A Brief Review of Atomistic Studies on BaTiO3 as a Photocatalyst for Solar Water Splitting
Abstract
1. Introduction
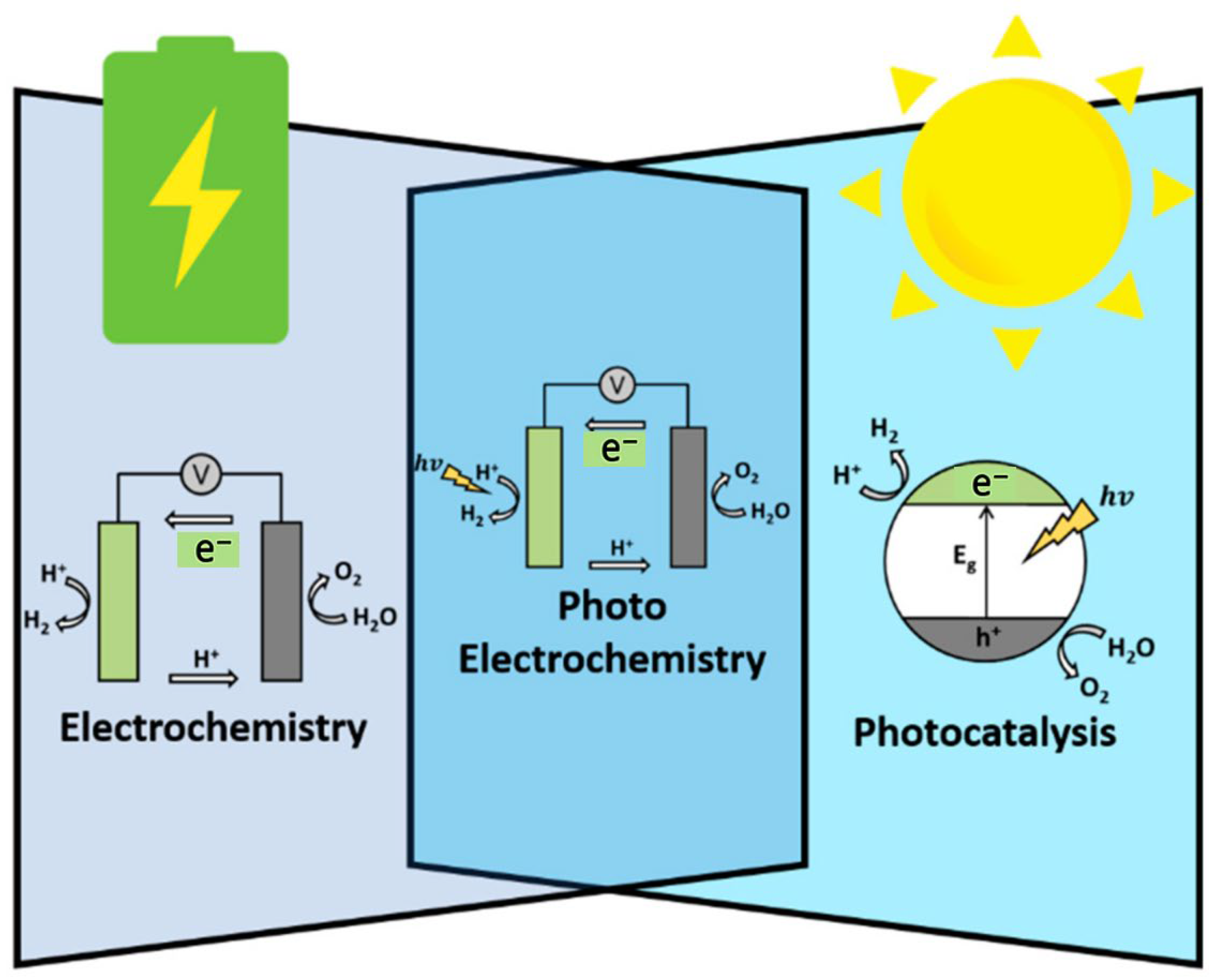
1.1. Photocatalytic Water Splitting and Its Challenges
1.2. Background Information Regarding the Use of BaTiO3 for Photocatalytic Water Splitting
- (i)
- Wide bandgap semiconductor
- (ii)
- Ferroelectricity and spontaneous polarization
- (iii)
- High charge separation efficiency
- (iv)
- Chemical stability
- (v)
- Favorable band edge positions
- (vi)
- Perovskite crystal structure
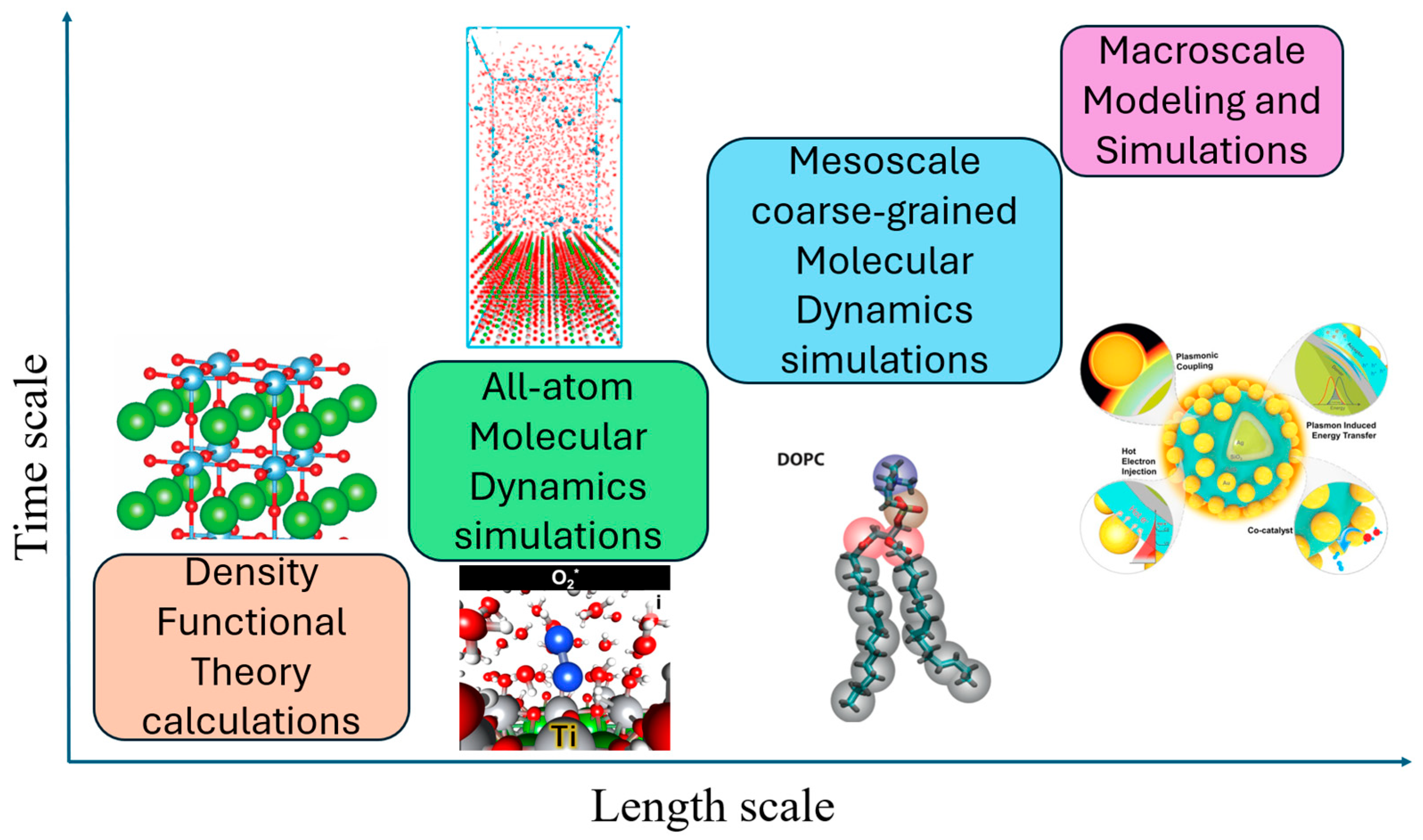
1.3. Atomistic Studies of the BaTiO3 Photocatalyst
1.4. Outline of Our Review
2. Main Body
2.1. DFT Calculations
2.2. Ab Initio MD Simulations
2.3. Classical All-Atom MD Simulations
2.4. Related Experimental Findings
3. Conclusions and Outlook
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| BaTiO3 | Barium titanate |
| BiFeO3 | Bismuth ferrite |
| BiVO4 | Bismuth vanadate |
| CB | Conduction band |
| CdS | Cadmium sulfide |
| CO | Carbon monoxide |
| CO2 | Carbon dioxide |
| CTF | Carbon triazine framework |
| DFT | Density functional theory |
| DFT + U | Density functional theory + Hubbard U correction |
| FDTD | Finite-difference time-domain |
| GGA | Generalized gradient approximation |
| HSE | Hybrid functional |
| HF | Hartree–Fock |
| LaAlO3 | Lanthanum aluminate |
| LDA | Local density approximation |
| MD | Molecular dynamics |
| MLP | Machine learning potentials |
| PAW | Projector augmented wave |
| PBE | Perdew–Burke–Ernzerhof |
| SrTiO3 | Strontium titanate |
| TiO2 | Titanium dioxide |
| VB | Valence band |
| VASP | Vienna Ab initio Simulation Package |
References
- Atilhan, S.; Park, S.; El-Halwagi, M.M.; Atilhan, M.; Moore, M.; Nielsen, R.B. Green hydrogen as an alternative fuel for the shipping industry. Curr. Opin. Chem. Eng. 2021, 31, 100668. [Google Scholar] [CrossRef]
- Qazi, U.Y. Future of hydrogen as an alternative fuel for next-generation industrial applications; challenges and expected opportunities. Energies 2022, 15, 4741. [Google Scholar] [CrossRef]
- Nurmanova, S.; Kolisnichenko, S.; Kokayev, U.; Kalmanova, D.; Karazhanov, A.; Alipbayev, Z.; Abuova, F.; Abdirashev, O.; Satanova, B. Optimizing waste motor oil recycling into diesel using novel deep eutectic solvents: An atomistic study. ES Mater. Manuf. 2025, 28, 1480. [Google Scholar] [CrossRef]
- Kaiwen, L.; Bin, Y.; Tao, Z. Economic analysis of hydrogen production from steam reforming process: A literature review. Energy Sources Part B Econ. Plan. Policy 2018, 13, 109–115. [Google Scholar] [CrossRef]
- Franchi, G.; Capocelli, M.; De Falco, M.; Piemonte, V.; Barba, D. Hydrogen production via steam reforming: A critical analysis of MR and RMM technologies. Membranes 2020, 10, 10. [Google Scholar] [CrossRef]
- Ranjekar, A.M.; Yadav, G.D. Steam reforming of methanol for hydrogen production: A critical analysis of catalysis, processes, and scope. Ind. Eng. Chem. Res. 2021, 60, 89–113. [Google Scholar] [CrossRef]
- Wang, M.; Wang, Z.; Gong, X.; Guo, Z. The intensification technologies to water electrolysis for hydrogen production—A review. Renew. Sustain. Energy Rev. 2014, 29, 573–588. [Google Scholar] [CrossRef]
- El-Shafie, M. Hydrogen production by water electrolysis technologies: A review. Results Eng. 2023, 20, 101426. [Google Scholar] [CrossRef]
- Shayan, E.; Zare, V.; Mirzaee, I.J.E.C. Hydrogen production from biomass gasification; A theoretical comparison of using different gasification agents. Energy Convers. Manag. 2018, 159, 30–41. [Google Scholar] [CrossRef]
- Tezer, Ö.; Karabağ, N.; Öngen, A.; Çolpan, C.Ö.; Ayol, A. Biomass gasification for sustainable energy production: A review. Int. J. Hydrogen Energy 2022, 47, 15419–15433. [Google Scholar] [CrossRef]
- Akhlaghi, N.; Najafpour-Darzi, G. A comprehensive review on biological hydrogen production. Int. J. Hydrogen Energy 2020, 45, 22492–22512. [Google Scholar] [CrossRef]
- Xu, X.; Zhou, Q.; Yu, D. The future of hydrogen energy: Bio-hydrogen production technology. Int. J. Hydrogen Energy 2022, 47, 33677–33698. [Google Scholar] [CrossRef]
- Razi, F.; Dincer, I.; Gabriel, K. Exergoenvironmental analysis of the integrated copper-chlorine cycle for hydrogen production. Energy 2021, 226, 120426. [Google Scholar] [CrossRef]
- Strušnik, D.; Avsec, J. Exergoeconomic machine-learning method of integrating a thermochemical Cu–Cl cycle in a multigeneration combined cycle gas turbine for hydrogen production. Int. J. Hydrogen Energy 2022, 47, 17121–17149. [Google Scholar] [CrossRef]
- Myrzakhmetov, B.; Akhmetova, A.; Bissenbay, A.; Karibayev, M.; Pan, X.; Wang, Y.; Bakenov, Z.; Mentbayeva, A. Chitosan-based biopolymers for anion-exchange membrane fuel cell application. R. Soc. Open Sci. 2023, 10, 230843. [Google Scholar] [CrossRef]
- Song, H.; Luo, S.; Huang, H.; Deng, B.; Ye, J. Solar-driven hydrogen production: Recent advances, challenges, and future perspectives. ACS Energy Lett. 2022, 7, 1043–1065. [Google Scholar] [CrossRef]
- Takeda, S.; Nam, H.; Chapman, A. Low-carbon energy transition with the sun and forest: Solar-driven hydrogen production from biomass. Int. J. Hydrogen Energy 2022, 47, 24651–24668. [Google Scholar] [CrossRef]
- Lim, Y.; Lee, D.-K.; Kim, S.M.; Park, W.; Cho, S.Y.; Sim, U. Low Dimensional Carbon-Based Catalysts for Efficient Photocatalytic and Photo/Electrochemical Water Splitting Reactions. Materials 2020, 13, 114. [Google Scholar] [CrossRef]
- Eidsvåg, H.; Bentouba, S.; Vajeeston, P.; Yohi, S.; Velauthapillai, D. TiO2 as a Photocatalyst for Water Splitting—An Experimental and Theoretical Review. Molecules 2021, 26, 1687. [Google Scholar] [CrossRef]
- Nadeem, M.A.; Khan, M.A.; Ziani, A.A.; Idriss, H. An Overview of the Photocatalytic Water Splitting over Suspended Particles. Catalysts 2021, 11, 60. [Google Scholar] [CrossRef]
- Arunachalam, P.; Nagai, K.; Amer, M.S.; Ghanem, M.A.; Ramalingam, R.J.; Al-Mayouf, A.M. Recent Developments in the Use of Heterogeneous Semiconductor Photocatalyst Based Materials for a Visible-Light-Induced Water-Splitting System—A Brief Review. Catalysts 2021, 11, 160. [Google Scholar] [CrossRef]
- Tian, L.; Guan, X.; Zong, S.; Dai, A.; Qu, J. Cocatalysts for Photocatalytic Overall Water Splitting: A Mini Review. Catalysts 2023, 13, 355. [Google Scholar] [CrossRef]
- Jakhar, M.; Kumar, A.; Ahluwalia, P.K.; Tankeshwar, K.; Pandey, R. Engineering 2D Materials for Photocatalytic Water-Splitting from a Theoretical Perspective. Materials 2022, 15, 2221. [Google Scholar] [CrossRef]
- Dauletbekova, A.; Abuova, F.; Piskunov, S. First-principles modeling of the H color centers in MgF2 crystals. Phys. Status Solidi C 2012, 10, 160–164. [Google Scholar] [CrossRef]
