Role of CF4 Addition in Gas-Phase Variations in HF Plasma for Cryogenic Etching: Insights from Plasma Simulation and Experimental Correlation
Abstract
1. Introduction
2. Methods
2.1. Simulation Model
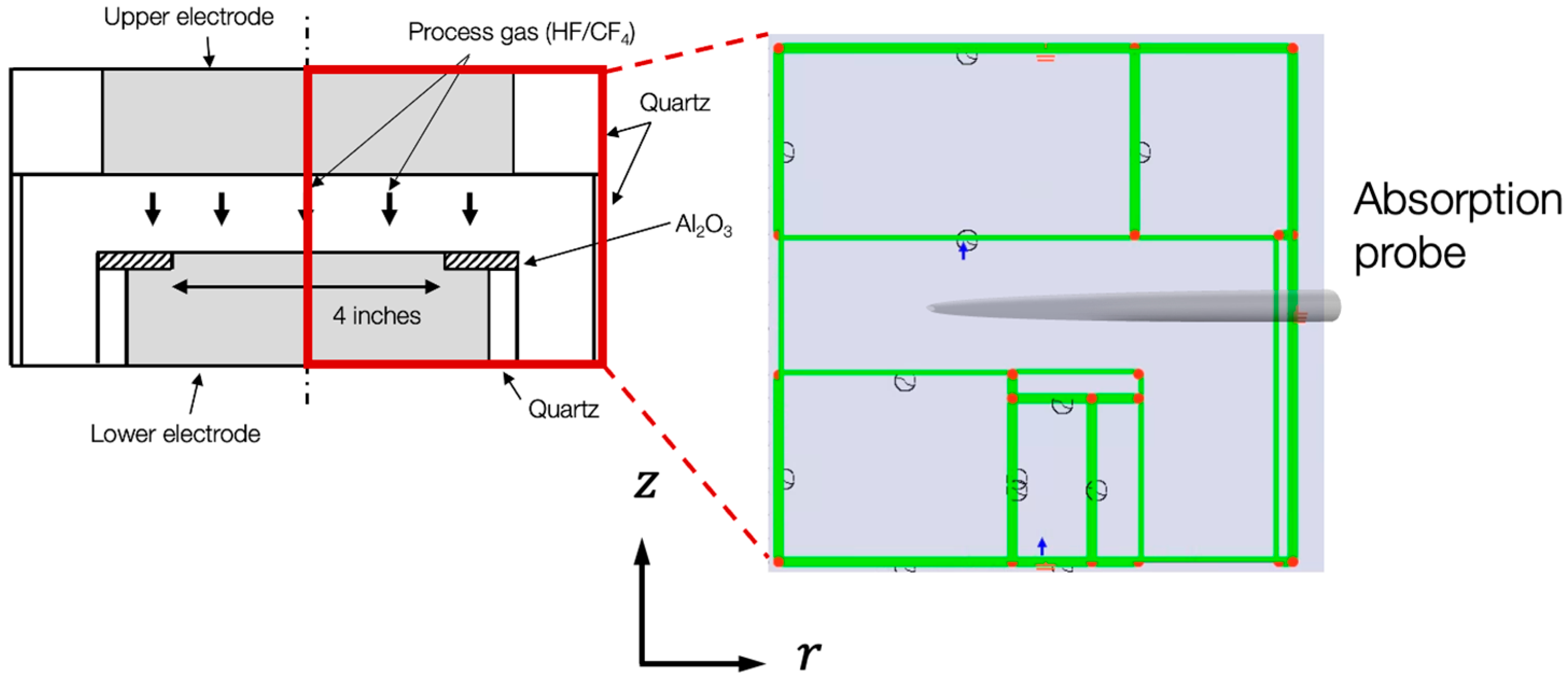
2.1.1. Geometric Model and Boundary Conditions
2.1.2. Plasma Simulation
2.1.3. Coupled Calculation of Plasma and Gas Flow Simulations
2.1.4. Gas-Phase Reaction Models
2.2. Experimental Procedures
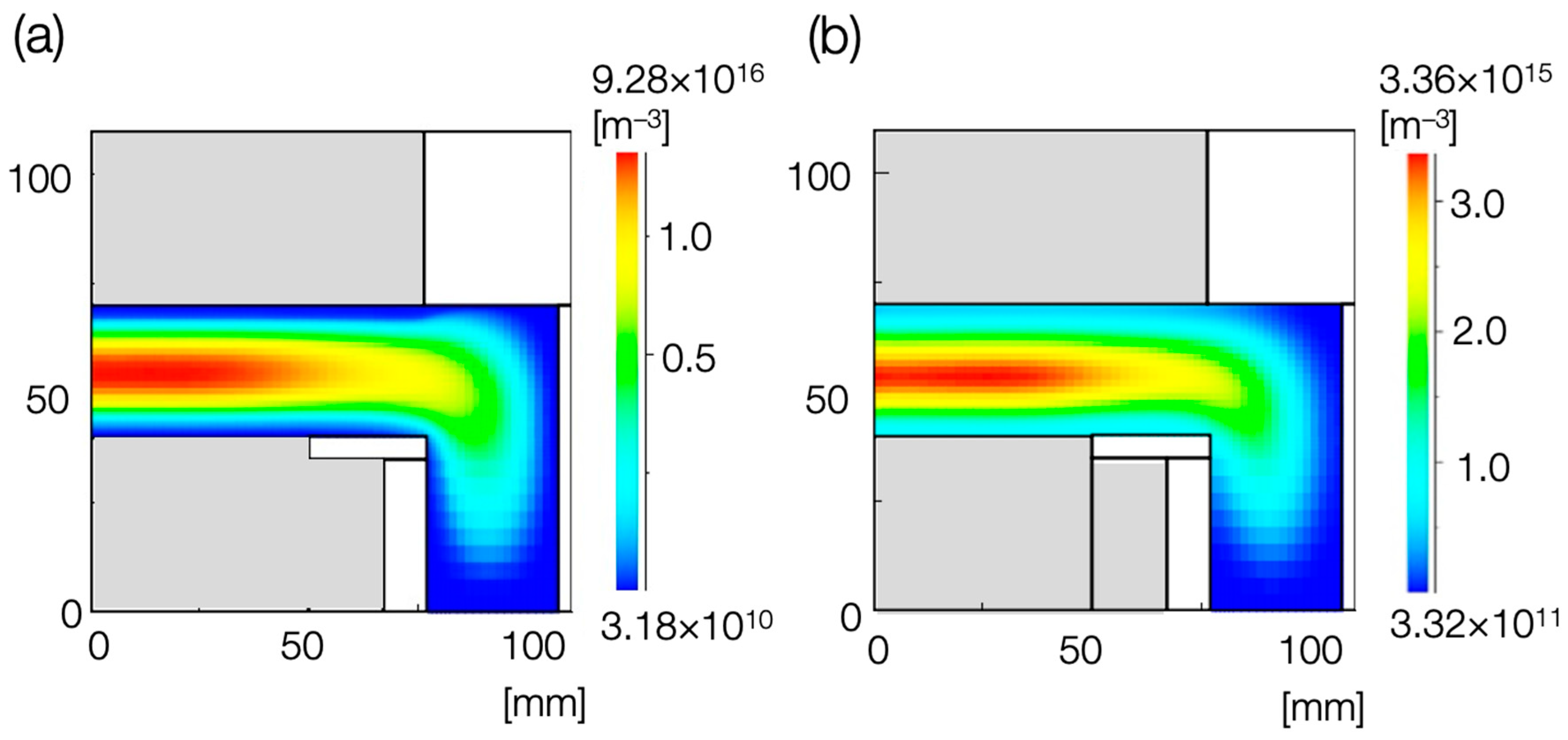
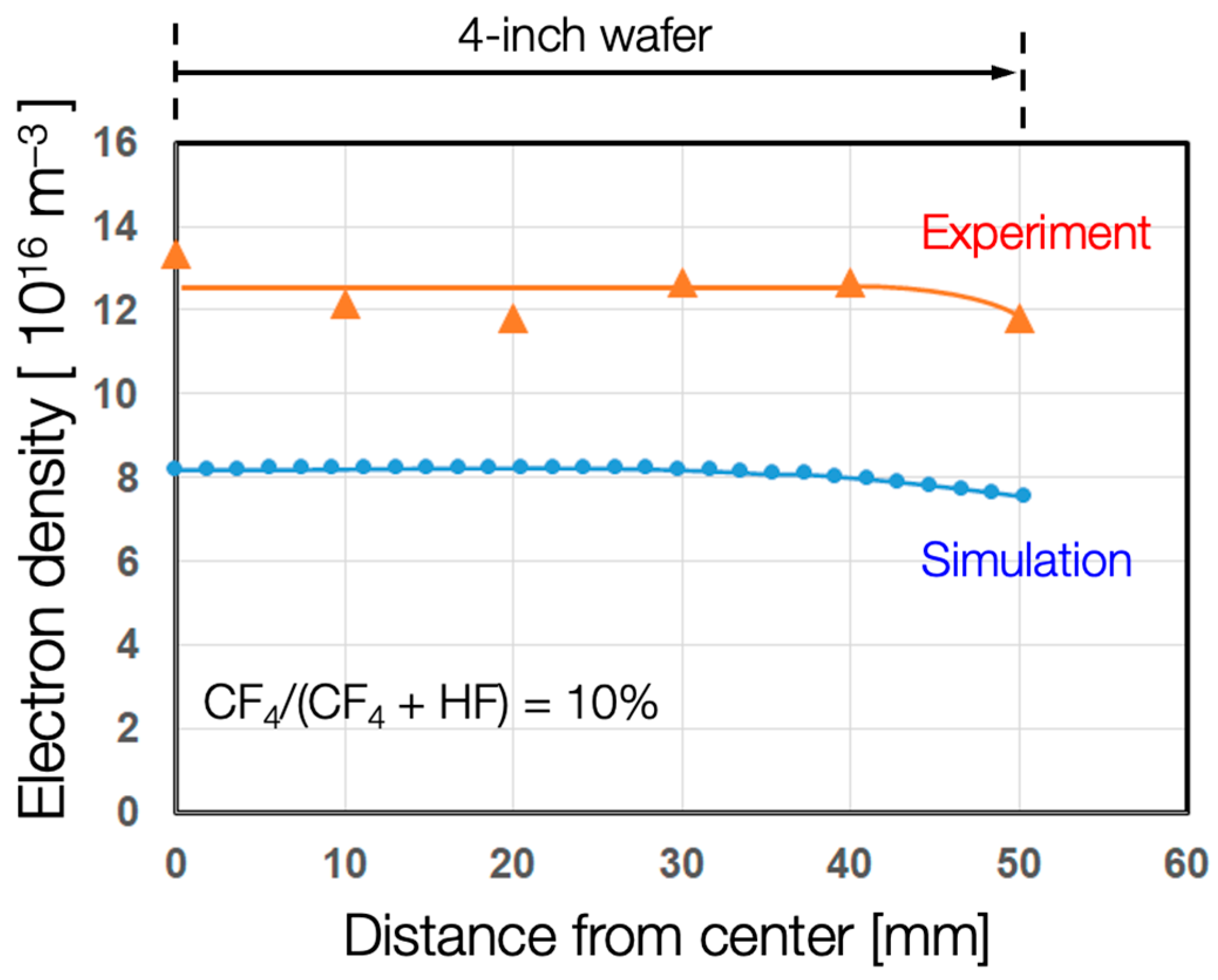
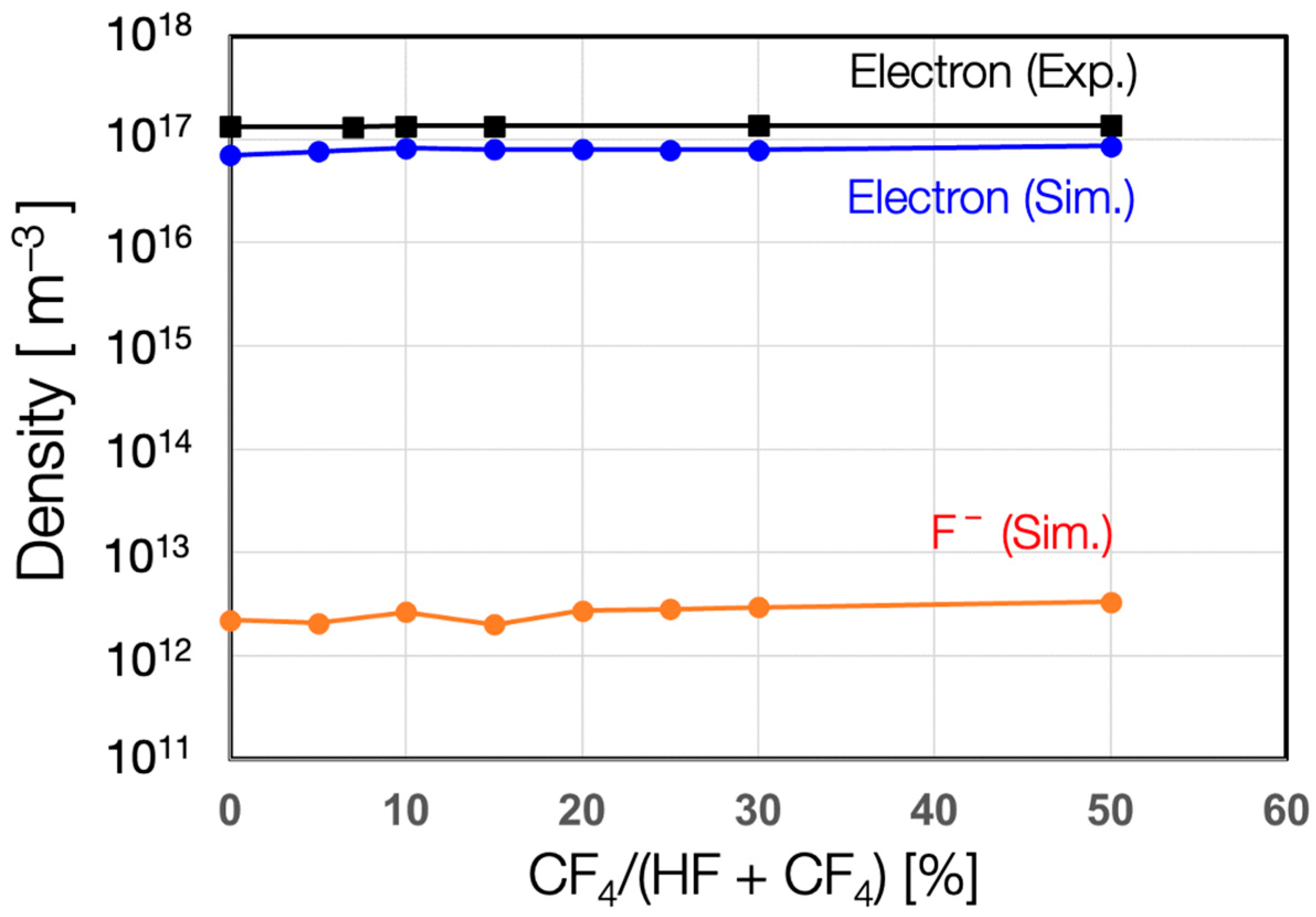
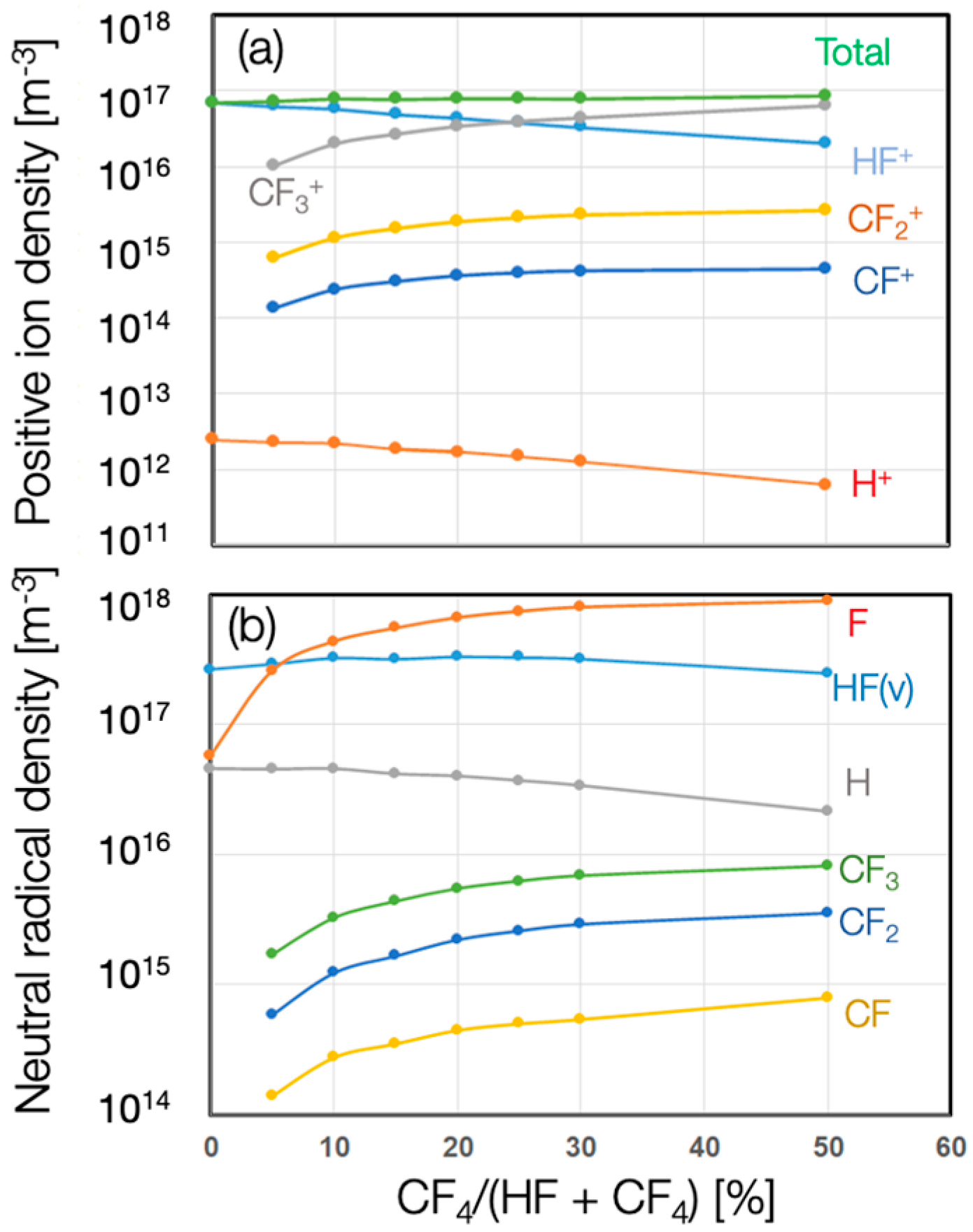
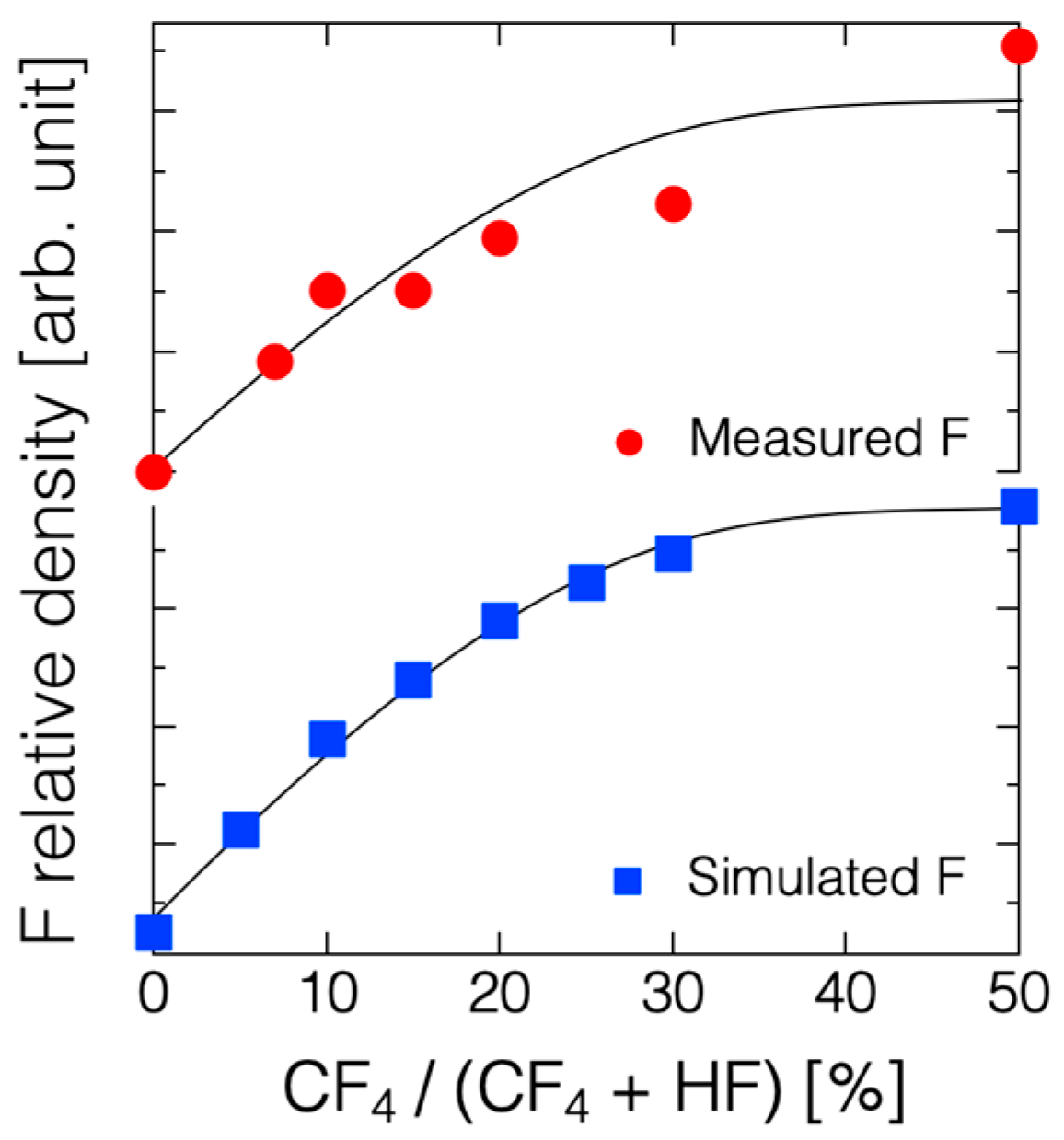
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Goda, A. 3-D NAND Technology Achievements and Future Scaling Perspectives. IEEE Trans. Electron. Devices 2020, 67, 1373–1381. [Google Scholar] [CrossRef]
- Jayachandran, D.; Sakib, N.U.; Das, S. 3D integration of 2D electronics. Nat. Rev. Electri. Eng. 2024, 1, 300–316. [Google Scholar] [CrossRef]
- Choi, K.S.; Kim, S.H.; Seo, J.W.; Kang, H.S.; Chu, S.W.; Bae, S.W.; Kwon, J.H.; Kim, G.S.; Park, Y.T.; Kwak, J.H.; et al. A Three Dimensional DRAM (3D DRAM) Technology for the Next Decades. In Proceedings of the 2024 IEEE Symposium on VLSI Technology and Circuits (VLSI Technology and Circuits), Honolulu, HI, USA, 16–20 June 2024; pp. 1–2. [Google Scholar]
- Ishikawa, K.; Karahashi, K.; Ishijima, T.; Cho, S.I.; Elliott, S.; Hausmann, D.; Mocuta, D.; Wilson, A.; Kinoshita, K. Progress in nanoscale dry processes for fabrication of high-aspect-ratio features: How can we control critical dimension uniformity at the bottom? Jpn. J. Appl. Phys. 2018, 57, 06JA01. [Google Scholar] [CrossRef]
- Cagomoc, C.M.D.; Isobe, M.; Hudson, E.A.; Hamaguchi, S. Molecular dynamics simulation of oxide-nitride bilayer etching with energetic fluorocarbon ions. J. Vac. Sci. Technol. A 2022, 40, 063006. [Google Scholar] [CrossRef]
- Chung, S.-J.; Luan, P.; Park, M.; Metz, A.; Oehrlein, G.S. Exploring oxide-nitride-oxide scalloping behavior with small gap structure and chemical analysis after fluorocarbon or hydrofluorocarbon plasma processing. J. Vac. Sci. Technol. B 2023, 41, 062201. [Google Scholar] [CrossRef]
- Krüger, F.; Lee, H.; Nam, S.K.; Kushner, M.J. Voltage waveform tailoring for high aspect ratio plasma etching of SiO2 using Ar/CF4/O2 mixtures: Consequences of low fundamental frequency biases. Phys. Plasmas 2024, 31, 033508. [Google Scholar] [CrossRef]
