1. Introduction
Fireworks are a beloved part of many cultural celebrations around the world, from New Year’s Eve to Independence Day to Diwali. Fireworks are composed of various chemicals that are carefully mixed together to create an impressive display of light and colour and/or sound [
1]. There are many different types of fireworks compositions, each of which produces a unique effect, among which the most common components are black powder, flash powder, colorants, stars and whistle powder.
Black powder is a mixture of potassium nitrate, charcoal and sulphur. It is the oldest and most widely used type of firework composition. It burns quickly and produces a bright flash of light. On the other hand, flash powder is a mixture of potassium perchlorate and aluminium or magnesium powder. It burns at very high temperatures and produces a bright and white flash. Colorants are added to firework compositions to create different colours (copper compounds produce blue colours; sodium compounds produce yellow colours; strontium compounds produce red colours) [
2,
3].
Although firework use is extensive worldwide, firework shows can produce significant amounts of greenhouse gas (GHG) emissions, primarily through the combustion of the pyrotechnic materials. According to studies measuring the influence of fireworks on ambient air quality, pyrotechnic articles release a variety of pollutants into the atmosphere, including gases such as carbon dioxide (CO
2), carbon monoxide (CO), sulphur dioxide (SO
2) and nitrogen oxides (NO
x) as well as solid particles such as soot [
4,
5,
6].
Air quality measurements in Vadodara (India) during the festival of Diwali for the consecutive years 2009, 2010 and 2011, indicate that during the festival day, the average levels of SO
2 and NO
x increased 23 and 3 times, respectively, compared to a regular day [
7]. Measurements during and after a Fourth of July fireworks display in downtown Minneapolis, Minnesota were carried out using a mix of low-cost sensors (CO and CO
2). The results showed that the concentration of CO increased 32% while that of CO
2 increased 17% overnight [
6]. In addition, tests about the influence of fireworks on CO
2 emissions were carried out in China. It was observed during the Spring Festival (or Chinese New Year) that the CO
2 mixing ratio was higher than the annual average value as well as CO, PM10, and PM2.5 [
8]. While SO
2 and NO
x contribute to the formation of acid rain and smog, CO
2 is a potent greenhouse gas that contributes to climate change by trapping heat in the atmosphere and is a significant greenhouse gas: CO
2 together with methane (CH
4), contribute between 80 and 85% of greenhouse gas emissions [
9]. In contrast, CO does not contribute directly to the greenhouse effect [
10], but favours the appearance of gases that do contribute directly to it [
11]. CO’s half-life in the atmosphere is about three months [
12], allowing it to slowly oxidize to form CO
2 and contribute to the formation of tropospheric ozone [
13,
14].
Firework displays contribute a relatively small fraction of greenhouse gas emissions compared to sectors such as transportation and energy production. In the European Union, fireworks emitted around 6500 metric tons of CO
2 equivalent in 2012, a fraction of the total emissions which exceeded 4 billion metric tons that year [
15]. However, their regular use can lead to ongoing air pollution and contribute to climate change. Despite their small contribution, efforts to reduce emissions from fireworks displays are important [
16]. Some manufacturers are exploring alternative materials such as nitrogen-rich compounds and perchlorate-free compositions to produce less pollution. There is evidence that the ClO
4− ion may affect the thyroid gland, as it makes iodide absorption difficult and may trigger hypothyroidism [
17,
18,
19]. In addition, groundwater contamination via the soil from rainfall, related to the production, handling and use of perchlorate, harms micro-organisms and aquatic fauna [
20,
21,
22]. According to Wilkin et al., measurements before a fireworks display recorded perchlorate concentrations in the surface water of a lake of between 0.005 and 0.081 μg/L [
23]. After the show, these concentrations reached values ranging from 24 to 1028 times the mean reference value. This highlights the need for sustainability measures in all sectors, including the use of environmentally friendly pyrotechnic materials.
Nitrocellulose is one of the main propellants used in various solid rockets such as ammunition. These nitrogen-rich compounds obtain their energy from the high heat of formation, which predominates over the energy released in the oxidation of carbon, from which energy is obtained in the case black powder [
24]. This results in combustion products that are less harmful to the environment, mainly due to the reduction in solid products (soot) and, therefore, produces effects with more clarity due to less smoke [
25]. Because of these characteristics, nitrogen-rich compounds have replaced black powder in ammunition. In addition, nitrocellulose is currently used in the pyrotechnic industry as a substitute for perchlorate in category F1 fountains for indoor use. Several studies have analysed different types of compositions used as perchlorate-free oxidant. Anhydrous 5-aminotetrazole [
26] and periodate salts [
27] have been studied as pyrotechnic oxidizers in mixtures with barium (green colour) and strontium (red colour), but these studies are not very detailed, as they only simulate a hypothetical situation.
Nevertheless, nitrocellulose might present a higher accident risk due to its instability, as it presents a natural autocatalytic process that leads to the natural degradation of nitrocellulose [
28]. Several studies have tested the thermal stability of nitrocellulose associated with ammunition [
29,
30] and others have analysed its specific application in fireworks as a propellant, binder or perchlorate-free compound [
31,
32,
33,
34], without taking into account the environmental impact of both compounds. On the other hand, Dahl et al. propose several methods of differentiation between black powder and nitrocellulose in firearms, where mass spectrometry is mentioned [
35,
36], but no study has carried out an environmental comparison between traditional powder and nitrocellulose in pyrotechnics by mass spectrometry. In order to address the thermal stability, thermogravimetric analysis (TGA) can be a useful tool as it allows an understanding of the thermal degradation process. Because of this, previously published literature has focused on performing thermogravimetric analysis using nitrocellulose [
37,
38], black powder [
39,
40] and other pyrotechnic compositions [
41,
42,
43,
44]. However, there is no comparison between black powder and nitrocellulose in pyrotechnics to find differences that might influence the possible use of nitrocellulose as a black powder substitute.
