2. Metals and Metal Fires
While ceramics are non-combustible, many other materials in building and construction, mechanical engineering and daily life can burn. Polymers, cardboard and wood are well-known to be susceptible to catching fire, and they can be equipped with fire-retardant additives or coatings, many of which bear detrimental side effects such as brominated compounds. Metals, too, can burn. Their proclivity to rusting, an oxidation process, alludes to this property. However, normally, one does not witness metal fires, because they are used in bulky objects with comparatively small surface areas. Three important light metals are Al, Mg and Ti. Titanium is difficult to process, and Mg is even lighter than Al, so it is very attractive particularly for mobility applications. A major disadvantage of Mg, though, is the imminent fire risk during machining [
2]. Molten magnesium spontaneously ignites when exposed to the atmosphere. Hence, magnesium die casting [
3] companies know that the magnesium melt has to be blanketed by heavy gases such as SO
2 or SF
6. Iron/steel dust must not be mixed with aluminum dust in metal processing workshops in order to avoid a dangerous “termite” reaction. Lithium-ion accumulators are also known to pose a fire risk. The magnesium processing industry has seen fires in chippings too. Metal fires demand fast reaction and special procedures to get under control. With light metals becoming more popular, e.g., in transportation, more awareness of their fire risks is needed along the entire value chain, from processing and manufacturing to recycling.
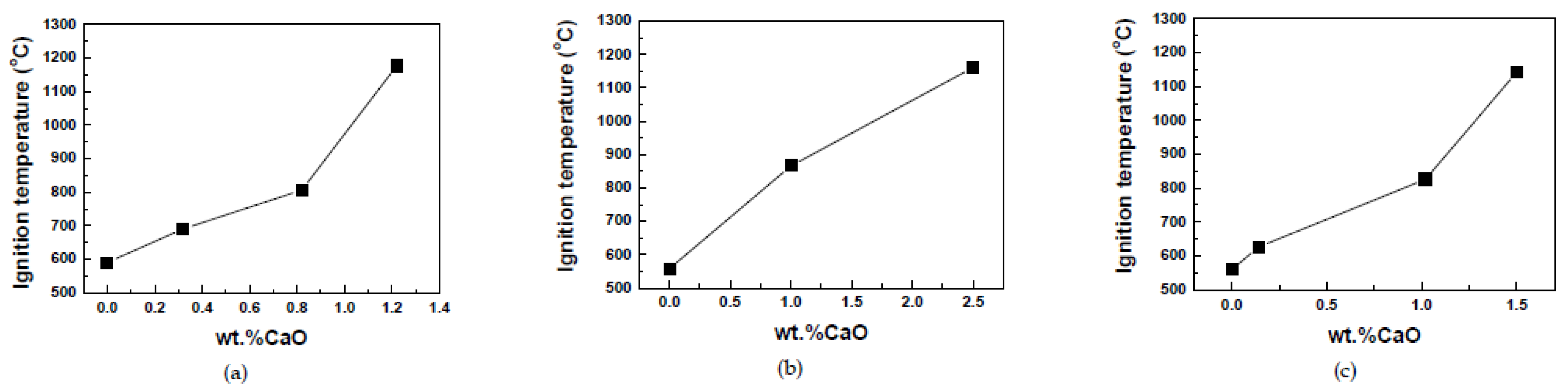
Magnesium can be made more robust against fire risk by alloying it with other metals or metal oxides, e.g., aluminum or small amounts of CaO, as in
Figure 1 and
Figure 2 [
4]. Such measures, however, are not always possible, because mechanical properties and other properties can be seriously affected. Therefore, shielding molten magnesium from contact with atmospheric oxygen is often required [
2].
Additionally, housekeeping must not be neglected in the magnesium processing industry [
2]. However, this is not always possible, so a certain residual risk of metal fire remains, and news reports more often than not bring to our attention the occurrence of magnesium fires, often with large impacts on the plants affected and even their surroundings. As metal fires are not “regular” fires, knowledge and experience of them tend to be lower than with traditional fires.
