Abstract
This study aimed to further explore the adsorption properties of different gases (CO2, O2, and CH4) on the coking coal surface by establishing a molecular model. Changes in the absolute adsorption capacity and the isosteric heat of adsorption of gases under different temperatures, pressures, and compositions were simulated using grand canonical Monte Carlo (GCMC) and molecular dynamics simulations. Interaction energy and energy distribution were used to analyze the adsorption behavior of gases, and the diffusion properties were investigated using the diffusion coefficient and diffusion activation energy. The absolute adsorption results fit well with the Langmuir–Freundlich model. The absolute adsorption capacity had a significant positive correlation with pressure and the corresponding mole fraction, and a significant negative correlation with temperature. The competitiveness, based on binary adsorption selectivity, was in the order of CO2 > O2 > CH4. The isosteric heat of adsorption of CH4 was slightly higher than that of O2, and that of CO2 was 1.49–1.64 times that of O2 and CH4. The isosteric heat of the adsorption of gases was also barely influenced by temperature and pressure. The interaction energy between CO2 and coal was greater than that of O2 or CH4, but the high pressure and high content were not conducive to the adsorption of O2 by CO2. The preferred adsorption site for CO2 was stronger than that for O2 and CH4, and its peak value negatively correlated with the molar fraction. The diffusion coefficient for single component gases initially increased and then decreased with increased pressure, showing a positive correlation with temperature. A close inverse correlation existed between diffusion activation energy and pressure. These results revealed the microscopic adsorption and diffusion regularities of CO2, O2, and CH4 in the coal model, indicating great significance in accurately predicting coal fires.
1. Introduction
Under the influence of the national characteristics of “poor oil, less gas, and rich coal”, coal dominates the energy system in China [1]. However, the complex geohydrological structure of mines and other disaster-inducing factors have conferred 56% of recoverable coal seams with self-combustion tendency [2], of which about 49% are affected by combined disasters [3]. Thus, preventing and controlling coal’s spontaneous combustion has become a research hotspot in coal mine safety [4].
The initiation of coal autoignition is an extremely intricate process of coal–oxygen physicochemical adsorption. The coal first physically adsorbs oxygen to saturation, at which point the functional groups on the coal surface chemically react with the oxygen to change the molecular structure and release heat. Heat build-up leads to an increase in temperature, triggering coal’s spontaneous combustion [5]. Coal–oxygen adsorption provides the early basis for coal self-ignition, a process of significant importance in revealing the theory of coal spontaneous combustion. However, the influence of CH4 and CO2 in the coal seam on coal–oxygen adsorption cannot be ignored. To this end, scholars have used molecular simulations to investigate the competitive adsorption regularity for CO2 and CH4 in coal. Lu et al. [6] studied the physisorption process of oxygen by functional groups in coal. Their results show that physisorption begins to change into chemisorption as the adsorption amount increases. Zhou et al. [7], Yang et al. [8], Zhang et al. [9], and Ren et al. [10] used a coal macromolecular model to adsorb CH4 and CO2 and found that the adsorption capacity of CO2 was stronger than that of CH4 [11]. Yu et al. [12], Liu X et al. [13], and Dang et al. [14] investigated the impact of oxygenic groups on the competitive adsorption of CO2, CH4, and N2 in coal using GCMC and density functional theory (DFT) simulations. They found that the strong quadrupole moment and polarization capacity of CO2 makes it more selectively adsorbed by functional groups than CH4 and N2. Cheng et al. [15], Xiang et al. [16], and Ding et al. [17] revealed the different adsorption and diffusion mechanisms of CH4, CO2, and N2 gases from three aspects: adsorption isotherms, adsorption heat, and diffusion coefficients using molecular simulation and quantum chemistry methods. They showed that the order of adsorption capacity for the three gases is CO2 > CH4 > N2, whereas the diffusion capacity follows the opposite sequence. Yu et al. [18] and Tang et al. [19] conducted experimental studies on the competitive adsorption of mixed gases under different volume fractions. They found that the adsorption amount in multicomponent gases was affected by the adsorption power, molecular properties, and the partial pressure. Wang et al. [20] and Zhang et al. [21] studied the adsorption of CH4, CO2, N2, O2, and their mixed gases on a coal surface model. The presence of CO2 was found to greatly reduce the adsorption amount of CH4 in the binary system, and the adsorption of oxygen was more likely to be affected by the volume fraction of methane.
In summarizing the competitive adsorption among multicomponent gases in coal, previous studies have primarily focused on improving the efficiency of CBM extraction, and most of them made comparisons with N2. Only a few studies have investigated the influence of the competitive adsorption behaviors of CH4, CO2, and O2 on coal’s spontaneous combustion. However, O2 is an essential gas for the spontaneous combustion of coal, so the study of its competitive relationship with CH4 and CO2 in coal is an essential prerequisite for ensuring the safe operation of coal mines and preventing the occurrence of fire accidents due to the spontaneous combustion of coal. Therefore, taking CO2, O2, and CH4 as the research objects, the present study used GCMC to simulate and analyze the adsorption behaviors of these gases in the molecular structure of coking coal. This research aimed to provide a theoretical basis for coal mine fire prevention at the microscopic level.
2. Construction and Simulation Method of the Coal Molecular Model
2.1. Coal Molecular Configuration
The coking coal was obtained from the 12th Coal Mine of Pingdingshan, Henan Province. For this research, elemental and industrial tests were used to analyze the coking coal, and the results are shown in Table 1. According to 13C-NMR, XRD, XPS, and other experiments, we obtained information on the distribution of hydrocarbon atoms, the arrangement of aromatic structures, and the presence of functional groups containing nitrogen, oxygen, and sulfur. By combining the results of the industrial analysis with the results of the elemental analysis, the molecular formula of coking coal was determined to be C209H140O17N4, which was derived from modeling methods described in the literature [22]. The two-dimensional structure diagram and the structural parameters are displayed in Figure 1 and Table 2, respectively.

Table 1.
The basic parameters of the coal sample.
