Tailoring the Chemistry of Plasma-Activated Water Using a DC-Pulse-Driven Non-Thermal Atmospheric-Pressure Helium Plasma Jet
Abstract
:1. Introduction
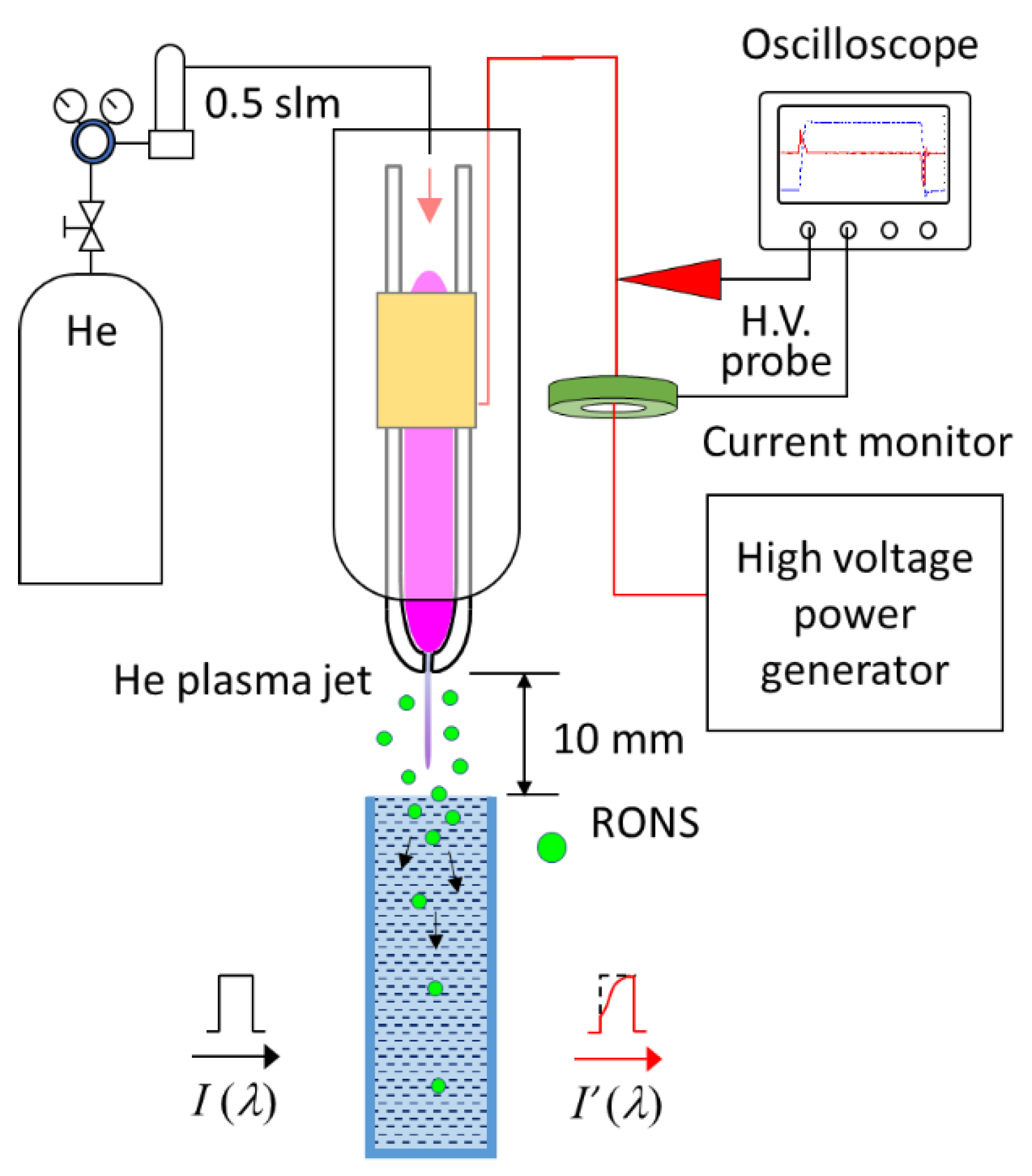
2. Experimental Setup
3. Results and Discussion
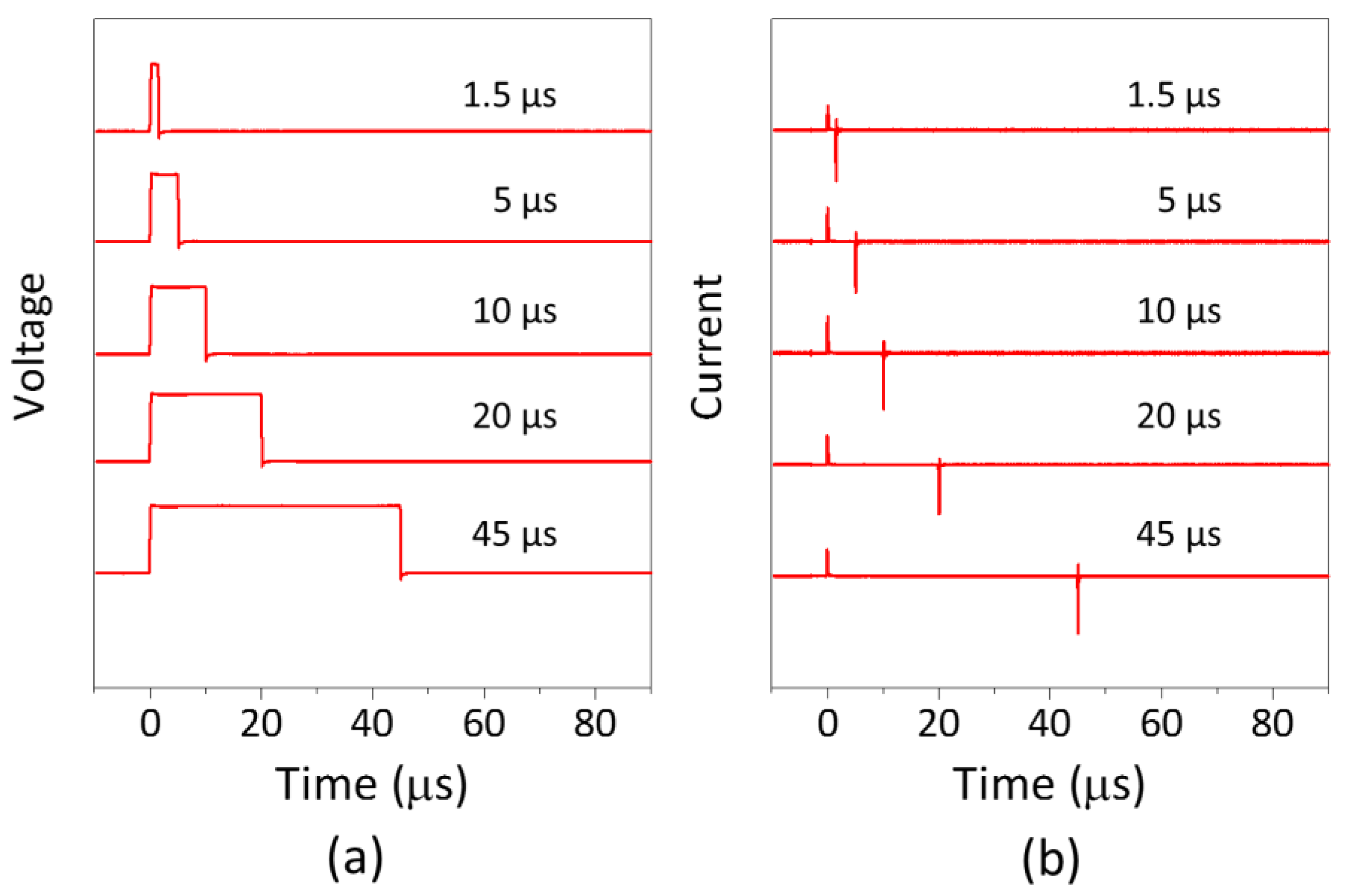
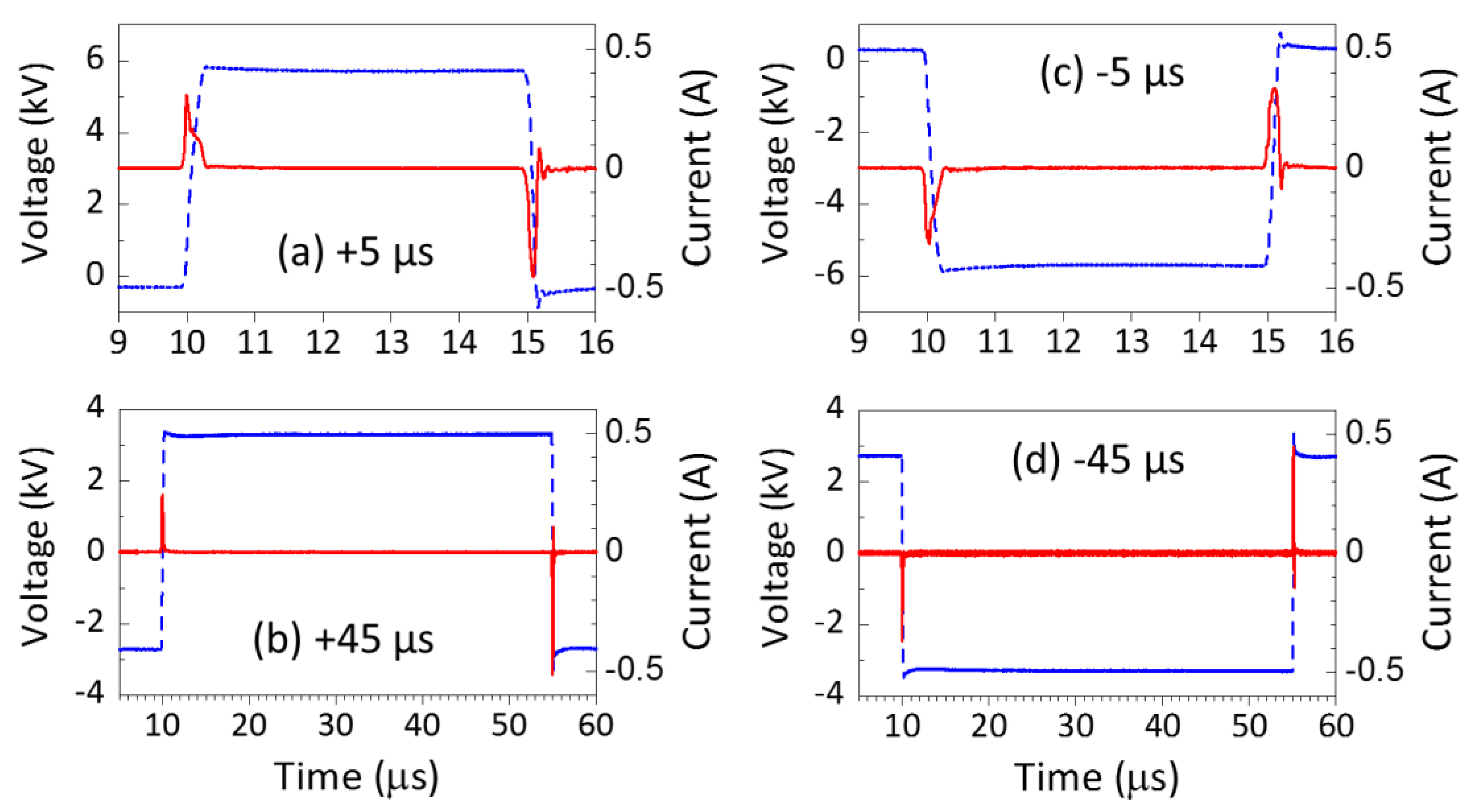
3.1. Voltage and Current Measurements
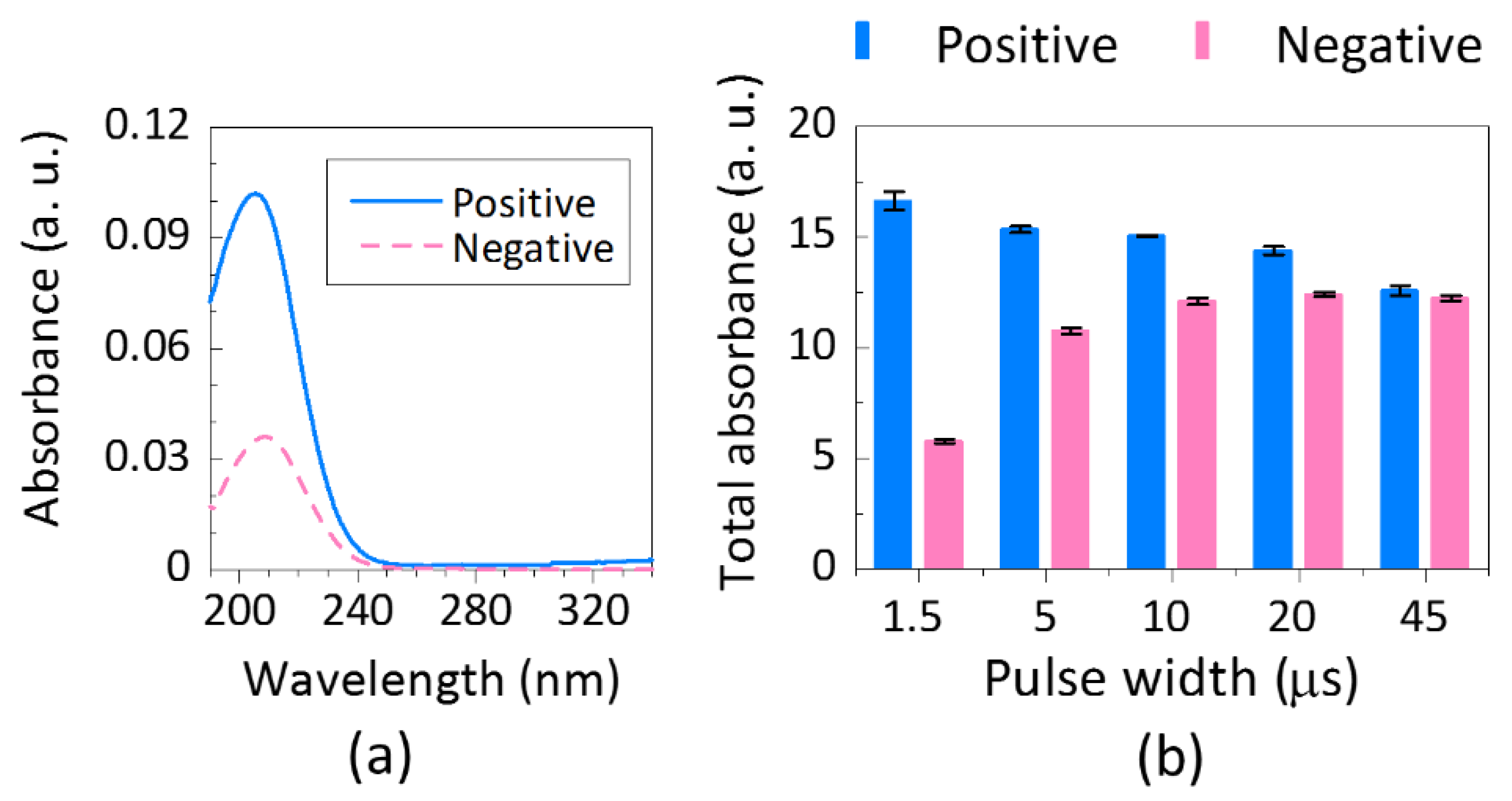
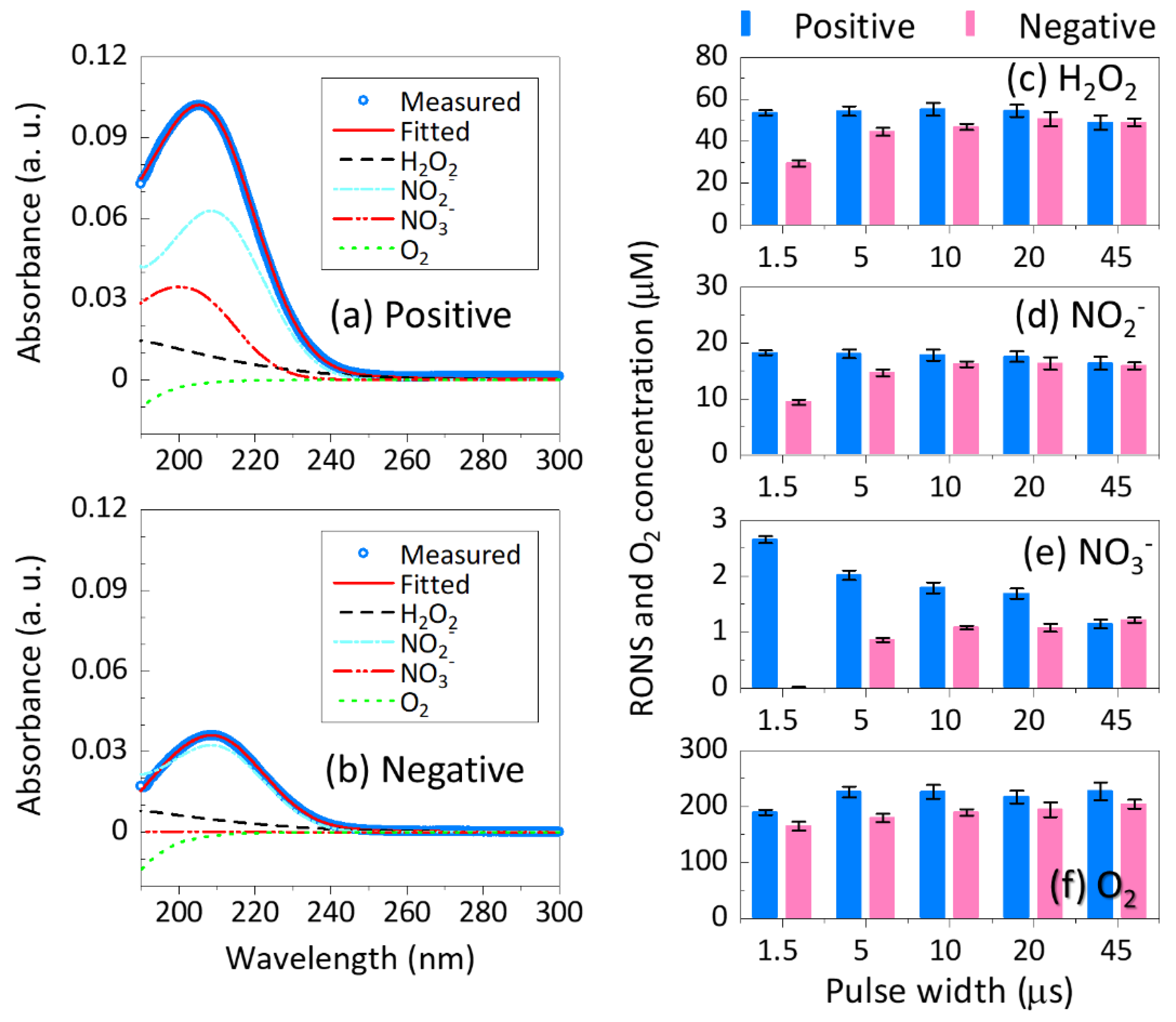
3.2. UV Absorption Spectroscopy
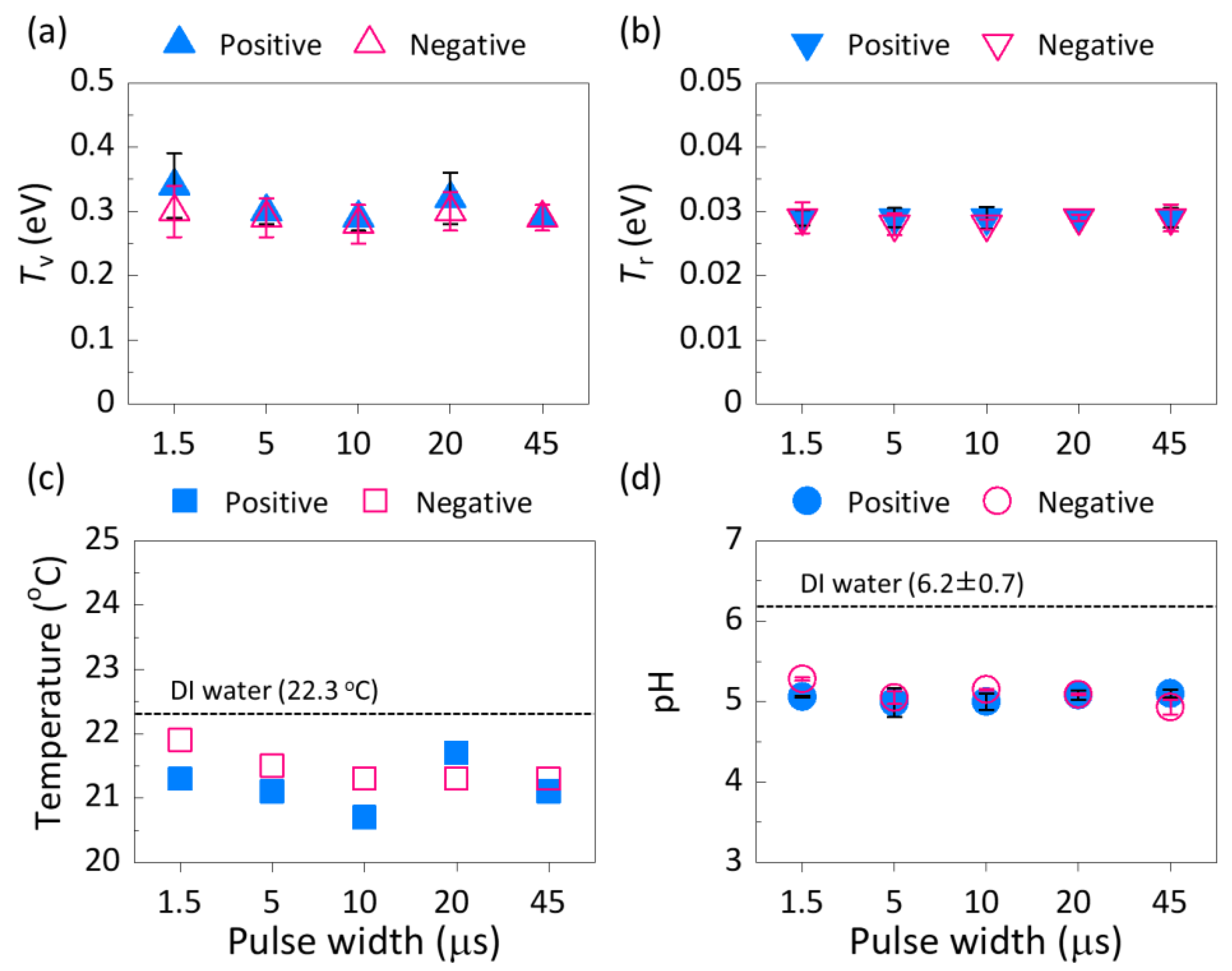
3.3. Plasma Parameters and pH
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Ikawa, S.; Kitano, K.; Hamaguchi, S. Effects of pH on bacterial inactivation in aqueous solutions due to low-temperature atmospheric pressure plasma application. Plasma Process. Polym. 2010, 7, 33–42. [Google Scholar] [CrossRef]
- Foster, J.; Sommers, B.S.; Gucker, S.N.; Blankson, I.M.; Adamovsky, G. Perspectives on the interaction of plasmas with liquid water for water purification. IEEE Trans. Plasma Sci. 2012, 40, 1311. [Google Scholar] [CrossRef]
- Jung, S.; Kim, H.J.; Park, S.; Yong, H.I.; Choe, J.H.; Jeon, H.-J.; Choe, W.; Jo, C. The use of atmospheric pressure plasma-treated water as a source of nitrite for emulsion-type sausage. Meat Sci. 2015, 108, 132–137. [Google Scholar] [CrossRef] [PubMed]
- Tasaki, T.; Ohshima, T.; Usui, E.; Ikawa, S.; Kitano, K.; Maeda, N.; Momo, Y. Plasma-treated water eliminates Streptococcus mutans in infected dentin model. Dent. Mater. J. 2016, 36, 422. [Google Scholar] [CrossRef]
