Non-Thermal Plasma-Assisted Synthesis of ZnO for Enhanced Photocatalytic Performance
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Characterization
2.3. Synthesis of Catalyst
2.4. Plasma Synthesis
2.5. Thermal Synthesis
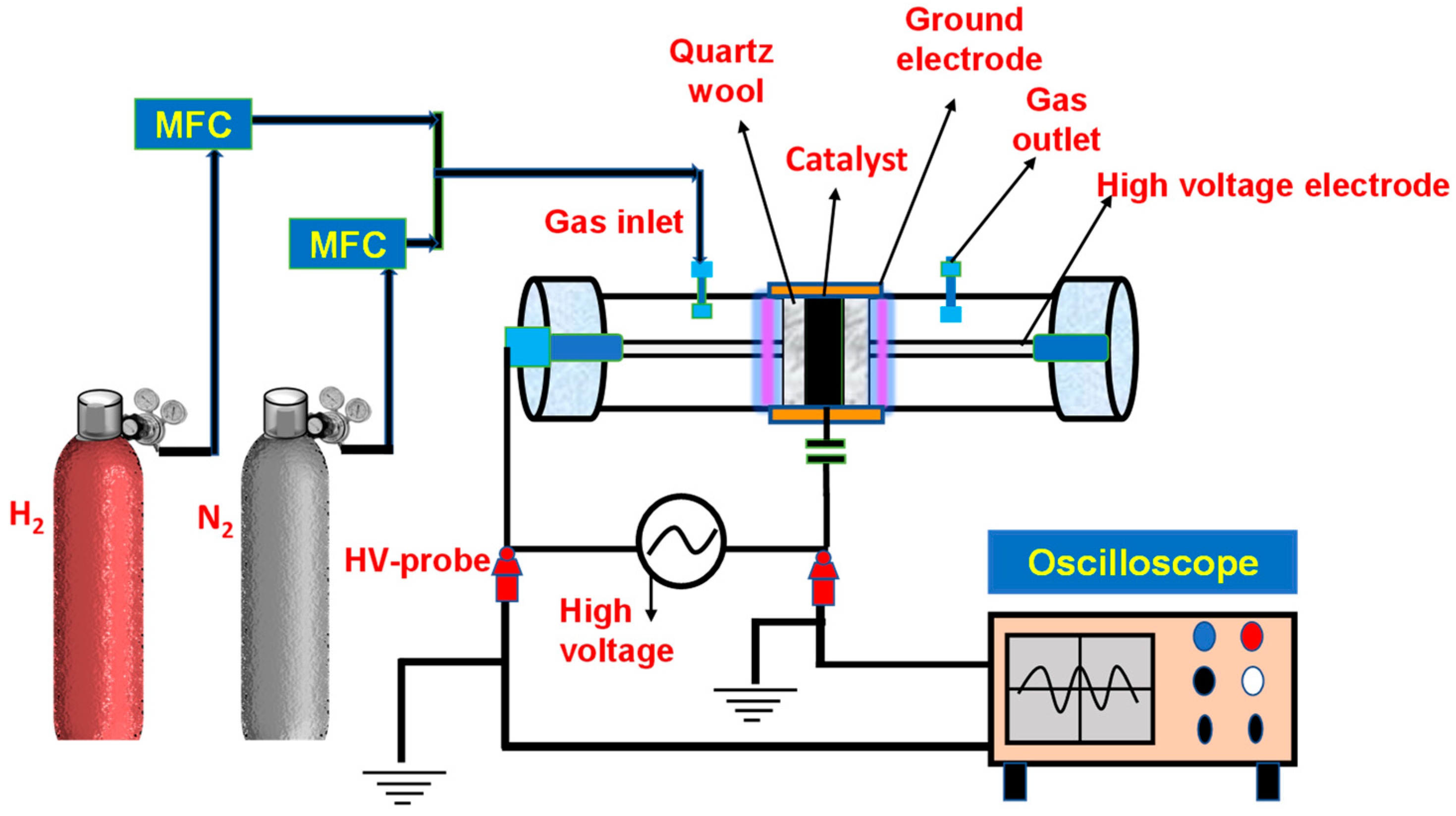
2.6. Plasma Reactor
2.7. Photocatalytic Studies
3. Results and Discussion
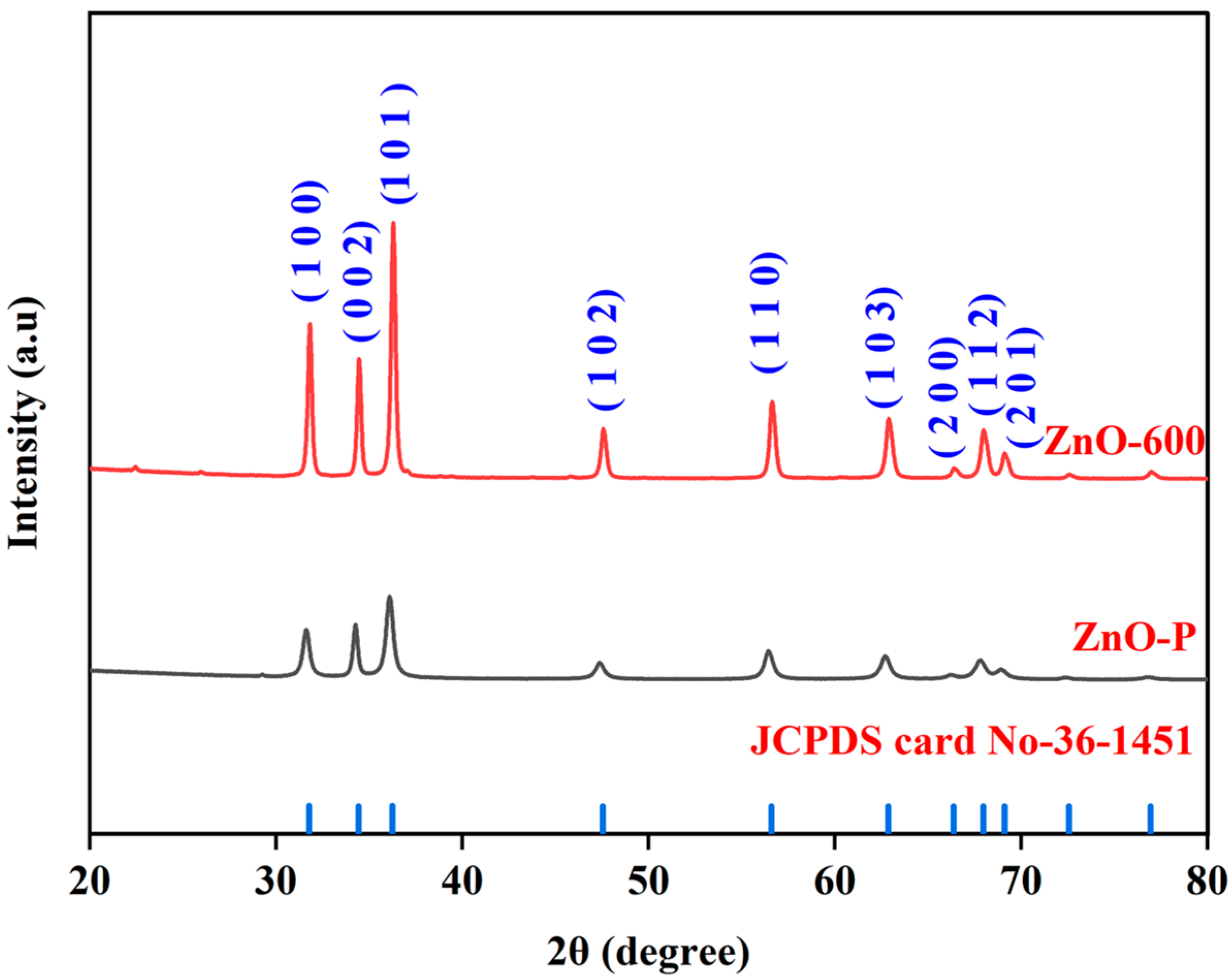
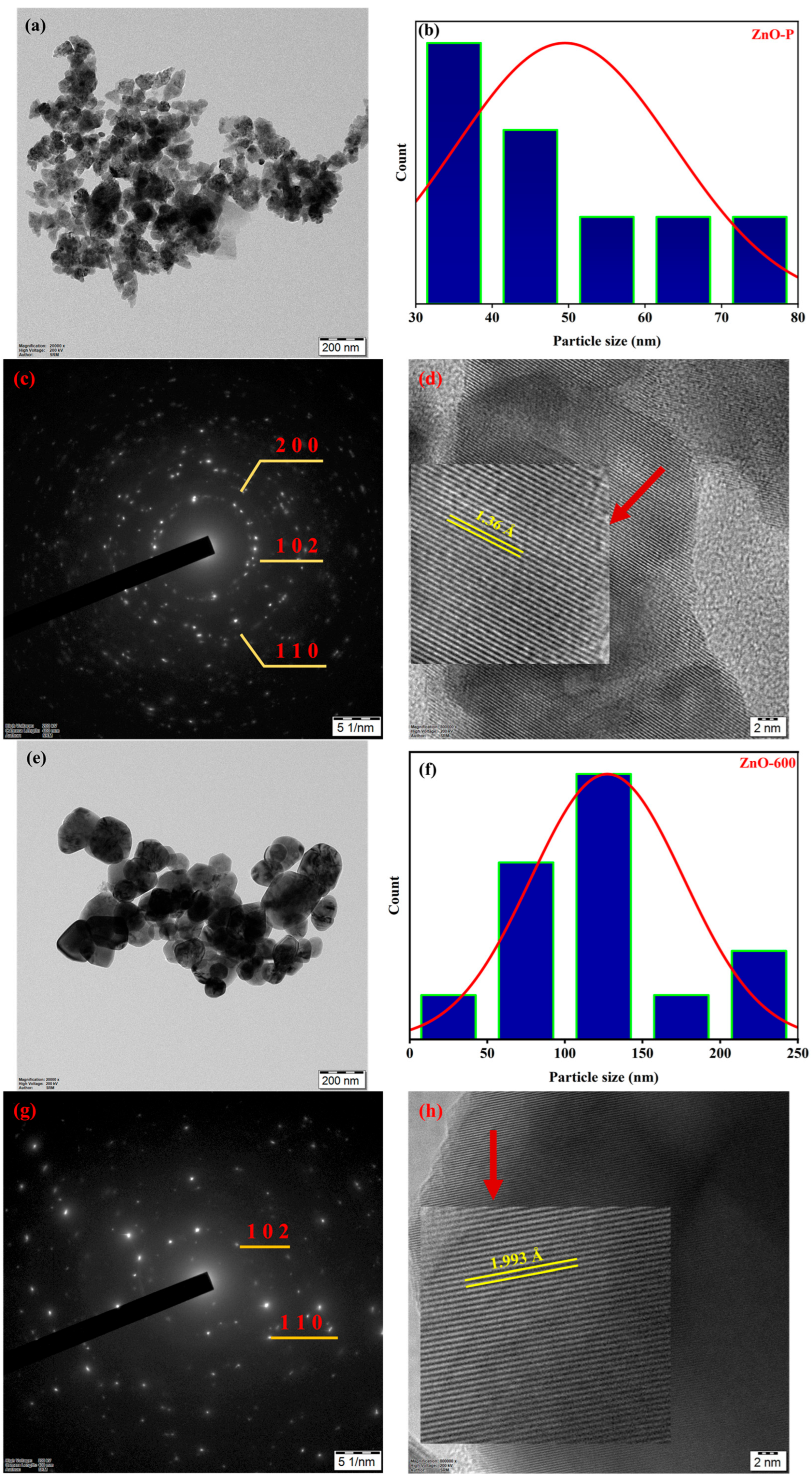
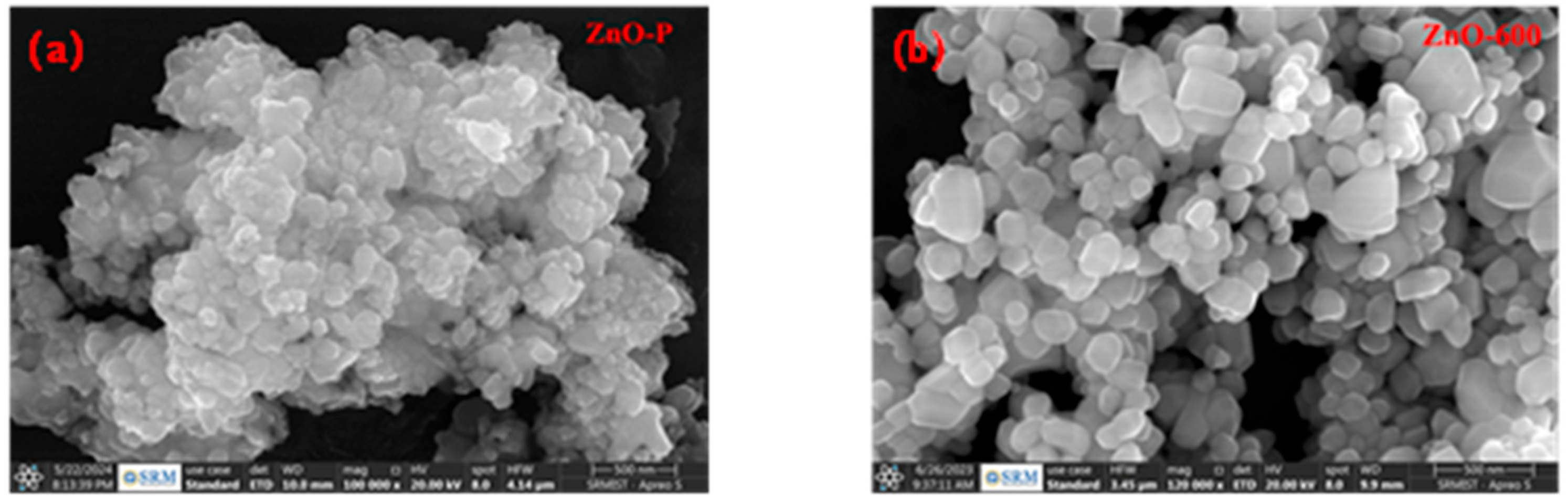
3.1. Structural Analysis
3.2. Catalytic Activity
3.3. Kinetics Studies
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- da Silva, G.T.; Carvalho, K.T.; Lopes, O.F.; Gomes, E.S.; Malagutti, A.R.; Mastelaro, V.R.; Mourão, H.A. Synthesis of ZnO nanoparticles assisted by N sources and their application in the photodegradation of organic contaminants. ChemCatChem 2017, 9, 3795–3804. [Google Scholar]
- Carvalho, K.T.; Fidelis, S.C.; Lopes, O.F.; Ribeiro, C. Effect of processing variables on the photocatalytic properties of ZnO thin films prepared using the polymeric precursor method. Ceram. Int. 2015, 41, 10587–10594. [Google Scholar]
- Russo, M.; Iervolino, G.; Vaiano, V.; Palma, V. Non-thermal plasma coupled with catalyst for the degradation of water pollutants: A review. Catalysts 2020, 10, 1438. [Google Scholar] [CrossRef]
- Mohan, H.; Mohandoss, S.; Prakash, A.; Balasubramaniyan, N.; Loganathan, S.; Assadi, A.A.; Khacef, A. Cold plasma assisted synthesis of spinel-CoFe2O4 nanoparticle with narrow bandgap and high magnetic activity. Inorg. Chem. Commun. 2024, 167, 112754. [Google Scholar]