3. Dealing with Metal Fires
Metal fires are dangerous and difficult to control. The high temperatures involved lead to long cooling times. Water, the general firefighting agent, cannot be used with metal, as the high temperatures lead to decomposition with hydrogen formation, resulting in a violent reaction [
5]. However, this approach bears some risks when the amount of metal involved is not known to the firefighters.
In
Table 1, different extinguishing agents for metal fires are shown. Some metals react with nitrogen, so only noble gases (helium, neon, or argon) are safe. Halocarbons should not be used when dealing with metal fires.
Although magnesium melts at 650 °C, it can auto-ignite at temperatures starting from 623 °C. However, fine particles will ignite even below that temperature, e.g., magnesium ribbons and shavings at approx. 510 °C, and fine magnesium powder at around 482 °C [
5]. Another source states approximately 680 °C as the ignition temperature for bulk magnesium [
6].
Fine, combustible particles bear the risk of causing a dust explosion. The danger scales with decreasing particle size. For magnesium powder, the lower explosive limit is reached with only 40 g/m
3 [
5].
Fine chips from metal working and dust are the most common reasons for fires in metal processing workshops [
7].
Table 2 lists some dust explosion data, reproduced from the database Gestis.
Magnesium dust belongs to class St1, with ignition energies on the order of 1J.
The former NFPA standard “NFPA 480, Standard for the Storage, Handling, and Processing of Magnesium Solids and Powders” has been integrated into the new “NFPA 484, Standard for Combustible Metals”. Both describe best practices related to Mg. Dust explosions are very dangerous, as demonstrated by the fatalities in two cases reported by the CSB [