- Nishizuka, T.; Igosawa, R.; Yokoyama, T.; Sako, K.; Moki, H.; Honda, M. Precise and practical 3D topography simulation of high aspect ratio contact hole etch by using model optimization algorithm. J. Vac. Sci. Technol. A 2024, 42, 043003. [Google Scholar] [CrossRef]
- Huard, C.M.; Zhang, Y.; Sriraman, S.; Paterson, A.; Kushner, M.J. Role of neutral transport in aspect ratio dependent plasma etching of three-dimensional features. J. Vac. Sci. Technol. A 2017, 35, 05C301. [Google Scholar] [CrossRef]
- Gottscho, R.A.; Jurgensen, C.W.; Vitkavage, D.J. Microscopic uniformity in plasma etching. J. Vac. Sci. Technol. B 1992, 10, 2133–2147. [Google Scholar] [CrossRef]
- Kihara, Y.; Tomura, M.; Sakamoto, W.; Honda, M.; Kojima, M. Beyond 10 m Depth Ultra-High Speed Etch Process with 84 Lower Carbon Footprint for Memory Channel Hole of 3D NAND Flash over 400 Layers. In Proceedings of the IEEE Symposium on (VLSI Technology and Circuits), Kyoto, Japan, 11–16 June 2023; pp. 1–2. [Google Scholar]
- Hsiao, S.-N.; Sekine, M.; Iijima, Y.; Hori, M. In Situ Monitoring Surface Reactions in Cryogenic Atomic Layer Etching of Silicon Nitride by Alternating Surface Modification with Hydrogen Fluoride Dose and Ar Plasmas. Chem. Mater. 2024, 36, 11042–11050. [Google Scholar] [CrossRef]
- Hsiao, S.-N.; Sekine, M.; Ishikawa, K.; Iijima, Y.; Ohya, Y.; Hori, M. An approach to reduce surface charging with cryogenic plasma etching using hydrogen-fluoride contained gases. Appl. Phys. Lett. 2023, 123, 212106. [Google Scholar] [CrossRef]
- Hsiao, S.N.; Sekine, M.; Britun, N.; Mo, M.K.T.; Imai, Y.; Tsutsumi, T.; Ishikawa, K.; Iijima, Y.; Suda, R.; Yokoi, M.; et al. Pseudo-Wet Plasma Mechanism Enabling High-Throughput Dry Etching of SiO2 by Cryogenic-Assisted Surface Reactions. Small Methods 2024, 8, 2400090. [Google Scholar] [CrossRef]
- Lill, T.; Wang, M.; Wu, D.; Oh, Y.-J.; Kim, T.W.; Wilcoxson, M.; Singh, H.; Ghodsi, V.; George, S.M.; Barsukov, Y.; et al. Low-temperature etching of silicon oxide and silicon nitride with hydrogen fluoride. J. Vac. Sci. Technol. A 2024, 42, 063006. [Google Scholar] [CrossRef]
- Dussart, R.; Tillocher, T.; Becerra, L.; Lefaucheux, P.; Overzet, L.J. Cryogenic etching of SiOxFy and SiO2 in SF6/H2 plasma. Jpn. J. Appl. Phys. 2025, 64, 05SP01. [Google Scholar] [CrossRef]