- Mohandoss, S.; Mohan, H.; Balasubramaniyan, N.; Assadi, A.A.; Loganathan, S. Pearl Millet seed surface modification and improved germination by non-thermal plasma discharge: Understanding the role of reactive species. Plasma Chem. Plasma Process. 2024, 44, 1031–1051. [Google Scholar]
- Chen, H.; Mu, Y.; Shao, Y.; Chansai, S.; Xu, S.; Stere, C.E.; Xiang, H.; Zhang, R.; Jiao, Y.; Hardacre, C.; et al. Coupling non-thermal plasma with Ni catalysts supported on BETA zeolite for catalytic CO2 methanation. Catal. Sci. Technol. 2019, 9, 4135–4145. [Google Scholar]
- Joshi, N.; Loganathan, S. Cold Plasma Techniques for Sustainable Material Synthesis and Climate Change Mitigation: A Review. Catalysts 2024, 14, 802. [Google Scholar] [CrossRef]
- Bjelajac, A.; Petrovic, R.; Stefanovic, M.; Phillipe, A.M.; Fleming, Y.; Guillot, J.; Chemin, J.-B.; Choquet, P.; Kadok, J.; Bulou, S. Au nanoparticle decoration of nanoparticular and nanotubular TiO2 using atmospheric pressure cold plasma for photocatalytic applications. Catal. Sci. Technol. 2024, 14, 5342–5351. [Google Scholar]
- Qi, X.F.; Zhang, F.; Chen, Z.P.; Chen, X.; Jia, M.C.; Ji, H.F.; Shi, Z.F. Hydrothermal synthesis of stable lead-free Cs4MnBi2Cl12 perovskite single crystals for efficient photocatalytic degradation of organic pollutants. J. Mater. Chem. C 2023, 11, 3715–3725. [Google Scholar]