10,
11] about the Hoeganaes Corporation fiasco in 2011 and a related incident as well as the Norsk Hydro incident in Canada [
12].
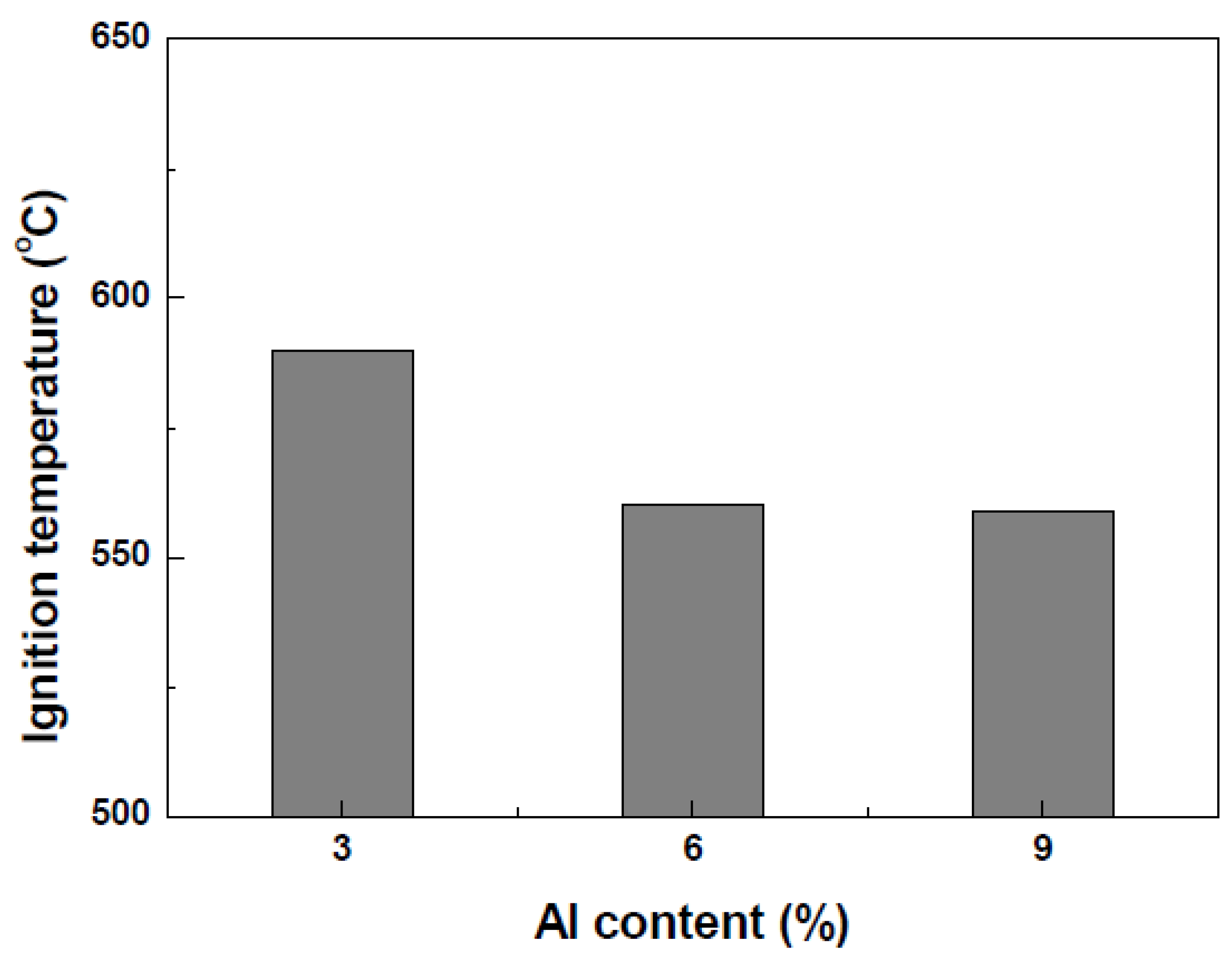
Figure 1 shows the ignition temperature of Mg-Al alloys. The higher the Al content, the lower the ignition temperature.
In
Figure 2, one can see how CaO added to Mg increases its ignition temperature.
One method to reduce the fire risk from magnesium chippings is to briquette them prior to recycling. This process, however, is expensive, even more so when there is a high fraction of lubricant [
13].
In most instances, the combustibility of magnesium is not desired, as it poses a safety risk. There are some applications, though, where this property is exploited:
“As magnesium ignites easily in air and burns with a bright light, it’s used in flares, fireworks, and sparklers” [
14].
Magnesium fires in industry are not a novel phenomenon and were recognized 150 years ago:
“In 1873 the German government in Berlin issued a first regulation, called Regulation for the Protection against Fires involving powders of Magnesium, Aluminum and their alloys in Foundries, Warehouses and other works” [
15].
Fear of magnesium fires still limits the use of this light metal in some industries, e.g., aviation, where additional weight savings compared to aluminum or titanium would be advantageous:
“Magnesium use, except for a few isolated examples” [
15,
16], is limited in the aviation industry.
5. Experimental
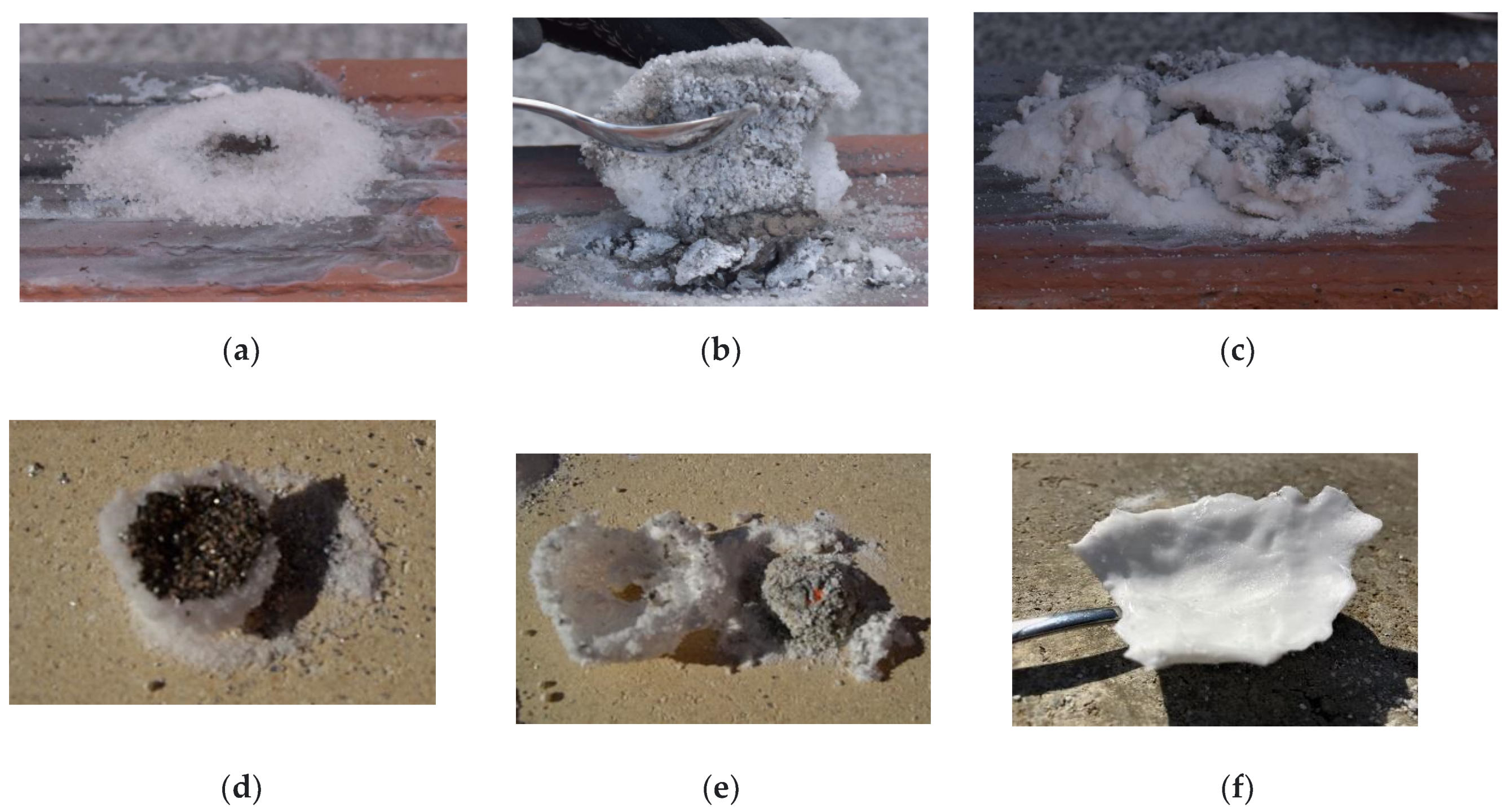
Flakes of pure Mg were placed on a refractory surface and ignited with a flame. In the first trials, 2 g of Mg flakes was used in each experiment. As
Figure 3 shows, the sand is able to cover and extinguish the flame (5 g). Cellulose flakes (5 g) were also able to smother the fire; see