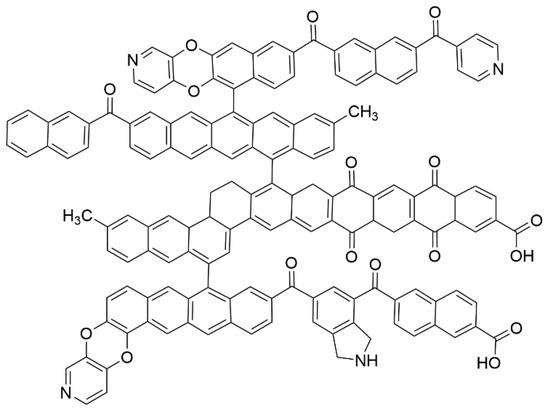
Figure 1.
The molecular configuration diagram of coal (two-dimensional).

Table 2.
The structural parameters of the coking coal.
2.2. Optimization of the Coal Macromolecule Model
Since only the structural model with the lowest energy represented the optimal configuration under study, the two-dimensional structure of coking coal was imported into Materials Studio software and the Forcite module was used to calculate MM and MD. The main purpose of MD calculation was to avoid excessive calculation of adsorption simulation due to the complex macromolecular structure. The MM parameters were [23] that the calculation method was a smart minimizer, the maximum number of iteration steps was set to 5000 steps, and the charge distribution and the force field were the charge equilibrium method and Dreiding. The electrostatic and Van der Waals values were calculated using the atom-based method. The MM calculation overcomes the disadvantage of only the local minimum value being obtained via MD. The minimum value of the whole potential energy surface was found using the annealing dynamics simulation. The MD parameters were the following [24]: the NVT ensemble (constant particle number N, volume V, and temperature T in the simulation system remain unchanged) was selected, the temperature range was 300–600 K, and it was heated up 60 K each time and cycled ten times. The temperature control program selected Nose, and the step size was 1 fs. The MM calculations were performed on the output configuration at the end of each cycle, and the parameters were set as described above. The optimized results of MM and MD are shown in Figure 2a,b, respectively. The adsorbent configuration after molecular optimization is shown in Figure 3.
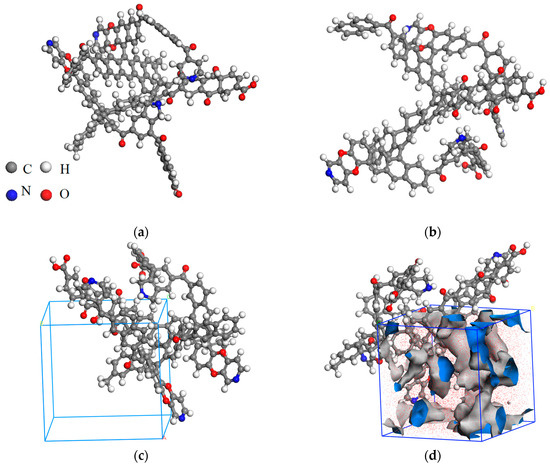
Figure 2.
(a) geometry optimization, (b) annealing treatment, (c) cubic cell model, (d) connelly surface.
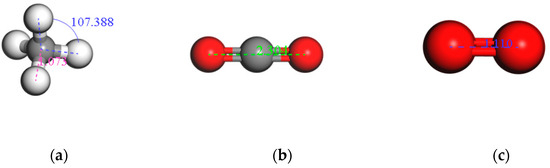
Figure 3.
The optimized molecular structure of an adsorbent: (a) CH4, (b) CO2, and (c) O2.
Density simulation not only added periodic boundary condition to coal molecules, but also explored the optimal configuration under periodic boundary conditions. Finally, the reliability of the modeling method was verified by comparing the cell density obtained after passing the minimum energy point with the actual coal sample density. The periodic boundary conditions were added to the model using the Amorphous Cell module. The following parameters were used to simulate the density [25]: the calculation used Medium, the Dreiding was used to force field, and the charge was calculated using the charge balance method (QEq). The initial density was 0.6 g/cm3, the final density was 1.8 g/cm3, and the interval was 0.05 g/cm3. By analyzing the relationship between density and potential energy, it was found that the structure had the lowest potential energy when the density was 1.35 g/cm3. The lattice parameters of the optimal configuration are nm, α = β = γ = 90°, as shown in Figure 2c.
After the NPT (P = 1 MPa, T = 298 K) ensemble simulation, the final density of the model was stabilized at 1.39 g/cm3, which was close to the measured value. The effective pore volume and surface area for the model were determined using the Atom Volumes and Surfaces tool, and were 2414 m2/g and 0.0209 cm3/g. The Connery surface of the model [26] is shown in Figure 2d.
To closely approach the real porous state of coal, a supercell molecule comprising 2 × 2 × 2 original molecules of coking coal was constructed to form pores of different sizes [27]. This paper used the GCMC method to simulate the relationship between the adsorption capacity of CO2, O2, and CH4 on coal surface and fugacity, wherein fugacity was converted from pressure using the Peng–Robinson equation [28]. The adsorption characteristics of gas molecules on the coal surface were investigated using the adsorption module to obtain the adsorption isotherm, adsorption site, action energy, diffusion coefficient, and adsorption selectivity. The parameters of the sorption module were set as follows [29]: the task item was Fix Pressure and the precision was Customized. The Metropolis method was used to calculate the energy change in adsorbed gas molecules on the surface of the coal molecular model. The number of molecules N in the equilibrium state of the simulated system was obtained using the μVT ensemble (chemical potential μ, volume V, and temperature T of the model system remained unchanged). To achieve thermodynamic equilibrium and save on computation time, the total number of simulation steps was determined to be 2 × 107 Monte Carlo steps. The number of equilibrium steps was set as 1 × 107 to ensure adsorption equilibrium and the number of production steps was set as 1 × 107 to sample the exact average date. The models obtained at different temperatures and pressures using the Fix pressure method were taken as the initial model, which was optimized using MD simulation. The Dynamic task was used to calculate the kinetic coefficients, such as diffusion coefficient and diffusion activation energy.