- Oehmigen, K.; Hähnel, M.; Brandenburg, R.; Wilke, C.; Weltmann, K.-D.; von Woedtke, T. The role of acidification for antimicrobial activity of atmospheric pressure plasma in liquids. Plasma Process. Polym. 2010, 7, 250–257. [Google Scholar] [CrossRef]
- Oehmigen, K.; Winter, J.; Hähnel, M.; Brandenburg, R.; Wilke, C.; Weltmann, K.-D.; von Woedtke, T. Estimation of possible mechanisms of Escherichia coli inactivation by plasma treated sodium chloride solution. Plasma Process. Polym. 2011, 8, 904–913. [Google Scholar] [CrossRef]
- Traylor, M.J.; Pavlovich, M.J.; Karim, S.; Hait, P.; Sakiyama, Y.; Clark, D.S.; Graves, D.B. Long-term antibacterial efficacy of air plasma-activated water. J. Phys. D Appl. Phys. 2011, 44, 472001. [Google Scholar] [CrossRef]
- Takai, E.; Kitano, K.; Kuwabara, J.; Shiraki, K. Protein inactivation by low-temperature atmospheric pressure plasma in aqueous solution. Plasma Processes Polym. 2012, 9, 77–82. [Google Scholar] [CrossRef]
- Burlica, R.; Grim, R.G.; Shih, K.-Y.; Balkwill, D.; Locke, B.R. Bacteria inactivation using low power pulsed gliding arc discharges with water spray. Plasma Process. Polym. 2012, 7, 640–649. [Google Scholar] [CrossRef]
- Julák, J.; Scholtz, V.; Kotúčová, S.; Janoušková, O. The persistent microbicidal effect in water exposed to the corona discharge. Phys. Med. 2012, 28, 230–239. [Google Scholar] [CrossRef]
- Kitano, K.; Ikawa, S.; Tani, A.; Nakashima, Y.; Yamazaki, H.; Ohshima, T.; Kaneko, K.; Ito, M.; Kuwata, T.; Yagishita, A. Freezing preservation of the plasma treated water for disinfection treatment in dental and surgical therapies based on the reduced pH method. In Proceedings of the 21st International Symposium Plasma Chemistry, Cairns, Australia, 4–9 August 2013; Cairns Convention Centre: Cairns City, Australia. [Google Scholar]