Figure 3b.
The sand in
Figure 3a covered and suffocated the flame. There was no stable crust. After 1 h of rest, the sand was pushed aside, and unburnt residual Mg flakes were found.
Figure 3b shows the core of the novel approach. Lightweight cellulose flakes are able to “hide” the glowing flame of Mg for several seconds; they prevent fresh oxygen from reaching the reaction zone and start charring (the oxygen below the flake layer is soon consumed, and the flake layer proves to be a sufficient barrier for fresh oxygen). The crust can break after some time, depending on the amount of burning/glowing metal below and the thickness of the crust. A high heat load will cause the cellulose flakes to react with the air and burn.
To make the cellulose flakes, old newspaper was used. The material was torn into flakes of approx. 5–8 mm in length, and had a fibrous appearance. Emerging dust was not removed.
With the cellulose flake treatment, several similar trials to the one depicted in
Figure 3b were conducted. Various flame-retardant materials were added to the flakes in concentrations from 1–50% (by weight). It was found that inorganic salts (NaCl, KCl, MgSO
4) and borax (sodium borate) were effective in completely suppressing the flames from the underlying burning Mg for at least several seconds. Borax (sodium borate) was chosen due to prior findings from the literature [
19].
Based on the example shown here in
Figure 3b, the hypothesis was born that cellulose flakes, in combination with inorganic salts, can effectively “seal” a magnesium fire. The flakes stop the reaction and begin the charring process, while the salts melt and place a suffocating, closed shield over the char, thus stabilizing it and allowing a regular attack by water spray to cool down the hearth of reaction.