- Abuova, F.U.; Kotomin, E.A.; Lisitsyn, V.M.; Akilbekov, A.T.; Piskunov, S. Ab initio modeling of radiation damage in MgF2 crystals. Nucl. Instrum. Methods Phys. Res. B 2014, 326, 314–317. [Google Scholar] [CrossRef]
- Abuova, A.U.; Mastrikov, Y.A.; Kotomin, E.A.; Kawazoe, Y.; Inerbaev, T.M.; Akilbekov, A.T. First principles modeling of Ag adsorption on the LaMnO3 (001) surfaces. Solid State Ionics 2015, 273, 46–50. [Google Scholar] [CrossRef]
- Guo, S.; Lin, H.; Hu, J.; Su, Z.; Zhang, Y. Computational Study of Novel Semiconducting Sc2CT2 (T = F, Cl, Br) MXenes for Visible-Light Photocatalytic Water Splitting. Materials 2021, 14, 4739. [Google Scholar] [CrossRef]
- Wang, G.; Xie, W.; Guo, S.; Chang, J.; Chen, Y.; Long, X.; Zhou, L.; Ang, Y.S.; Yuan, H. Two-Dimensional GeC/MXY (M = Zr, Hf; X, Y = S, Se) Heterojunctions Used as Highly Efficient Overall Water-Splitting Photocatalysts. Molecules 2024, 29, 2793. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, H.; Li, Y.; Zhang, M.; Zheng, Y. Designing a 0D/1D S-Scheme Heterojunction of Cadmium Selenide and Polymeric Carbon Nitride for Photocatalytic Water Splitting and Carbon Dioxide Reduction. Molecules 2022, 27, 6286. [Google Scholar] [CrossRef]
- Yan, Y.; Chen, Z.; Cheng, X.; Shi, W. Research Progress of ZnIn2S4-Based Catalysts for Photocatalytic Overall Water Splitting. Catalysts 2023, 13, 967. [Google Scholar] [CrossRef]
- Zhang, H.; Liu, J.; Xu, T.; Ji, W.; Zong, X. Recent Advances on Small Band Gap Semiconductor Materials (≤2.1 eV) for Solar Water Splitting. Catalysts 2023, 13, 728. [Google Scholar] [CrossRef]
- Morante, N.; Folliero, V.; Dell’Annunziata, F.; Capuano, N.; Mancuso, A.; Monzillo, K.; Galdiero, M.; Sannino, D.; Franci, G. Characterization and Photocatalytic and Antibacterial Properties of Ag- and TiOx-Based (x = 2, 3) Composite Nanomaterials under UV Irradiation. Materials 2024, 17, 2178. [Google Scholar] [CrossRef]
- Zhao, Z.; Li, R.; Zhao, C.; Pei, J. A Study on the Effect of Conductive Particles on the Performance of Road-Suitable Barium Titanate/Polyvinylidene Fluoride Composite Materials. Materials 2025, 18, 1185. [Google Scholar] [CrossRef]
- Mamani Flores, E.; Vera Barrios, B.S.; HuillcaHuillca, J.C.; Chacaltana García, J.A.; Polo Bravo, C.A.; Nina Mendoza, H.E.; Quispe Cohaila, A.B.; Gamarra Gómez, F.; Tamayo Calderón, R.M.; Fora Quispe, G.d.L.; et al. Cr3+ Doping Effects on Structural, Optical, and Morphological Characteristics of BaTiO3 Nanoparticles and Their Bioactive Behavior. Crystals 2024, 14, 998. [Google Scholar] [CrossRef]
- Abidin, M.Z.U.; Ikram, M.; Moeen, S.; Nazir, G.; Kanoun, M.B.; Goumri-Said, S. A comprehensive review on the synthesis of ferrite nanomaterials via bottom-up and top-down approaches: Advantages, disadvantages, characterizations, and computational insights. Coord. Chem. Rev. 2024, 520, 216158. [Google Scholar] [CrossRef]
- Jiang, Q.; Cui, X.F.; Zhao, M. Size effects on Curie temperature of ferroelectric particles. Appl. Phys. A Mater. Sci. Process. 2004, 78, 703–704. [Google Scholar] [CrossRef]
- Sood, A.; Desseigne, M.; Dev, A.; Maurizi, L.; Kumar, A.; Millot, N.; Han, S.S. A Comprehensive Review on Barium Titanate Nanoparticles as a Persuasive Piezoelectric Material for Biomedical Applications: Prospects and Challenges. Small 2023, 19, e2206401. [Google Scholar] [CrossRef] [PubMed]
- Lang, X.Y.; Jiang, Q. Size and interface effects on Curie temperature of perovskite ferroelectric nanosolids. J. Nanopart. Res. 2007, 9, 595–603. [Google Scholar] [CrossRef]
- Wada, S.; Hoshina, T.; Yasuno, H.; Ohishi, M.; Kakemoto, H.; Tsurumi, T.; Yashima, M. Size Effect of Dielectric Properties for Barium Titanate Particles and Its Model. Key Eng. Mater. 2006, 301, 27–30. [Google Scholar] [CrossRef]
- Chakraborty, A.; Liton, M.; Sarker, M.; Rahman, M.; Khan, M. A comprehensive DFT evaluation of catalytic and optoelectronic properties of BaTiO3 polymorphs. Phys. B Condens. Matter 2023, 648, 414418. [Google Scholar] [CrossRef]
- Navas, D.; Fuentes, S.; Castro-Alvarez, A.; Chavez-Angel, E. Review on Sol-Gel Synthesis of Perovskite and Oxide Nanomaterials. Gels 2021, 7, 275. [Google Scholar] [CrossRef]
- Lu, W.; Quilitz, M.; Schmidt, H. Nanoscaled BaTiO3 powders with a large surface area synthesized by precipitation from aqueous solutions: Preparation, characterization and sintering. J. Eur. Ceram. Soc. 2007, 27, 3149–3159. [Google Scholar] [CrossRef]
- Suherman, B.; Nurosyid, F.; Khairuddin; Sandi, D.K.; Irian, Y. Impacts of low sintering temperature on microstructure, atomic bonds, and dielectric constant of barium titanate (BaTiO3) prepared by co-precipitation technique. J. Phys. Conf. Ser. 2022, 2190, 012006. [Google Scholar] [CrossRef]
- Hayashi, H.; Hakuta, Y. Hydrothermal Synthesis of Metal Oxide Nanoparticles in Supercritical Water. Materials 2010, 3, 3794–3817. [Google Scholar] [CrossRef] [PubMed]
- Khort, A.A.; Podbolotov, K.B. Preparation of BaTiO3 nanopowders by the solution combustion method. Ceram. Int. 2016, 42, 15343–15348. [Google Scholar] [CrossRef]
- Choi, G.J.; Kim, H.S.; Cho, Y.S. BaTiO3 particles prepared by microwave-assisted hydrothermal reaction using titanium acylate precursors. Mater. Lett. 1999, 41, 122–127. [Google Scholar] [CrossRef]
- Buscaglia, V.; Buscaglia, M.T.; Canu, G. BaTiO3-Based Ceramics: Fundamentals, Properties and Applications. In Encyclopedia of Materials: Technical Ceramics and Glasses; Elsevier: Amsterdam, The Netherlands, 2021; pp. 311–344. ISBN 9780128222331. [Google Scholar]
- Ramakanth, S.; James Raju, K.C. Band gap narrowing in BaTiO3 nanoparticles facilitated by multiple mechanisms. J. Appl. Phys. 2014, 115, 173507. [Google Scholar] [CrossRef]
- Tewatia, K.; Sharma, A.; Sharma, M.; Kumar, A. Factors affecting morphological and electrical properties of Barium Titanate: A brief review. Mater. Today Proc. 2021, 44, 4548–4556. [Google Scholar] [CrossRef]
- Kumar, A.; Gori, Y.; Kumar, A.; Meena, C.S.; Dutt, N. (Eds.) Advanced Materials for Biomedical Applications, 1st ed.; Taylor and Francis: Boca Raton, FL, USA, 2023; ISBN 9781032356068. [Google Scholar]
- Benyoussef, M.; Mura, T.; Saitzek, S.; Azrour, F.; Blach, J.-F.; Lahmar, A.; Gagou, Y.; El Marssi, M.; Sayede, A.; Jouiad, M. Nanostructured BaTi1−xSnxO3 ferroelectric materials for electrocaloric applications and energy performance. Curr. Appl. Phys. 2022, 38, 59–66. [Google Scholar] [CrossRef]
- Qiao, L.; Bi, X. Microstructure and grain size dependence of ferroelectric properties of BaTiO3 thin films on LaNiO3 buffered Si. J. Eur. Ceram. Soc. 2009, 29, 1995–2001. [Google Scholar] [CrossRef]
- Buscaglia, M.T.; Buscaglia, V.; Viviani, M.; Nanni, P.; Hanuskova, M. Influence of foreign ions on the crystal structure of BaTiO3. J. Eur. Ceram. Soc. 2000, 20, 1997–2007. [Google Scholar] [CrossRef]