- Takai, E.; Kitamura, T.; Kuwabara, J.; Ikawa, S.; Yoshzawa, S.; Shiraki, K.; Kawasaki, H.; Arakawa, R.; Kitano, K. Chemical modification of amino acids by atmospheric-pressure cold plasma in aqueous solution. J. Phys. D Appl. Phys. 2014, 47, 285403. [Google Scholar] [CrossRef]
- Lukes, P.; Dolezalova, E.; Sisrova, I.; Clupek, M. Aqueous-phase chemistry and bactericidal effects from an air discharge plasma in contact with water: Evidence for the formation of peroxynitrite through a pseudo-second-order post-discharge reaction of H2O2 and HNO2. Plasma Sour. Sci. Technol. 2014, 23, 015019. [Google Scholar] [CrossRef]
- Kobayashi, T.; Iwata, N.; Oh, J.-S.; Hashizume, H.; Ohta, T.; Takeda, K.; Ishikawa, K.; Hori, M.; Ito, M. Bactericidal pathway of Escherichia coli in buffered saline treated with oxygen radicals. J. Phys. D Appl. Phys. 2017, 50, 155208. [Google Scholar] [CrossRef]
- Naïtali, M.; Kamgang-Youbi, G.; Herry, J.-M.; Bellon-Fontaine, M.-N.; Brisset, J.-L. Combined effects of long-living chemical species during microbial inactivation using atmospheric plasma-treated water. Appl. Environ. Microbiol. 2010, 76, 7662. [Google Scholar] [CrossRef] [PubMed]
- Oh, J.-S.; Aranda-Gonzalvo, Y.; Bradley, J.W. Time-resolved mass spectroscopic studies of an atmospheric-pressure helium microplasma jet. J. Phys. D Appl. Phys. 2011, 44, 365202. [Google Scholar] [CrossRef]