3. Simulation Result Analysis
3.1. Absolute Adsorption Capacity
3.1.1. Single Component Gas Adsorption Capacity
The simulation data were fitted using Langmuir, Freundlich, and Langmuir–Freundlich models [30]. The Langmuir–Freundlich model could describe the entire adsorption for gases very well, and its fitting formula [31] was as follows:
where is the adsorption amount (mL/g), is the gas maximum single layer adsorption amount, is the adsorption pressure (MPa), is the adsorption equilibrium constant (MPa−1), and is the surface heterogeneity of the adsorbent. The fitting curves of the adsorption isotherm are shown in Figure 4.
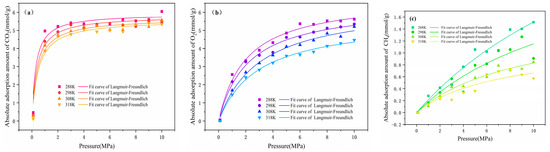
Figure 4.
The adsorption isotherm and Langmuir–Freundlich fits of (a) CO2, (b) O2, and (c) CH4.
With increased pressure, the adsorption isotherm for CO2 initially increased quickly and remained stable, O2 increased slowly at the low pressure stage and then gradually became saturated, and CH4 increased linearly. These phenomena not only showed that there was a critical value of the influence of pressure on the adsorption capacity [32], but the molecular weight of the gas also affected the adsorption amount because coal molecules had a limited effective adsorption point. Therefore, CO2 had a high adsorption amount due to its greater molecular weight, compared to CH4 and O2. In comparison with the simulation results of Qiang [33], the absolute adsorption isotherm for CO2 in this work was found to be qualitatively in agreement, but quantitatively higher. The reason was that the hydrogen, oxygen, and nitrogen content in coking coal was higher, indicating that there were more hydroxyl, carboxyl, and methoxyl groups interacting with CO2. The relationship between the adsorption amount of gases under the same condition was CO2 > O2 > CH4. The interaction energy between gases and coal molecules was an important basis for sequencing. The adsorption capacity decreased with the temperature increase. This was because the initial stage of adsorption was physisorption caused by intermolecular suction, and the increase in temperature reduced the original weak binding force and led to desorption.
The fitting parameters and correlation coefficient of the Langmuir–Freundlich models are summarized in Table 3. All R2 values exceeded 0.98, confirming the reliability of the Langmuir–Freundlich model. The relationship of the parameter was a(CO2) > a(O2) > a(CH4), which means that CO2 was the first gas to attain stability and had the maximum adsorption capacity. The parameter b was inversely proportional to the pressure required for saturation, indicating that the magnitude of the pressure required by the gas was CH4 > O2 > CO2, so that the adsorption rate for CO2 increased the fastest under a low pressure [34]. With increased temperature, the fitted parameters gradually decreased. This suggests that the temperature was not favorable to gas adsorption, but provided energy for the gas to escape from the coal surface.

Table 3.
The Langmuir–Freundlich fitting parameters.
3.1.2. Multi-Component Gas Adsorption Capacity
To investigate the adsorption capacity between different gases, the adsorption isotherms for multi-component gases on coal at 298 K were simulated. To analyze the effect of molar fraction on adsorption behavior in the binary component system and the difference in competitive adsorption of gases in the ternary component system, the component ratios were set to 1:4, 2:3, 3:2, 4:1, and 1:1:1. The simulation results are shown in Figure 5a–d.
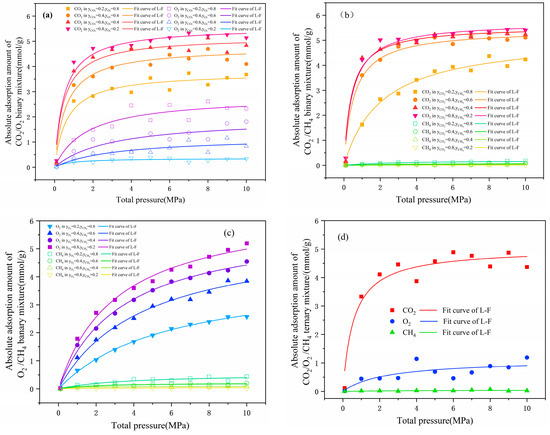
Figure 5.
Absolute adsorption isotherm of binary (a–c) and ternary (d) systems.
Figure 5 shows that the adsorption amount of multi-component gas increased with increased molar fraction. In the CO2/O2 and CO2/CH4 binary system, the adsorption amounts of CO2 were always greater than those of O2 and CH4 at different molar fractions, manifesting that CO2 had a more competitive capacity than O2 and CH4. A comparison of Figure 5a,b revealed that the maximum values of CO2 in the CO2/O2 and CO2/CH4 systems were 5.32 and 7.57 mmol/g, respectively, demonstrating that O2 was more competitive than CH4 and consistent with Figure 5c. By comparing the adsorption quantities within the pure-gas system and the CO2/O2/CH4 system, it could be found that the adsorption amount for O2 dramatically decreased. It has been shown that the presence of CO2 affected the physical adsorption of O2 in coal and reduced the possibility of the spontaneous combustion of coal from the source.
3.1.3. Adsorptive Selectivity
To further research the preferential adsorption ability of the gases on coal, adsorption selectivity was used to describe the competition hierarchy for multicomponent gas. could be defined as follows:
where (or ) and (or ) are the mole fraction of species (or ) in the adsorbed phase and bulk phase, respectively. The adsorption selectivity was larger than 1, indicating that the competitive adsorption of adsorbate in the multi-component was stronger than that of adsorbate , and greater selectivity corresponded with stronger adsorption.
decreased with the increased pressure and molar fraction of CO2, as shown in Figure 6a, indicating that high pressure and high content reduced the competitiveness of CO2. This finding was primarily due to the CO2 saturation being reached during the high-pressure phase, whereas O2 was always on the rise. Figure 6b showed that was inversely proportional to pressure and positively proportional to the molar fraction of CO2, indicating that a higher CO2 content corresponded with a stronger the competitive adsorption. As shown in Figure 6c, was proportional to the pressure and the molar fraction of O2 because the amount and rate of adsorption for O2 was far beyond that of CH4. The analysis of adsorption selectivity revealed that the competitiveness remained at CO2 > O2 > CH4, and this conclusion was confirmed by the tri-component system shown in Figure 6d.