- Khedhri, M.H.; Abdelmoula, N.; Khemakhem, H.; Douali, R.; Dubois, F. Structural, spectroscopic and dielectric properties of Ca-doped BaTiO3. Appl. Phys. A Mater. Sci. Process. 2019, 125, 193. [Google Scholar] [CrossRef]
- Da Lu, Y.; Han, D.D.; Liu, Q.L.; Wang, Y.D.; Sun, X.Y. Structure and Dielectric Properties of Ce and Ca Co-Doped BaTiO3 Ceramics. Key Eng. Mater. 2016, 680, 184–188. [Google Scholar] [CrossRef]
- Rached, A.; Wederni, M.A.; Belkahla, A.; Dhahri, J.; Khirouni, K.; Alaya, S.; Martín-Palma, R.J. Effect of doping in the physico-chemical properties of BaTiO3 ceramics. Phys. B Condens. Matter 2020, 596, 412343. [Google Scholar] [CrossRef]
- Banerjee, T.; Balasubramanian, G. Predictive Modeling of Molecular Mechanisms in Hydrogen Production and Storage Materials. Materials 2023, 16, 6050. [Google Scholar] [CrossRef]
- Inerbaev, T.M.; Abuova, A.U.; Zakiyeva, Z.Y.; Abuova, F.U.; Mastrikov, Y.A.; Sokolov, M.; Gryaznov, D.; Kotomin, E.A. Effect of Rh doping on optical absorption and oxygen evolution reaction activity on BaTiO3 (001) surfaces. Molecules 2024, 29, 2707. [Google Scholar] [CrossRef]
- Chen, L.; Yu, Z.; Ji, X.; Huang, R.; Luo, L.; Tang, Z.; Zhang, Y. Insight into the effect of pH on the ferroelectric polarization field applied in photoelectrochemical water oxidation. Mater. Sci. Semicond. Process. 2022, 147, 106729. [Google Scholar] [CrossRef]
- Boonpalit, K.; Artrith, N. Mechanistic Insights into the Oxygen Evolution Reaction on Nickel-Doped Barium Titanate via Machine Learning-Accelerated Simulations. arXiv 2024, arXiv:2412.15452. [Google Scholar] [CrossRef]
- Bradley, R.; Radhakrishnan, R. Coarse-Grained Models for Protein-Cell Membrane Interactions. Polymers 2013, 5, 890–936. [Google Scholar] [CrossRef]
- Ren, H.; Yang, J.; Yang, W.; Zhong, H.; Lin, J.; Radjenovic, P.M.; Sun, L.; Zhang, H.; Xu, J.; Tian, Z.; et al. Core–Shell–Satellite plasmonic photocatalyst for broad-spectrum photocatalytic water splitting. ACS Mater. Lett. 2020, 3, 69–76. [Google Scholar] [CrossRef]
- Goga, N.; Mayrhofer, L.; Tranca, I.; Nedea, S.; Heijmans, K.; Ponnuchamy, V.; Vasilateanu, A. A Review of Recent Developments in Molecular Dynamics Simulations of the Photoelectrochemical Water Splitting Process. Catalysts 2021, 11, 807. [Google Scholar] [CrossRef]
- Abdikarimova, U.; Bissenova, M.; Matsko, N.; Issadykov, A.; Khromushin, I.; Aksenova, T.; Munasbayeva, K.; Slyamzhanov, E.; Serik, A. Visible Light-Driven Photocatalysis of Al-Doped SrTiO3: Experimental and DFT Study. Molecules 2024, 29, 5326. [Google Scholar] [CrossRef] [PubMed]
- Eglitis, R.I.; Piskunov, S.; Popov, A.I.; Purans, J.; Bocharov, D.; Jia, R. Systematic Trends in Hybrid-DFT Computations of BaTiO3/SrTiO3, PbTiO3/SrTiO3 and PbZrO3/SrZrO3 (001) Hetero Structures. Condens. Matter 2022, 7, 70. [Google Scholar] [CrossRef]
- Eglitis, R.I.; Purans, J.; Jia, R. Comparative Hybrid Hartree-Fock-DFT Calculations of WO2-Terminated Cubic WO3 as Well as SrTiO3, BaTiO3, PbTiO3 and CaTiO3 (001) Surfaces. Crystals 2021, 11, 455. [Google Scholar] [CrossRef]
- Sikam, P.; Thirayatorn, R.; Kaewmaraya, T.; Thongbai, P.; Moontragoon, P.; Ikonic, Z. Improved Thermoelectric Properties of SrTiO3 via (La, Dy and N) Co-Doping: DFT Approach. Molecules 2022, 27, 7923. [Google Scholar] [CrossRef] [PubMed]
- Eglitis, R.I.; Jia, R. Review of Systematic Tendencies in (001), (011) and (111) Surfaces Using B3PW as Well as B3LYP Computations of BaTiO3, CaTiO3, PbTiO3, SrTiO3, BaZrO3, CaZrO3, PbZrO3 and SrZrO3 Perovskites. Materials 2023, 16, 7623. [Google Scholar] [CrossRef]
- Jouybar, S.; Naji, L.; Sarabadani Tafreshi, S.; de Leeuw, N.H. A Density Functional Theory Study of the Physico-Chemical Properties of Alkali Metal Titanate Perovskites for Solar Cell Applications. Molecules 2024, 29, 3355. [Google Scholar] [CrossRef] [PubMed]
- Elegbeleye, I.F.; Maluta, N.E.; Maphanga, R.R. Density Functional Theory Study of Optical and Electronic Properties of (TiO2)n=5,8,68 Clusters for Application in Solar Cells. Molecules 2021, 26, 955. [Google Scholar] [CrossRef]
- Yan, Z.; Zhang, Y.; Kang, W.; Deng, N.; Pan, Y.; Sun, W.; Ni, J.; Kang, X. TiO2 Gas Sensors Combining Experimental and DFT Calculations: A Review. Nanomaterials 2022, 12, 3611. [Google Scholar] [CrossRef]
- Gustavsen, K.R.; Feng, T.; Huang, H.; Li, G.; Narkiewicz, U.; Wang, K. DFT Calculation of Carbon-Doped TiO2 Nanocomposites. Materials 2023, 16, 6117. [Google Scholar] [CrossRef]
- Wang, J.; Zhou, T.; Zhang, Y.; Chen, S.; Bai, J.; Li, J.; Zhou, B. The design of high-performance photoanode of CQDs/TiO2/WO3 based on DFT alignment of lattice parameter and energy band, and charge distribution. J. Colloid Interface Sci. 2021, 600, 828–837. [Google Scholar] [CrossRef]
- Thongyong, N.; Chanlek, N.; Srepusharawoot, P.; Takesada, M.; Cann, D.P.; Thongbai, P. Experimental study and DFT calculations of improved giant dielectric properties of Ni2⁺/Ta5⁺ co-doped TiO2 by engineering defects and internal interfaces. J. Eur. Ceram. Soc. 2022, 42, 4944–4952. [Google Scholar] [CrossRef]
- Amrhar, O.; Lee, H.S.; Lgaz, H.; Berisha, A.; Ebenso, E.E.; Cho, Y. Computational insights into the adsorption mechanisms of anionic dyes on the rutile TiO2 (110) surface: Combining SCC-DFT tight binding with quantum chemical and molecular dynamics simulations. J. Mol. Liq. 2023, 377, 121554. [Google Scholar] [CrossRef]
- Zeng, Z.; Wodaczek, F.; Liu, K.; Stein, F.; Hutter, J.; Chen, J.; Cheng, B. Mechanistic insight on water dissociation on pristine low-index TiO2 surfaces from machine learning molecular dynamics simulations. Nat. Commun. 2023, 14, 6131. [Google Scholar] [CrossRef]
- Boboriko, N.E.; Dzichenka, Y.U. Molecular dynamics simulation as a tool for prediction of the properties of TiO2 and TiO2: MoO3-based chemical gas sensors. J. Alloys Compd. 2021, 855, 157490. [Google Scholar] [CrossRef]
- Raffaini, G. Surface chemistry, crystal structure, size, and topography role in the albumin adsorption process on TiO2 anatase crystallographic faces and its 3D-nanocrystal: A molecular dynamics study. Coatings 2021, 11, 420. [Google Scholar] [CrossRef]
- Maleki, F.; Di Liberto, G.; Pacchioni, G. pH-and facet-dependent surface chemistry of TiO2 in aqueous environment from first principles. ACS Appl. Mater. Interfaces 2023, 15, 11216–11224. [Google Scholar] [CrossRef]
- Nosaka, Y. Water Photo-Oxidation over TiO2—History and Reaction Mechanism. Catalysts 2022, 12, 1557. [Google Scholar] [CrossRef]
- Estévez Ruiz, E.P.; Lago, J.L.; Thirumuruganandham, S.P. Experimental Studies on TiO2 NT with Metal Dopants through Co-Precipitation, Sol–Gel, Hydrothermal Scheme and Corresponding Computational Molecular Evaluations. Materials 2023, 16, 3076. [Google Scholar] [CrossRef]