- McKay, K.; Oh, J.-S.; Walsh, J.L.; Bradley, J.W. Mass spectrometric diagnosis of an atmospheric pressure helium microplasma jet. J. Phys. D Appl. Phys. 2013, 46, 464018. [Google Scholar] [CrossRef]
- Oh, J.-S.; Furuta, H.; Hatta, A.; Bradley, J.W. Investigating the effect of additional gases in an atmospheric-pressure helium plasma jet using ambient mass spectrometry. Jpn. J. Appl. Phys. 2015, 54, 01AA03. [Google Scholar] [CrossRef]
- Tani, A.; Ono, Y.; Fukui, S.; Ikawa, S.; Kitano, K. Free radicals induced in aqueous solution by non-contact atmospheric-pressure cold plasma. Appl. Phys. Lett. 2012, 100, 254103. [Google Scholar] [CrossRef]
- Uchida, G.; Nakajima, A.; Ito, T.; Takenaka, K.; Kawasaki, T.; Koga, K.; Shiratani, M.; Setsuhara, Y. Effects of nonthermal plasma jet irradiation on the selective production of H2O2 and NO2− in liquid water. J. Appl. Phys. 2016, 120, 203302. [Google Scholar] [CrossRef]
- Ito, T.; Uchida, G.; Nakajima, A.; Takenaka, K.; Setsuhara, Y. Control of reactive oxygen and nitrogen species production in liquid by nonthermal plasma jet with controlled surrounding gas. Jpn. J. Appl. Phys. 2017, 56, 01A06. [Google Scholar] [CrossRef]
- Oh, J.-S.; Szili, E.J.; Ogawa, K.; Short, R.D.; Ito, M.; Furuta, H.; Hatta, A. UV–vis spectroscopy study of plasma-activated water: Dependence of the chemical composition on plasma exposure time and treatment distance. Jpn. J. Appl. Phys. 2018, 57, 0102B9. [Google Scholar] [CrossRef]
- Ogawa, K.; Yajima, H.; Oh, J.-S.; Furuta, H.; Hatta, A. Effects of sheath gas flow on He atmospheric pressure plasma jet. Appl. Phys. Express. 2019, 12, 036001. [Google Scholar] [CrossRef]