- Hsiao, S.N.; Imai, Y.; Sekine, M.; Suda, R.; Iijima, Y.; Kihara, Y.; Ishikawa, K.; Hori, M. Revolutionizing reactive ion etching: Ion-enhanced surface autocatalytic reactions enabling ultra-high throughput using cryogenic hydrogen-fluoride plasma. Chem. Eng. J. 2025, 522, 167517. [Google Scholar] [CrossRef]
- Hidayat, R.; Kim, H.L.; Khumaini, K.; Chowdhury, T.; Mayangsari, T.R.; Cho, B.; Park, S.; Lee, W.J. Selective etching mechanism of silicon oxide against silicon by hydrogen fluoride: A density functional theory study. Phys. Chem. Chem. Phys. 2023, 25, 3890–3899. [Google Scholar] [CrossRef]
- Takagi, S.; Hsiao, S.-N.; Ma, C.-Y.; Sekine, M.; Matsunaga, F. Plasma simulation of HF plasma generated in dual-frequency chamber for high aspect ratio dielectric etching. Jpn. J. Appl. Phys. 2024, 63, 09SP21. [Google Scholar] [CrossRef]
- Kim, I.S.; Shim, C.E.; Kim, S.W.; Lee, C.S.; Kwon, J.; Byun, K.E.; Jeong, U. Amorphous Carbon Films for Electronic Applications. Adv. Mater. 2023, 35, e2204912. [Google Scholar] [CrossRef]
- Jiang, Z.; Zhu, H.; Sun, Q. Process Optimization of Amorphous Carbon Hard Mask in Advanced 3D-NAND Flash Memory Applications. Electronics 2021, 10, 1374. [Google Scholar] [CrossRef]
- Park, S.J.; Kim, D.; Baek, S.Y.; Lee, C.; Kim, J.; Roh, S.; Park, J.; Kyung, S.; Choi, C. Nitrogen doped high selectivity amorphous carbon film for high aspect ratio etch process. Thin Solid Film. 2025, 809, 140582. [Google Scholar] [CrossRef]
- Yeom, H.J.; Yoon, M.Y.; Choi, D.; Lee, Y.; Kim, J.H.; You, S.J.; Lee, H.C. Role of Oxygen in Amorphous Carbon Hard Mask Plasma Etching. ACS Omega 2023, 8, 32450–32457. [Google Scholar] [CrossRef]
- Son, H.J.; Efremov, A.; Choi, G.; Kwon, K.-H. Individual Effects of Various Plasma-Related Factors on the High Aspect Ratio Oxide Etching Process at Low-Frequency Bias Power Using an Inductively Coupled Plasma System. Plasma Chem. Plasma Proc. 2023, 44, 635–649. [Google Scholar] [CrossRef]
- Hsiao, S.-N.; Britun, N.; Nguyen, T.-T.-N.; Tsutsumi, T.; Ishikawa, K.; Sekine, M.; Hori, M. Manipulation of etch selectivity of silicon nitride over silicon dioxide to a-carbon by controlling substrate temperature with a CF4/H2 plasma. Vacuum 2023, 210, 111863. [Google Scholar] [CrossRef]
- Choi, G.; Efremov, A.; Kwon, K.H. Comparative study of CF4 + X + He (X = C4F8 or C4H2F6) plasmas for high aspect ratio etching of SiO2 with ACL mask. Plasma Pro. Polym. 2024, 21, 2400046. [Google Scholar] [CrossRef]