To further develop the material system, several salts were cast onto burning flakes of Mg, approx. 20 g of salt onto 2 g of metal flakes; see
Figure 4 for NaCl, KCl, borax (sodium borate, Na₂[B₄O₅(OH)₄]), boric acid (H₃BO₃) and MgSO
4.
From the pure materials, KCl and boric acid seem to be the best suited candidate materials for the enhanced cellulose flake system.
In the next step, different formulations of cellulose flakes (supplied by Isocell, 5–8 mm in dimension, dry) were mixed with varying portions of NaCl, KCl, MgSO4, boric acid and borax, ranging from 10 to 30% by weight. These mixtures were obtained merely by dry blending the powders with cellulose flakes (hand mixing in pots and machine mixing in a cement mixer (90 L)). In subsequent experiments, these mixtures were cast onto small piles of burning magnesium to test their “suffocating” properties.
After lab experiments, field trials took place where larger amounts of up to 2 kg of Mg flakes were lit and subjected to different mixtures of cellulose flakes with salt additions. These larger trials were carried out in an abandoned quarry under the supervision of a local fire brigade. Ambient conditions were without rain or wind and took place at 22 °C. The ground for the experiments was dry, level dolomite gravel. After the application of cellulose flake mixes to the fires, a firefighter administered a water spray. It was discovered that no oxyhydrogen formation and no subsequent explosions took place, and that the water spray attack was therefore possible. The effect was a rapid cooling of the “tranquilized” covered Mg heaps. Due to the partly charred and “solidified” cellulose material, the impinging water spray did not “wash away” this cover from the top of the Mg piles. Neither could the water travel through the cellulose flake layer and get in touch with the underlying molten metal, since the heat from beneath led to evaporation within the layer.
The NFPA 750 “Standard on Water Mist Fire Protection Systems” [
20] is not applicable to our process and hence not discussed.
Without the cooling water, the flames would eventually eat though the charred structure and continue to burn, as verified in a series of experiments. That burning, however, was not as vigorous as it was prior to the cover, because the inorganic salts were still resting on top of the burning metal. The difference was that most of the charred cellulose flakes were consumed without continued cooling by water spray. The water spray cooling was continued until the temperature of the heaps dropped considerably. No thermoelectric camera was used to determine the “end” of the Mg fires clearly; when using a spade, it could be seen that even after 30 min of cooling, some unreacted magnesium flakes were still at the bottom and could be ignited instantly upon air contact. This implies that the cooling must be continued until the heat has been entirely extracted to avoid reignition.
In the next step, the composition of the mixture was optimized (
Figure 5 and
Table 3).
In
Figure 5, one can see the crust upside down, with white MgO in top. Below is the charred cellulose mixed with the 30% salts.
The final large-scale trials involved up to 150 kg of magnesium flakes. In order to apply the cellulose flakes to such vigorous fires, a safe distance is of utmost importance, a requirement difficult to meet with a standard extinguishing agent such as sand; see
Figure 6.
As can be seen from
Figure 6 (right part), the cellulose flakes are lightweight, so they can easily reach the spot of action (fire) without perturbation of the underlying material. In the left section, a spade was used to remove the charred cover, exposing the Mg flakes to air, causing reignition.
Table 3 compares the different mixtures that were prepared. The assessment was made qualitatively using school grades for three aspects: mechanical stability of the crust, thickness of the crust and water compatibility.
By mechanical stability, it was assessed whether the crust can be lifted intact or is too brittle. No instrumented measurements were carried out; 5 = can be lifted and takes force for disintegration, 1 = no cohesion in the crust.
By thickness, the height of the crust was assessed for a trial series with 5 kg of Mg flakes covered by approx. 10 kg of flakes; 5 = more than 3 cm, 1 = less than 1 cm.
Using water compatibility, whether the stability of the crust was affected by the water spray was assessed; 1 = complete run-off and 5 = “fully stable”.