- Oh, J.-S.; Walsh, J.L.; Bradley, J.W. Influence of pulse width on the creation of ions in an atmospheric-pressure plasma jet. In Proceedings of the 2012 IEEE International Conference on Plasma Science, Edinburgh, UK, 8–13 July 2012. [Google Scholar]
- Uchida, G.; Takenaka, K.; Setsuhara, Y. Influence of voltage pulse width on the discharge characteristics in an atmospheric dielectric-barrier-discharge plasma jet. Jpn. J. Appl. Phys. 2016, 55, 01AH03. [Google Scholar] [CrossRef]
- Liu, Z.; Zhou, C.; Liu, D.; Xu, D.; Xia, W.; Cui, Q.; Wang, B.; Kong, M.G. Production and correlation of reactive oxygen and nitrogen species in gas- and liquid-phase generated by helium plasma jets under different pulse widths. Phys. Plasmas 2018, 25, 013528. [Google Scholar] [CrossRef]
- Uchida, G.; Takenaka, K.; Takeda, K.; Ishikawa, K.; Hori, M.; Setsuhara, Y. Selective production of reactive oxygen and nitrogen species in the plasma-treated water by using a nonthermal high-frequency plasma jet. Jpn. J. Appl. Phys. 2018, 57, 0102B4. [Google Scholar] [CrossRef]
- Bruggeman, P.J.; Kushner, M.J.; Locke, B.R.; Gardeniers, J.G.E.; Graham, W.G.; Graves, D.B.; Hofman-Caris, R.C.H.M.; Maric, D.; Reid, J.P.; Ceriani, E.; et al. Plasma–liquid interactions: A review and roadmap. Plasma Sour. Sci. Technol. 2016, 25, 053002. [Google Scholar] [CrossRef]
- Tachibana, K. Current status of microplasma research. IEEJ Trans. Electr. Electron. Eng. 2006, 1, 145–155. [Google Scholar] [CrossRef]