- Bhat, S.V.; Deepak, F.L. Tuning the bandgap of ZnO by substitution with Mn2+, Co2+ and Ni2+. Solid State Commun. 2005, 135, 345–347. [Google Scholar]
- Raha, S.; Ahmaruzzaman, M. ZnO nanostructured materials and their potential applications: Progress, challenges and perspectives. Nanoscale Adv. 2022, 4, 1868–1925. [Google Scholar] [PubMed]
- Noman, M.T.; Amor, N.; Petru, M. Synthesis and applications of ZnO nanostructures (ZONSs): A review. Crit. Rev. Solid State Mater. Sci. 2022, 47, 99–141. [Google Scholar] [CrossRef]
- Hewlett, R.M.; McLachlan, M.A. Surface structure modification of ZnO and the impact on electronic properties. Adv. Mater. 2016, 28, 3893–3921. [Google Scholar] [PubMed]
- Sedaghat, S.; Kasi, V.; Nejati, S.; Krishnakumar, A.; Rahimi, R. Improved performance of printed electrochemical sensors via cold atmospheric plasma surface modification. J. Mater. Chem. C 2022, 10, 10562–10573. [Google Scholar]
- Yadawa, Y.; Jha, D.; Joshi, N. Cost-effective efficient materials for dye degradation using non-aqueous sol–gel route. Environ. Sci. Pollut. Res. 2024, 31, 740–756. [Google Scholar]
- Puneetha, J.; Kottam, N.; Rathna, A. Investigation of photocatalytic degradation of crystal violet and its correlation with bandgap in ZnO and ZnO/GO nanohybrid. Inorg. Chem. Commun. 2021, 125, 108460. [Google Scholar]
- Kotresh, M.G.; Patil, M.K.; Inamdar, S.R. Reaction temperature-based synthesis of ZnO nanoparticles using co-precipitation method: Detailed structural and optical characterization. Optik 2021, 243, 167506. [Google Scholar] [CrossRef]