The best recipe was found to be “mix 16” with 15% KCl and 15% sodium borate (Na₂[B₄O₅(OH)₄], borax). When we compare the water solubilities of the tested salts, we can see that sodium borate has a lower value than boric acid (H3BO3). Additionally, the corrosiveness and inhalative toxicity of borax is less than that of boric acid. For these reasons, borax is preferred over boric acid. Mix 9 and 10 were the reference materials dry sand and dry cement, respectively.
7. Results and Discussion
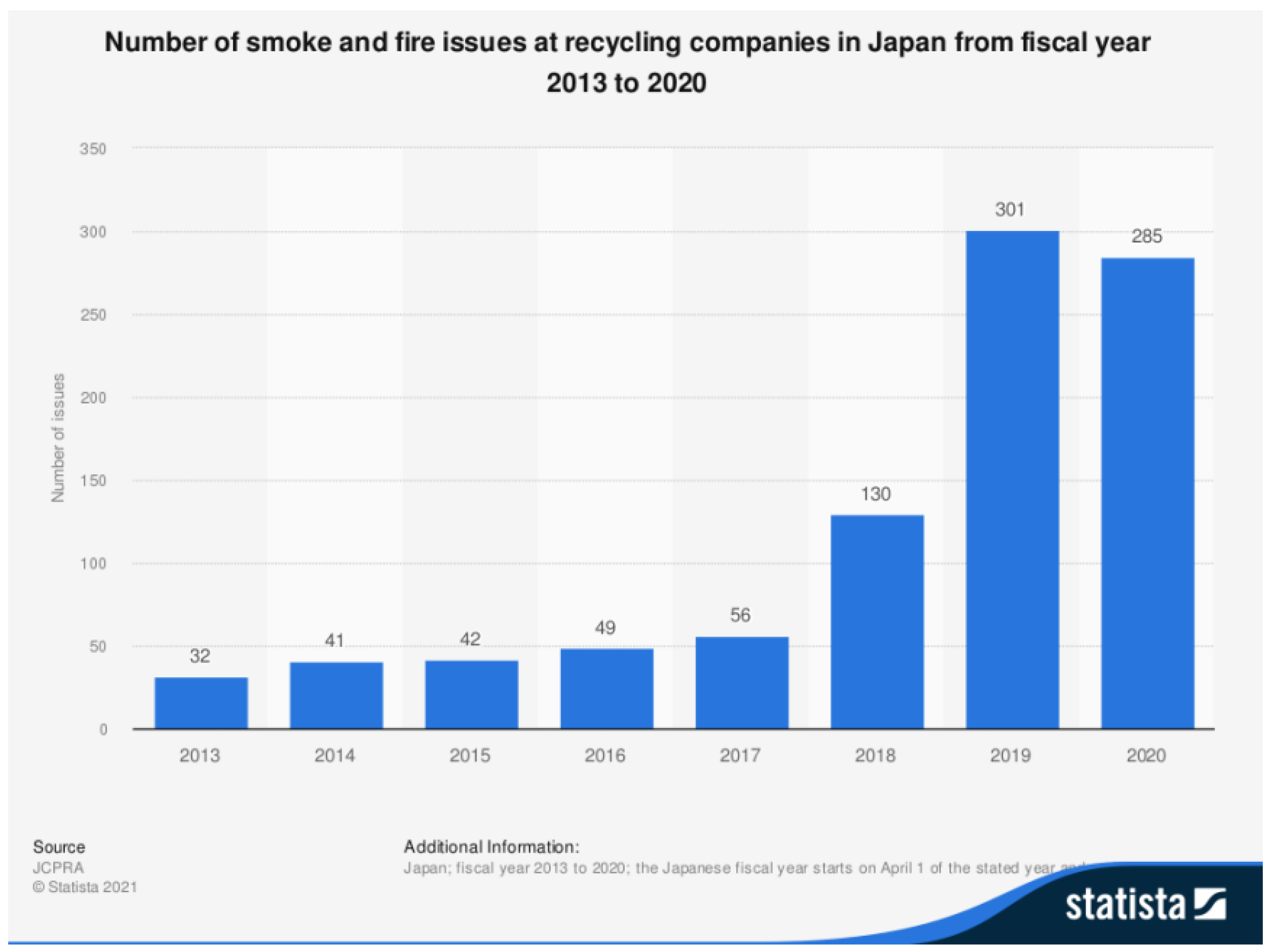
In this work, a counter-intuitive approach was developed to control metal fires. With the increase in lithium-ion batteries, it is expected that fires due to reactive metals will increase in the future, particularly in recycling and waste treatment plants.