- Choi, M.; Lee, Y.; You, Y.; Cho, C.; Jeong, W.; Seong, I.; Choi, B.; Kim, S.; Seol, Y.; You, S.; et al. Characterization of SiO2 Plasma Etching with Perfluorocarbon (C4F8 and C6F6) and Hydrofluorocarbon (CHF3 and C4H2F6) Precursors for the Greenhouse Gas Emissions Reduction. Materials 2023, 16, 5624. [Google Scholar] [CrossRef]
- Karahashi, K.; Li, H.; Yamada, K.; Ito, T.; Numazawa, S.; Machida, K.; Ishikawa, K.; Hamaguchi, S. Etching yields and surface reactions of amorphous carbon by fluorocarbon ion irradiation. Jpn. J. Appl. Phys. 2017, 56, 06HB09. [Google Scholar] [CrossRef]
- Standaert, T.E.F.M.; Hedlund, C.; Joseph, E.A.; Oehrlein, G.S.; Dalton, T.J. Role of fluorocarbon film formation in the etching of silicon, silicon dioxide, silicon nitride, and amorphous hydrogenated silicon carbide. J. Vac. Sci. Technol. A 2004, 22, 53–60. [Google Scholar] [CrossRef]
- Engelmann, S.U.; Bruce, R.L.; Joseph, E.A.; Fuller, N.C.M.; Graham, W.S.; Sikorski, E.M.; Kohjasteh, M.; Zhu, Y.; Nakamura, M.; Ito, A.; et al. Nitride etching with hydrofluorocarbons. I. Selective etching of nitride over silicon and oxide materials by gas discharge optimization and selective deposition of fluorocarbon polymer. J. Vac. Sci. Technol. B 2017, 35, 051803. [Google Scholar] [CrossRef]
- Takagi, S.; Ishii, K.; Hsiao, S.-N.; Sekine, M. Comparison of distributions of etching rate and calculated plasma parameters in dual-frequency capacitively coupled plasma. Jpn. J. Appl. Phys. 2023, 62, SN1011. [Google Scholar] [CrossRef]
- Takagi, S.; Nakaegawa, T.; Hsiao, S.-N.; Sekine, M. Estimations of secondary electron emission coefficients of Si, SiO2, and polyimide electrodes in dual-frequency capacitively coupled discharge. Jpn. J. Appl. Phys. 2022, 62, SA1009. [Google Scholar] [CrossRef]
- Kinoshita, S.; Takagi, S.; Kai, T.; Shiozawa, J.; Maki, K. Multiscale Analysis of Silicon Low-Pressure Chemical Vapor Deposition. Jpn. J. Appl. Phys. 2005, 44, 7855. [Google Scholar] [CrossRef]
- Musallam, M.; Johnson, C.M. Real-Time Compact Thermal Models for Health Management of Power Electronics. IEEE Trans. Power Electron. 2010, 25, 1416–1425. [Google Scholar] [CrossRef]