- Tzvetkov, G.; Tsvetkov, M.; Mihaylov, L.; Spassov, T. Ultrasound-assisted vs traditional wet-chemical method to fabricate ZnO/ε-Zn (OH)2 nanocomposites. Compos. Commun. 2024, 49, 101983. [Google Scholar]
- Wang, Z.; Zhang, Y.; Neyts, E.C.; Cao, X.; Zhang, X.; Jang, B.W.L.; Liu, C.J. Catalyst preparation with plasmas: How does it work? ACS Catal. 2018, 8, 2093–2110. [Google Scholar]
- Vekselman, V.; Raitses, Y.; Shneider, M.N. Growth of nanoparticles in dynamic plasma. Phys. Rev. E 2019, 99, 063205. [Google Scholar]
- Zhou, J.; Wei, T.; An, X. Combining non-thermal plasma technology with photocatalysis: A critical review. Phys. Chem. Chem. Phys. 2023, 25, 1538–1545. [Google Scholar] [CrossRef] [PubMed]
- Nunomura, S. A review of plasma-induced defects: Detection, kinetics and advanced management. J. Phys. D Appl. Phys. 2023, 56, 363002. [Google Scholar] [CrossRef]
- Liu, H.; Hu, Y.; Zhang, Z.; Liu, X.; Jia, H.; Xu, B. Synthesis of spherical Ag/ZnO heterostructural composites with excellent photocatalytic activity under visible light and UV irradiation. Appl. Surf. Sci. 2015, 355, 644–652. [Google Scholar] [CrossRef]
- Sone, B.T.; Makamu, E.; Mohamed, H.E.A.; Oputu, O.; Fester, V. Green-synthesized ZnO via Hyphaene thebaica fruit extracts: Structure & catalytic effect on the Ozonation of Coralene Rubine-S2G azo disperse dye. Environ. Nanotechnol. Monit. Manag. 2021, 16, 100515. [Google Scholar]