- Kydyrbay, N.; Zhazitov, M.; Abdullah, M.; Duisebayev, T.; Tezekbay, Y.; Aldongarov, A.; Karibayev, M.; Nuraje, N.; Toktarbaiuly, O. Structural, surface, and theoretical investigation of hydrophobic-modified nanodiamond powders. Scientific Reports 2025, 15, 24329. [Google Scholar] [CrossRef]
- Yang, M.; Bonati, L.; Polino, D.; Parrinello, M. Using metadynamics to build neural network potentials for reactive events: The case of urea decomposition in water. Catal. Today 2022, 387, 143–149. [Google Scholar] [CrossRef]
- Ismagambetov, O.; Aldiyarov, N.; Almas, N.; Irgibaeva, I.; Baitassova, Z.; Piskunov, S.; Aldongarov, A.; Abdirashev, O. Atomistic Modeling of Natural Gas Desulfurization Process Using Task-Specific Deep Eutectic Solvents Supported by Graphene Oxide. Molecules 2024, 29, 5282. [Google Scholar] [CrossRef]
- Nulimu, A.; Aldongarov, A.; Sarsenova, S.; Ibrayeva, A.; Karibayev, M. Unraveling the Role of Functional Groups in Polyaniline for Ammonia Sensing: A Theoretical Approach. Engineered Science 2025, 36, 1616. [Google Scholar] [CrossRef]
- Yao, N.; Chen, X.; Fu, Z.H.; Zhang, Q. Applying classical, ab initio, and machine-learning molecular dynamics simulations to the liquid electrolyte for rechargeable batteries. Chem. Rev. 2022, 122, 10970–11021. [Google Scholar] [CrossRef]
- Chen, Y.; Liu, Y.; Xu, Y.; Guo, X.; Cao, Y.; Ming, W. Review: Modeling and Simulation of Membrane Electrode Material Structure for Proton Exchange Membrane Fuel Cells. Coatings 2022, 12, 1145. [Google Scholar] [CrossRef]
- Samantaray, S.; Mohanty, D.; Satpathy, S.K.; Hung, I.-M. Exploring Recent Developments in Graphene-Based Cathode Materials for Fuel Cell Applications: A Comprehensive Overview. Molecules 2024, 29, 2937. [Google Scholar] [CrossRef]
- Shah, D.; Karibayev, M.; Adotey, E.K.; Amouei Torkmahalleh, M. Impact of Volatile Organic Compounds on Chromium Containing Atmospheric Particulate: Insights from Molecular Dynamics Simulations. Sci. Rep. 2020, 10, 17387. [Google Scholar] [CrossRef]
- Wang, W.; Qu, Z.; Wang, X.; Zhang, J. A Molecular Model of PEMFC Catalyst Layer: Simulation on Reactant Transport and Thermal Conduction. Membranes 2021, 11, 148. [Google Scholar] [CrossRef]
- Chen, X.; Hou, W.; Zhai, F.; Cheng, J.; Yuan, S.; Li, Y.; Wang, N.; Zhang, L.; Ren, J. Reversible Hydrogen Storage Media by g-CN Monolayer Decorated with NLi4: A First-Principles Study. Nanomaterials 2023, 13, 647. [Google Scholar] [CrossRef]
- Li, Q.; Li, Q.; Wang, F.; Wu, J.; Wang, Y. The Carrying Behavior of Water-Based Fracturing Fluid in Shale Reservoir Fractures and Molecular Dynamics of Sand-Carrying Mechanism. Processes 2024, 12, 2051. [Google Scholar] [CrossRef]
- Karibayev, M.; Shah, D. Comprehensive Computational Analysis Exploring the Formation of Caprolactam-Based Deep Eutectic Solvents and Their Applications in Natural Gas Desulfurization. Energy Fuels 2020, 34, 9894–9902. [Google Scholar] [CrossRef]
- Shelyapina, M.G. Hydrogen Diffusion on, into and in Magnesium Probed by DFT: A Review. Hydrogen 2022, 3, 285–302. [Google Scholar] [CrossRef]
- Mutisya, S.M.; Kalinichev, A.G. Carbonation reaction mechanisms of portlandite predicted from enhanced Ab Initio molecular dynamics simulations. Minerals 2021, 11, 509. [Google Scholar] [CrossRef]
- Kohmuean, P.; Inthomya, W.; Wongkoblap, A.; Tangsathitkulchai, C. Monte Carlo Simulation and Experimental Studies of CO2, CH4 and Their Mixture Capture in Porous Carbons. Molecules 2021, 26, 2413. [Google Scholar] [CrossRef]
- Torkmahalleh, M.A.; Karibayev, M.; Konakbayeva, D.; Fyrillas, M.M.; Rule, A.M. Aqueous Chemistry of Airborne Hexavalent Chromium during Sampling. Air Qual. Atmos. Health 2018, 11, 1059–1068. [Google Scholar] [CrossRef]
- Dong, K.; Niu, Z.; Kong, S.; Jia, B. Impact of Supercritical Carbon Dioxide on Pore Structure and Gas Transport in Bituminous Coal: An Integrated Experiment and Simulation. Molecules 2025, 30, 1200. [Google Scholar] [CrossRef] [PubMed]
- Filipe, H.A.L.; Loura, L.M.S. Molecular Dynamics Simulations: Advances and Applications. Molecules 2022, 27, 2105. [Google Scholar] [CrossRef]
- Smith, A.; Dong, X.; Raghavan, V. An Overview of Molecular Dynamics Simulation for Food Products and Processes. Processes 2022, 10, 119. [Google Scholar] [CrossRef]
- Celik, I.; Yadav, R.; Duzgun, Z.; Albogami, S.; El-Shehawi, A.M.; Fatimawali; Idroes, R.; Tallei, T.E.; Emran, T.B. Interactions of the Receptor Binding Domain of SARS-CoV-2 Variants with hACE2: Insights from Molecular Docking Analysis and Molecular Dynamic Simulation. Biology 2021, 10, 880. [Google Scholar] [CrossRef]
- SdfLiu, W.D.; Yu, Y.; Dargusch, M.; Liu, Q.; Chen, Z.G. Carbon allotrope hybrids advance thermoelectric development and applications. Renew. Sustain. Energy Rev. 2021, 141, 110800. [Google Scholar] [CrossRef]
- Zhang, Y.Q.; Liu, Y.J.; Liu, Y.L.; Zhao, J.X. Boosting sensitivity of Boron Nitride Nanotube (BNNT) to nitrogen dioxide by Fe encapsulation. J. Mol. Graph. Model. 2014, 51, 1–6. [Google Scholar] [CrossRef]
- Rodríguez-Quintana, R.; Carbajal-Franco, G.; Rojas-Chávez, H. DFT study of the H2 molecules adsorption on pristine and Ni doped graphite surfaces. Mater. Lett. 2021, 293, 129660. [Google Scholar] [CrossRef]
- Zhang, W.-S.; Liu, Y.-T.; Yao, T.-T.; Wu, G.-P.; Liu, Q. Oxygen defect engineering toward the length-selective tailoring of carbon nanotubes via a two-step electrochemical strategy. J. Phys. Chem. C 2020, 124, 27097–27106. [Google Scholar] [CrossRef]
- Elias, A.; Uddin, N.; Hossain, A.; Saha, J.K.; Siddiquey, I.A.; Sarker, D.R.; Diba, Z.R.; Uddin, J.; Choudhury, M.H.R.; Firoz, S.H. An experimental and theoretical study of the effect of Ce doping in ZnO/CNT composite thin film with enhanced visible light photo-catalysis. Int. J. Hydrogen Energy 2019, 44, 20068–20078. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, H.; Zhang, Z.; Jia, X.; An, L. CO adsorption on Fe-doped vacancy-defected CNTs–A DFT study. Chem. Phys. Lett. 2019, 730, 316–320. [Google Scholar] [CrossRef]
- Myrzakhmetov, B.; Karibayev, M.; Wang, Y.; Mentbayeva, A. Density functional theory investigation of intermolecular interactions for hydrogen-bonded deep eutectic solvents. Eurasian Chem.-Technol. J. 2024, 26, 29–36. [Google Scholar] [CrossRef]
- Xu, J.; Wan, Q.; Anpo, M.; Lin, S. Bandgap opening of graphdiyne monolayer via B, N-codoping for photocatalytic overall water splitting: Design strategy from DFT studies. J. Phys. Chem. C 2020, 124, 6624–6633. [Google Scholar] [CrossRef]
- Loh, G.; Pandey, R.; Yap, Y.K.; Karna, S.P. MoS2 quantum dot: Effects of passivation, additional layer, and h-BN substrate on its stability and electronic properties. J. Phys. Chem. C 2015, 119, 1565–1574. [Google Scholar] [CrossRef]
- Pandey, D.; Kumar, A.; Chakrabarti, A.; Pandey, R. Stacking-dependent electronic properties of aluminene based multilayer van der Waals heterostructures. Comput. Mater. Sci. 2020, 185, 109952. [Google Scholar] [CrossRef]