The parameters obtained from the TGA can be a starting point to assess the risks associated with nitrocellulose and black powder. The HAZOP (Hazard and Operability) methodology has been used for years to address this type of risk. Indeed, previously published literature has used HAZOP as a tool to define the risks associated with the production, storage, transport and use of both black powder and nitrocellulose [
45,
46,
47]. An analysis that considers both components and finds similarities and differences between these processes applied to black powder and nitrocellulose may provide significant insights that help the transition to a more sustainable component in pyrotechnic devices.
The present study focuses on the use of nitrocellulose instead of black powder in usual pyrotechnic devices, as is already the case in ammunition, and measures greenhouse gas emissions produced by their use. On the other hand, nitrocellulose is analysed as a possible substitute of pyrotechnic perchlorate oxidants. This composition is already used for this purpose in some fountains, as it is established in the European standard EN 15947-5:2015 [
48]. However, the present study aims to show the advantages of the introduction of nitrocellulose as a propellant or perchlorate-free compound for the rest of the fireworks available for sale to the general public and aerial fireworks. The study will provide insight for inclusion of nitrocellulose in the European Standards for more devices.
Therefore, taking into account that nitrocellulose is already used as a substitute for perchlorate oxidants, an experimental environmental comparison was envisaged. In this way, results could be obtained for the GHG emitted by both compositions so that their sustainable viability could be confirmed. The use of nitrocellulose as a substitute for black powder and as a propellant for large aerial devices has not yet developed in the same way in European regulations. Therefore, a theoretical environmental study and a risk assessment of both components was carried out by means of thermogravimetric analysis and HAZOP methodology. This provided data to enable an estimate of whether inclusion of nitrocellulose is feasible, both sustainably and at safe levels.
2. Materials and Methods
2.1. Theoretical Emissions Comparison by Using Nitrocellulose as a Propellant
Aerial fireworks can be seen mainly in firework shows, where shells predominate over the rest. This type of show is included in a multitude of festivities and celebrations worldwide and, nowadays, is a tourist attraction for many visitors. The Macy’s Independence Day show in New York is one of the most important world-famous firework shows. The case study focuses on the 2022 edition. According to information provided by the show’s organiser, 50 pyrotechnic experts were responsible for firing approximately 1920 shells per minute for 25 min, among other pyrotechnic devices [
49]. This represents a total of 48,000 shells fired from five barges across the East River.
All the pyrotechnic devices used in this kind of show present a common characteristic: the article composition rises to a certain height and the effect is produced in the air. To achieve this type of effect, it is necessary to use a chemical mixture that works as a propellant and lifts the firework to the desired height. This propellant composition, known as lift powder, is nowadays composed by black powder. In addition, a certain amount of pressure needs to be generated inside a mortar to achieve the lift. These mortars, external or included in the article itself (depending on the generic type) determine the calibre of the article.
The exact compositions of the shells fired during the show are not publicly available, but for the case study a design commonly used in fireworks shows and shells is assumed, as can be seen in
Table 1. According to this design, two different calibres of shells were fired, each representing half of the total number of shells. Based on these calibres, an average NEC (Net Explosive Content) can be estimated.
Each of the mixtures contains different components and different mass percentages [
50]. Black powder, consisting of potassium nitrate (KNO
3), carbon and sulphur appears in lift, sound effects and burst powders. However, the percentages of the individual components vary depending on the desired effect. In the case of black powder, used as a propellant, 78% is potassium nitrate, 16% carbon and 6% sulphur. The pyrotechnic reaction that occurs for this composition is [
2,
3]:
Once the data regarding the shells studied are known, the total emissions released into the atmosphere during the display of the pyrotechnic devices can be estimated and compared to nitrocellulose use.
According to previously published literature [
25,
29,
51], nitrogen-rich compounds can be used as propellants. In fact, this compound is currently used as a propellant in most firearms ammunition. This can be a solution to reduce the environmental pollution generated by pyrotechnic articles during their use. Here, the emissions released by the black powder for lifting shells fired during the Macy’s Independence Day show in 2022 are compared with a second scenario in which black powder is replaced by nitrocellulose.
Currently, according to standard EN 15947-5:2015 [
48], nitrocellulose can be found in the pyrotechnic sector in different articles such as fountains for indoor use of category F1 (fireworks considered to be low risk) or table bombs. According to standard EN 15947-2:2015 [
52], table bombs are devices which produce the ejection of streamers, confetti and/or fantasy elements located inside the device. In this type of article, nitrocellulose is used as propellant to achieve the ejection of the other items contained inside. This is the same principle of operation that is intended to be applied to the rest of the aerial items in this case study, replacing the lifting powder with nitrocellulose.
The nitrocellulose used in this study, in accordance with European regulations for articles for sale to the general public, must contain less than 12.6% nitrogen content, as higher concentrations mean excessive energy released during combustion and greater instability at room temperature [
53]. It is assumed that the same amount of nitrocellulose is sufficient to replace black powder and achieve the same explosion heights, due to a 1:1 substitution ratio making it easier to compare the environmental footprint, which is the main objective. The nitrocellulose thermal decomposition reaction, based on its main products [
2,
3,
54,
55], can be defined as:
The total amount of lift powder used in the shells during the 4 July 2022 show was 6528 kg of black powder. Therefore, it is assumed that this total amount is replaced by nitrocellulose.
2.2. Experimental Emissions Tests
To validate the theoretical calculations, experiments were carried out measuring carbon monoxide and carbon dioxide emissions produced by some pyrotechnic devices.
2.2.1. Samples
According to standard EN 15947-5:2015 [
48], category F1 fountains for indoor use are the only articles for sale to the general public that can be found on the market with both nitrocellulose and conventional powder. Conventional powder fountains are mainly composed of potassium perchlorate (KClO
4) and metallic elements that are responsible for the production of sparks (mainly Mg, Al or Ti). Perchlorates are highly polluting and harmful during production, handling and use [
24], so reducing the use of this type of composition would be a step towards the sustainability of the pyrotechnic sector. Nitrocellulose fountains, on the other hand, are composed of approximately 85–90% of the nitrogen-rich compound. The remaining composition is made by titanium to achieve the sparking effect.
