1. Introduction
Wildfires have become an increasingly common phenomenon due to changing climatic conditions [
1,
2] and earlier policies of fire suppression which have led to fuel accumulation and created a potential risk of large-scale fires [
3,
4]. The abandonment of agricultural areas and pastoral activities or plots with solar power plants contributes to fire occurrence as well [
4,
5]. The increased frequency of wildfires has substantial environmental and socio-economic impacts [
6]. Fires have a lasting impact on the environment from the moment of their occurrence and over their whole duration, and their impact can be evident even several decades afterwards [
7].
During combustion, harmful substances such as heavy metals (HM), potentially toxic metal elements, polycyclic aromatic hydrocarbons (PAH), gases, and carbon emissions are released. These substances affect the fire-affected area itself, nearby ecosystems, water supplies [
8], adjacent agricultural systems, and humans due to the resulting air pollution and possible entry of potentially toxic elements into the food chain [
9]. The biodiversity and wildlife habitat are altered and destroyed during a wildfire [
7]. The fire-affected area is especially vulnerable, as the soil cover is partially or completely combusted and does not protect the soil against water and wind erosion. Water and wind erosion are responsible for spreading potentially toxic substances released during combustion to both the surrounding and very distant ecosystems, exposing them to contamination.
It is not always necessary to intervene in the fire-affected area, as a species-rich and stable ecosystem can be created through natural recovery and succession [
10,
11]. On the other hand, in certain cases, depending on many factors (as mentioned later in this paper), it is advisable to initiate a post-fire treatment as soon as possible. Among those factors are, for example, the size of the fire-affected area, if and how many human lives, properties, and water supplies are endangered, and the extent of expected erosion in the fire-affected area is [
12].
A possible method of post-fire management is phytoremediation. It is an eco-friendly, low-cost, plant-based method the principle of which consists in re-vegetating contaminated soils using plants capable of sequestering trace elemental pollutants in various ways [
13], thereby preventing their spread through the environment. The advantage of phytoremediation is that it uses organisms in a natural manner and maintains the ecological balance of the environment, making it less damaging than conventional alternatives [
14]. Plants can extract pollutants by translocating them from soil to the aboveground harvestable biomass (phytoextraction), reduce their bioavailability in soil (phytostabilization) or convert them into less toxic form, and release them via transpiration through their foliage system (phytovolatilization) [
15]. Simultaneously with revegetation, the soil surface is consolidated and runoff and soil erosion are mitigated. Other types of phytoremediation include phytodegradation and rhizodegradation; however, these apply to organic pollution. Rhizodegradation uses microorganisms in the rhizosphere to decompose organic pollutants [
16], while, during phytodegradation, plants degrade organic pollutants with the help of enzymes instead of rhizospheric microorganisms [
17,
18].
The efficiency of phytoremediation can be enhanced by the addition of soil amendments; therefore, it often combined with their application, which is called “aided phytoremediation”. Thanks to the addition of soil amendments, the physico-chemical properties of soil are improved, the contaminant bioavailability is lowered, and a better environment for the reintroduction of vegetation cover is facilitated [
19], which results in a reduced soil recuperation period. Soil amendments can be materials of organic (e.g., compost, biochar) or inorganic (e.g., bentonite, diatomite) origin. Based on previous studies carried out by Barroso et al. [
20,
21], the following organic soil amendments have been chosen here: biochar, compost, a combination of the two, and a combination of biochar and NPK fertilizer with
Lolium perenne L., (a grass species that is commonly used for phytoremediation owing to its global geographical distribution in both cold to humid regions). Grass species are used in phytoremediation thanks to properties such as rapid growth, tolerance to contaminants, and the capability to regrow shoots after cutting [
22,
23].
The efficiency of phytoremediation, particularly of phytoaccumulation/phytoextraction, can be indicated by the Bioconcentration Factor (BCF) and Translocation Factor (TF) [
24]. Both are indicators of plants’ ability to accumulate or translocate heavy metals and other metal elements from the soil. In the case of BCF, the number indicates a ratio of concentration of HM in plant tissue against the concentration of HM in the surrounding environment [
25,
26], while TF demonstrates the efficiency of translocating HM from the underground biomass (UGB) to the aboveground biomass (AGB) [
27].
Wildfires are a process in which radical changes occur throughout a whole ecosystem, including the availability of selected metal elements for vegetation. Therefore, our hypothesis is that the addition of soil amendments to the soil after a fire does not change the availability of selected metal elements for uptake by plants. To confirm or refute this hypothesis, the following sub-objectives were set: (i) to determine the effect of applying soil amendments to burnt soil (BS) on the growth of model plant biomass; (ii) to determine the effect of applying soil amendments to the BS on the proportion of selected metal elements in soil and biomass; and (iii) to determine the effect of applying soil amendments to the BS on the mobility of selected metal elements between the burnt soil and the biomass of the model plants.