- Lin, Y.-P.; Bocharov, D.; Isakoviča, I.; Pankratov, V.; Popov, A.A.; Popov, A.I.; Piskunov, S. Chlorine Adsorption on TiO2(110)/Water Interface: Nonadiabatic Molecular Dynamics Simulations for Photocatalytic Water Splitting. Electron. Mater. 2023, 4, 33–48. [Google Scholar] [CrossRef]
- Tada, K.; Sakata, K.; Yamada, S.; Okazaki, K.; Kitagawa, Y.; Kawakami, T.; Yamanaka, S.; Okumura, M. DFT calculations for Au adsorption onto a reduced TiO2 (110) surface with the coexistence of Cl. Mol. Phys. 2014, 112, 365–378. [Google Scholar] [CrossRef]
- Li, Y.; Gao, D.; Peng, S.; Lu, G.; Li, S. Photocatalytic hydrogen evolution over Pt/Cd0.5Zn0.5S from saltwater using glucose as electron donor: An investigation of the influence of electrolyte NaCl. Int. J. Hydrogen Energy 2011, 36, 4291–4297. [Google Scholar] [CrossRef]
- Alghamdi, H.; Idriss, H. Study of the modes of adsorption and electronic structure of hydrogen peroxide and ethanol over TiO2 rutile (110) surface within the context of water splitting. Surf. Sci. 2018, 669, 103–113. [Google Scholar] [CrossRef]
- Vu, N.H.; Le, H.V.; Cao, T.M.; Pham, V.V.; Le, H.M.; Nguyen-Manh, D. Anatase–rutile phase transformation of titanium dioxide bulk material: A DFT+U approach. J. Phys. Condens. Matter 2012, 24, 405501. [Google Scholar] [CrossRef]
- Kolesov, G.; Grånäs, O.; Hoyt, R.; Vinichenko, D.; Kaxiras, E. Real–time TD–DFT with classical ion dynamics: Methodology and applications. J. Chem. Theory Comput. 2016, 12, 466–476. [Google Scholar] [CrossRef]
- You, P.; Chen, D.; Lian, C.; Zhang, C.; Meng, S. First–principles dynamics of photoexcited molecules and materials towards a quantum description. Wiley Interdiscip. Rev. Comput. Mol. Sci. 2021, 11, e1492. [Google Scholar] [CrossRef]
- Pham, T.A.; Ping, Y.; Galli, G. Modelling heterogeneous interfaces for solar water splitting. Nat. Mater. 2017, 16, 401–408. [Google Scholar] [CrossRef]
- Agosta, L.; Brandt, E.G.; Lyubartsev, A.P. Diffusion and reaction pathways of water near fully hydrated TiO2 surfaces from ab initio molecular dynamics. J. Chem. Phys. 2017, 147, 024704. [Google Scholar] [CrossRef]
- Balzaretti, F.; Gupta, V.; Ciacchi, L.C.; Aradi, B.; Frauenheim, T.; Köppen, S. Water reactions on reconstructed rutile TiO2: A density functional theory/density functional tight binding approach. J. Phys. Chem. C 2021, 125, 13234–13246. [Google Scholar] [CrossRef]
- Gao, M.; Connor, P.K.N.; Ho, G.W. Plasmonic photothermic directed broadband sunlight harnessing for seawater catalysis and desalination. Energy Environ. Sci. 2016, 9, 3151–3160. [Google Scholar] [CrossRef]
- Schilling, M.; Luber, S. Computational Modeling of Cobalt-Based Water Oxidation: Current Status and Future Challenges. Front. Chem. 2018, 6, 100. [Google Scholar] [CrossRef]
- VandeVondele, J.; Mohamed, F.; Krack, M.; Hutter, J.; Sprik, M.; Parrinello, M. The influence of temperature and density functional models in ab initio molecular dynamics simulation of liquid water. J. Chem. Phys. 2005, 122, 014515. [Google Scholar] [CrossRef]
- Sinha, V.; Govindarajan, N.; de Bruin, B.; Meijer, E.J. How Solvent Affects C–H Activation and Hydrogen Production Pathways in Homogeneous Ru-Catalyzed Methanol Dehydrogenation Reactions. ACS Catal. 2018, 8, 6908–6913. [Google Scholar] [CrossRef]
- Cheng, J.; Liu, X.; VandeVondele, J.; Sulpizi, M.; Sprik, M. Redox Potentials and Acidity Constants from Density Functional Theory Based Molecular Dynamics. Acc. Chem. Res. 2014, 47, 3522–3529. [Google Scholar] [CrossRef]
- Liu, X.; Jiang, B.; Liu, Y.; Liu, L.; Xia, T.; Zhang, X.; Ye, C.; Yu, Y.; Wang, B. Two-Dimensional As/BlueP van der Waals Hetero-Structure as a Promising Photocatalyst for Water Splitting: A DFT Study. Coatings 2020, 10, 1160. [Google Scholar] [CrossRef]
- Singh, A.K.; Mathew, K.; Zhuang, H.L.; Henning, R.G. Computational screening of 2D materials for photocatalysis. J. Phys. Chem. Lett. 2015, 6, 1087–1098. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.G.; Lv, Y.H.; Xu, J.; Liu, Y.F.; Zhang, R.Q.; Zhu, Y.F. A strategy of enhancing the photoactivity of g-C3N4 via doping of nonmetal elements: A first-principles study. J. Phys. Chem. C 2012, 116, 23485–23493. [Google Scholar] [CrossRef]
- Li, G.; Zhao, Y.C.; Zeng, S.M.; Ni, J. The realization of half-metal and spin-semiconductor for metal adatoms on arsenene. Appl. Surf. Sci. 2016, 390, 60–67. [Google Scholar] [CrossRef]
- Li, X.H.; Wang, B.J.; Cai, X.L.; Zhang, L.W.; Wang, G.D.; Ke, S.H. Tunable electronic properties of arsenene/GaS van der Waals heterostructures. RSC Adv. 2017, 7, 28393. [Google Scholar] [CrossRef]
- Fang, L.Z.; Li, X.P.; Geng, Z.D.; Wang, T.X.; Xia, C.X. Band alignment tuning in GeS/arsenene staggered hetero-structures. J. Alloy. Compd. 2019, 793, 283–288. [Google Scholar] [CrossRef]
- Jamdagni, P.; Thakur, A.; Kumar, A.; Ahluwalia, P.K.; Pandey, R. Two dimensional allotropes of arsenene with a wide range of high and anisotropic carrier mobility. Phys. Chem. Chem. Phys. 2018, 20, 29939. [Google Scholar] [CrossRef]
- Wang, B.J.; Li, X.H.; Cai, X.L.; Yu, W.Y.; Zhang, L.W.; Zhao, R.Q.; Ke, S.H. Blue Phosphorus/Mg(OH)2 van der Waals hetero-structures as Promising Visible-Light Photocatalysts for Water Splitting. J. Phys. Chem. C 2018, 122, 7075–7080. [Google Scholar] [CrossRef]
- Li, Q.F.; Ma, X.F.; Lei, Z.; Wan, X.G.; Rao, W.F. Theoretical design of blue phosphorene/arsenene lateral heterostructures with superior electronic properties. J. Phys. D Appl. Phys. 2018, 51, 255304. [Google Scholar] [CrossRef]
- Inerbaev, T.M.; Graupner, D.R.; Abuova, A.U.; Abuova, F.U.; Kilin, D.S. Optical properties of BaTiO3 at room temperature: DFT modelling. RSC Adv. 2025, 15, 5405–5412. [Google Scholar] [CrossRef]
- Ogunkunle, S.A.; Mortier, F.; Bouzid, A.; Hinsch, J.J.; Zhang, L.; Wu, Z.; Bernard, S.; Zhu, Y.; Wang, Y. Navigating Alkaline Hydrogen Evolution Reaction Descriptors for Electrocatalyst Design. Catalysts 2024, 14, 608. [Google Scholar] [CrossRef]
- Miran, H.A.; Jaf, Z.N.; Altarawneh, M.; Jiang, Z.-T. An Insight into Geometries and Catalytic Applications of CeO2 from a DFT Outlook. Molecules 2021, 26, 6485. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Chen, Y.; Chen, T.; Rajendran, S.; Zeng, Z.; Qin, J.; Zhang, X. A long-standing polarized electric field in TiO2@BaTiO3/CdS nanocomposite for effective photocatalytic hydrogen evolution. Fuel 2021, 314, 122758. [Google Scholar] [CrossRef]
- Cai, W.; Ma, X.; Chen, J.; Shi, R.; Wang, Y.; Yang, Y.; Jing, D.; Yuan, H.; Du, J.; Que, M. Synergy of oxygen vacancy and piezoelectricity effect promotes the CO2 photoreduction by BaTiO3. Appl. Surf. Sci. 2023, 619, 156773. [Google Scholar] [CrossRef]
