–570 C, metal elements come into contact with oxygen and the C–C bonds with PVDF are broken. In the third stage (570–670 C), Al and B are oxidised. In the last stage, the residue B is oxidised (670–770 C). However, the TG-DSC analysis provided information about the temperature and masses only, and not about any chemical structures.
The combustion characteristics of the obtained composites were also examined. During these measurements, intense burning of the sample with yellow light was observed, and green light appeared, which may have indicated the appearance of BO
2 [
32]. As the quantity of Al NPs increased, a more intense combustion process was observed.
4. Use of Measurement Methods
The investigation of the mechanisms of reactions taking place in energetic materials relies on the application of a range of instrumental analysis methods, as shown for the individually discussed cases. Due to the extremely rapid nature of some of the investigated processes, most classical methods of investigating reaction mechanisms are not directly applicable and need to be modified for the needs of a particular reacting system, utilised in a non-standard way, and employed ex situ for post-reaction analysis or even replaced by other methods.
It should be noted that the experimental methods used to study the reaction mechanisms of EMs can be classified based on multiple criteria, i.e., the nature of the information obtained about the reacting system (structural vs. non-structural, direct vs. indirect, and confirming vs. excluding), the regime of analysis (ex situ vs. in situ), and the mode of measurement (continuous during reaction vs. multi-point vs. post facto analysis). Moreover, depending on the investigated process and the participating chemical species, some methods may fall into different categories. To exemplify, a method considered continuous for a deflagrating system may be unable to provide even a single data point during the detonation of another system. That said, for the purpose of this review, we have organized this section in terms of whether an analytical method provides information directly about the chemical structure and transformations taking place or not.
4.1. Methods Providing Structural Data
IR spectroscopy provides a wealth of information about the presence and transformation of functional groups in the investigated samples. The key limitations of this measurement method are listed below:
susceptibility to the presence of water—significant amounts of moisture in the sample can overlap with and obscure the signals originating from the functional groups present in the tested sample;
significant time required for acquiring data—even in the case of Fourier transform (FT) instruments, the acquisition time of a single spectrum is of the order of seconds, making it unsuitable for studying rapid (i.e., deflagration and detonation) processes;
highly limited utility in investigating inorganic species, unless instruments capable of measuring into the far IR range are used—in the classical 4000–400 cm
−1 range, signals of species such as sulfates, carbonates, nitrates, metal carbonyls, interhalogen compounds, nitrogen oxides, and some coordination compounds can be observed [
33];
Consequently, the method is best suited to studying the thermal decomposition (particularly with the availability of FT IR spectrometers offering temperature control of the work area) and ageing of organic energetic materials. Nevertheless, IR spectroscopy can be used to study rapid processes; however, it will likely be limited to post facto analysis of the reaction products.
Raman spectroscopy can be seen as a method complementary to IR spectroscopy, sharing many limitations and advantages. The key features of Raman spectroscopy, in comparison with IR are as follows:
lack of susceptibility to the presence of water—Raman spectroscopy can be conducted even for aqueous solutions;
high utility in investigating organic, inorganic, hybrid, and coordination species;
viability of using multiple laser sources for sample excitation, allowing fine-tuning towards specific sample types;
X-ray diffractometry (XRD) is a source of crystallographic information about investigated samples. Although it can be debated whether the method truly offers structural information, comparison of experimental diffractograms with those included in the vast available databases allows facile identification of the majority of species encountered in the reactions of EMs.
XRD offers the ability to distinguish between even very complex substances and between the individual polymorphs of a specific substance. The key drawbacks of XRD are the significant measurement time that precludes following even relatively slow reactions, and in the case of investigating unknown substances, the need for the sample to be highly homogeneous and to consist primarily of a single phase.
Nuclear magnetic resonance (NMR) spectroscopy is most commonly conducted for liquid samples, but can also be conducted for solid samples (i.e., magic angle spinning NMR spectroscopy). NMR spectroscopy enables investigation of the chemical surroundings of magnetic spin-bearing nuclei (e.g., 1H, 13C, 15N, 29Si and 31P), with 1H NMR and 13C NMR spectroscopic measurements being by far the most common. As such, the method produces relatively extensive information about the structure of the investigated compounds, although it has less applicability to simple inorganic compounds and the products of their reactions. Similar to the abovementioned methods, NMR spectroscopy can be used as either a post facto analysis method or as a method for investigating slow processes that can preferably be conducted in solution. It is also worth noting that the chemical shifts of some energetic functional groups show similar chemical shifts, complicating the analysis of the recorded spectra in some cases. In order to conduct the most common 1H NMR spectroscopy for liquids, cost-intensive deuterated solvents need to be used and the investigated systems should be soluble in them, which is applicable mostly to organic compounds. This issue can be avoided through the use of solid-state NMR spectroscopy, often referred to as “magic angle spinning” (MAS) NMR spectroscopy. The rotation of anisotropic samples in the case of MAS NMR, however, leads to loss of some information about their structure.