- Ito, Y.; Urabe, K.; Takano, N.; Tachibana, K. High speed deposition of SiO2 films with plasma jet based on capillary dielectric barrier discharge at atmospheric pressure. Appl. Phys. Express 2008, 1, 067009. [Google Scholar] [CrossRef]
- Oh, J.-S.; Olabanji, O.T.; Hale, C.; Mariani, R.; Kontis, K.; Bradley, J.W. Imaging gas and plasma interactions in the surface-chemical modification of polymers using micro-plasma jets. J. Phys. D Appl. Phys. 2011, 44, 155206. [Google Scholar] [CrossRef]
- Kitano, K.; Hamaguchi, S.; Aoki, H.U.S. Plasma Producing Apparatus and Method of Plasma Production. U.S. Patent 8,232,729 B2, 2012. [Google Scholar]
- Oh, J.-S.; Kakuta, M.; Furuta, H.; Akatsuka, H.; Hatta, A. Effect of plasma jet diameter on the efficiency of reactive oxygen and nitrogen species generation in water. Jpn. J. Appl. Phys. 2016, 55, 06HD01. [Google Scholar] [CrossRef]
- Oh, J.-S.; Szili, E.J.; Gaur, N.; Hong, S.-H.; Furuta, H.; Kurita, H.; Mizuno, A.; Hatta, A.; Short, R.D. How to assess the plasma delivery of RONS into tissue fluid and tissue. J. Phys. D Appl. Phys. 2016, 49, 304005. [Google Scholar] [CrossRef]
- Szili, E.J.; Gaur, N.; Hong, S.-H.; Kurita, H.; Oh, J.-S.; Ito, M.; Mizuno, A.; Hatta, A.; Cowin, A.J.; Graves, D.B.; et al. The assessment of cold atmospheric plasma treatment of DNA in synthetic models of tissue fluid, tissue and cells. J. Phys. D Appl. Phys. 2017, 50, 274001. [Google Scholar] [CrossRef]
- Oh, J.-S.; Walsh, J.L.; Bradley, J.W. Plasma bullet current measurements in a free-stream helium capillary jet. Plasma Sour. Sci. Technol. 2012, 21, 034020. [Google Scholar] [CrossRef]