- Messai, R.; Ferhat, M.F.; Belmekki, B.; Alam, M.W.; Al-Othoum, M.A.S.; Sadaf, S. GAD plasma-assisted synthesis of ZnO nanoparticles and their photocatalytic activity. Mater. Res. Express 2024, 11, 015006. [Google Scholar] [CrossRef]
- Reddy, N.K.; Devika, M.; Hahn, Y.B. Excellent enhancement in the device performance of nitrogen plasma treated ZnO nanorods based diodes. Nano Converg. 2014, 1, 26. [Google Scholar] [CrossRef]
- Di, L.; Zhang, J.; Zhang, X.; Wang, H.; Li, H.; Li, Y.; Bu, D. Cold plasma treatment of catalytic materials: A review. J. Phys. D Appl. Phys. 2021, 54, 333001. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, Q.; Xu, M.; Yuan, H.; Chen, Y.; Zhang, J.; Luo, K.; Zhang, J.; You, B. Novel and efficient synthesis of Ag-ZnO nanoparticles for the sunlight-induced photocatalytic degradation. Appl. Surf. Sci. 2019, 476, 632–640. [Google Scholar] [CrossRef]
- Klok, L.A.; Steffen, T.T.; Sabedra, H.R.; Fontana, L.C.; Hammer, P.; Marega, F.M.; Costa, L.C.; Pessan, L.A.; Becker, D. ZnO surface modification with maleic anhydride using plasma treatment. Plasma Process. Polym. 2024, 21, 2300165. [Google Scholar] [CrossRef]
- Muhammad, B.L.; Cummings, F. Nitrogen plasma irradiation of Fe doped ZnO nanowire arrays for improved optical properties. Mater. Today Proc. 2021, 36, 383–389. [Google Scholar] [CrossRef]
- Ananth, A.; Dharaneedharan, S.; Seo, H.J.; Heo, M.S.; Boo, J.H. Soft jet plasma-assisted synthesis of Zinc oxide nanomaterials: Morphology controls and antibacterial activity of ZnO. Chem. Eng. J. 2017, 322, 742–751. [Google Scholar] [CrossRef]