4.2. Methods Providing Non-Structural or Indirect Data
Thermal analysis methods, such as differential scanning calorimetry (DSC), differential thermal analysis (DTA), thermogravimmetry (TG), and ignition/explosion temperature measurements can provide a variety of information about the behavior of investigated samples upon thermal conditioning. Although this information cannot directly identify an on-going process, it can provide insight into both its thermodynamics and kinetics. Similarly, thermal analysis typically cannot be used to follow the progress of deflagration or detonation, but may provide data about the conditions required to initiate a given reaction, as well as data related to the overall process, e.g., the net heat of combustion.
In the case of DSC and DTA, information about the thermal effects of the processes occurring can be obtained. Due to the fact that very few cases of chemical or physical transitions take place without a corresponding thermal response, the two methods have significant utility in determining the number (often requiring thermogram deconvolution) and nature (exothermic vs. endothermic) of the observed processes. Many theoretical methods, such as Kissinger’s or Ozawa’s, allow information about the activation energy of a process to be obtained by repeating experiments at multiple heating rates.
In turn, TG provides information about a more narrow group of processes, as many physical transitions (particularly transitions between polymorphs of a given substance) take place without a change in sample mass. If combined with DSC or DTA, TG allows this type of physical transition to be differentiated from among other processes. While DTA/TG, for example, does not allow direct identification of these transitions, for a known system, the temperatures at which these transitions take place can provide strong evidence for the occurrence of a particular transition. Identification of the on-going processes is, however, possible with the use of “hyphenated” methods, such as coupled differential thermal analysis and mass spectrometry (MS), as the products of the observed process can be identified qualitatively and quantitatively. The use of “hyphenated” methods for this purpose is not very common and their exact utility in terms of studying the reactions taking place in EMs has not yet been fully explored.
Elemental analysis is another highly useful analysis method, even though limited to post facto analysis of the composition of reaction products. Although the method provides useful data that can compliment other methods in identifying the reaction products and determining their amounts, the key limitation of the method is that most existing instruments are only capable of quantifying the amounts of several elements (typically C, H, N, O, S). Unless primarily organic EMs are investigated, the post facto analysis of reaction products, solid products in particular, is more favorable with the use of traditional instrumental analysis methods, such as, for example, atomic absorption spectroscopy (AAS).
Fast, time-resolved
pressure and
temperature measurements can be utilised to follow the progress of both deflagration and detonation. When applied directly, these measurements can be used to determine the main descriptors of the process, such as the deflagration temperature and the explosion pressure. The use of specialized experimental set-ups (e.g., manometric bomb), however, allows obtaining more informative data, such as the rate of pressurization in a constant volume, which can be directly correlated with the evolution of gaseous products and the increase in temperature within the set-up. Although both types of measurements often require both ingenuity in designing the experiments and repetition of experiments under a wide array of experimental conditions, they can provide significant insight into the on-going processes [
34].
5. Conclusions
The investigation of EMs using thermal analysis methods is an important aspect of investigating the mechanisms of reactions taking place within those materials. utilising data produced by such methods allows determining some aspects of the reaction mechanism and to provide more fundamental data, i.e., determining the reaction activation energy and the reaction induction temperature. These data, however, are not sufficient for determining the complete mechanisms of the reaction pathways underlying the decomposition, combustion, or ageing of EMs. Thermal analysis methods are unable to provide structural information about the chemical species occurring in the observed reactions, and as such, should be supported by additional experimental evidence if the on-going reactions are to be reliably identified. Determining the mechanism of even a single reaction is a complex endeavor, necessitating the use of complementary methods and corroborating their results. As such, analysis of the mechanism of the complex processes taking place in EMs should not be conducted based on a single method.
Piecing together a mechanism for the processes taking place in EMs is an important aspect of work on these materials, as it enables rational design, control over the performance, and understanding of the potential safety issues caused by such materials. In this work, we have shown that the selection of methods used to provide experimental evidence on which a process mechanism would be based, is crucial to allowing a reliable investigation. In many cases, this selection is sub-optimal, as discussed above. An important group of methods, which can provide highly useful, time-resolved data, includes the use of high-speed optics (visible light and IR cameras, including streak methods). Similarly, pyrometric methods and analysis of the emission spectra of combusting/deflagrating/detonating EMs may also provide significant insight into the mechanisms of the processes.