- Wang, M.; Wang, C.; Liu, Y.; Zhou, X. Hybrid density functional theory description of non-metal doping in perovskite BaTiO3 for visible-light photocatalysis. J. Solid State Chem. 2019, 280, 121018. [Google Scholar] [CrossRef]
- Rizwan, M.; Hajra, N.; Zeba, I.; Shakil, M.; Gillani, S.; Usman, Z. Electronic, structural, and optical properties of BaTiO3 doped with lanthanum (La): Insight from DFT calculation. Optik 2020, 211, 164611. [Google Scholar] [CrossRef]
- Xu, H.; Wang, P.; Luan, S.; Cheng, L.; Fu, Z.; Cao, X.; Zhang, L.; Yu, S.; Sun, R. Vacancy engineering for high tetragonal BaTiO3 synthesized by solid-state approaches. Powder Technol. 2024, 444, 119955. [Google Scholar] [CrossRef]
- Xie, P.; Yang, F.; Li, R.; Ai, C.; Lin, C.; Lin, S. Improving hydrogen evolution activity of perovskite BaTiO3 with Mo doping: Experiments and first-principles analysis. Int. J. Hydrogen Energy 2019, 44, 11695–11704. [Google Scholar] [CrossRef]
- Usman, M.; Rehman, J.U.; Tahir, M.B.; Hussain, A. First-principles calculations to investigate the effect of Cs-doping in BaTiO3 for water-splitting application. Solid State Commun. 2022, 355, 114920. [Google Scholar] [CrossRef]
- Chun, H.; Lee, Y.; Kim, S.; Yoon, Y.; Kim, Y.; Park, S. Surface termination of BaTiO3(111) single crystal: A combined DFT and XPS study. Appl. Surf. Sci. 2021, 578, 152018. [Google Scholar] [CrossRef]
- Dahbi, S.; Tahiri, N.; Bounagui, O.E.; Ez-Zahraouy, H. Effects of oxygen group elements on thermodynamic stability, electronic structures, and optical properties of the pure and pressed BaTiO3 perovskite. Comput. Condens. Matter 2022, 32, e00728. [Google Scholar] [CrossRef]
- Dahbi, S.; Tahiri, N.; Bounagui, O.E.; Ez-Zahraouy, H. Electronic, optical, and thermoelectric properties of perovskite BaTiO3 compound under the effect of compressive strain. Chem. Phys. 2021, 544, 111105. [Google Scholar] [CrossRef]
- Fo, Y.; Zhou, X. A theoretical study on tetragonal BaTiO3 modified by surface co-doping for photocatalytic overall water splitting. Int. J. Hydrogen Energy 2022, 47, 19073–19085. [Google Scholar] [CrossRef]
- Jensen, S.J.; Inerbaev, T.M.; Abuova, A.U.; Kilin, D.S. Spin unrestricted nonradiative relaxation dynamics of cobalt-doped anatase nanowire. J. Phys. Chem. C 2017, 121, 16110–16125. [Google Scholar] [CrossRef]
- Bhat, D.K.; Bantawal, H.; Pi, U.; Shenoy, U.S. Enhanced photoresponse and efficient charge transfer in porous graphene-BaTiO3 nanocomposite for high-performance photocatalysis. Diam. Relat. Mater. 2023, 139, 110312. [Google Scholar] [CrossRef]
- Bashir, M.Z.; Naqvi, S.A.Z.; Naeem, M.A.; Munir, R.; Noreen, S. Theoretical study of optoelectronic, elastic, and mechanical properties of gallium-modified barium titanate (Ba1−xGaxTiO3) perovskite ceramics by DFT. Mater. Sci. Semicond. Process. 2024, 182, 108734. [Google Scholar] [CrossRef]
- Wang, S.; Ge, K.; Cui, H.; Li, S.; Yang, Y.; Pan, M.; Zhu, L. Self-polarization-enhanced oxygen evolution reaction by flower-like core–shell BaTiO3@NiFe-layered double hydroxide heterojunctions. Chem. Eng. J. 2023, 479, 147831. [Google Scholar] [CrossRef]
- Chen, G.; Ji, Y.; Shi, X.; An, P.; Zhang, J.; Li, Y.; Liu, S.F.; Yan, J. Oxygen-deficient BaTiO3 loading sub-nm PtOx for photocatalytic biological wastewater splitting to green hydrogen production. Chem. Eng. J. 2024, 496, 154261. [Google Scholar] [CrossRef]
- Guo, M.; Zhong, J.; Li, W.; Hou, H.; Bowen, C.R.; Zhan, X.; Yang, H.; Yang, M.; Chen, Z.; Chen, D.; et al. Highly efficient photocatalytic hydrogen evolution enabled by piezotronic effects in SrTiO3/BaTiO3 nanofiber heterojunctions. Nano Energy 2024, 127, 109745. [Google Scholar] [CrossRef]
- Zulfiqar, W.; Alay-e-Abbas, S.M. Improved thermodynamic stability and visible light absorption in Zr+X codoped (X = S, Se, and Te) BaTiO3 photocatalysts: A first-principles study. Mater. Today Commun. 2022, 32, 103867. [Google Scholar] [CrossRef]
- Kovač, I.; Mužević, M.; Pajtler, M.V.; Lukačević, I. Charge carrier dynamics across the metal oxide/BaTiO3 interfaces toward photovoltaic applications from the theoretical perspective. Surf. Interfaces 2023, 39, 102974. [Google Scholar] [CrossRef]
- Kaptagay, G.A.; Satanova, B.M.; Abuova, A.U.; Konuhova, M.; Zakiyeva, Z.; Tolegen, U.Z.; Koilyk, N.O.; Abuova, F.U. Effect of rhodium doping for photocatalytic activity of barium titanate. Opt. Mater. X 2025, 25, 100382. [Google Scholar] [CrossRef]
- Opoku, F.; Akoto, O.; Kwaansa-Ansah, E.E.; Asare-Donkor, N.K.; Adimado, A.A. Role of BaTiO3 crystal surfaces on the electronic properties, charge separation, and visible light–response of the most active (001) surface of LaAlO3: A hybrid density functional study. Chem. Phys. Impact 2023, 6, 100236. [Google Scholar] [CrossRef]
- Abbasi, P.; Barone, M.R.; Cruz-Jáuregui, M.d.l.P.; Valdespino-Padilla, D.; Paik, H.; Kim, T.; Kornblum, L.; Schlom, D.G.; Pascal, T.A.; Fenning, D.P. Ferroelectric Modulation of Surface Electronic States in BaTiO3 for Enhanced Hydrogen Evolution Activity. Nano Lett. 2022, 22, 4276–4284. [Google Scholar] [CrossRef]
- Gunawan, M.; Bowdler, O.; Zhou, S.; Fang, X.; Zhang, Q.; Sakamoto, Y.; Sun, K.; Gunawan, D.; Chang, S.L.; Amal, R.; et al. Ferroelectric Polarization-Induced Performance Enhancements in BiFeO3/BiVO4 Photoanodes for Photoelectrochemical Water Splitting. Adv. Funct. Mater. 2025, 35, 2417651. [Google Scholar] [CrossRef]
- Goumri-Said, S.; Kanoun, M.B. Insight into the Effect of Anionic–Anionic Co-Doping on BaTiO3 for Visible Light Photocatalytic Water Splitting: A First-Principles Hybrid Computational Study. Catalysts 2022, 12, 1672. [Google Scholar] [CrossRef]
- Chandrappa, S.; Galbao, S.J.; Krishnan, P.S.S.R.; Koshi, N.A.; Das, S.; Myakala, S.N.; Lee, S.; Dutta, A.; Cherevan, A.; Bhattacharjee, S.; et al. Iridium-Doping as a Strategy to Realize Visible-Light Absorption and P-Type Behavior in BaTiO3. J. Phys. Chem. C 2023, 127, 12383–12393. [Google Scholar] [CrossRef]
- Bhat, D.K.; Bantawal, H.; Shenoy, U.S. Rhodium Doping Augments Photocatalytic Activity of Barium Titanate: Effect of Electronic Structure Engineering. Nanoscale Adv. 2020, 2, 5688–5698. [Google Scholar] [CrossRef]
- Qiu, H.; Yang, T.; Zhou, J.; Yang, K.; Ying, Y.; Ding, K.; Yang, M.; Huang, H. Tunable Hydrogen Evolution Activity by Modulating Polarization States of Ferroelectric BaTiO3. J. Mater. Chem. A 2023, 11, 7034–7042. [Google Scholar] [CrossRef]
- Wang, Y.; Zhou, Q.; Zhang, Q.; Ren, Y.; Cui, K.; Cheng, C.; Wu, K. Effects of La-N Co-Doping of BaTiO3 on Its Electron-Optical Properties for Photocatalysis: A DFT Study. Molecules 2024, 29, 2250. [Google Scholar] [CrossRef]
- Saadon, N.M.Q.; Miran, H.A. Optoelectronic Tuning of Barium Titanate Doped with Pt: A Systematic First-Principles Study. Pap. Phys. 2024, 16, 160002. [Google Scholar] [CrossRef]