- Swan, I.; Bryant, A.; Mawby, P.A.; Ueta, T.; Nishijima, T.; Hamada, K. A Fast Loss and Temperature Simulation Method for Power Converters, Part II: 3-D Thermal Model of Power Module. IEEE Trans. Power Electron. 2012, 27, 258–268. [Google Scholar] [CrossRef]
- Joshipura, K.N.; Vinodkumar, M. Electron scattering cross sections with HF, OH, NH and CH molecules. Phys. Lett. A 1997, 224, 361. [Google Scholar] [CrossRef]
- Gauyacq, J.P. Associative detachment and vibrational excitation in the e−-HF system. J. Phys. B Atom. Mol. Phys. 1983, 16, 4049. [Google Scholar] [CrossRef]
- Volynets, V.; Barsukov, Y.; Kim, G.; Jung, J.-E.; Nam, S.K.; Han, K.; Huang, S.; Kushner, M.J. Highly selective Si3N4/SiO2 etching using an NF3/N2/O2/H2 remote plasma. I. Plasma source and critical fluxes. J. Vac. Sci. Technol. A 2020, 38, 023008. [Google Scholar] [CrossRef]
- Hayashi, M.; Nimura, T. Calculation of electron swarm parameters in fluorine. J. Appl. Phys. 1983, 54, 4879–4882. [Google Scholar] [CrossRef]
- Ho, P.; Johannes, J.E.; Buss, R.J.; Meeks, E. Modeling the plasma chemistry of C2F6 and CHF3 etching of silicon dioxide, with comparisons to etch rate and diagnostic data. J. Vac. Sci. Technol. A 2001, 19, 2344–2367. [Google Scholar] [CrossRef]
- Mao, M.; Wang, Y.N.; Bogaerts, A. Numerical study of the plasma chemistry in inductively coupled SF6 and SF6/Ar plasmas used for deep silicon etching applications. J. Phys. D Appl. Phy. 2011, 44, 435202. [Google Scholar] [CrossRef]
- Bordage, M.C.; Ségur, P.; Christophorou, L.G.; Olthoff, J.K. Boltzmann analysis of electron swarm parameters in CF4 using independently assessed electron-collision cross sections. J. Appl. Phys. 1999, 86, 3558–3566. [Google Scholar] [CrossRef]
- Christophorou, L.G.; Olthoff, J.K.; Rao, M.V.V.S. Electron Interactions with CF4. J. Phys. Chem. Ref. Data 1996, 25, 1341–1388. [Google Scholar] [CrossRef]
- Tarnovsky, V.; Kurunczi, P.; Rogozhnikov, D.; Becker, K. Absolute cross sections for the dissociative electron impact ionization of the CF, (x = l–3) free radicals. Int. J. Mass Spectrom. Ion Process. 1993, 128, 181. [Google Scholar] [CrossRef]
- Vasenkov, A.V.; Li, X.; Oehrlein, G.S.; Kushner, M.J. Properties of c-C4F8 inductively coupled plasmas. II. Plasma chemistry and reaction mechanism for modeling of Ar/c-C4F8/O2 discharges. J. Vac. Sci. Technol. A 2004, 22, 511–530. [Google Scholar] [CrossRef]
- Bonham, R.A. Electron Impact Cross Section Data for Carbon Tetrafluoride. Jpn. J. Appl. Phys. 1994, 33, 4157. [Google Scholar] [CrossRef]