4. Discussion
The application of soil amendments to burnt soil modifies the soil properties (pH, distribution of substances) and changes the conditions for vegetation recovery [
20,
21]. Limiting factors of phytoremediation include bad root growth and development in contaminated soil. The addition of soil amendments can precondition the soil and thereby reduce possible limitations posed by potentially toxic metal elements, resulting in successful establishment of vegetation cover [
41,
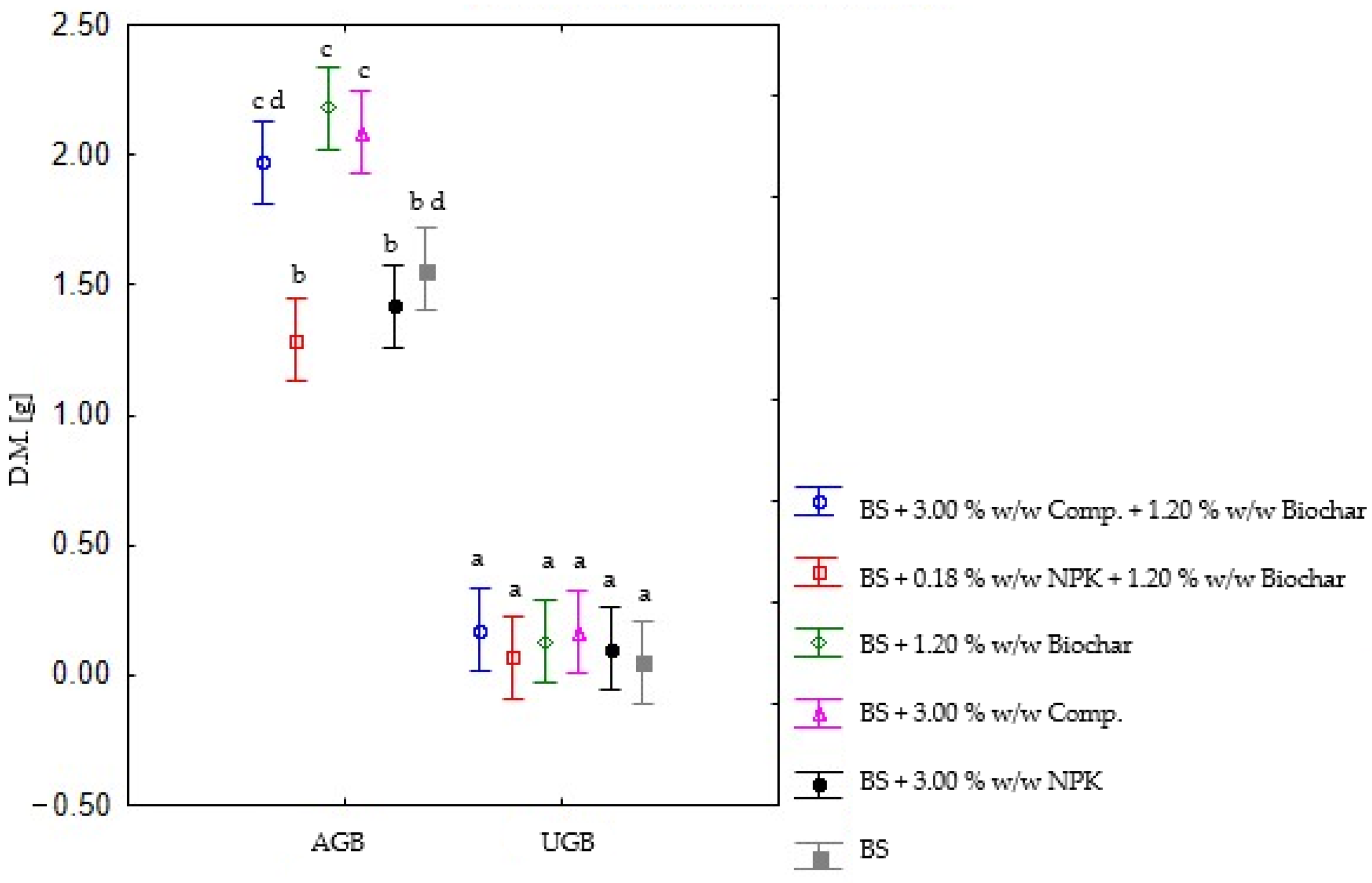
42]. The effect of soil amendments on biomass growth differed between aboveground and underground biomass. Soil amendments showed a more pronounced effect on AGB growth than on UGB, where a statistical difference in growth was not observed. The fact that UGB growth is more sensitive to the effects induced by fire than AGB growth has previously been described in a 2005 study by Snyman et al. [
43] where changes in AGB and UGB growth were monitored. The most successful amendments for promoting AGB growth were biochar and compost. Yields of
Lolium perenne AGB in these variants were significantly higher than the AGB yield obtained from the unamended BS. This can be attributed to their capacity to boost microbial activity in the soil, which contributes to increased available nutrient content for uptake by plants. Nutrient cycling is influenced by microorganism activity [
44]. Low AGB yield in the unamended BS might be explained by a reduction in the microbial population in the BS by the effect of fire. The most dramatic change is perceptible in the first year after the fire [
45]. With time, the microbial population tends to recover; however, the negative effects can persist for years [
46].