- Koike, S.; Sakamoto, T.; Kobori, S.; Matsuura, H.; Akatsuka, H. Spectroscopic study on vibrational nonequilibrium of a microwave discharge nitrogen plasma. Jpn. J. Appl. Phys. 2004, 43, 5550–5557. [Google Scholar] [CrossRef]
- Oh, J.-S.; Strudwick, X.; Short, R.D.; Ogawa, K.; Hatta, A.; Furuta, H.; Gaur, N.; Hong, S.-H.; Cowin, A.J.; Fukuhara, H.; et al. How plasma induced oxidation, oxygenation, and de-oxygenation influences viability of skin cells. Appl. Phys. Lett. 2016, 109, 203701. [Google Scholar] [CrossRef]
- Szili, E.J.; Oh, J.-S.; Fukuhara, H.; Bhatia, R.; Gaur, N.; Nguyen, C.K.; Hong, S.-H.; Ito, S.; Ogawa, K.; Kawada, C.; et al. Modelling the helium plasma jet delivery of reactive species into a 3D cancer tumour. Plasma Sour. Sci. Technol. 2018, 27, 014001. [Google Scholar]
- Kurihara, J.; Iwagami, N.; Oyama, K.-I. N2 temperature of vibration instrument for sounding rocket observation in the lower thermosphere. In An Introduction to Space Instrumentation; Terrapub: Tokyo, Japan, 2003. [Google Scholar]
- Ogawa, K.; Oh, J.-S.; Gaur, N.; Hong, S.-H.; Kurita, H.; Mizuno, A.; Hatta, A.; Short, R.D.; Szili, E.J. Modulating the concentrations of reactive oxygen and nitrogen species and oxygen in water with helium and argon gas and plasma jets. Jpn. J. Appl. Phys. 2019, 58, SAAB01. [Google Scholar] [CrossRef]
- Ikawa, S.; Tani, A.; Nakashima, Y.; Kitano, K. Physicochemical properties of bactericidal plasma-treated water. J. Phys. D Appl. Phys. 2016, 49, 425401. [Google Scholar] [CrossRef]