Figure 8 shows the development in this respect in Japan.
Magnesium and other reactive metals pose a fire risk, and traditional methods of combatting these fires have significant downsides. This study demonstrated that cellulose flakes containing approx. 30% inorganic salts can be an effective means of controlling a metal fire in a safe and cost-effective way, so that subsequent cooling with water spray becomes feasible. As
Table 3 shows, a mix of 30% cellulose flakes, 15% KCl and 15% sodium borate gave the best results. The cellulose flakes can be transported to the fire through pneumatic conveying, so that a gentle flow of material “rains” onto the fire, from a safe distance. By contrast, traditional firefighting media for metal fire, such as dry sand or cement, have to be poured onto the fire, which puts the firefighters at increased risk from splashes of metal. The novel method [
22,
23,
24,
25] was found to be suitable to control open and enclosed magnesium fires, with the benefit of low-cost materials and increased safety for firefighters. The amount of cellulose flakes necessary to extinguish a fire is given in
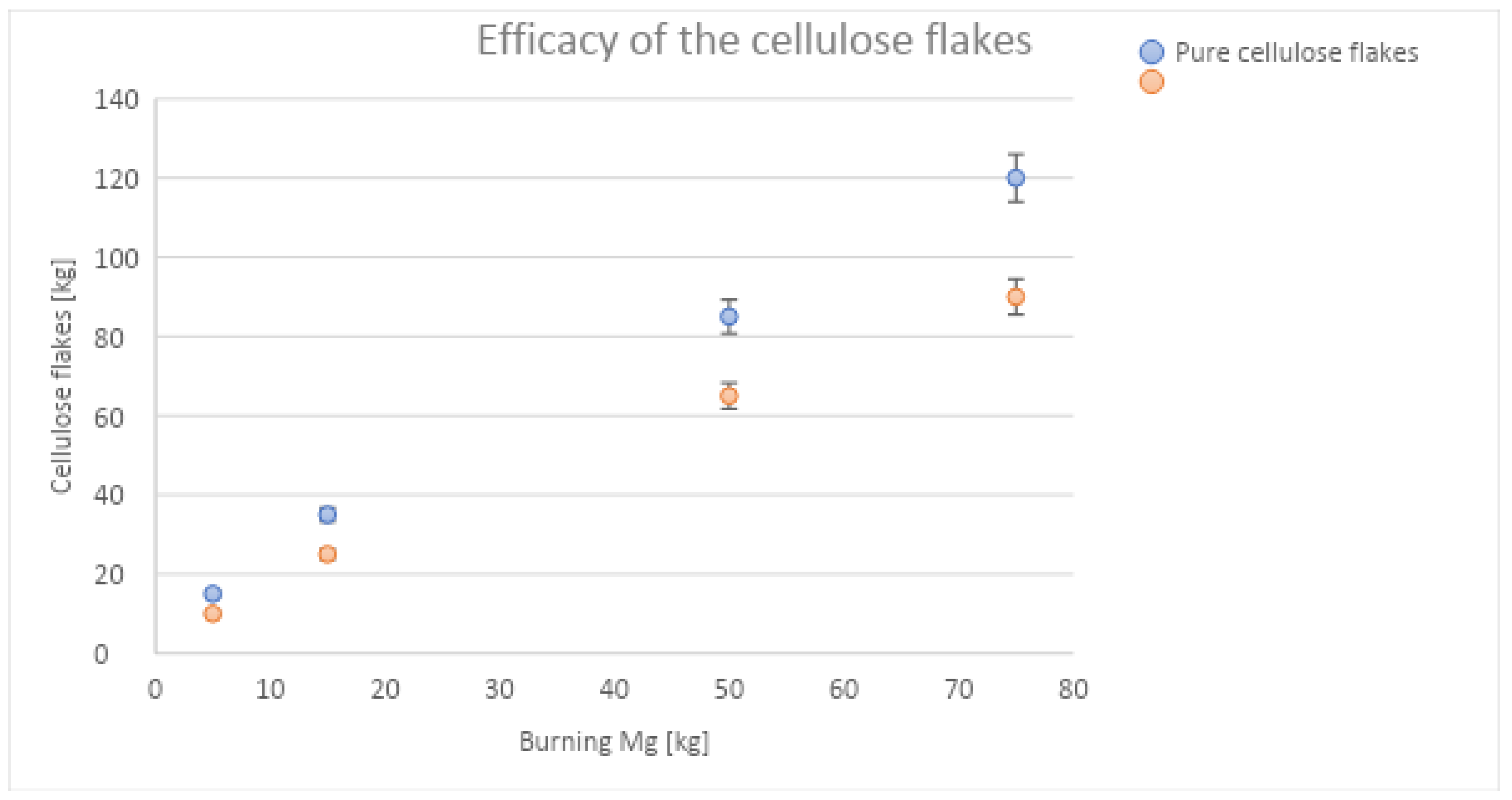
Figure 9.
Using uncoated cellulose flakes, approx. 1.6 times the mass of the burning magnesium is needed to control the flames. With cellulose flakes containing 30% (by weight) inorganic salts, that ratio is reduced to approx. 1.2. It was found that smaller fires need a slightly higher ratio of extinguishing agent. For instance, for 15 kg of magnesium, 25 kg of flames were needed, which is a factor of 1.7. For practical firefighting, one should try to blow as much cellulose flake material as possible onto the flames, preferably at a rate of ~50 kg/min or higher, and then, as soon as the flames cease, application of the water spray should begin. The authors did not conduct a variation of throughput cellulose flow, but it was found that slower covering of the flames results in a faster burn-through by the still-hot magnesium, so that more flakes are needed to re-cover the material. Although the material may be partially charred, the water spray attack can start safely only when the material is fully covered with cellulose flakes. The largest fire that the authors have controlled with the proposed technology was 120 kg of magnesium flakes in a fully developed fire. No significant difference between clean flakes and flakes contaminated by oil or oil/water emulsions from machining operations was found. It is assumed that the high flame temperature immediately evaporates the adjacent contamination, provided that the oil and, particularly, the water content does not become too high; however, here, further studies should be carried out.