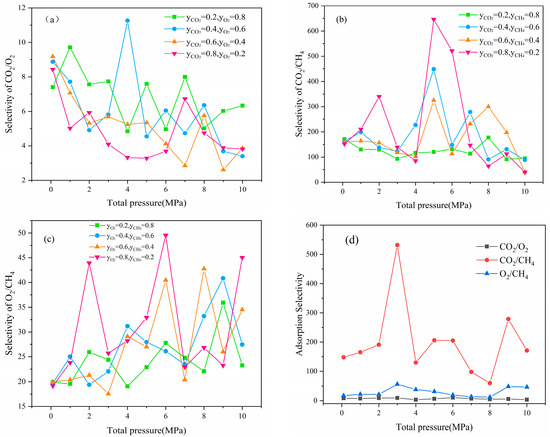
Figure 6.
Adsorption selectivity of binary (a–c) and ternary (d) systems.
3.2. Isosteric Heat of Adsorption
3.2.1. Single Component Gas Adsorption Heat
To some extent, the adsorption capacity could be reflected by the magnitude of the isosteric heat of adsorption. A stronger interaction energy between the gas and coal corresponded with greater isosteric heat [35]. The relationship between the adsorption heat and pressure of CO2, O2, and CH4 at different temperatures is shown in Figure 7. The adsorption heat of each gas was only slightly affected by temperature and pressure. The adsorption heat of CO2 initially decreased and then increased at around 8.625 kcal/mol. This finding may be due to the energy of the adsorbate–adsorbent interaction dominating at low pressure, whereas the adsorbate–adsorbate interaction contributed more at high pressure. Conversely, the isosteric heat for O2 and CH4 showed an overall decreasing trend of about 0.5–1 kcal/mol, indicating that adsorption was dominated by adsorbate–adsorbent interaction. The isosteric heat of CH4 was a little superior to that of O2, and that of CO2 (8.44–8.73 kcal/mol) was about 1.49–1.56 and 1.55–1.64 times that of O2 and CH4, respectively. Thus, the order of adsorption heat was CO2 > CH4 > O2, inconsistent with the order of the adsorption amount. This indicated that the isosteric heat of adsorption was only one of many factors affecting the adsorption capacity, which reflected the adsorption capacity to a certain extent. Because the heat levels of CO2, O2, and CH4 were less than 10 kcal/mol, the adsorption on the coal surface was a physical process [36].
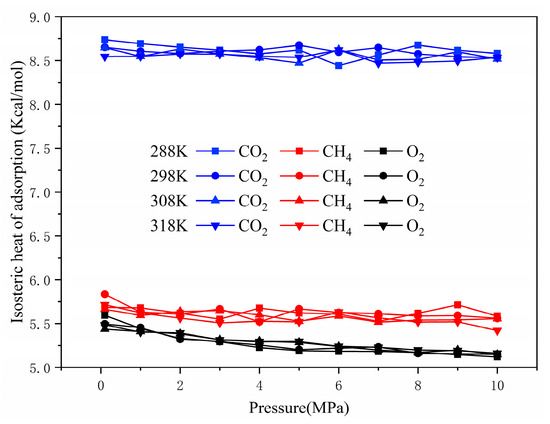
Figure 7.
The isosteric adsorption heat of single component gas.
Henry’s law of adsorption describes the linear relationship between adsorption amount and equilibrium pressure at low pressure, and the formula was as follows:
where is the adsorbing capacity, is the Henry constant, and is the adsorption pressure. The Henry constant could characterize the adsorbate affinity, and it decreased with decreased . The relationship of adsorption affinity for three gases was CO2 > O2 > CH4, as shown in Figure 8. This result was due to the permanent quadrupole moment of the CO2 molecule, which created a stronger electrostatic force on the surface of coal molecules [37]. The negative correlation showed that the affinity of these gases could be reduced by the creasing temperature. The values of CO2 were more sensitive to temperature changes, and that of CH4 slowly decreased with increased temperature.
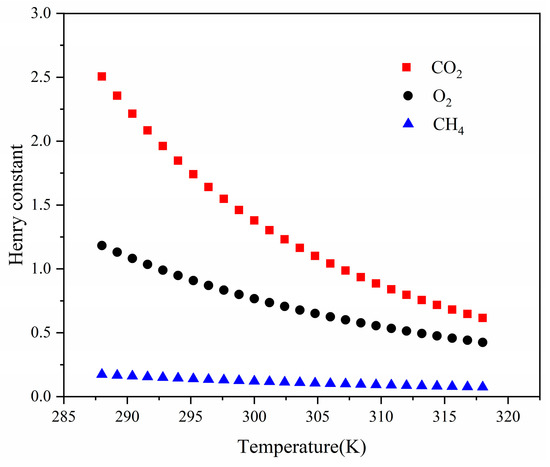
Figure 8.
The Henry constant for single component gas.
3.2.2. Multi-Component Gas Adsorption Heat
The isosteric heat of adsorption in multi-component systems is shown in Figure 9, and it depended primarily on gas species and the system associated with it. Figure 9a shows that the adsorption heat for CO2 was proportional to its molar coefficient in the CO2/CH4 system, but inversely proportional in the CO2/O2 system, consistent with the trend of adsorption selectivity in binary systems. Influenced by the competition between CO2 and CH4, the adsorption heat of O2 in the CO2/O2 and O2/CH4 systems differed. The adsorption heat of O2 decreased with increased molar fraction, which was contrary to that of CH4, indicating that the high CH4 content was more competitive than that of O2. By comparison with Figure 9b and c, it could be seen that the adsorption heat of O2 and CH4 in the O2/CH4 system followed the same trend as that of pure gas, whereas it fluctuated more when CO2 was involved. This phenomenon was due to the adsorption heat of pure CO2, showing a local minimum that could be found only in strongly adsorbed gas [38]. The order of adsorption heat of gases in the CO2/O2/CH4 system was compatible with that of pure gases. The change trends for O2 and CH4 showed more obvious fluctuations than those of CO2, as shown in Figure 9d. This finding indicated a strong competition between O2 and CH4 for adsorption heat.