- Zhang, Y.; Xia, Y.; Wang, L.; Cheng, B.; Yu, J. Influence of calcination temperature on photocatalytic H2O2 productivity of hierarchical porous ZnO microspheres. Nanotechnology 2021, 32, 415402. [Google Scholar] [CrossRef] [PubMed]
- Camarda, P.; Messina, F.; Vaccaro, L.; Agnello, S.; Buscarino, G.; Schneider, R.; Popescu, R.; Gerthsen, D.; Lorenzi, R.; Gelardi, F.M.; et al. Luminescence mechanisms of defective ZnO nanoparticles. Phys. Chem. Chem. Phys. 2016, 18, 16237–16244. [Google Scholar] [CrossRef]
- Knust, S.; Ruhm, L.; Kuhlmann, A.; Meinderink, D.; Bürger, J.; Lindner, J.K.; de los Arcos de Pedro, M.T.; Grundmeier, G. In situ backside Raman spectroscopy of zinc oxide nanorods in an atmospheric-pressure dielectric barrier discharge plasma. J. Raman Spectrosc. 2021, 52, 1237–1245. [Google Scholar] [CrossRef]
- Dong, J.J.; Zhang, X.W.; You, J.B.; Cai, P.F.; Yin, Z.G.; An, Q.; Ma, X.B.; Jin, P.; Wang, Z.G.; Chu, P.K. Effects of hydrogen plasma treatment on the electrical and optical properties of ZnO films: Identification of hydrogen donors in ZnO. ACS Appl. Mater. Interfaces 2010, 2, 1780–1784. [Google Scholar] [CrossRef]
- Wang, D.; Zhao, D.; Wang, F.; Yao, B.; Shen, D. Nitrogen-doped ZnO obtained by nitrogen plasma treatment. Phys. Status Solidi a 2015, 212, 846–850. [Google Scholar] [CrossRef]
- Nandi, P.; Das, D. Photocatalytic degradation of Rhodamine-B dye by stable ZnO nanostructures with different calcination temperature induced defects. Appl. Surf. Sci. 2019, 465, 546–556. [Google Scholar] [CrossRef]