The pneumatic conveying was best conducted in “dilute phase” mode, since with dense-phase conveying, the risk for clogging was increased significantly. Having a blocked conveying line would cost the firefighters valuable time to get the system up and running again. It is advisable to have a second conveying hose available in case of line blockage. For the front part of the conveying hose, there is no diameter reduction, as this could lead to a blocked line. A metallic pipe could be used for the front part to provide extra distance for the firefighters.
When mixing the inorganic salts with the cellulose flakes, dry mixing and wet mixing were tested. Wet mixing was shown to soak the flakes and consume high amounts of energy, so this method was not used. In order to avoid de-mixing of the salt and flakes, small particle sizes were advantageous. The best results were achieved with cellulose flakes of 5–8 mm and salt with fine-ground particles of approx. 1 mm or less. The mix of 15% KCl, 15% borax and 70% cellulose flakes was found to be non-hygroscopic at an ambient temperature and 50–70% relative humidity over a storage period of 2 weeks.
Extinguishers containing AVD (Aqueous Vermiculite Dispersion) have been developed for extinguishing traction batteries (used in heavy industrial electrical applications such as electric forklift trucks and electric tractors) as well as light metals [
26]. Future research could compare AVD with the cellulose-flake based technology described in this paper. Additionally, direct field trial comparisons with expandable graphite [
21] could be made. In this work, a preliminary feasibility was to be demonstrated. Since this is a purely experimental study, the authors have not determined the heat release rate or any other parameters that would be interesting for modelling work. More research should be conducted along the described firefighting approach—we have made some reports here to contribute to increased safety for firefighters in the context of metal fires.