- Reuter, S.; Tresp, H.; Wende, K.; Hammer, M.U.; Winter, J.; Masur, K.; Schmidt-Bleker, A.; Weltmann, K. From RONS to ROS: Tailoring plasma jet treatment of skin cells. IEEE Trans. Plasma Sci. 2012, 40, 2986. [Google Scholar] [CrossRef]
- Szili, E.J.; Oh, J.-S.; Hong, S.-H.; Hatta, A.; Short, R.D. Probing the transport of plasma-generated RONS in an agarose target as surrogate for real tissue: Dependency on time, distance and material composition. J. Phys. D Appl. Phys. 2015, 48, 202001. [Google Scholar] [CrossRef]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Oh, J.-S.; Szili, E.J.; Hatta, A.; Ito, M.; Shirafuji, T. Tailoring the Chemistry of Plasma-Activated Water Using a DC-Pulse-Driven Non-Thermal Atmospheric-Pressure Helium Plasma Jet. Plasma 2019, 2, 127-137. https://doi.org/10.3390/plasma2020010
Oh J-S, Szili EJ, Hatta A, Ito M, Shirafuji T. Tailoring the Chemistry of Plasma-Activated Water Using a DC-Pulse-Driven Non-Thermal Atmospheric-Pressure Helium Plasma Jet. Plasma. 2019; 2(2):127-137. https://doi.org/10.3390/plasma2020010
Chicago/Turabian StyleOh, Jun-Seok, Endre J. Szili, Akimitsu Hatta, Masafumi Ito, and Tatsuru Shirafuji. 2019. "Tailoring the Chemistry of Plasma-Activated Water Using a DC-Pulse-Driven Non-Thermal Atmospheric-Pressure Helium Plasma Jet" Plasma 2, no. 2: 127-137. https://doi.org/10.3390/plasma2020010
APA StyleOh, J.-S., Szili, E. J., Hatta, A., Ito, M., & Shirafuji, T. (2019). Tailoring the Chemistry of Plasma-Activated Water Using a DC-Pulse-Driven Non-Thermal Atmospheric-Pressure Helium Plasma Jet. Plasma, 2(2), 127-137. https://doi.org/10.3390/plasma2020010