Three different types of F1 category fountains for indoor use with nitrocellulose were analysed: two of them with the same composition but different NECs (samples A and B) and a third one with a different nitrocellulose (sample C). To compare these results with perchlorate fountains, three different types of conventional powder fountains were also tested (samples D, E and F). Detailed information on each of the test articles can be found in
Table 2.
Each sample analysed in the tests was selected from all the samples in the primary pack, choosing those that were in an ideal state, without scratches, breaks or signs that part of the composition may have been lost.
2.2.2. Equipment
Tests were carried out using an Emerson XStreamIR continuous gas analyser that measured carbon monoxide and dioxide, CO and CO2, from an inlet flow rate. The gas analyser was correctly calibrated before the measurements were carried out. Moreover, as a quality control measure, before carrying out the tests, the values measured by the analyser were checked using a 300 ppm CO gas bottle and a 20% vol. CO2 gas bottle. The gas analyser was a mobile tower whose operation is based on physicochemical principles. The analyser sensors react to changes in the measured parameter, from which they emit a measurement signal.
2.2.3. Tests
Two tests were carried out for each sample to obtain reliable measurements. Each test was carried out with one unit of each of the samples, except for the tests on sample D, for which the test was carried out using two units to produce enough gas stream, thereby increasing the NEC to 2.8 g.
The inlet flow of the gas analyser was connected via a suction tube to the cover of a hermetically sealed reactor through its only outlet. The samples to be tested were placed inside the reactor and once the protruding fuse or the sealing paper of the pyrotechnic article was started, the reactor was closed before the device began releasing gas. In this way, as the test articles were inside a hermetically sealed container, the gases generated by the devices were not released into the environment but remained inside the reactor until the pump of the analyser was started and they were extracted through the suction tube.
The reactor in which the tests were carried out had a volume of 10 L. Once the pump was started, the gases emitted by the fountains flowed into the analyser, where the CO and CO2 sensors measured the concentrations of both gases in the inlet flow received, in ppm and % vol., respectively. The measurement indicated by the analyser was considered valid once the measurement had stabilized.
The inlet flow received by the analyser did not correspond only to the gases released by the combustion reaction of the fountain, since the tests were carried out in an atmosphere of air that was trapped in the reactor when closed. This simulated a real situation in which pyrotechnic articles are fired in an open environment.
At the end of each test, the reactor was opened and cleaned with an air compressor so that the following measurements were not influenced.
2.3. Thermogravimetric Analysis and Mass Spectrometry
Thermogravimetric analysis (TGA) was carried out using a STA 449F5 Jupiter® apparatus, manufactured by NETZSCH, Selb (Germany), that allows the determination of the mass changes as the time and temperature vary. The test was performed as simultaneous thermal analysis (STA), which means that differential thermal analysis (DTA) is performed together with the TGA. The derivative of the mass loss curve (DTG) allows the thermal decomposition behaviour of the sample to be defined. DTA is similar to differential scanning calorimetry (DSC) and allows the definition of the energy effects (exothermic and endothermic peaks) of the sample degradation within the considered temperature range.
In the present study, nitrocellulose was tested using STA, and the test was performed twice to avoid deviations. The performed tests were non-isothermal, applying a constant heating rate of 10 K/min from 50 °C up to 600 °C. The nitrocellulose mass was set at 1.5 mg ± 0.5 mg, and the tests were performed using 50 mL/min argon flow as carrier gas to produce an inert atmosphere.
The experiments were conducted simultaneously with a thermal analyser NETZSCH STA 449F5 Jupiter®, coupled with quadrupole mass spectrometer QMS403D Aëolos®, manufactured by NETZSCH, Selb (Germany). Coupling is accomplished using a 75 µm capillary made of quartz that is placed within the stainless steel transfer line, which is then heated at 270 °C to prevent condensation of evolved species. To detect all gases that were of interest, atomic mass units (amu) within a range from 12 to 46 were scanned, where each of them was scanned for 0.2 s in a total of 177 bargraph cycles.
Mass spectrometry identifies species by using differences in mass-to-charge ratio (
m/
z) of ionized atoms or molecules. Each molecule identified consists of different fragments which are included in the respective molecule in different ratios. The intensity of each of the fragments and their ratios are available in the NIST database [
56].
2.4. Comparative Risk Analysis between Nitrocellulose and Black Powder
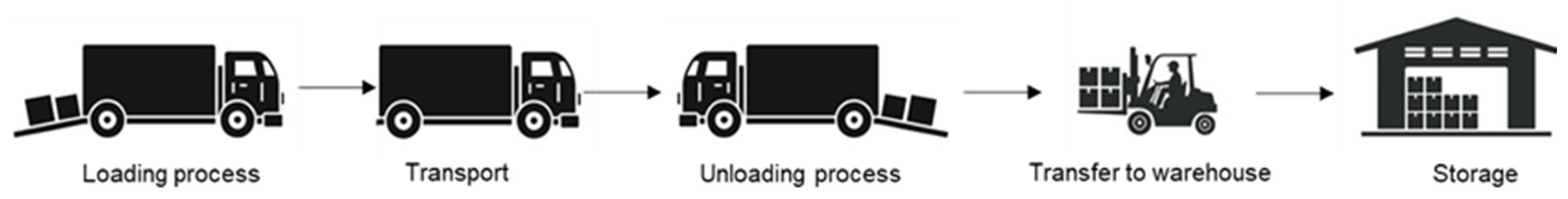
Although nitrocellulose may be a more environmentally friendly solution than black powder, it is more inherently instable. Black powder implies a latent risk in handling, so it is essential to analyse whether nitrocellulose would increase this risk. For the HAZOP analysis the focus was on the transport from the factory to the warehouse, and its storage.