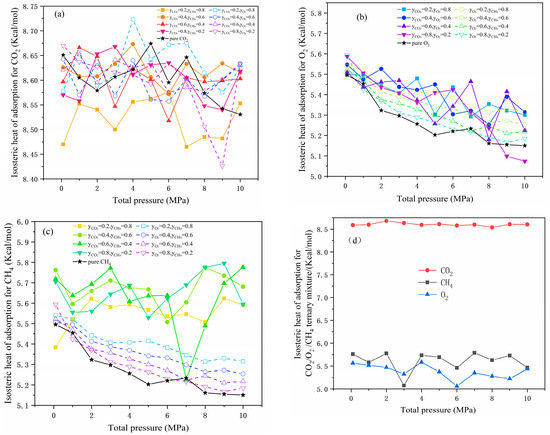
Figure 9.
The isosteric heat of adsorption of CO2, O2, and CH4 in binary (a–c) and ternary (d) systems.
3.3. Interaction Energy
The interaction energies of CO2, O2, and CH4, including van der Waals energy and electrostatic energy, were analyzed at different molar fractions in multi-component systems to further investigate the effect of competitive adsorption of gases for the interaction energy [39]. The results are shown in Figure 10.
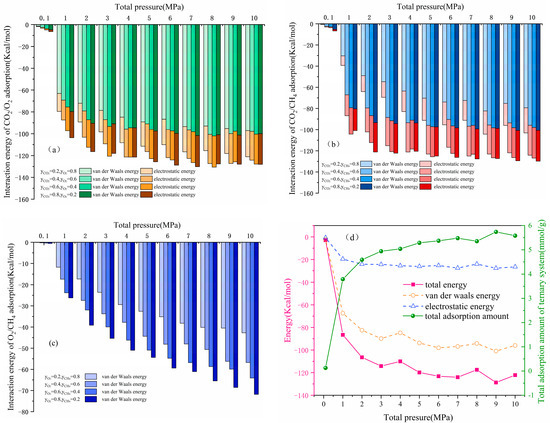
Figure 10.
Interaction energy: (a) in the CO2/O2 system; (b) in the CO2/CH4 system; (c) in the O2/CH4 system; and (d) the total energy and adsorption amount in the CO2/O2/CH4 system.
As shown in Figure 10a,b, the van der Waals energy accounted for more than 79% and 77% of the total interaction energy in the CO2/O2 and CO2/CH4 systems, respectively, whereas the rest of the electrostatic energy originated from CO2. This was because CO2 was electrically charged and generated electrostatic energy when adsorbed. Meanwhile, the higher molar fraction for CO2 corresponded with stronger interaction energy, indicating that it was more likely to adsorb than O2 and CH4, and the adsorption system was more stable. For the CO2/O2 system, the interaction energy was slightly reduced when the pressure exceeded 8 MPa and the molar fraction of CO2 exceeded 60%, demonstrating that the high pressure and high content reduced the competitiveness of CO2. For O2/CH4 binary systems, up to 98% of the total energy of interaction was van der Waals energy, and only a small amount of energy originated from electrostatic energy. The interaction energy was proportional to the pressure and the molar fraction of O2, indicating that O2 was more stable than CH4. The results showed that the relationship of the adsorption stability of the gases was CO2 > O2 > CH4, which was consistent with the relationship of adsorption amount.
As shown in Figure 10d, the interaction energy in the CO2/O2/CH4 system decreased from −2.962 kcal/mol to −122.163 kcal/mol with the pressure from 0.1 MPa to 10 MPa. The van der Waals energy accounted for more than 73%. The larger the absolute value of the interaction energy, the more prone it was to adsorb. The van der Waals energy, electrostatic energy, and total energy initially increased rapidly and then slowly with increased pressure, which was consistent with the increasing trend of adsorption capacity under the CO2/O2/CH4 system [40].
3.4. Energy Distribution
The adsorbed sites could be identified using the energy distribution and be used to analyze competitive adsorption. Larger negative values indicated a stronger interaction energy and more favorable adsorption sites [41]. The energy distribution of pure CO2, O2, and CH4 at different temperatures was shown in Figure 11a. The preferential adsorption site for CO2 was lower than O2 or CH4, and the peak for O2 was almost equal to that for CH4. This was consistent with the order of equal heat of adsorption. The distinct potential energy peak was around −10.5 kcal/mol, corresponding with favorable adsorption sites for CO2. Its peak decreased with increased temperature, which was because the temperature stimulated the activity of CO2 molecules, so that the adsorbed gas molecules began to diffuse. Another peak at around −6.5 kcal/mol represented the favorable adsorption sites for O2 and CH4. The peaks of the preferential adsorption sites for O2 and CH4 moved toward the lower energy region with increased pressure, and a new peak at −4 kcal/mol formed at the secondary adsorption site. This finding was primarily due to the increase in adsorption volume caused by pressure, so that the priority adsorption sites gradually became saturated. Then, a large number of gas molecules shifted to the secondary adsorption sites, resulting in a movement in the relative importance of the adsorption sites.
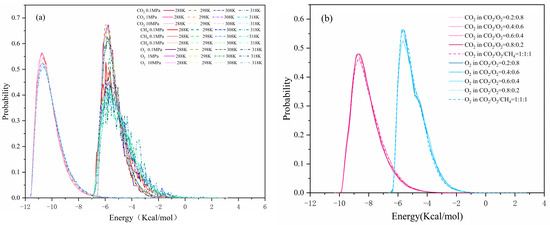
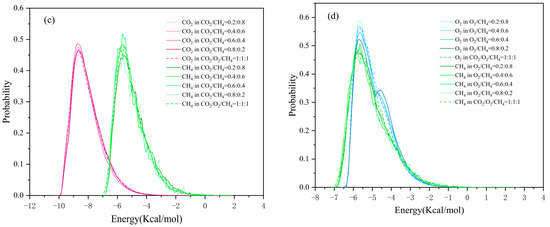
Figure 11.