Although there are few studies dedicated to the long-term effects of biochar application on soil biota and microbial communities [
47], many researchers [
48,
49] have performed experiments confirming that its application to soil evokes a change in the structure of the soil microbial community and increases enzyme activity, which both support biomass production. Nutrient availability during biochar application was researched in a study by Vahedi et al. (2022) [
50]; the authors recommended combining biochar application and inoculation with growth-promoting bacteria. Another study in which nutrient-poor soils conditioned by biochar were examined was Alburquerque et al. 2015 [
51], who used a pot-grown experiment. Biochar alone did not mitigate the nutrient deficiency, and its potential benefits were mainly seen in its combination with other fertilizers. These findings were not confirmed by this study, as the AGB yield in a variant enriched with biochar was one of the highest, showing that
Lollium perenne L. prospered well. This might have been caused by a low dosage of biochar.
The stimulating effect of compost on the microbial effect is well known, and emerges from the nature of its formation. Compost contributes to nutrient cycling in the soil and supports soil life [
52,
53], which results in better biomass development. The availability of metal elements in compost-amended soils was studied by Kubná et al. 2015 [
54], and it was concluded that increasing the dose of compost enhances immobility, and thus the bioavailability of HM is decreased. This does not apply to all trace elements, e.g., Zn, which is able to form chelates with the organic compounds that are introduced to the soil thanks to compost application.
The effects of the application of organic (poultry manure) and inorganic (NPK fertilizer) soil amendments on burnt soil were studied by Villar et al. 2004 [
42], resulting in similar findings, in that changes in biomass production induced by the organic amendments were more evident than those evoked by NPK fertilizer, and the effect was more evident on AGB.
Lolium perenne is a commonly used grass species for phytostabilization and aids phytostabilization, especially for Cd, Zn, Pb, and Cu [
40]; therefore, it can be expected that metal elements will be primarily concentrated in UGB.
Lolium perenne, along with other phytostabilizers, develops an abundant root system, produces rich AGB, and does not translocate metal elements to shoots, which is a required condition in phytostabilization that prevents contaminants from entering the food chain [
13]. Phytostabilization offers more positives for the fire-affected area, namely, faster recovery of vegetation, which protects the soil from erosion and increases its infiltration capacity [
55].
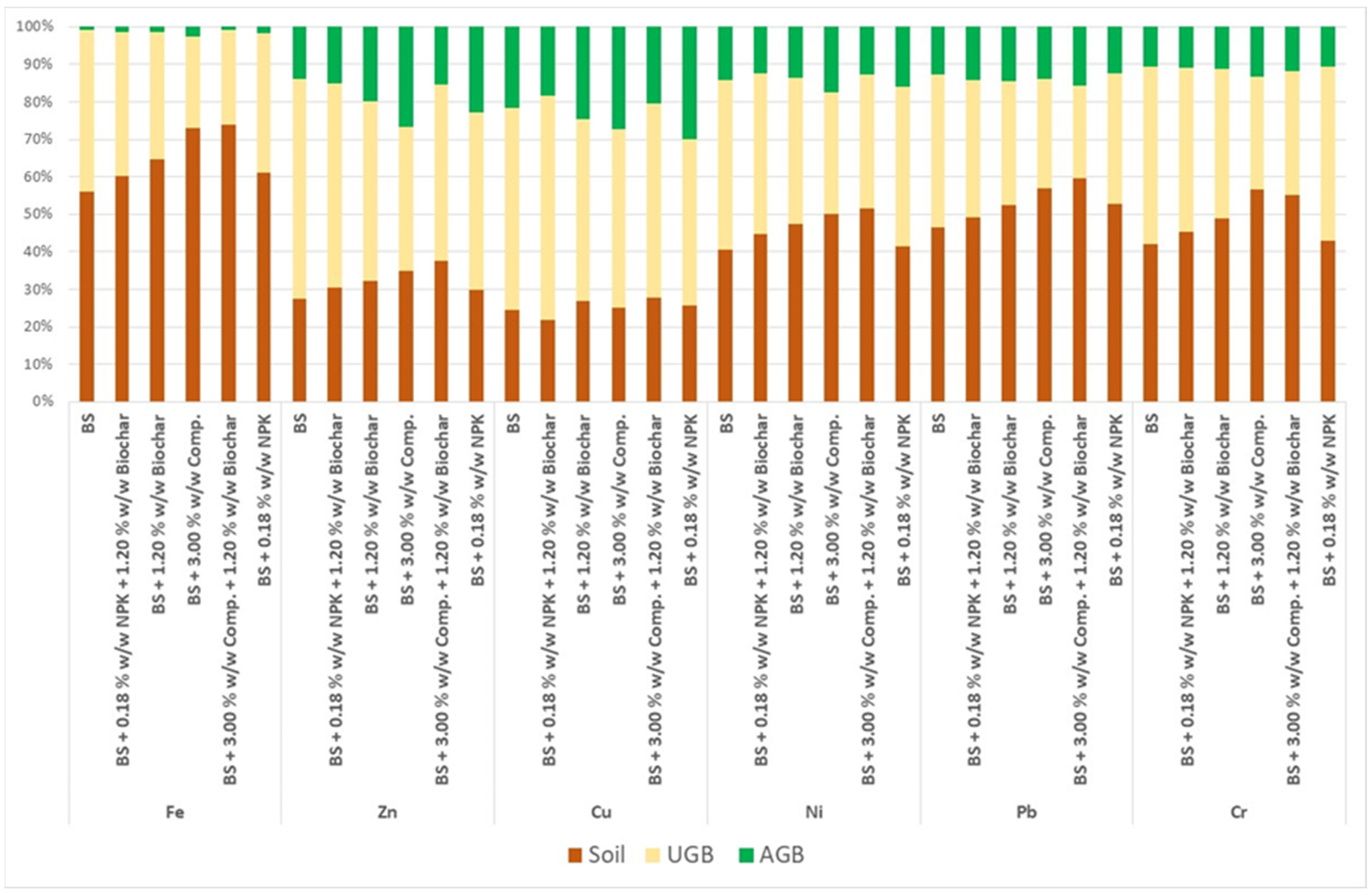