- Gorobchuk, A. Numerical modeling of silicon processing technology in CF4/H2 plasma. In Proceedings of the 2015 International Siberian Conference on Control and Communications (SIBCON), Omsk, Russia, 21–23 May 2015; pp. 1–4. [Google Scholar]
- Kokura, H.; Nakamura, K.; Ghanashev, I.P.; Sugai, H. Plasma Absorption Probe for Measuring Electron Density in an Environment Soiled with Processing Plasmas. Jpn. J. Appl. Phys. 1999, 38, 5262. [Google Scholar] [CrossRef]
- Nakamura, K.; Ohata, M.; Sugai, H. Highly sensitive plasma absorption probe for measuring low-density high-pressure plasmas. J. Vac. Sci. Technol. A 2003, 21, 325–331. [Google Scholar] [CrossRef]
- Britun, N.; Mo, M.K.T.; Hsiao, S.-N.; Arellano, F.J.T.; Sekine, M.; Hori, M. Optical actinometry for number density measurements in low-pressure plasmas: Advantages, error sources, and method validation. J. Appl. Phys. 2024, 136, 111101. [Google Scholar] [CrossRef]
- Li, B.; Li, H.; Chen, Z.; Xie, J.; Feng, G.; Liu, W. Experimental and Simulational Studies on the Theoretical Model of the Plasma Absorption Probe. Plasma Sci. Technol. 2010, 12, 513. [Google Scholar] [CrossRef]
- Hsiao, S.-N.; Nguyen, T.-T.-N.; Tsutsumi, T.; Ishikawa, K.; Sekine, M.; Hori, M. On the Etching Mechanism of Highly Hydrogenated SiN Films by CF4/D2 Plasma: Comparison with CF4/H2. Coatings 2021, 11, 1535. [Google Scholar] [CrossRef]
- Itikawa, Y. Electron Collisions with Hydrogen Fluoride. J. Phys. Chem. Ref. Data 2017, 46, 013105. [Google Scholar] [CrossRef]
- Wolff, W.; Dogan, M.; Luna, H.; Coutinho, L.H.; Mootheril, D.; Baek, W.; Pfeifer, T.; Dorn, A. Absolute electron impact ionization cross-sections for CF4: Three dimensional recoil-ion imaging combined with the relative flow technique. Rev. Sci. Instrum. 2024, 95, 095103. [Google Scholar] [CrossRef]
- Liu, Y.; Booth, J.-P.; Chabert, P. Effect of frequency on the uniformity of symmetrical RF CCP discharges. Plasma Sources Sci. Technol. 2018, 27, 055012. [Google Scholar] [CrossRef]
- Kushner, M.J. A kinetic study of the plasma-etching process. I. A model for the etching of Si and SiO2 in CnFm/H2 and CnFm/O2 plasmas. J. Appl. Phys. 1982, 53, 2923–2938. [Google Scholar] [CrossRef]
- Wei, J.; Woo, B.; Lee, D.; Jeong, K.H.; Kwon, K.H. C4F6 Etching Characteristics for High-Aspect-Ratio Etching of SiO2 Films Using an Inductively Coupled Plasma Etching System with Low-Frequency Bias Power. Plasma Proc. Polym. 2025, 22, e70012. [Google Scholar] [CrossRef]