Energy distribution: (a,b) in a single component adsorption system; (c,d) in different adsorption systems.
The energy distribution of gases in the multi-component systems was compared at different molar fractions in order to study competition among adsorption sites, as shown in Figure 11b–d. In the CO2/O2 system, the peak value of the preferential adsorption site increased with increased molar fraction, implying that CO2 and O2 gradually reached saturation with increased adsorption capacity. The potential energy shifted to the right and the peak decreased with increased molar fraction in the CO2/CH4 system, showing that the high content did not facilitate the competitive adsorption of gas molecules at the preferred site. By comparing the energy–distribution curve of O2 in the CO2/O2 and O2/CH4 systems, we found that adding CH4 increased the peak at the second adsorption site. This finding indicated that the presence of CH4 forced the O2 molecules to diffuse away from the preferred adsorption site, thereby inhibiting O2 from reaching saturation. The molecular proportion of the preferred adsorption site for O2 (−5 kcal/mol) in the binary components was reduced.
3.5. Diffusion Property
When gas molecules made contact with the coal surface, different pressures and concentration gradients were formed by different adsorption capacities. The gas diffused from high to low under the gradient, which follows the microscopic principle of diffusion [42]. The self-diffusion coefficients of CO2, O2, and CH4 were calculated using MD simulation to reveal the diffusion regularity of the gas on coal [43]. From the Einstein diffusion equation, the Ds could be calculated as follows [44]:
where is the slope of the fitting curve of , and ; is the number of gas molecules; is the Cartesian position vector of gas molecule in the microcrystallite at the time ; and is the initial position vector of the gas molecule. The diffusion coefficient and diffusion activation energy for gases on coal at different pressures are depicted in Figure 12.
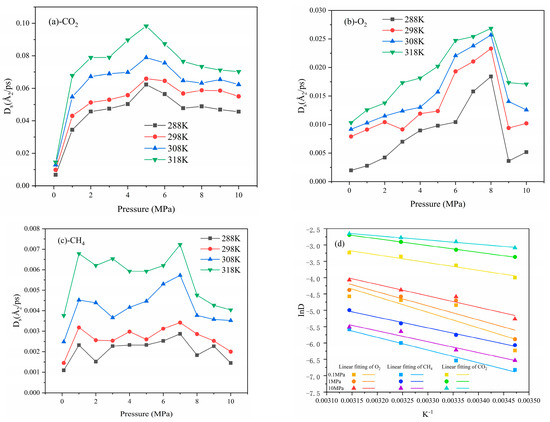
Figure 12.
The self-diffusion coefficient (a–c) and the diffusion activation energy (d) of CO2, O2, and CH4.
The self-diffusion coefficient of gases was positively correlated with temperature because the higher temperature corresponded with the higher internal energy of the molecules. According to the law of conserving energy, the internal energy between molecules could be converted into kinetic energy, thereby intensifying the movement of molecules and making it easier for them to diffuse. The relationship between the self-diffusion coefficients of the gas was CO2 > O2 > CH4 at the same temperature, consistent with the results of Kelemen et al. [45]. The self-diffusion coefficient initially increased and then decreased with pressure. The main reason for the reduction was that gas molecules stacked up more tightly and interacted more strongly under high pressure.
The diffusion of gas in coal molecules was an activation process. The diffusion activation energy could be reckoned using the Arrhenius equation [46], and the specific formula is as follows:
where is the pre-exponential factor; is the apparent activation energy, kcal/mol; is the ideal gas constant; and T is the temperature, K. InD is fitted well to the reciprocal of temperature, and the calculated result is listed in Table 4. The activation energy of gas diffusion was found to be negatively correlated with pressure, and the activation energy of O2 was approximately twice that of CO2.

Table 4.
The diffusion activation energy of CO2, O2, and CH4.
4. Conclusions
To research the microcosm mechanism of CO2, O2, and CH4 adsorption and diffusion on coal, a realistic macromolecular coal model was established. GCMC and MD molecular simulations were performed in single, binary, and ternary systems, considering the effect of temperature, pressure, and molar fraction.
(1) Adsorption isotherms were well fitted with the Langmuir–Freundlich model. The absolute adsorption amount was directly proportional to the pressure and inversely to the temperature. The adsorption of multi-component gases showed that adsorption amount was proportional to the molar fraction, but high pressure and high content reduced the competitiveness for CO2. The competitive capacities were CO2 > O2 > CH4, based on adsorption selectivity. By comparing the adsorption amount of O2 under different component systems, we found that CO2 significantly reduced the adsorption amount of O2.
(2) The isosteric heat of adsorption of CO2 (8.44–8.73 kcal/mol) was much greater than that of O2 or CH4 (5.12–5.83 kcal/mol). The difference in order between the adsorption quantity and the adsorption heat for the three gases meant that the adsorption amount was influenced by the adsorption heat, and many other factors. The adsorption heat was affected by the molar fraction and competition from other gases in mixed adsorption systems, which changed the adsorption sites and adsorption spaces and influenced the interaction energy. The presence of CO2 affected the trend of the equivalent heat of adsorption of another gas with which it competed, and the existence of CH4 caused large fluctuations in the adsorption heat of O2.
(3) The electrostatic energy and high van der Waals energy between CO2 and coal resulted in an interaction greater than with O2 and CH4. The greater interaction energy corresponded with the greater adsorption amount. In a competitive adsorption system, CO2 and CH4 changed the relative importance of the competitive adsorption sites for O2, thereby inhibiting O2 adsorption. The gas diffusion coefficient was inversely correlated with the temperature under the same pressure. The diffusion coefficient increased and then decreased with increased pressure at the same temperature. The order of diffusion activation energy was O2 > CH4 > CO2, which was negatively correlated with pressure.