Figure 1 details the process under study.
HAZOP methodology consists of four phases. In the first one, collection of information about the process takes place. Since this is not an industrial process, a total of 21 accidents in the pyrotechnics industry were analysed for this risk analysis [
57,
58,
59,
60,
61,
62]. Afterwards, the second phase proceeds with the location of the nodes. Each node is systematically analysed by assessing the possible deviations of each parameter. In this case, the sub-processes and nodes were identified as shown in
Table 3.
After this analysis, the third phase consists of assessing the frequency and severity of each deviation. To conduct this analysis, a severity vs. frequency matrix was considered. Severity was evaluated as “low”, “serious”, “severe”, “very severe” and “catastrophic”. Frequency was evaluated as “every week”, “every month”, “every year”, “has happened” and “has never happened”. For this analysis, the values obtained in the thermogravimetry tests were considered. The last phase is the conclusion of the study.
3. Results and Discussion
3.1. Comparison of Black Powder and Nitrocellulose in the Macy’s Independence Day Show
3.1.1. Emissions Released from the Shells of Macy’s Fireworks Show
Based on the shell composition and the units under study, the total emissions released from the lift powder were calculated according to Equation (1). It is well known that the products released by fireworks range from greenhouse gas emissions (GHG) to perchlorate/metal compositions. The theoretically obtained results are shown in
Table 4.
It is important to highlight the emissions of gases that contribute to the greenhouse effect. As for CO
2, a gas that contributes directly to the greenhouse effect, 1662 kg is generated in the fireworks display. On the other hand, 1058 kg of CO is emitted, a gas that although it does not contribute directly to the greenhouse effect, favours the creation of other gases that have a direct influence [
11].
3.1.2. Emissions Released by Using Nitrocellulose as a Propellant
By replacing black powder used in the fireworks display for lift powder with nitrocellulose and calculating the theoretical emissions generated, is it possible to prove the GHG reduction. Taking into account the pyrotechnic reaction that occurs in pyrotechnic articles using nitrocellulose as a propellant and assuming that the same amount of nitrocellulose as black powder is sufficient to achieve the same effects, the emissions generated can be calculated. The calculated emissions are given in
Table 5.
The results obtained show a significant reduction in carbon dioxide emissions, around 42%. Nevertheless, the carbon monoxide released to the atmosphere increases around 190% due to the chemical reactions that take place. However, it has to be considered that CO do not contribute directly to the greenhouse effect, as it only affects the atmosphere by reacting and producing other compounds [
13,
14], and that its half-life in the atmosphere is much shorter than that of CO
2 [
12].
Moreover, the black powder does not only produce GHG but also potassium compounds that at high levels can produce health problems such as skin and eye irritation, and even severe health problems if their concentration significantly increases [
63]. Nitrocellulose compositions remove the use of perchlorate compounds; therefore, the problems associated with the potassium compounds do not have to be considered. In this way, it has been shown that by using nitrocellulose as a propellant in pyrotechnic products, it is possible to reduce emissions of gases that have a direct impact on the greenhouse effect, such as CO
2, and which present a greater risk than those that contribute indirectly [
9]. Specifically, nitrocellulose reduces carbon dioxide emissions by 42%, i.e., it emits approximately half as much CO
2. Nevertheless, it has to be noticed that the use of nitrocellulose produces nitrogen dioxide, which was not found in black powder combustion. Nitric oxides lead to important environmental problems such as acidification, eutrophication and toxicity, among other effects [
64].
3.2. Gas Analyser Measurements
After analysing the use of nitrocellulose as a substitute for black powder as a propellant, the use of nitrocellulose as a substitute for perchlorate/metal-based compounds was analysed experimentally. These types of substances are one of the most harmful in pyrotechnics, which is why they are one of the main objectives of the sector, in order to move towards sustainable development [
65].
Currently, F1 category fountains for indoor use are manufactured as a variant with a nitrocellulose composition that aims to eliminate perchlorate compositions, thus reducing the environmental problems they cause.
The gas analyser measures the concentrations of both direct (carbon dioxide) and indirect (carbon monoxide) greenhouse gases generated. Therefore, nitrocellulose fountains and perchlorate fountains were tested. The results obtained in the tests include emissions from the protruding fuse of the perchlorate fountains. However, these emissions are negligible if compared to the total emission amounts. These results are shown in
Table 6.
As can be seen, the differences between Test 1 and Test 2 are not significant and can be attributed to the fact that the samples used in the tests have a small variation in NEC, which leads to this small difference in CO and CO2 concentrations.