- Krüger, F.; Zhang, D.; Luan, P.; Park, M.; Metz, A.; Kushner, M.J. Autonomous hybrid optimization of a SiO2 plasma etching mechanism. J. Vac. Sci. Technol. A 2024, 42, 043008. [Google Scholar] [CrossRef]
- Kushner, M.J. Hybrid modelling of low temperature plasmas for fundamental investigations and equipment design. J. Phys. D Appl. Phys. 2009, 42, 194013. [Google Scholar] [CrossRef]
- Kim, G.; Kwon, J.-W.; Lee, I.; Seo, H.; Park, J.-B.; Shin, J.-H.; Kim, G.-H. Application of Plasma Information-Based Virtual Metrology (PI-VM) for Etching in C4F8/Ar/O2 Plasma. IEEE Trans. Semicond. Manuf. 2024, 37, 602–614. [Google Scholar] [CrossRef]
- Lill, T.; Berry, I.L.; Shen, M.; Hoang, J.; Fischer, A.; Panagopoulos, T.; Chang, J.P.; Vahedi, V. Dry etching in the presence of physisorption of neutrals at lower temperatures. J. Vac. Sci. Technol. A 2023, 41, 023005. [Google Scholar] [CrossRef]
- Dussart, R.; Ettouri, R.; Nos, J.; Antoun, G.; Tillocher, T.; Lefaucheux, P. Cryogenic etching of silicon compounds using a CHF3 based plasma. J. Appl. Phys. 2023, 133, 113306. [Google Scholar] [CrossRef]
- Jong, W.H.; Hyun, W.T.; Nam, I.C.; Hyeong, J.E.; Chan, H.K.; Jun, W.J.; Kyung, L.K.; Hee, J.Y.; Hyun, M.C.; Yu, G.J.; et al. Reactive ion etching of indium gallium zinc oxide (IGZO) and chamber cleaning using low global warming potential gas. Appl. Surf. Sci. 2024, 671, 160692. [Google Scholar] [CrossRef]
- Tran, T.N.; Ishikawa, K. Reaction surface analysis of plasma etching of SiN, SiO2, and poly-Si films using low-global warming potential CF3CHCF2 gas. Appl. Surf. Sci. 2025, 710, 163955. [Google Scholar] [CrossRef]
- You, S.; Kim, M.; Cho, I.; Kim, J.; Lee, S.; Kim, C.-K. Cyclic etching of SiO2 contact holes using heptafluoropropyl methyl ether having low global-warming potential. Mater. Des. 2025, 259, 114797. [Google Scholar] [CrossRef]

| Reaction Number | Reaction | Reference |
|---|---|---|
| G1 | e + HF → HF + e | [26] |
| G2 | e + HF → HF+ + 2e | [26] |
| G3 | e + HF → HF (v = 1) + e | [27] |
| G4 | e + HF → HF (v = 2) + e | [27] |
| G5 | e + HF → H + F + e | Calibration [17] |
| G6 | e + HF+ → H + F | [28] |
| G7 | e + HF (v = 1) → HF+ + 2e | Estimation |
| G8 | e + HF (v = 2) → HF+ + 2e | Estimation |