- Sharma, D.; Upadhyay, S.; Satsangi, V.R.; Shrivastav, R.; Waghmare, U.V.; Dass, S. Nanostructured BaTiO3/Cu2O Heterojunction with Improved Photoelectrochemical Activity for H2 Evolution: Experimental and First-Principles Analysis. Appl. Catal. B Environ. 2016, 189, 75–85. [Google Scholar] [CrossRef]
- Inerbaev, T.; Akilbekov, A.; Kenbayev, D.; Dauletbekova, A.; Shalaev, A.; Polisadova, E.; Konuhova, M.; Piskunov, S.; Popov, A.I. Color Centers in BaFBr Crystals: Experimental Study and Theoretical Modeling. Materials 2024, 17, 3340. [Google Scholar] [CrossRef]
- Tymińska, N.; Wu, G.; Dupuis, M. Water Oxidation on Oxygen-Deficient Barium Titanate: A First-Principles Study. J. Phys. Chem. C 2017, 121, 8378–8389. [Google Scholar] [CrossRef]
- Fan, X.T.; Wen, X.J.; Zhuang, Y.B.; Cheng, J. Molecular insight into the GaP (110)-water interface using machine learning accelerated molecular dynamics. J. Energy Chem. 2023, 82, 239–247. [Google Scholar] [CrossRef]
- Miao, L.; Jia, W.; Cao, X.; Jiao, L. Computational chemistry for water-splitting electrocatalysis. Chem. Soc. Rev. 2024, 53, 2771–2807. [Google Scholar] [CrossRef]
- Zhang, M.; Hou, Y.C.; Jiang, Y.; Ni, X.; Wang, Y.; Zou, X. Rational design of water splitting electrocatalysts through computational insights. Chem. Commun. 2024, 60, 14521–14536. [Google Scholar] [CrossRef]
- Orhan, I.B.; Zhao, Y.; Babarao, R.; Thornton, A.W.; Le, T.C. Machine Learning Descriptors for CO2 Capture Materials. Molecules 2025, 30, 650. [Google Scholar] [CrossRef]
- Ma, K.; Yang, C.; Zhang, J.; Li, Y.; Jiang, G.; Chai, J. Machine Learning-Assisted Hartree–Fock Approach for Energy Level Calculations in the Neutral Ytterbium Atom. Entropy 2024, 26, 962. [Google Scholar] [CrossRef]
- Tereshchenko, A.; Pashkov, D.; Guda, A.; Guda, S.; Rusalev, Y.; Soldatov, A. Adsorption Sites on Pd Nanoparticles Unraveled by Machine-Learning Potential with Adaptive Sampling. Molecules 2022, 27, 357. [Google Scholar] [CrossRef]
- Biswas, M.; Desai, R.; Mannodi-Kanakkithodi, A. Screening of novel halide perovskites for photocatalytic water splitting using multi-fidelity machine learning. Phys. Chem. Chem. Phys. 2024, 26, 23177–23188. [Google Scholar] [CrossRef]
- Allam, O.; Maghsoodi, M.; Jang, S.S.; Snow, S.D. Unveiling competitive adsorption in TiO2 photocatalysis through machine-learning-accelerated molecular dynamics, DFT, and experimental methods. ACS Appl. Mater. Interfaces 2024, 16, 36215–36223. [Google Scholar] [CrossRef]
- Agrawal, S.; Wang, B.; Wu, Y.; Casanova, D.; Prezhdo, O.V. Photocatalytic activity of dual defect modified graphitic carbon nitride is robust to tautomerism: Machine learning assisted ab initio quantum dynamics. Nanoscale 2024, 16, 8986–8995. [Google Scholar] [CrossRef]
- Gao, Y.; Zhang, Q.; Hu, W.; Yang, J. First-principles computational screening of two-dimensional polar materials for photocatalytic water splitting. ACS Nano 2024, 18, 19381–19390. [Google Scholar] [CrossRef]
- Raman, A.S.; Vojvodic, A. Providing atomistic insights into the dissolution of rutile oxides in electrocatalytic water splitting. J. Phys. Chem. C 2022, 126, 922–932. [Google Scholar] [CrossRef]
- Li, Z.; Wang, J.; Yang, C.; Liu, L.; Yang, J.Y. Thermal transport across TiO2–H2O interface involving water dissociation: Ab initio-assisted deep potential molecular dynamics. J. Chem. Phys. 2023, 159, 144701. [Google Scholar] [CrossRef]
- Jia, M.; Zhuang, Y.B.; Wang, F.; Zhang, C.; Cheng, J. Water-mediated proton hopping mechanisms at the SnO2 (110)/H2O interface from ab initio deep potential molecular dynamics. Precis. Chem. 2024, 2, 644–654. [Google Scholar] [CrossRef]
- Schienbein, P.; Blumberger, J. Data-Efficient Active Learning for Thermodynamic Integration: Acidity Constants of BiVO4 in Water. ChemPhysChem 2025, 26, e202400490. [Google Scholar] [CrossRef]
- Park, H.; Kim, Y.; Choi, S.; Kim, H.J. Data-driven computational design of stable oxygen evolution catalysts by DFT and machine learning: Promising electrocatalysts. J. Energy Chem. 2024, 91, 645–655. [Google Scholar] [CrossRef]
- Wang, W.; Zhang, M.; Li, X.; Zhang, S.; Yu, F.; Li, S.; Comini, E.; Wang, Z.L.; Ren, K. Boosting efficiency in piezo-photocatalysis process using POLED Ba0.7Sr0.3TiO3 nanorod arrays for pollutant degradation and hydrogen production. ACS Appl. Mater. Interfaces 2024, 16, 20497–20509. [Google Scholar] [CrossRef]
- Shao, Y.; de Ruiter, J.M.; de Groot, H.J.M.; Buda, F. Photocatalytic Water Splitting Cycle in a Dye-Catalyst Supramolecular Complex: Ab Initio Molecular Dynamics Simulations. J. Phys. Chem. C 2019, 123, 21403–21414. [Google Scholar] [CrossRef]
- Zhang, S.; Ye, H.; Hua, J.; Tian, H. Recent Advances in Dye-Sensitized Photoelectrochemical Cells for Water Splitting. EnergyChem 2019, 1, 100015. [Google Scholar] [CrossRef]
- Nada, H.; Kobayashi, M.; Kakihana, M. Anisotropy in Conformation and Dynamics of a Glycolate Ion Near the Surface of a TiO2 Rutile Crystal Between Its {001} and {110} Planes: A Molecular Dynamics Study. J. Phys. Chem. C 2016, 120, 6502–6514. [Google Scholar] [CrossRef]
- YazdanYar, A.; Aschauer, U.; Bowen, P. Interaction of Biologically Relevant Ions and Organic Molecules with Titanium Oxide (Rutile) Surfaces: A Review on Molecular Dynamics Studies. Colloids Surf. B Biointerfaces 2018, 161, 563–577. [Google Scholar] [CrossRef]
- Harmon, K.J.; Chen, Y.; Bylaska, E.J.; Catalano, J.G.; Bedzyk, M.J.; Weare, J.H.; Fenter, P. Insights on the Alumina–Water Interface Structure by Direct Comparison of Density Functional Simulations with X-ray Reflectivity. J. Phys. Chem. C 2018, 122, 26934–26944. [Google Scholar] [CrossRef]
- Biriukov, D.; Kroutil, O.; Předota, M. Modeling of Solid–Liquid Interfaces Using Scaled Charges: Rutile (110) Surfaces. Phys. Chem. Chem. Phys. 2018, 20, 23954–23966. [Google Scholar] [CrossRef]
- Futera, Z.; English, N.J. Exploring Rutile (110) and Anatase (101) TiO2 Water Interfaces by Reactive Force-Field Simulations. J. Phys. Chem. C 2017, 121, 6701–6711. [Google Scholar] [CrossRef]
- Cheng, J.; Sprik, M. Aligning Electronic Energy Levels at the TiO2/H2O Interface. Phys. Rev. B 2010, 82, 081406. [Google Scholar] [CrossRef]
- Asproulis, N.; Drikakis, D. An Artificial Neural Network-Based Multiscale Method for Hybrid Atomistic-Continuum Simulations. Microfluid. Nanofluidics 2013, 15, 559–574. [Google Scholar] [CrossRef]
- Smith, E.R.; Müller, E.A.; Craster, R.V.; Matar, O.K. A Langevin Model for Fluctuating Contact Angle BehaviourParametrised Using Molecular Dynamics. Soft Matter 2016, 12, 9604–9615. [Google Scholar] [CrossRef]