Author Contributions
Conceptualization, L.Q. and Z.W.; methodology, Z.W.; software, L.Q.; validation, L.Q., Z.W. and L.L.; formal analysis, Z.W.; investigation, Z.W.; resources, L.Q.; data curation, Z.W.; writing—original draft preparation, Z.W.; writing—review and editing, Z.W.; supervision, L.Q. All authors have read and agreed to the published version of the manuscript.
Funding
This work is supported by the National Science Foundation of China (51804355) and the Research and Innovation Project of Zhongyuan University of Technology (YKY2023ZK13).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data unavailable due to privacy.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Wu, K. Simulation of Characteristics of Coal and Oxygen Adsorption with Quantum Chemistry Methods. Master’s Thesis, Xi’an University of Science and Technology, Xi’an, China, 2011. [Google Scholar]
- Cui, L. Present Status and Countermeasure Consideration on Comprehensive Utilization of China Coal Resources. Coal Econ. Res. 2013, 33, 46–47+66. [Google Scholar]
- Yuan, S. Gas Management Concept and Coal and Gas Co-mining Technology. Energy Energy Conserv. 2018, 10, 151–152. [Google Scholar]
- Xu, Y. Research on Macro Featured Parameters and Test Methods of Coal Spontaneous Combustion Character. Master’s Thesis, Xi’an University of Science and Technology, Xi’an, China, 2014. [Google Scholar]
- Chen, L.; Zhang, Y. Study on influence laws of oxygen concentration on thermal effect of coal low-temperature oxidation. J. Saf. Sci. Technol. 2020, 16, 49–54. [Google Scholar]
- Lu, J.; He, Y.; Cheng, G. Construction of Lignite Macromolecular Model and its Analysis on Physisorption Oxygen at Low Temperatures. Min. Res. Dev. 2021, 41, 58–66. [Google Scholar]
- Zhou, W.; Wang, H.; Zhang, Z.; Chen, H.; Liu, X. Molecular simulation of CO2/CH4/H2O competitive adsorption and diffusion in brown coal. RSC Adv. 2019, 9, 3004–3011. [Google Scholar] [CrossRef]
- Yang, S. Study on the Microstructure of Water-Bearing Tectonic Coal and Its Adsorption Characteristics of CH4 and CO2. Master’s Thesis, Qingdao University of Technology, Qingdao, China, 2021. [Google Scholar]
- Zhang, J.; Liu, K.; Clennell, M.; Dewhurst, D.; Pervukhina, M. Molecular simulation of CO2–CH4 competitive adsorption and induced coal swelling. Fuel 2015, 160, 309–317. [Google Scholar] [CrossRef]
- Ren, Z. The Studies on Adsorption of CH4, CO2 Gas on the Antracite Coal of Yang Quan. Master’s Thesis, Henan Polytechnic University, Jiaozuo, China, 2010. [Google Scholar]
- Allen, M.P.; Tildesley, D.J. Computer Simulation of Liquids; Oxford University Press: Oxford, UK, 2017. [Google Scholar]
- Yu, S.; Bo, J.; Fengjuan, L. Competitive adsorption of CO2/N2/CH4 onto coal vitrinite macromolecular: Effects of electrostatic interactions and oxygen functionalities. Fuel 2019, 235, 23–38. [Google Scholar] [CrossRef]
- Liu, X.-Q.; He, X.; Qiu, N.-X.; Yang, X.; Tian, Z.-Y.; Li, M.-J.; Xue, Y. Molecular simulation of CH4, CO2, H2O and N2 molecules adsorption on heterogeneous surface models of coal. Appl. Surf. Sci. 2016, 389, 894–905. [Google Scholar] [CrossRef]
- Dang, Y.; Zhao, L.; Lu, X.; Xu, J.; Sang, P.; Guo, S.; Zhu, H.; Guo, W. Molecular simulation of CO2/CH4 adsorption in brown coal: Effect of oxygen-, nitrogen-, and sulfur-containing functional groups. Appl. Surf. Sci. 2017, 423, 33–42. [Google Scholar] [CrossRef]
- Cheng, G.; Li, Y.; Zhang, M.; Cao, Y. Simulation of the adsorption behavior of CO2/N2/O2 and H2O molecules in lignite. J. China Coal Soc. 2021, 46, 960–969. [Google Scholar]
- Xiang, J.; Zheng, F.; Liang, H. Molecular simulation of the CH4/CO2/H2O adsorption onto the molecular structure of coal. Sci. China Earth Sci. 2014, 44, 1418–1428. [Google Scholar] [CrossRef]
- Yi, D. Research on the Mechanism of CH4, CO2, H2O and O2 Adsorption on Coal Molecule. Master’s Thesis, School of Energy, Power and Mechanical Engineering, Beijing, China, 2018. [Google Scholar]
- Yu, B. Experimental Study of Yang Quan anthracite’s adsorption behavior of binary-component mixtures(N2-CH4); Henan Polytechnic University: Jiaozuo, China, 2010. [Google Scholar]
- Tang, S.; Han, D. Adsorption and desorption of multi element gas by coal. Coal Sci. Technol. 2002, 30, 58–60. [Google Scholar]
- Wang, S.; Hu, Y.; Yang, X.; Liu, G.; He, Y. Examination of adsorption behaviors of carbon dioxide and methane in oxidized coal seams. Fuel 2020, 273, 117599. [Google Scholar] [CrossRef]
- Zhang, Q.; Liu, W.; Li, N.; Duan, C. Experimental Study on Adsorption Characteristics of CH4, CO2 and Their Multi-component Gases in Huainan C13 Coal. Saf. Coal Mines 2019, 50, 14–17. [Google Scholar]
- Qu, L.; Liu, L.; Chen, J.; Wang, Z. Molecular Model Construction and Optimization Study of Gas Coal in the Huainan Mining Area. Processes 2022, 11, 73. [Google Scholar] [CrossRef]