| G9 | e + H → H+ + 2e | [28] |
| G10 | e + F2 → F2 + e | [29] |
| G11 | e + F2 → F + F− | [29] |
| G12 | F− + F → F2 + e | [30] |
| G13 | F− + F → 2F + e | [31] |
| G14 | F− + F2 → F + F2 + e | [32] |
| G15 | H + F + HF → HF + HF | [28] |
| G16 | H + F + HF → HF (v = 1) + HF | [28] |
| G17 | H + F + HF → HF (v = 2) + HF | [28] |
| G18 | HF + F → F2 + H | [28] |
| G19 | HF (v = 1) + F → F2+ H | [28] |
| G20 | HF (v = 2) + F → F2 + H | [28] |
| Reaction Number | Reaction | Reference |
|---|---|---|
| G21 | e + CF4 → CF4 + e | [33] |
| G22 | e + CF4 → CF3+ + 2e + F | [34] |
| G23 | e + CF4 → CF2+ + 2e + 2F | [34] |
| G24 | e + CF4 → CF+ + 2e + 3F | [34] |
| G25 | e + CF4 → CF3 + e + F | [34] |
| G26 | e + CF4 → CF2 + e + 2F | [34] |
| G27 | e + CF4 → CF + e + 3F | [34] |
| G28 | e + CF3 → CF3+ + 2e | [35] |
| G29 | e + CF3 → CF2+ + 2e + F | [35] |
| G30 | e + CF3 → CF+ + 2e + 2F | [35] |
| G31 | e + CF3 → CF2 + e + F | [34] |
| G32 | e + CF3+ → CF2 + F | [36] |
| G33 | e + CF2 → CF2+ + 2e | [35] |
| G34 | e + CF2 → CF+ + 2e + F | [35] |
| G35 | e + CF2 → CF + e + F | [31] |
| G36 | e + CF → CF+ + 2e | [37] |
| G37 | F + CF3 → CF4 | [36] |
| G38 | F + CF2 → CF3 | [36] |
| G39 | F + CF → CF2 | [36] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Takagi, S.; Hsiao, S.-N.; Imai, Y.; Sekine, M.; Matsunaga, F. Role of CF4 Addition in Gas-Phase Variations in HF Plasma for Cryogenic Etching: Insights from Plasma Simulation and Experimental Correlation. Plasma 2025, 8, 48. https://doi.org/10.3390/plasma8040048
Takagi S, Hsiao S-N, Imai Y, Sekine M, Matsunaga F. Role of CF4 Addition in Gas-Phase Variations in HF Plasma for Cryogenic Etching: Insights from Plasma Simulation and Experimental Correlation. Plasma. 2025; 8(4):48. https://doi.org/10.3390/plasma8040048
Chicago/Turabian StyleTakagi, Shigeyuki, Shih-Nan Hsiao, Yusuke Imai, Makoto Sekine, and Fumihiko Matsunaga. 2025. "Role of CF4 Addition in Gas-Phase Variations in HF Plasma for Cryogenic Etching: Insights from Plasma Simulation and Experimental Correlation" Plasma 8, no. 4: 48. https://doi.org/10.3390/plasma8040048
APA StyleTakagi, S., Hsiao, S.-N., Imai, Y., Sekine, M., & Matsunaga, F. (2025). Role of CF4 Addition in Gas-Phase Variations in HF Plasma for Cryogenic Etching: Insights from Plasma Simulation and Experimental Correlation. Plasma, 8(4), 48. https://doi.org/10.3390/plasma8040048