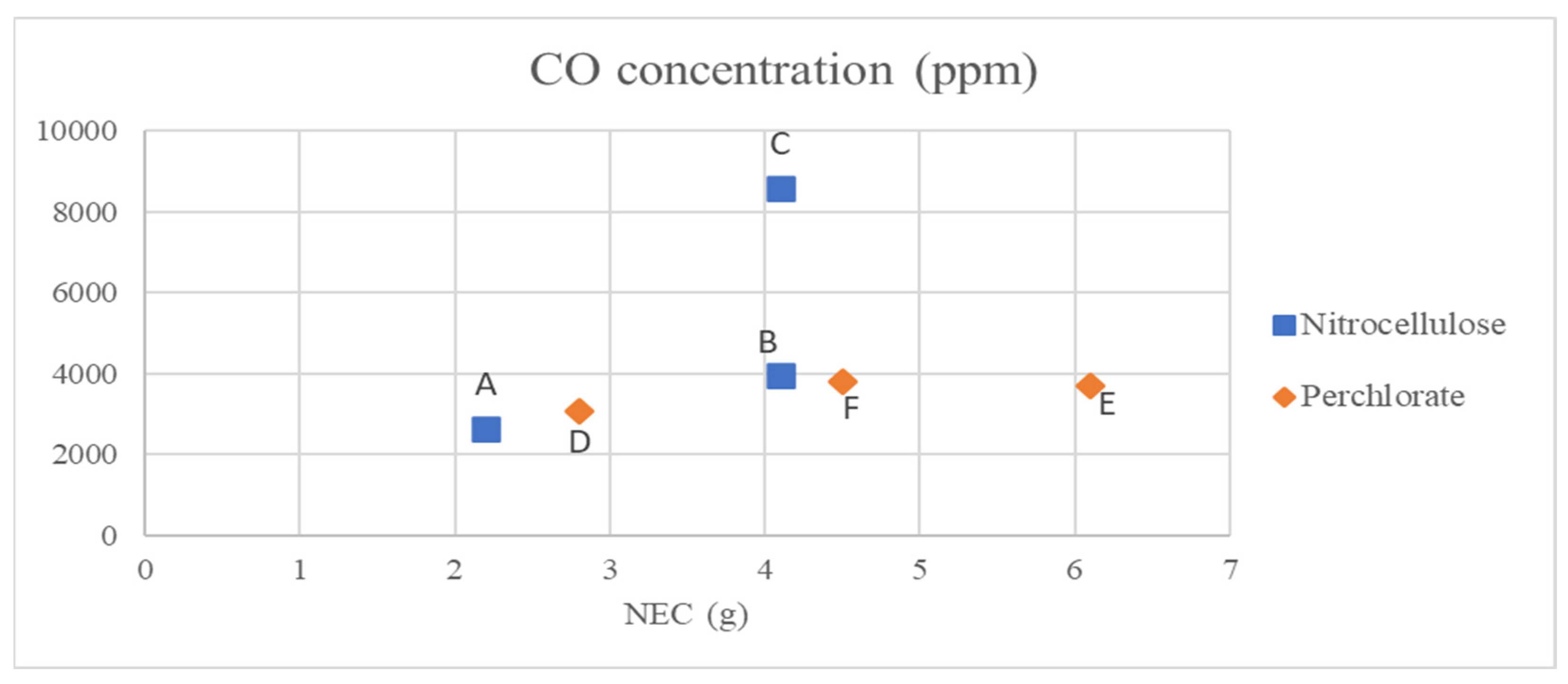
To properly compare the results obtained, the average of the concentrations measured in the tests carried out for each of the samples was calculated. The concentrations of CO in ppm and CO
2 in % vol. are graphically represented, as a function of the NEC of the test for each sample, in
Figure 2 and
Figure 3, respectively.
It can be seen that the nitrocellulose used by the second manufacturer (sample C) generates more emissions of both carbon monoxide and carbon dioxide than the first of the nitrocellulose samples under study. When compared to sample B, which contains the same NEC, concentrations of both gases are approximately twice as high. This indicates that despite being the same type of article and using the same composition, the specific characteristics of each nitrocellulose (percentage of nitrogen, quality of the components and/or the method of synthesis) influence the emissions generated. The same occurs when comparing the perchlorate fountains, as can be seen from sample D. Despite having the lowest NEC, sample D has the highest CO2 content of the three perchlorate fountains tested.
A general comparison of nitrocellulose fountains with those using perchlorate gives results similar to those obtained theoretically when comparing nitrocellulose with black powder. For CO concentration, they follow a slightly higher trend than those of perchlorate compositions. However, for CO2 concentration, those of the perchlorate samples are slightly above the nitrocellulose samples. These trends are less significant when compared to sample C, due to the poorer quality of the composition.
This means that, comparing nitrocellulose with perchlorate, both compositions emit similar or slightly higher CO2 values for perchlorate, but the opposite occurs for CO emissions, which are slightly higher for nitrocellulose compositions.
Table 7 shows the corresponding normalized emissions of each of the gases studied, i.e., per one gram of NEC. It can be seen that the CO emissions for the nitrocellulose samples are higher than those of the perchlorate samples, as deduced in the previous
Figure 2. In the case of CO
2, the emissions from the perchlorate composition are slightly above those of the nitrocellulose composition in general. This difference is significant for sample D.
It should also be added that, apart from these two compositions that directly or indirectly contribute to the greenhouse effect, perchlorate compounds are considered one of the most harmful in pyrotechnics, due to the environmental pollution they produce during their production, handling and use, mainly in groundwater [
23]. Therefore, the use of perchlorate-free compositions, such as nitrocellulose, would help to reduce greenhouse gas emissions from pyrotechnics and avoid pollution from perchlorate-based compounds [
17,
18,
19,
20,
21,
22].