- Li, Z.; Wang, Y.; Li, P.; Li, H.; Bai, H.; Guo, Q. Macromolecular model construction and quantum chemical calculation of Ningdong Hongshiwan coal. CIESC J. 2018, 69, 2208–2216. [Google Scholar]
- Jianbo, J. Construction of Structural Model and Molecular Simulation of Methane Formation Mechanism during Coal Pyrolysis for Shendong Vitrinite. Ph.D. Dissertation, Taiyuan University of Technology, Taiyuan, China, 2010. [Google Scholar]
- Zhu, H.; He, X.; Huo, Y.; Xie, Y.; Wang, W.; Fang, S. Construction and optimization of lignite molecular structure model. J. Min. Sci. Technol. 2021, 6, 429–437. [Google Scholar]
- Gelb, L.D.; Gubbins, K. Pore size distributions in porous glasses: A computer simulation study. Langmuir 1999, 15, 305–308. [Google Scholar] [CrossRef]
- Gao, D.; Hong, L.; Wang, J.; Zheng, D. Adsorption simulation of methane on coals with different metamorphic grades. AIP Adv. 2019, 9, 095108. [Google Scholar] [CrossRef]
- Gao, D.; Hong, L.; Wang, J.; Zheng, D. Molecular simulation of gas adsorption characteristics and diffusion in micropores of lignite. Fuel 2020, 269, 117443. [Google Scholar] [CrossRef]
- Wu, S.; Jin, Z.; Deng, C. Molecular simulation of coal-fired plant flue gas competitive adsorption and diffusion on coal. Fuel 2019, 239, 87–96. [Google Scholar] [CrossRef]
- Zheng, Y.; Li, Q.; Yuan, C.; Tao, Q.; Zhao, Y.; Zhang, G.; Liu, J.; Qi, G. Thermodynamic analysis of high-pressure methane adsorption on coal-based activated carbon. Fuel 2018, 230, 172–184. [Google Scholar] [CrossRef]
- Li, J.; Li, B.; Ren, C.; Zhang, Y.; Wang, B. An adsorption model for evaluating methane adsorption capacity in shale under various pressures and moisture. J. Nat. Gas Sci. Eng. 2020, 81, 103426. [Google Scholar] [CrossRef]
- Billemont, P.; Coasne, B.; De Weireld, G. An experimental and molecular simulation study of the adsorption of carbon dioxide and methane in nanoporous carbons in the presence of water. Langmuir 2011, 27, 1015–1024. [Google Scholar] [CrossRef] [PubMed]
- Qiang, Q. Study on Competitive Adsorption Mechanism of Pingdingshan Bituminous Coal for Gas under Different Moisture Conditions. Master’s Thesis, Inner Mongolia University of Science and Technology, Baotou, China, 2022. [Google Scholar]
- Li, S.-G.; Bai, Y.; Lin, H.-F.; Shu, C.-M.; Yan, M.; Laiwang, B. Molecular simulation of adsorption of gas in coal slit model under the action of liquid nitrogen. Fuel 2019, 255, 115775. [Google Scholar] [CrossRef]
- Wen, H.; Tang, R.; Zhang, D.; Dai, A.; Fan, S.; Zhai, X. Molecular simulation study on adsorption and diffusion of CO in bituminous coal. J. Saf. Sci. Technol. 2022, 18, 95–101. [Google Scholar]
- Wu, S. Molecular Simulation Research on Mechanism of Coal Spontaneous Combustion Prevention and Storage of Flue Gas. Ph.D. Dissertation, Liaoning Technical University, Jinzhou, China, 2019. [Google Scholar]
- Wang, L.; Wang, Z.; Li, X.; Yang, Y. Molecular dynamics mechanism of CH4 diffusion inhibition by low temperature in anthracite microcrystallites. ACS Omega 2020, 5, 23420–23428. [Google Scholar] [CrossRef]
- Sui, H.; Yao, J. Effect of surface chemistry for CH4/CO2 adsorption in kerogen: A molecular simulation study. J. Nat. Gas Sci. Eng. 2016, 31, 738–746. [Google Scholar] [CrossRef]
- Wiser, W.H. Conversion of bituminous coal to liquids and gases: Chemistry and representative processes. In Magnetic Resonance: Introduction, Advanced Topics and Applications to Fossil Energy; Springer: Dordrecht, The Netherlands, 1984; pp. 325–350. [Google Scholar]
- Zhang, J.; Clennell, M.B.; Liu, K.; Dewhurst, D.N.; Pervukhina, M.; Sherwood, N. Molecular dynamics study of CO2 sorption and transport properties in coal. Fuel 2016, 177, 53–62. [Google Scholar] [CrossRef]
- Düren, T.; Bae, Y.-S.; Snurr, R.Q. Using molecular simulation to characterise metal–organic frameworks for adsorption applications. Chem. Soc. Rev. 2009, 38, 1237–1247. [Google Scholar] [CrossRef]
- Liu, Z.-X.; Feng, Z.-C. Theoretical study on adsorption heat of methane in coal. J. China Coal Soc. 2012, 37, 647–653. [Google Scholar]
- Wang, D. Study on Gas Adsorption and Diffusion of Soft and Hard Coal Based on Molecular Simulation. Master’s Thesis, Henan Polytechnic University, Jiaozuo, China, 2018. [Google Scholar]
- Krishna, R. Diffusion in porous crystalline materials. Chem. Soc. Rev. 2012, 41, 3099–3118. [Google Scholar] [CrossRef] [PubMed]
- Kelemen, S.; Kwiatek, L. Physical properties of selected block Argonne Premium bituminous coal related to CO2, CH4, and N2 adsorption. Int. J. Coal Geol. 2009, 77, 2–9. [Google Scholar] [CrossRef]
- Hu, H.; Du, L.; Xing, Y.; Li, X. Detailed study on self-and multicomponent diffusion of CO2-CH4 gas mixture in coal by molecular simulation. Fuel 2017, 187, 220–228. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).