Although several of the soil amendments positively affected AGB growth, their BCF capacity was not increased. Therefore, it is essential to assess the main priority for the fire-affected area, whether it is rapid recovery of vegetation or the immobilization of contaminants. Our chemical analysis of the generated ash showed that the ash did not surpass the preventive limits (
Table A3) in the Decree on Protection of Agricultural Land, thus, the priority should be erosion protection of the soil, which implies the promotion of AGB growth through the application of compost and biochar. The best capacity concentration of metal elements in roots (meaning highest BCF) was in a variant with unamended BS. The only exception was Cu, an element in which the uptake by plants is regulated well even in soils with different Cu concentrations [
56,
57].
The ability of
Lolium perenne to translocate was higher in variants BS + 3.00%
w/
w Comp. for Fe and Zn and in variant BS + 0.18%
w/
w NPK for Ni and Cu. Cr usually accumulates in roots, and the concentration of Cr depends on the content of dissolved substances in soil. An undetectable concentration of Cr in AGB confirms that absorbed Cr is hardly translocated to the AGB [
58,
59].
5. Conclusions
The application of soil amendments to burnt soil can affect the growth of aboveground biomass. After the application of biochar and compost, aboveground biomass growth increased. The growth of underground biomass of Lolium perenne was not affected by the application of soil amendments. The occurrence of selected metal elements in the soil after performing the pot experiment did not exceed the preventive limits set by law, which allows for the possibility of employing phytostabilization instead of phytoaccumulation in this particular study. The monitored metal elements had the lowest concentrations in aboveground biomass of Lolium perenne.
The highest bioconcentration factor values for most of the analyzed elements (Fe, Zn, Ni, Pb, and Cr) were recorded in the variant with untreated burnt soil. Soil amendments limit the bioconcentration capacity of Lolium perenne. An exception was seen with Cu in certain soil amendments. These elements can be biologically blocked in Lolium perenne roots by applying the mentioned soil amendments. The ability of Lolium perenne to translocate was supported by burnt soil + 3.00% w/w Comp. for Fe and Zn and by BS + 0.18% w/w NPK for Ni and Cu. The addition of certain soil amendments (biochar and compost) to the soil promoted the formation of Lolium perenne biomass, which can be used for rapid revegetation of burnt areas, thereby reducing soil erosion. The experiment did not manage to demonstrate the ability of Lolium perenne to phytoaccumulate selected metal elements. However, certain soil amendments support the translocation of certain metal elements, and thus their temporary biological blocking in root biomass.