- Cuscó, R.; Alarcón-Lladó, E.; Ibáñez, J.; Artús, L.; Jiménez, J.; Wang, B.; Callahan, M.J. Temperature dependence of Raman scattering in ZnO. Phys. Rev. B—Condens. Matter Mater. Phys. 2007, 75, 165202. [Google Scholar] [CrossRef]
- Al-Hada, N.M.; Saion, E.B.; Shaari, A.H.; Kamarudin, M.A.; Flaifel, M.H.; Ahmad, S.H.; Gene, S.A. A facile thermal-treatment route to synthesize ZnO nanosheets and effect of calcination temperature. PLoS ONE 2014, 9, e103134. [Google Scholar] [CrossRef]
- Lee, H.U.; Lee, Y.C.; Lee, S.C.; Park, S.Y.; Son, B.; Lee, J.W.; Lim, C.-H.; Choi, C.-J.; Choi, M.-H.; Lee, S.Y.; et al. Visible-light-responsive bicrystalline (anatase/brookite) nanoporous nitrogen-doped TiO2 photocatalysts by plasma treatment. Chem. Eng. J. 2014, 254, 268–275. [Google Scholar] [CrossRef]
- Bhat, A.H.; Chopan, N.A.; Chisti, H.-T.-N. Enhanced photocatalytic degradation of crystal violet dye and high-performance electrochemical supercapacitor applications of hydrothermally synthesised magnetic bifunctional nanocomposite (Fe3O4/ZnO). Nanotechnology 2023, 34, 495604. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Yang, C.; Liu, Y.; Fan, Y.; Dang, F.; Qiu, Y.; Zhou, H.; Wang, W.; Liu, Y. Solvothermal synthesis of ZnO nanoparticles for photocatalytic degradation of methyl orange and p-nitrophenol. Water 2021, 13, 3224. [Google Scholar] [CrossRef]
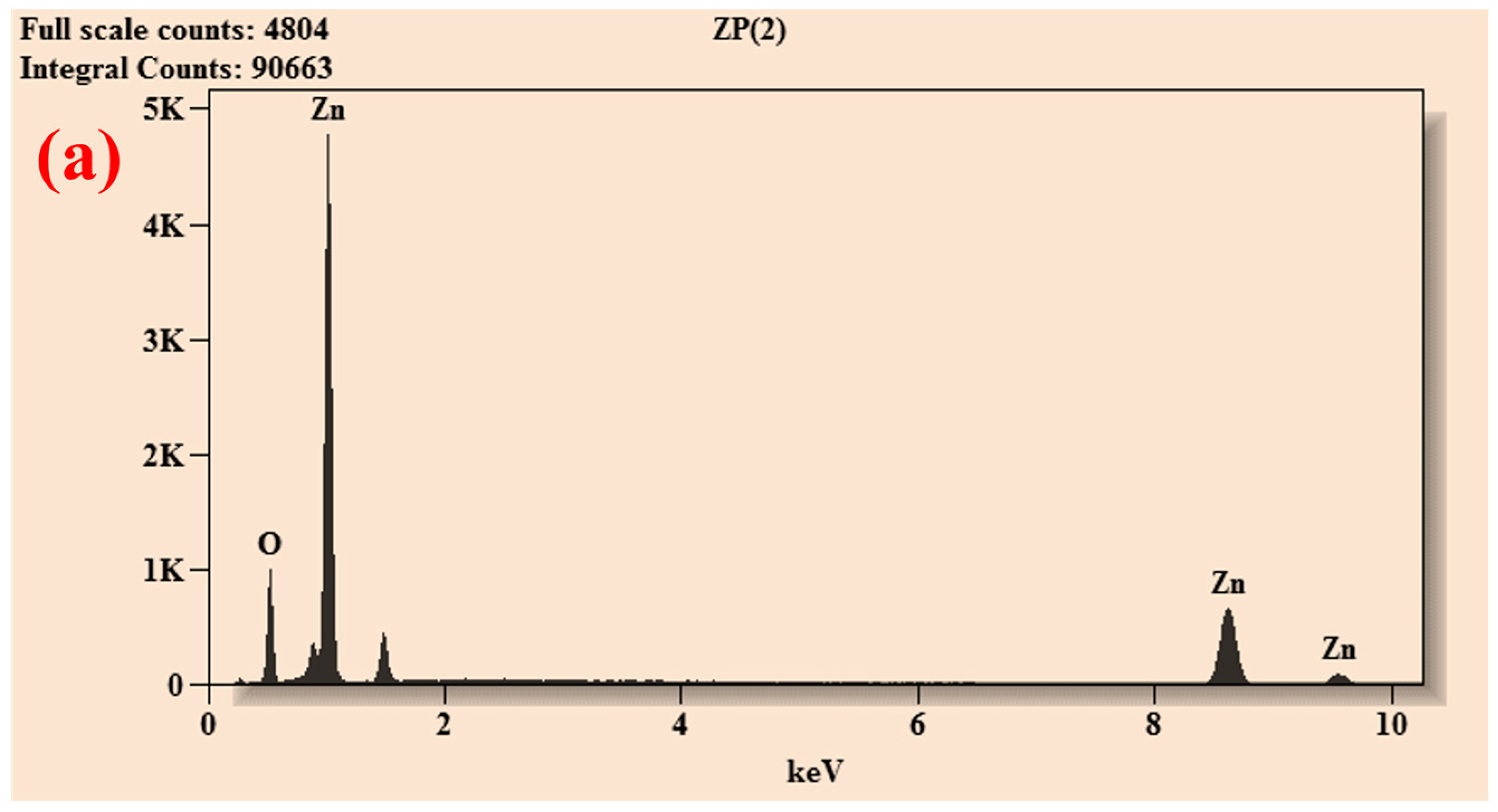
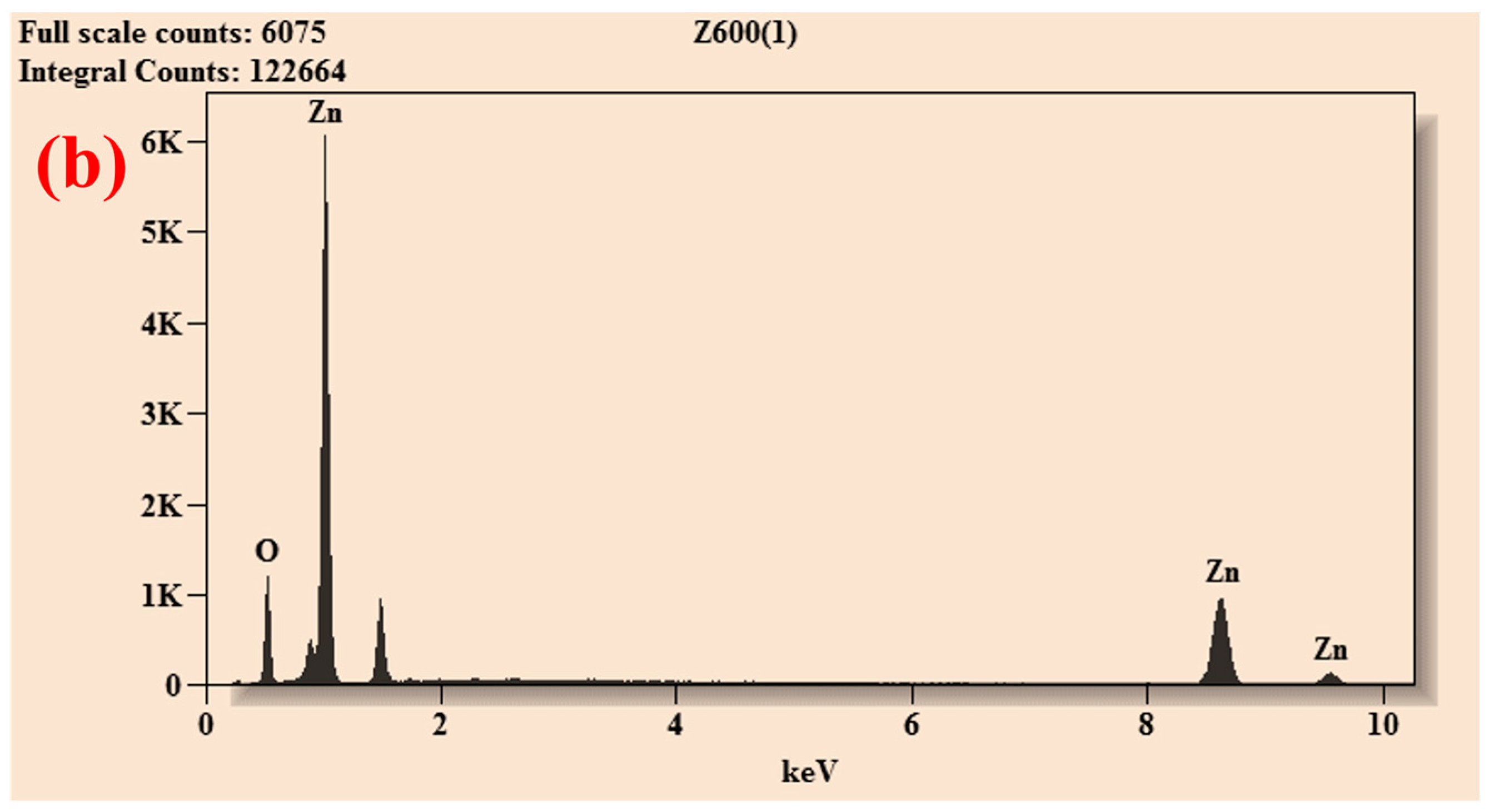