3.3. Thermogravimetric Analysis and Mass Spectrometry
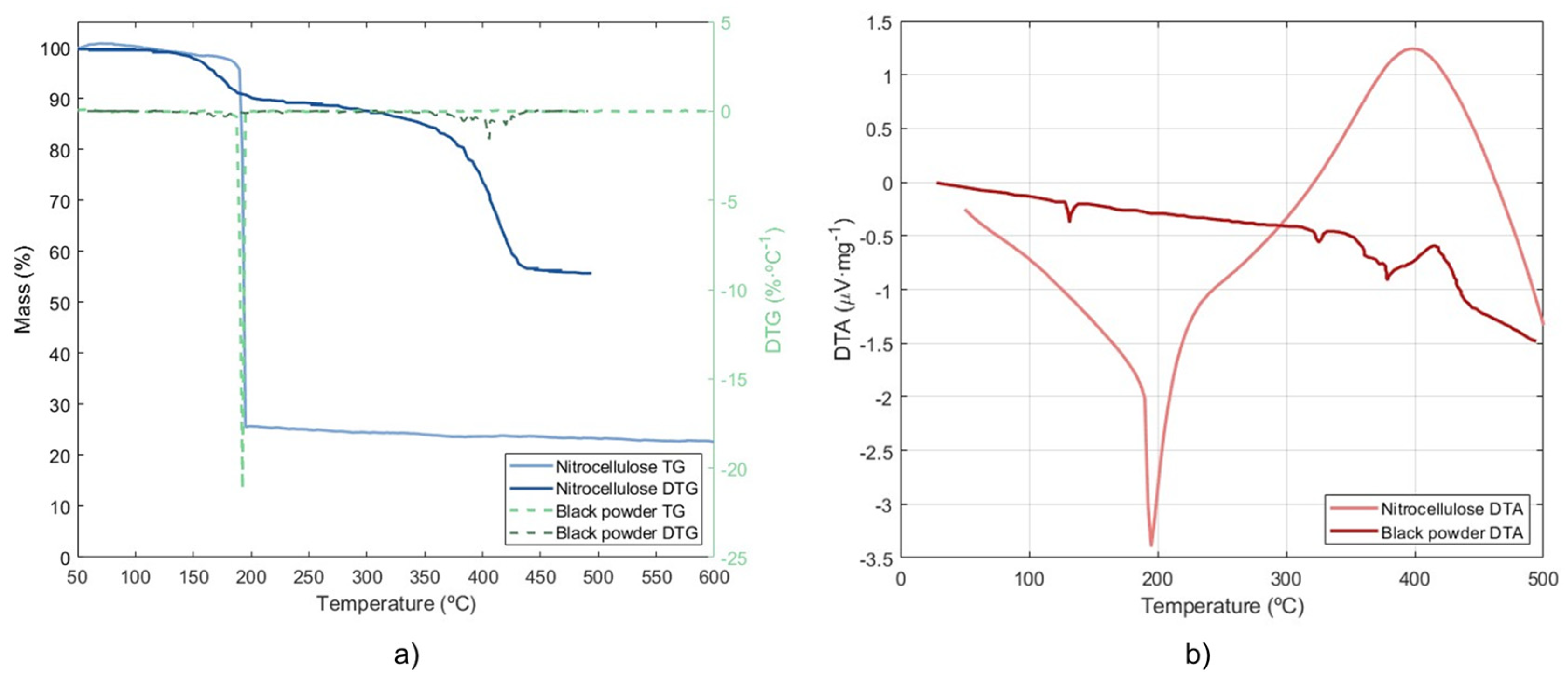
The TGA and DTG results are plotted in
Figure 4a, while
Figure 4b shows the DTA curve. In this figure, curves from black powder results under helium atmosphere reported by Turcotte et al. [
39] are plotted in order to properly compare the results between nitrocellulose and black powder. From the nitrocellulose TGA curve, it is possible to see that the moisture content can be neglected as it does not produce a significant mass loss when moisture is released. This suggests that an increase in moisture content will not influence their combustion characteristics but will reduce their ignition risk. When reaching 200 °C a reaction takes place producing a drastic mass loss up to approximately 77% of initial mass. This reaction corresponds to the decomposition of the nitrocellulose which previously published literature has already located around 200 °C [
38,
66]. Indeed, at 192.5 °C the reaction reaches its maximum mass loss rate, showing a peak in the DTG curve. On the other hand, when addressing black powder results it can be noticed that decomposition requires higher temperatures, as the mass loss produced due to the combustion reaction begins around 350 °C. There is also a small mass loss at the beginning of the reaction (~10% mass loss around 200–250 °C) that correspond to sulphur vaporization. The final residue is greater than 50%, which is consistent with the fact that the black powder produces a greater amount of ash than nitrocellulose. Because of this, the black powder DTG peak is significantly lower and, because of the temperature at which the fast oxidation that characterizes the combustion takes place, the maximum mass loss rate is shifted to the right close to 400 °C. When addressing black powder TG curves, it can be noticed that there are two different stages.
When DTA is addressed, there is a steady tendency for values to decrease from the beginning of the test until 187.5 °C; after which there is a drastic drop until 195 °C, and after that a tendency to increase, leading to positive DTA values after 300 °C. It indicates that there is an endothermic process at the beginning of the test but, once the sample reaction takes place an exothermic phase takes place. On the other hand, black powder shows a small endothermic peak at the beginning due to moisture release and a second group of peaks between 300 °C and 400 °C is produced when the reaction accelerates.
It has to be pointed out that nitrocellulose has an inherent instability due to the oxygen and chemical bonds present in its composition, characteristics that allows nitrocellulose to react even under inert conditions. Previous studies have reported thermogravimetric curves for nitrocellulose in which decomposition occurs with a progressive mass loss under inert conditions. However, the present study shows a rapid oxidation, which is more indicative of combustion than thermal degradation. This observation aligns with the known behaviour of nitrocellulose, and can be explained due to the oxygen released during the thermal degradation of the sample, which can be shown in
Figure 5, represented by amu 32. This exothermic stage continues up to 400 °C when the DTA begins to decrease again.
If the TGA results for the nitrocellulose are compared to black powder results from previously published literature, important differences can be noticed [
39,
40]. When the heating rate is not low enough it might happen that the different volatilization stages are not clearly seen in the TG curve. Although in the present study a 10 K/min heating rate was used, the results are similar to previously published nitrocellulose TG using lower heating rates (such as 2, 4 or 6 K/min) [
67]. As mentioned before, the temperature at which nitrocellulose decomposition takes place is significantly lower than the temperature at which the KNO
3 oxidizes the charcoal in the black powder, which means that in terms of safety, nitrocellulose requires lower energies to explode. Indeed, it is well known that nitrocellulose presents less thermal stability than black powder, and it is heavily affected by the moisture content [
68,
69].
Other differences can be found when addressing the ash content and the reaction time. As mentioned before, the black powder TG curves revealed an ash content around 60% as only 40% of the mass was volatilized in the two stages. On the other hand,
Figure 4 shows that the nitrocellulose ash content was set around 20%, which is significantly lower. Nevertheless, the 20% ash content seems to be high if compared to previously published literature [
37,
38,
66] in which the residue after TG is usually lower than 20%. This fact can be explained due to the sample composition and the carrier gas during TGA tests. Regarding the reaction time, the differences are not so relevant, as the sudden mass drop that can be noticed in
Figure 4 has been also reported in previous studies regarding black powder fast oxidation. In the present study, it was found that the reaction lasted for 26.4 s.