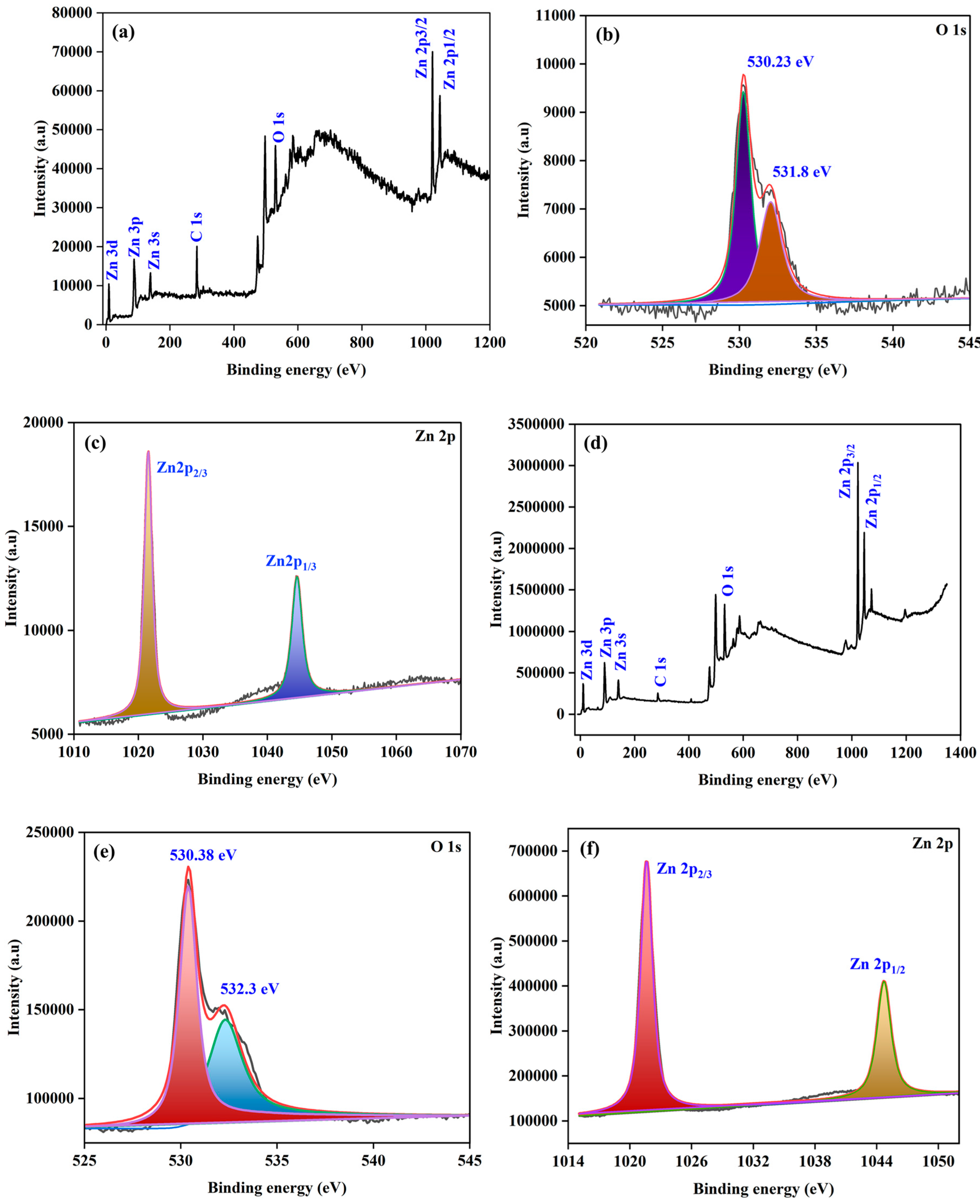
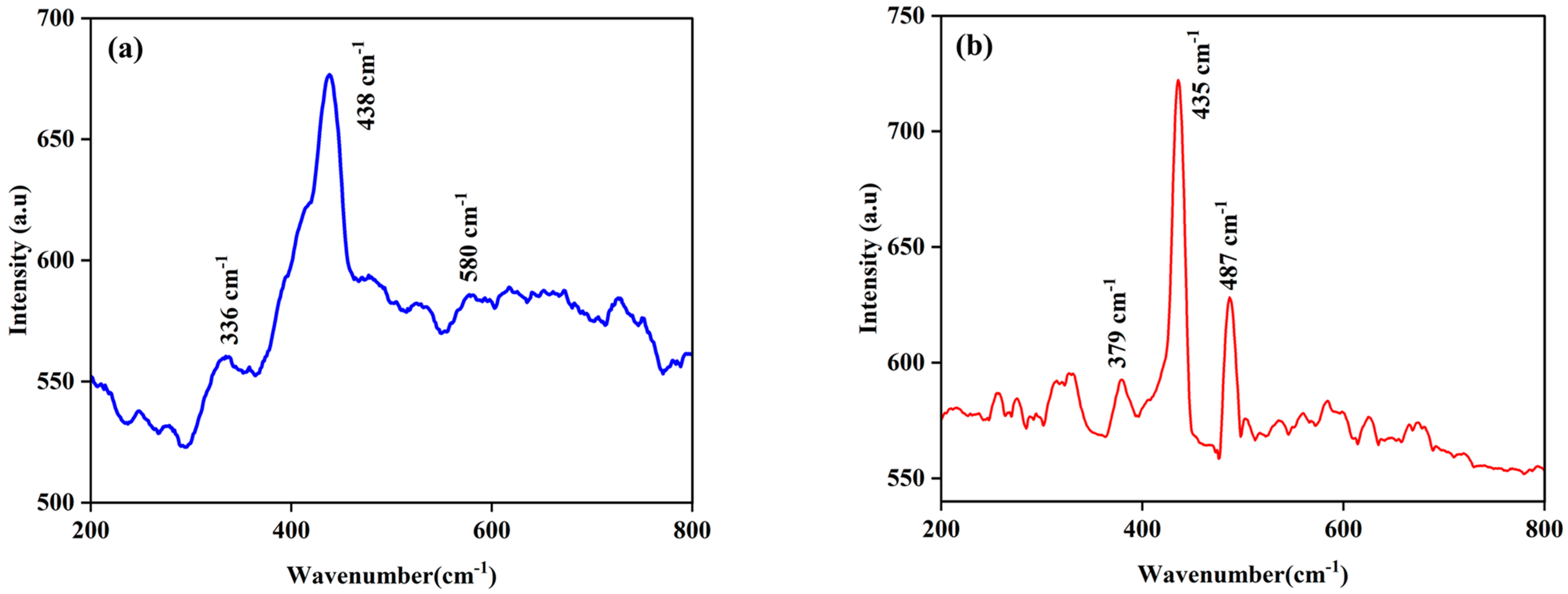
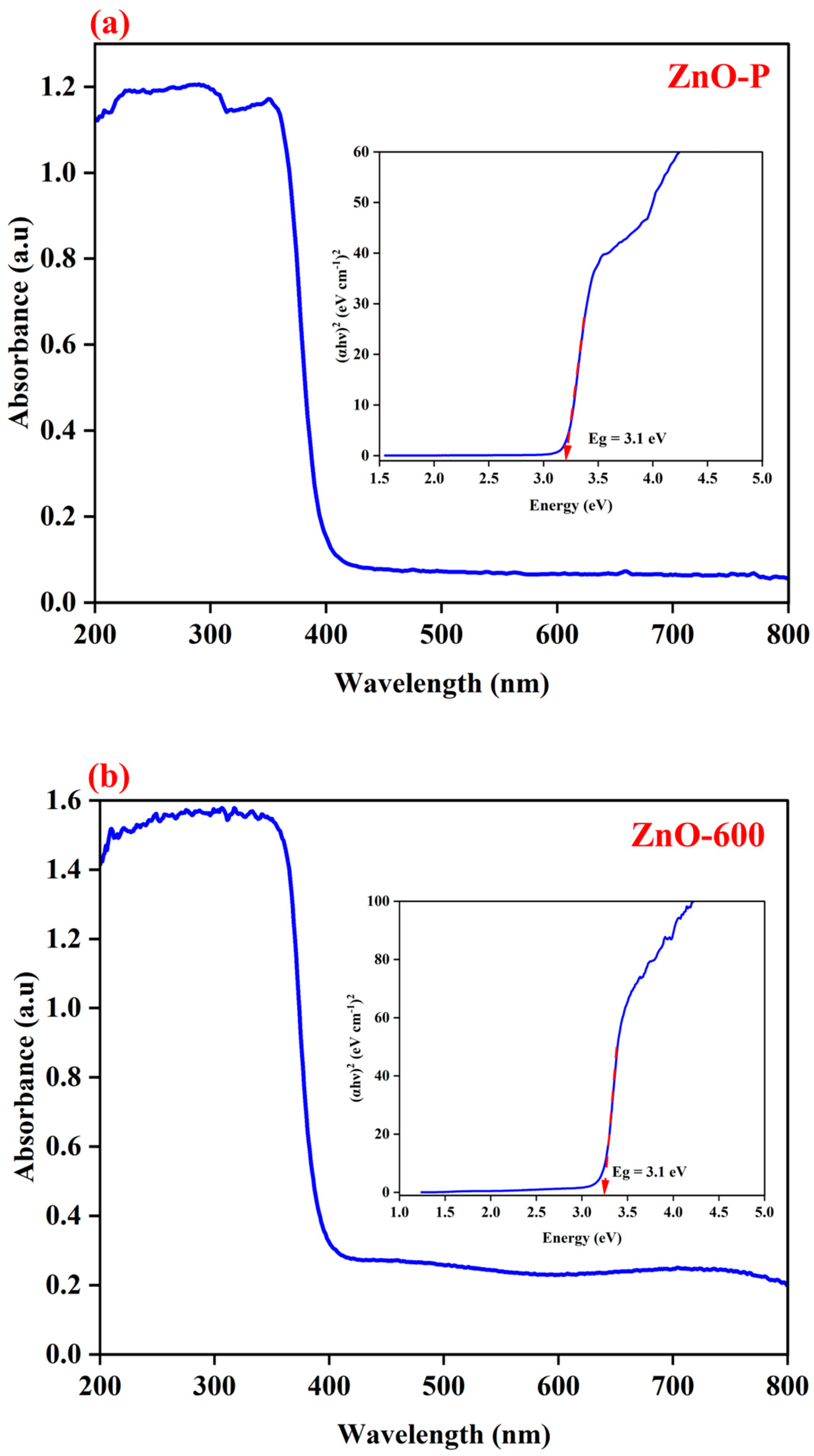
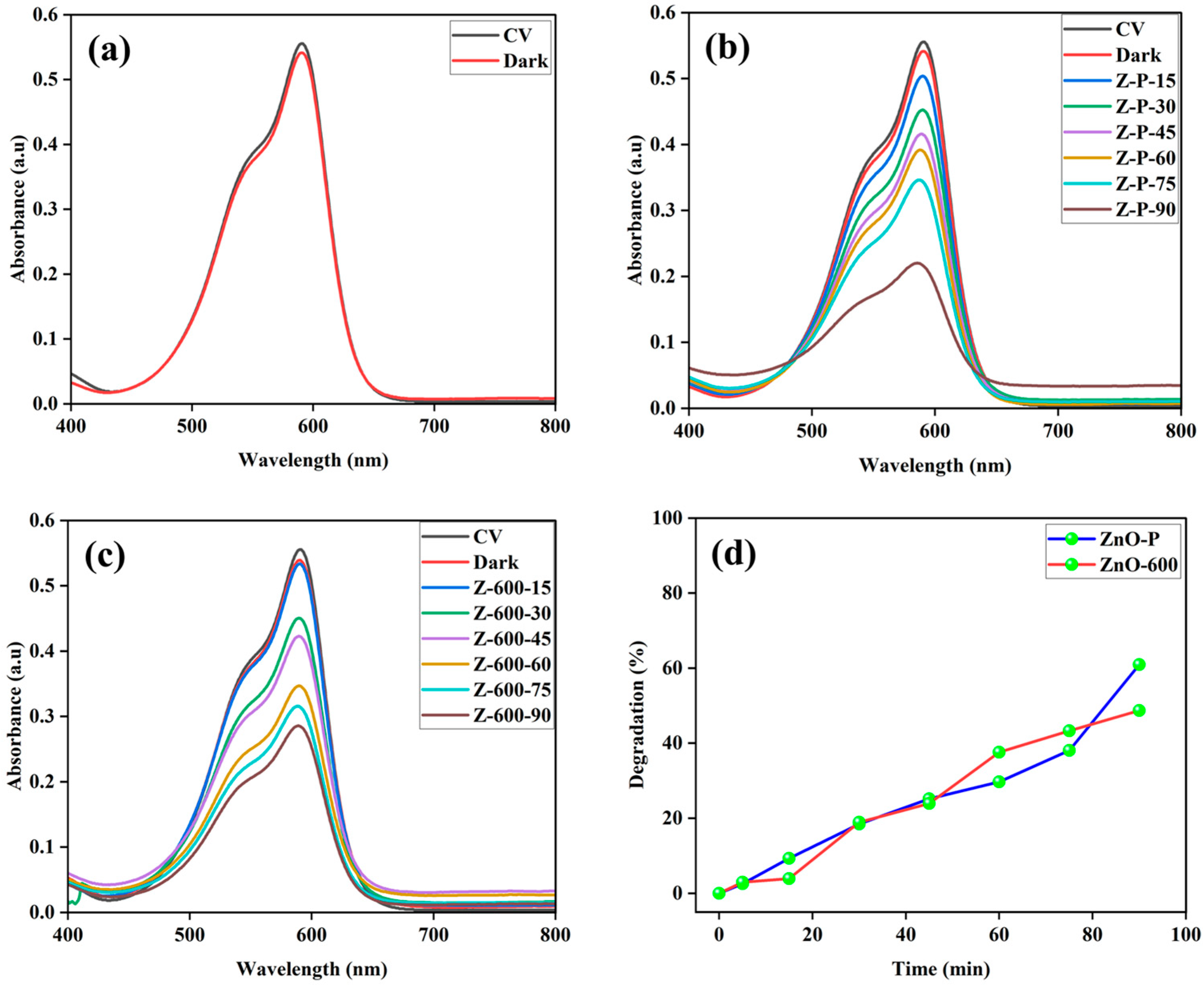
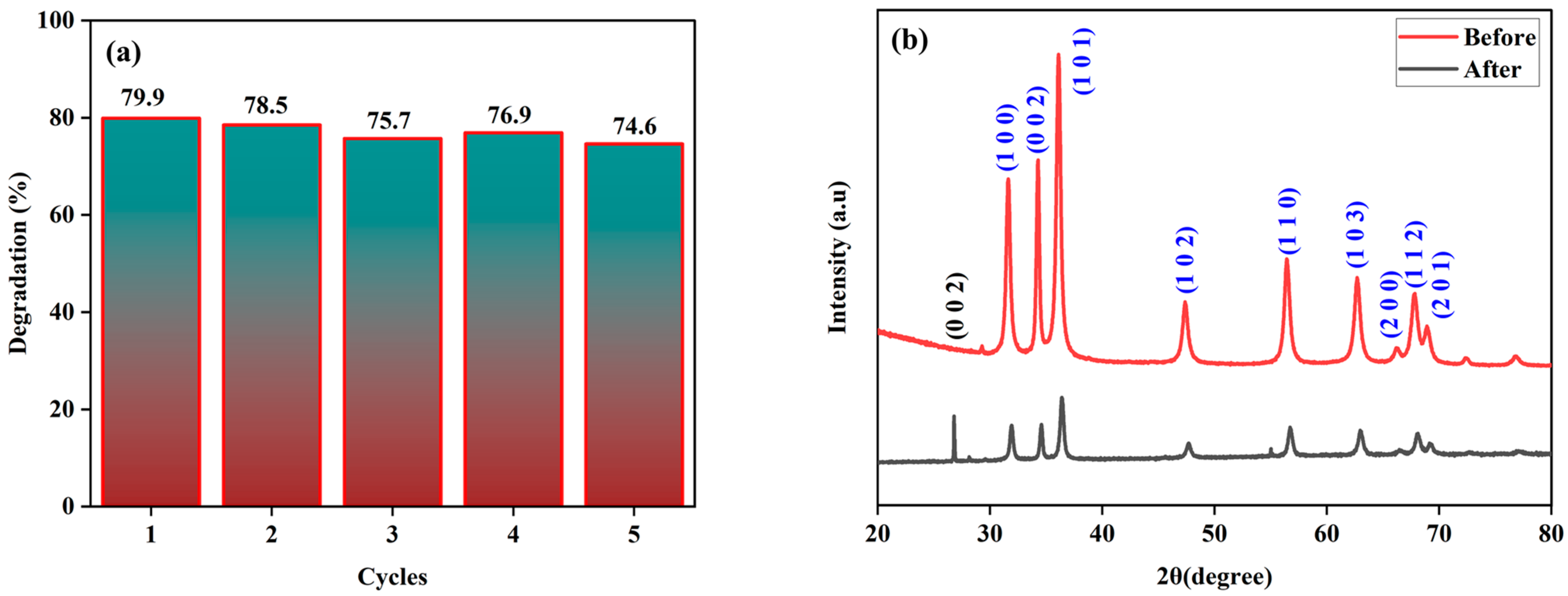
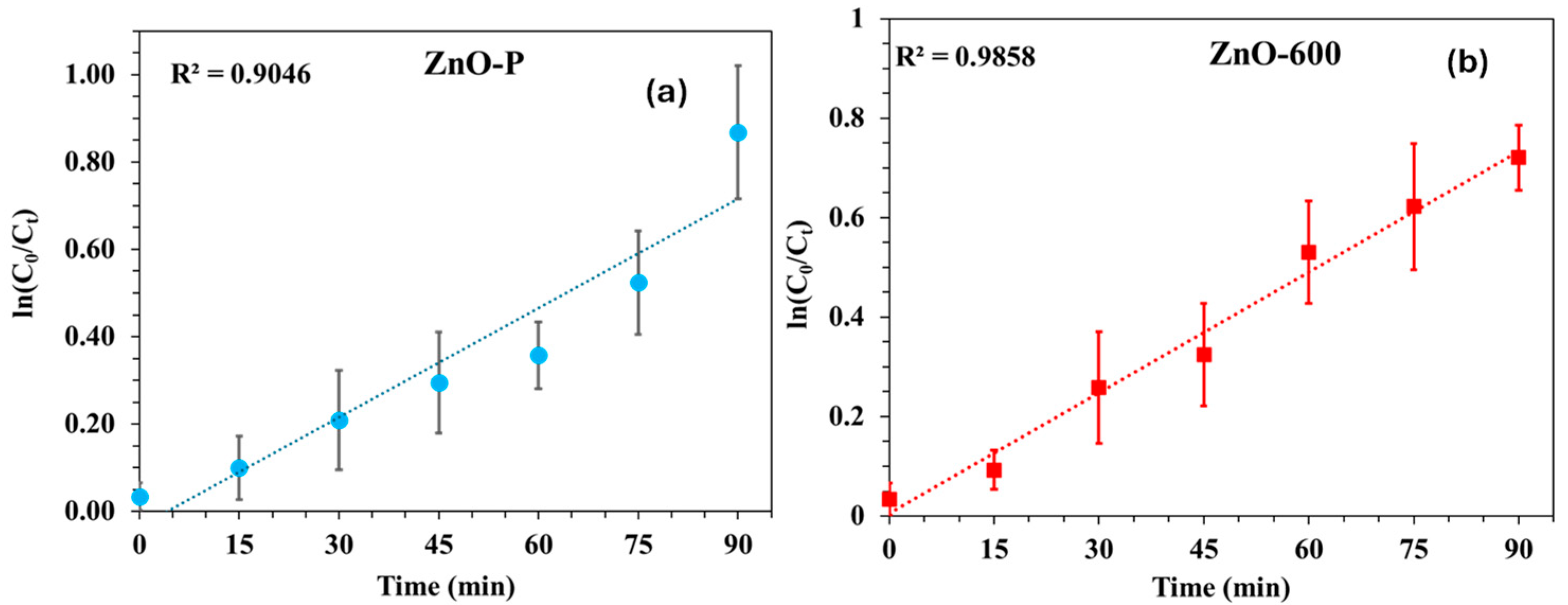
| Element | Net Counts | Weight% | Atom% | Atom% | |
|---|---|---|---|---|---|
| ZnO-600 | O | 6405 | 20.01 | 50.55 | ±0.66 |
| Zn | 17,455 | 79.99 | 49.45 | ±0.71 | |
| Total | 100.00 | 100.00 | |||
| ZnO-P | O | 5441 | 22.68 | 54.52 | ±0.76 |
| Zn | 12,325 | 77.32 | 45.48 | ±0.78 | |
| Total | 100.00 | 100.00 | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mohan, H.; Mohandoss, S.; Balasubramaniyan, N.; Loganathan, S. Non-Thermal Plasma-Assisted Synthesis of ZnO for Enhanced Photocatalytic Performance. Plasma 2025, 8, 25. https://doi.org/10.3390/plasma8020025
Mohan H, Mohandoss S, Balasubramaniyan N, Loganathan S. Non-Thermal Plasma-Assisted Synthesis of ZnO for Enhanced Photocatalytic Performance. Plasma. 2025; 8(2):25. https://doi.org/10.3390/plasma8020025
Chicago/Turabian StyleMohan, Harshini, Subash Mohandoss, Natarajan Balasubramaniyan, and Sivachandiran Loganathan. 2025. "Non-Thermal Plasma-Assisted Synthesis of ZnO for Enhanced Photocatalytic Performance" Plasma 8, no. 2: 25. https://doi.org/10.3390/plasma8020025
APA StyleMohan, H., Mohandoss, S., Balasubramaniyan, N., & Loganathan, S. (2025). Non-Thermal Plasma-Assisted Synthesis of ZnO for Enhanced Photocatalytic Performance. Plasma, 8(2), 25. https://doi.org/10.3390/plasma8020025