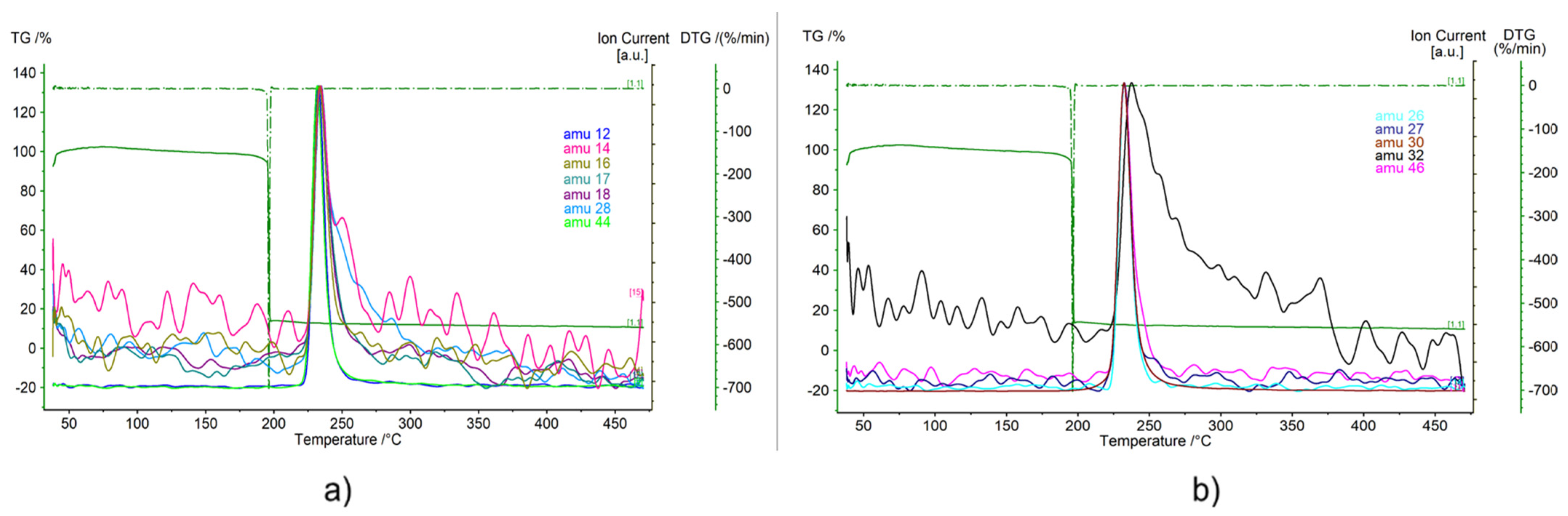
Figure 5a,b presents the mass spectrometry (MS) results superimposed to the TGA and DTG curves; nevertheless, due to the workflow of the mass spectrometer, a certain delay between DTG peak and amu peaks is present. The MS curves are divided into two different plots so the results can be seen more clearly. As the experiment was conducted using argon as a carrier gas, the thermal degradation of the nitrocellulose was simulated and so the products from this thermal decomposition are represented in the MS.
The main components are water, carbon monoxide, carbon dioxide and nitrogen and they are undoubtedly detected. Amu 18 is the molecular ion for H
2O, while amu 17 is a fragment ion. Because of an almost perfect match of those two curves, amu 18 confidently represents the release of H
2O. Carbon monoxide is detected in amu 12, 16, 28 and 29. However, amu 12, 16 and 28 do not only represent CO but also CO
2. Carbon dioxide is not only one of the main products of nitrocellulose combustion but also a product if its decomposition [
70]. The sharp peak at amu 44 represents its release. It is confirmed by its fragment ions, amu 16 and amu 12. Amu 12 follows the sharp shape of amu 44 with a very short release interval, while amu 16 is slightly wider, due to the influence of oxygen’s fragment ion.
Regarding nitrogen, it can be found both in amu 14 and 28. Nitrogen release is not only present during the combustion process of the nitrocellulose but also during pyrolysis [
70,
71,
72]. As mentioned above, amu 28 represents the release of carbon monoxide and dioxide together with nitrogen, while amu 14 can be attributed only to nitrogen. If looking carefully at
Figure 5, it can be noticed that amu 14 presents an additional peak around 250 °C, which can explain the fact that the peak of amu 28 is wider than the other component signals.
Besides these, there is another clear signal represented by amu 32, which corresponds to oxygen. The fact that this signal is wider than any other amu curve means that there is a continuous release of oxygen in several phases after the decomposition of nitrocellulose. Nevertheless, oxygen is not among the main products of nitrocellulose pyrolysis, no mass changes are present during the oxygen release, and there is no effect on amu 16, which could also be oxygen. Some authors have reported oxygen release in nanothermites that include nitrocellulose in its composition [
73]; however, the release temperatures are close to 500 °C. On the other hand, Chai et al. [
74] reported that the decomposition of nitrocellulose involves the fracture of the oxygen bridge, which could be a possible explanation for amu 32. Nevertheless, it is clear that this is an anomaly and further tests should be carried out in order to properly assess it.
The presence of HCN in combustion and pyrolysis products of triple-base propellants (composites of nitrocellulose, nitroglycerine and nitroguanidine) [
74,
75] might be related to peak shapes of amu 27, which is a molecular ion for HCN, and amu 26, which is fragment ion for HCN, because those two curve shapes have almost a perfect match. The remaining signals (amu 30 and amu 46) correspond to nitrogen oxides. It was found that during thermal decomposition and combustion of nitrocellulose, the predominant oxide of nitrogen is nitric oxide NO [
55]. This is confirmed by amu 30 which is depicted with a very clear curve with no “noise” disturbance and a very high peak. Nevertheless, nitrogen dioxide NO
2 is also found [
53,
76], and it is represented by amu 46.
The TGA and MS results have provided significant information regarding the nitrocellulose thermal behaviour and degradation process. Nitrocellulose data obtained from the TG results suggest that the explosion reaction is easier to achieve when compared to black powder, mainly due to the lower temperatures and the greater amount of volatile matter. This can be translated into a more severe risk, but this could be reduced if their moisture content is increased. Indeed, previous research has already reported improvements in nitrocellulose thermal stability by increasing the moisture content [
68]. Nevertheless, increasing the moisture content significantly deteriorates pyrotechnic devices [
77,
78]. Therefore, nitrocellulose should undergo a drying process before being incorporated into the devices. This would maintain the correct functioning of the fireworks, reducing the risk of ignition during transport and handling of the nitrocellulose
Moreover, due to the fact that the nitrocellulose presented low thermal stability, it is necessary to properly address the associated risk to determine if nitrocellulose is a viable substitute for black powder in pyrotechnic devices.
3.4. Risk Analysis Results
The full results of the study can be found in
Table S1 of Annex I of the Supplementary Material. The assessment was carried out independently for both compounds, nitrocellulose and black powder, but both compounds obtained the same result in the risk analysis. The reason is that both are sensitive to the same parameters, which are described in
Figure 6a. The most critical points of the process are those involving impact, friction and contact with an ignition source. In addition, the correct functioning of the safety systems is very relevant. A total of 21 maximum-risk situations and 51 high-risk situations were assessed. The number of situations is higher than in other sectors as the explosive material is highly reactive [
79].
Figure 6b shows that most risk situations occur during storage and during transfer to storage.
Although both compounds share the same risk situations, thermogravimetric analysis allows differences to be discerned. Some differences can be seen in the results of the thermogravimetric analysis. The risk of self-ignition is low for both compounds, as their self-ignition temperatures are high. However, when inert atmospheres are used, nitrocellulose reacts at 200 °C and gunpowder at around 400 °C as shown in the thermogravimetric analysis, so it is easier to start the reaction in nitrocellulose than in black powder. Therefore, although the severity of the consequences in the event of an accident are very similar for both compounds, it is easier for nitrocellulose to cause them. This does not mean that it cannot be used, but that it will be necessary to adapt some safeguards and protective measures to the use of nitrocellulose.
Throughout the study, a 1:1 substitution of black powder by nitrocellulose was considered, to estimate a direct comparison in environmental emissions that can be taken as a reference. However, when analysing previous studies [
80,
81], it is observed that the ratio of nitrocellulose necessary to reach the same heights in aerial fireworks should probably increase, as higher nitrogen content is required to achieve the correct explosiveness for aerial devices. Future studies should consider estimating the real ratio necessary to be able to use this type of nitrogen-rich compound and achieve the same effects as with black powder. Increasing the amount of nitrocellulose or the nitrogen content would directly increase the risks associated with pyrotechnic devices and, according to published literature [
82,
83], one of the problems associated with this increase in composition may be greater confinement inside the containers, which may lead to necessary design modifications. In addition, increasing the nitrogen content of nitrocellulose increases its ignition risk, so it should be considered whether increasing the moisture content for transport and handling, prior to incorporation into the pyrotechnic device, would make its use safer. These issues need to be analysed in depth in future research.
Once the accurate ratio between black powder and nitrocellulose is defined, the economic viability of this substitute should be analysed to properly address how the costs are influenced. It is known that nitrocellulose presents a greater cost than black powder (depending on the manufacturer up to ~65% more); therefore, since the amount of nitrocellulose will have to be increased, the economic costs are likely to increase in terms of production. Nevertheless, carbon markets and GHG emission-neutral regulations should be also considered.
4. Conclusions
The use of nitrocellulose as a substitute for traditional powders in pyrotechnic compositions was investigated with the aim of evaluating its viability as a more sustainable and safer alternative. The results were obtained through thermogravimetric analysis, mass spectrometry and experimental emission tests.
It was shown that nitrocellulose can significantly reduce greenhouse gas emissions, particularly carbon dioxide, compared to black powder. However, carbon monoxide emissions are higher when using nitrocellulose, due to the reactions that take place during combustion. Taking into account the residence time in the atmosphere of both gases and their influence on the greenhouse effect, it can be confirmed that nitrocellulose is a composition with advantages in environmental sustainability, as far as the greenhouse effect is concerned. By using nitrocellulose as a substitute for perchlorate oxidizers, the same trend in CO2 and CO emissions was obtained. Additionally, by eliminating perchlorates from the pyrotechnic compositions, groundwater contamination via soil from rainfall related to the production, handling and use of these compounds is reduced.
By comparing nitrocellulose from different suppliers, it was found that despite being the same type of article and using the same composition, the specific characteristics of each nitrocellulose (percentage of nitrogen, quality of the components and/or the method of synthesis) influence the emissions generated; therefore, standards should consider not only nitrogen percentage but the total composition.
When performing thermogravimetric analysis of nitrocellulose, it was noted that the moisture content can be neglected, as it does not produce a significant mass loss when moisture is released. This suggests that an increase in moisture content will not influence its combustion characteristics, but will reduce its risk of ignition during transport and handling of nitrocellulose. It must then undergo a drying process before being incorporated into the devices, thus avoiding deterioration of the pyrotechnic products.
Both thermogravimetric and HAZOP analyses confirmed that nitrocellulose is less thermally stable than black powder, as already reported by other authors. This does not mean that it cannot be used, but that some safeguards and protective measures will need to be adapted to the use of nitrocellulose.
Future studies should consider studying real prototypes to estimate the real ratio needed to be able to use this type of nitrogen-rich compound and achieve the same effects as with black powder, thus analysing not only its environmental feasibility but also its real application and the associated economic analysis.