- Smith, E.R.; Theodorakis, P.E.; Craster, R.V.; Matar, O.K. Moving Contact Lines: Linking Molecular Dynamics and Continuum-Scale Modeling. Langmuir 2018, 34, 12501–12518. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Bieberle-Hütter, A. Modeling and Simulations in Photoelectrochemical Water Oxidation: From Single Level to Multiscale Modeling. ChemSusChem 2016, 9, 1223–1242. [Google Scholar] [CrossRef]
- Jung, C.K.; Braunwarth, L.; Jacob, T. Grand Canonical ReaxFF Molecular Dynamics Simulations for Catalytic Reactions. J. Chem. Theory Comput. 2019, 15, 5810–5816. [Google Scholar] [CrossRef] [PubMed]
- Islam, M.M.; Kolesov, G.; Verstraelen, T.; Kaxiras, E.; van Duin, A.C.T. EReaxFF: A Pseudoclassical Treatment of Explicit Electrons within Reactive Force Field Simulations. J. Chem. Theory Comput. 2016, 12, 3463–3472. [Google Scholar] [CrossRef] [PubMed]
- Tranca, D.C.; Zimmerman, P.M.; Gomes, J.; Lambrecht, D.; Keil, F.J.; Head-Gordon, M.; Bell, A.T. Hexane Cracking on ZSM-5 and Faujasite Zeolites: A QM/MM/QCT Study. J. Phys. Chem. C 2015, 119, 28836–28853. [Google Scholar] [CrossRef][Green Version]
- Rusevich, L.L.; Kotomin, E.A.; Zvejnieks, G.; Popov, A.I. Ab initio calculations of structural, electronic and vibrational properties of BaTiO3 and SrTiO3 perovskite crystals with oxygen vacancies. Low Temp. Phys. 2020, 46, 1185–1195. [Google Scholar] [CrossRef]
- Eglitis, R.; Purans, J.; Popov, A.I.; Jia, R. Systematic trends in YAlO3, SrTiO3, BaTiO3, BaZrO3 (001) and (111) surface ab initio calculations. Int. J. Mod. Phys. B 2019, 33, 1950390. [Google Scholar] [CrossRef]
- Eglitis, R.I.; Purans, J.; Popov, A.I.; Bocharov, D.; Chekhovska, A.; Jia, R. Ab initio computations of O and AO as well as ReO2, WO2 and BO2-terminated ReO3, WO3, BaTiO3, SrTiO3 and BaZrO3 (001) surfaces. Symmetry 2022, 14, 1050. [Google Scholar] [CrossRef]
- Eglitis, R.I.; Kotomin, E.A.; Popov, A.I.; Kruchinin, S.P.; Jia, R. Comparative ab initio calculations of SrTiO3, BaTiO3, PbTiO3 and SrZrO3 (001) and (111) surfaces as well as oxygen vacancies. Low. Temp. Phys. 2022, 48, 80–88. [Google Scholar] [CrossRef]
- Eglitis, R.I.; Popov, A.I. Comparative ab initio calculations for ABO3 perovskite (001), (011) and (111) surfaces as well as YAlO3 (001) surfaces and F centers. J. Nano Electron. Phys. 2019, 11, 01001. [Google Scholar] [CrossRef]
- Panthi, G.; Park, M. Approaches for enhancing the photocatalytic activities of barium titanate: A review. J. Energy Chem. 2022, 73, 160–188. [Google Scholar] [CrossRef]
- Liu, X.; Lv, S.; Fan, B.; Xing, A.; Jia, B. Ferroelectric Polarization-Enhanced Photocatalysis in BaTiO3-TiO2 Core-Shell Heterostructures. Nanomaterials 2019, 9, 1116. [Google Scholar] [CrossRef]
- Rauf, A.; Wang, W.; Zheng, D.; Feng, S.; Khan, U.; Akbar, A.R.; Peng, G.; Wu, Z.; Liu, F. Ferroelectric polarization induced charge separation in BaTiO3/Si: A pathway for non-PN junction photovoltaics. Ceram. Int. 2022, 48, 28413–28418. [Google Scholar] [CrossRef]
- Abuova, A.U.; Mastrikov, Y.A.; Kotomin, E.A.; Piskunov, S.N.; Inerbaev, T.M.; Akilbekov, A.T. First-principles modeling of oxygen adsorption on Ag-doped LaMnO3 (001) surface. J. Electron. Mater. 2020, 49, 1421–1434. [Google Scholar] [CrossRef]
- Fan, J.; Song, Z.; Tan, B.; Wang, H.; Chen, Z.; Xu, H.; Yan, J. Enhanced hydrogen production via piezo-photocatalytic water splitting using BaTiO3 crystal phase engineering. J. Solid State Chem. 2025, 345, 125251. [Google Scholar] [CrossRef]
- Chandrasekaran, S.; Wang, Q.; Liu, Q.; Wang, H.; Qiu, D.; Lu, H.; Liu, Y.; Bowen, C.; Huang, H. Dynamic regulation of ferroelectric polarization using external stimuli for efficient water splitting and beyond. Chem. Soc. Rev. 2025, 54, 2275–2343. [Google Scholar] [CrossRef]
- Nagarajarao, S.H.; Mokshanatha, P.B.; Servottam, S. Photocatalytic water treatment by perovskite materials. Synth. Met. 2025, 312, 117875. [Google Scholar] [CrossRef]
- Assavachin, S.; Sawangphruk, M.; Osterloh, F.E. Ferroelectric BiFeO3 and BaTiO3 photocatalysts for photoelectrochemical water splitting. Curr. Opin. Chem. Eng. 2025, 48, 101123. [Google Scholar] [CrossRef]
- Chandrappa, S.; Myakala, S.N.; Koshi, N.A.; Galbao, S.J.; Lee, S.-C.; Bhattacharjee, S.; Eder, D.; Cherevan, A.; Murthy, D.H.K. Unveiling valence state-dependent photocatalytic water splitting activity and photocathodic behavior in visible light-active iridium-doped BaTiO3. ACS Appl. Mater. Interfaces 2024, 16, 8763–8771. [Google Scholar] [CrossRef] [PubMed]
- Parangusan, H.; Bhadra, J.; Ahmad, Z.; Karuppasamy, K.; Mallick, S.; Touati, F.; Al-Thani, N. Hierarchical BaTiO3/NiFe2O4 nanocomposite as an efficacious photoanode for photoelectrochemical water splitting. Ceram. Int. 2022, 48, 29136–29143. [Google Scholar] [CrossRef]


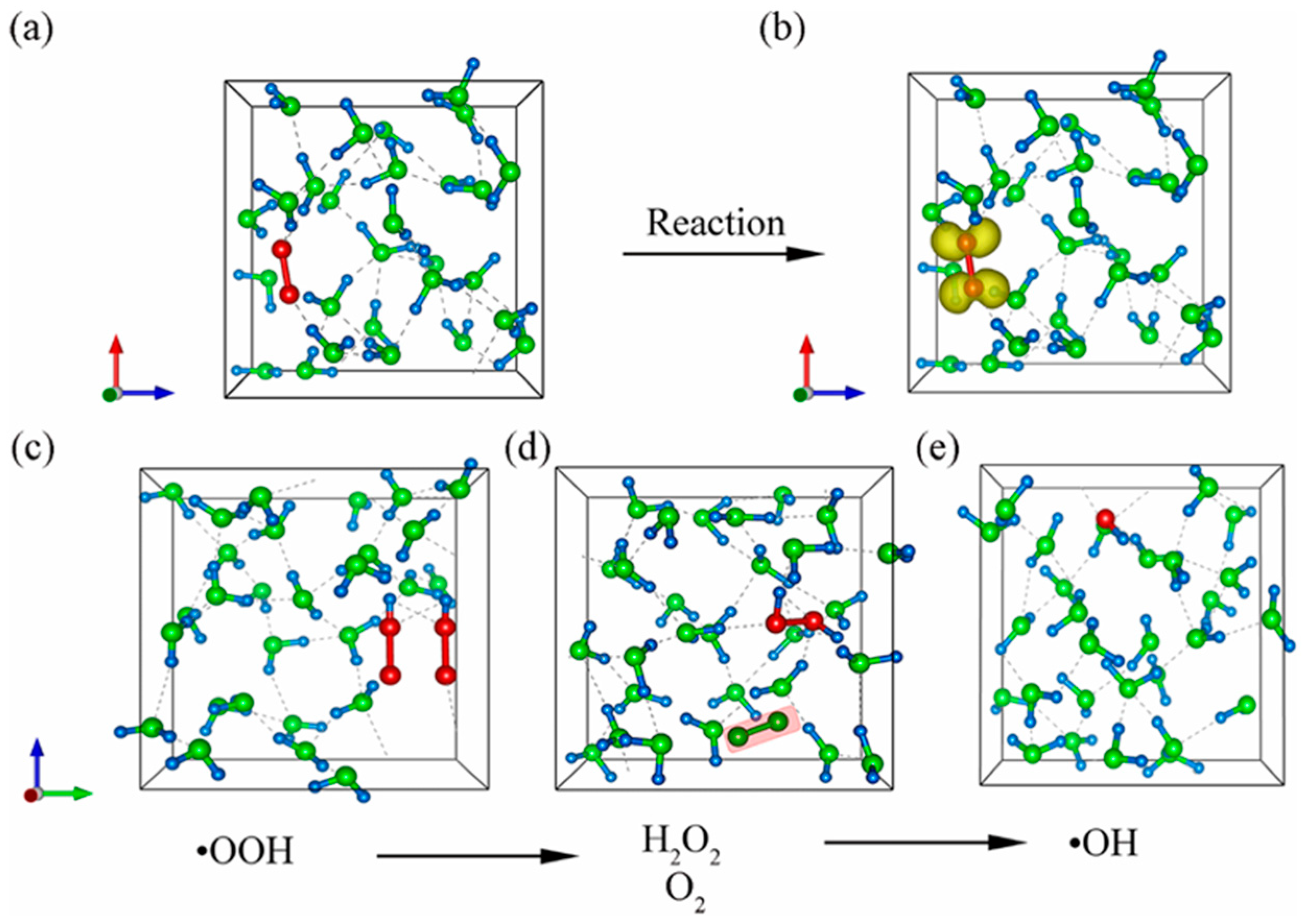
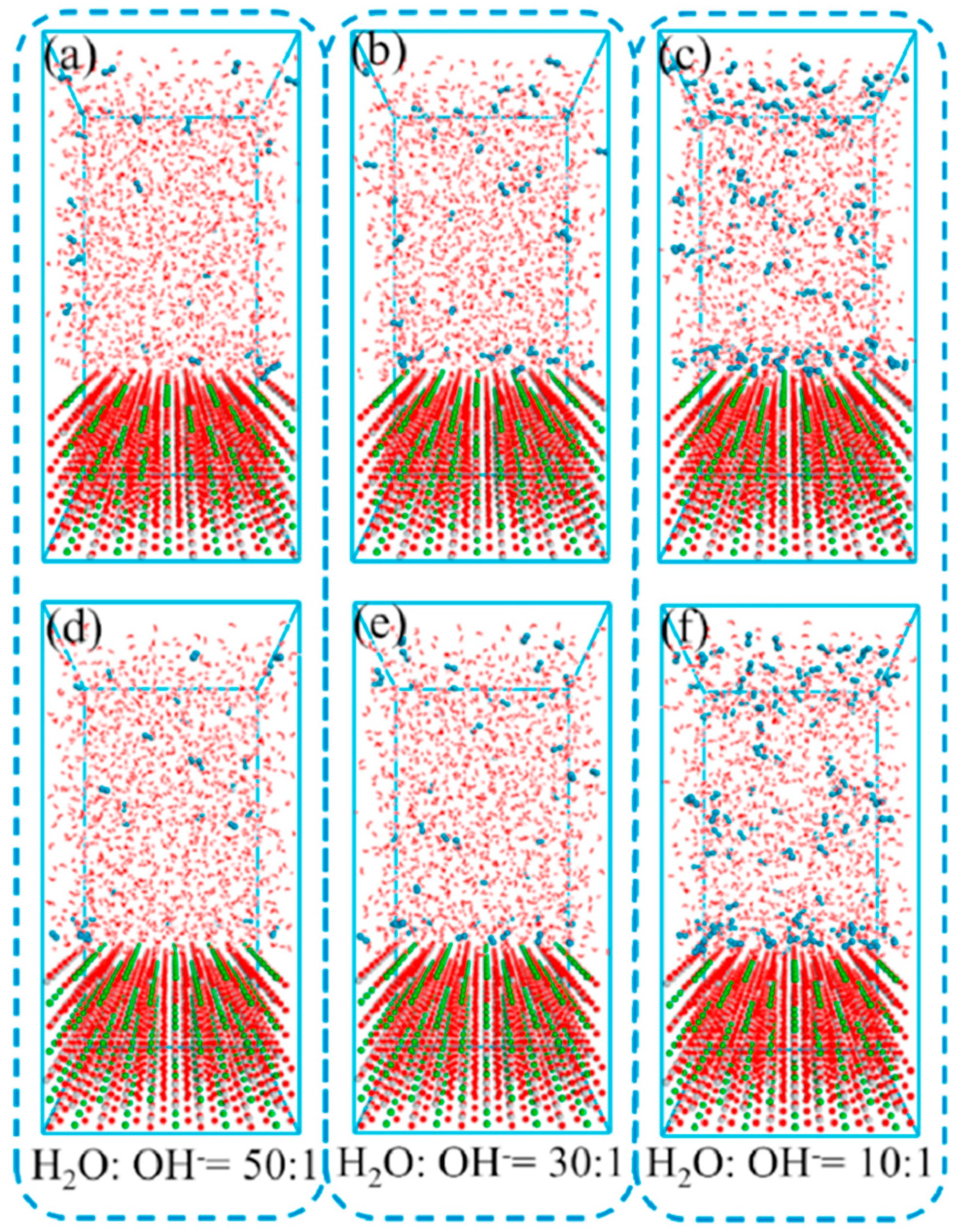
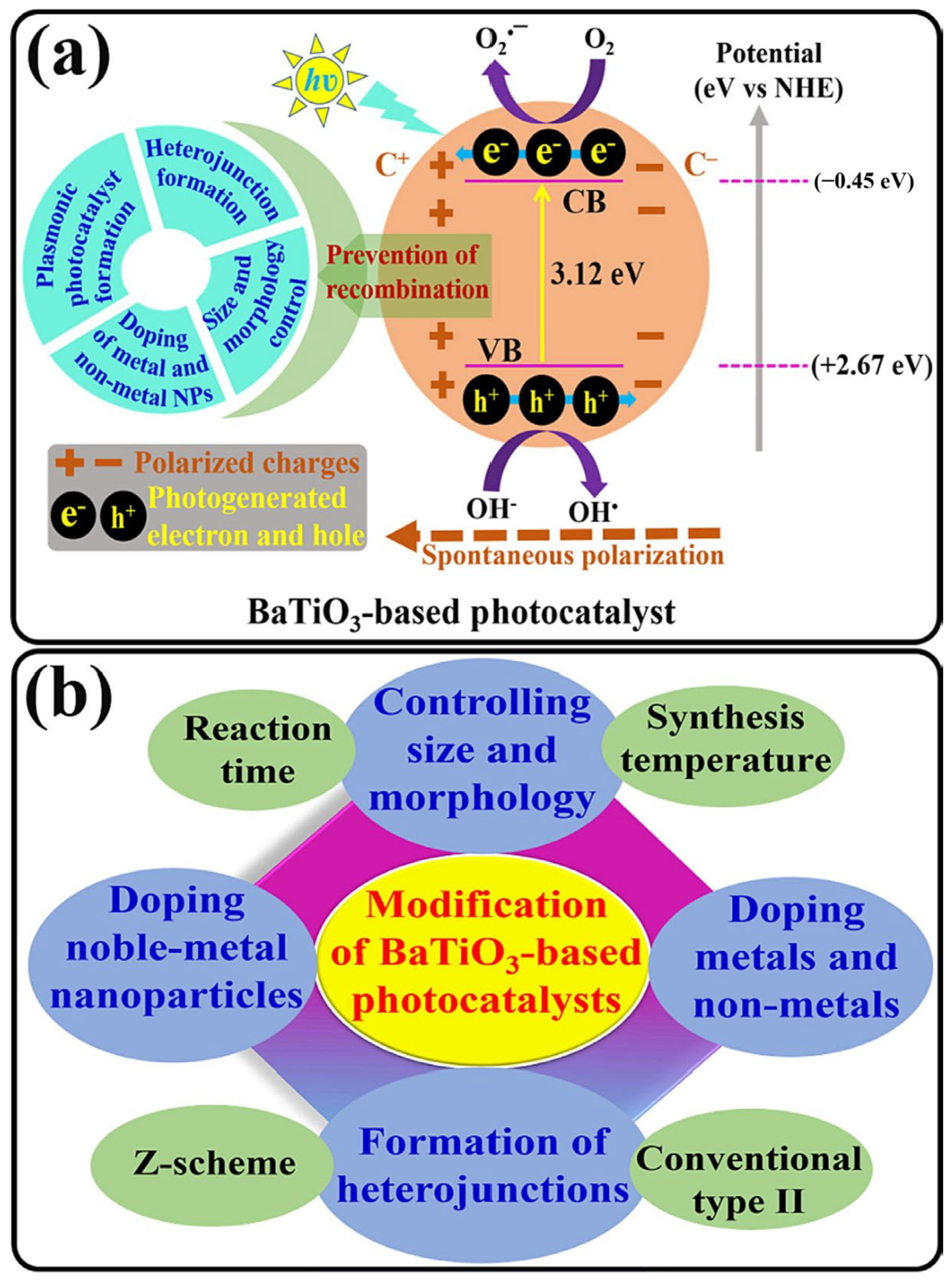
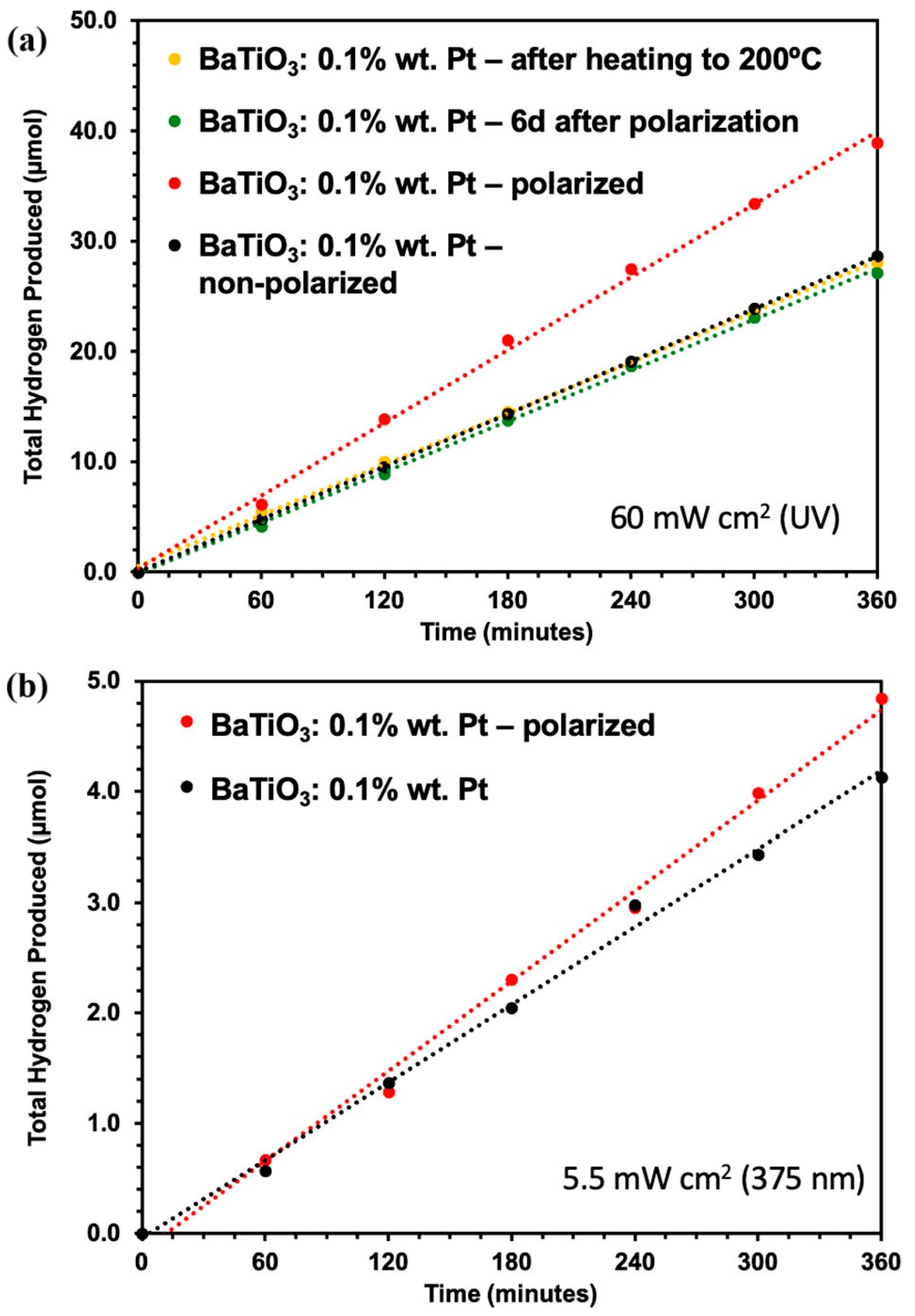
| Designed Systems | Methods | Main Findings |
|---|---|---|
| TiO2, TiO2/BaTiO3, TiO2@ BaTiO3/CdS [139] | DFT calculations using Vienna Ab initio Simulation Package (VASP) with Generalized Gradient Approximation-Perdew Burke Ernzerhof (GGA-PBE) functional. Projector-augmented wave (PAW) method for ion–electron interactions (cutoff energy: 400 eV). DFT + Hubbard U correction (DFT+U) approach for d-electron correlation correction. Finite-Difference Time-Domain (FDTD) method for electric field distribution simulations. | Calculated bandgap of TiO2: 3.22 eV. CB primarily composed of O(p) orbitals. VB primarily composed of Ti(d) orbitals. Photogenerated charge likely accumulates in these orbitals. After combining with BaTiO3, CB and VB compositions remain similar to TiO2. Calculated bandgap decreases. Adding CdS clusters to TiO2/BaTiO3 caused slight crystal distortion in BaTiO3, potentially inducing spontaneous polarization. Density of states at CB and VB formed by S(p), Ba(d), and Cd(d) orbitals. Bandgap further decreased. However, the authors noted that this significant reduction in bandgap contradicts experimental results due to known limitations of standard DFT. TiO2/BaTiO3/CdS nanosheet exhibits an intrinsic electric field, facilitating charge separation and diffusion to the surface. |
| Wheat-heading BaTiO3, wheat-heading BaTiO3-oxygen vacancy [140] | DFT calculations using Materials Studio 2017, with GGA-PBE functional. Plane wave cutoff energy: 400 eV. K-point mesh: 3 × 3 × 3. Maximum force tolerance: 0.05 eV/Å. Cleaved along [001] direction and vacuum thickness of 10 Å in z-direction. | Bandgap for wheat-heading BaTiO3: 3.05 eV. CB mainly composed of Ti 3d and O 2p orbitals. VB dominated by O 2p orbitals. Charge transfer from O 2p to Ti 3d. After oxygen vacancy, bandgap reduced to 2.71 eV. VB remains dominated by O 2p orbitals. CB contributions shift to O 2p, Ba 3d, and Ti 3d. Enhanced charge transfer between Ti and O vacancy. Higher charge density improves piezo-photocatalytic performance. |
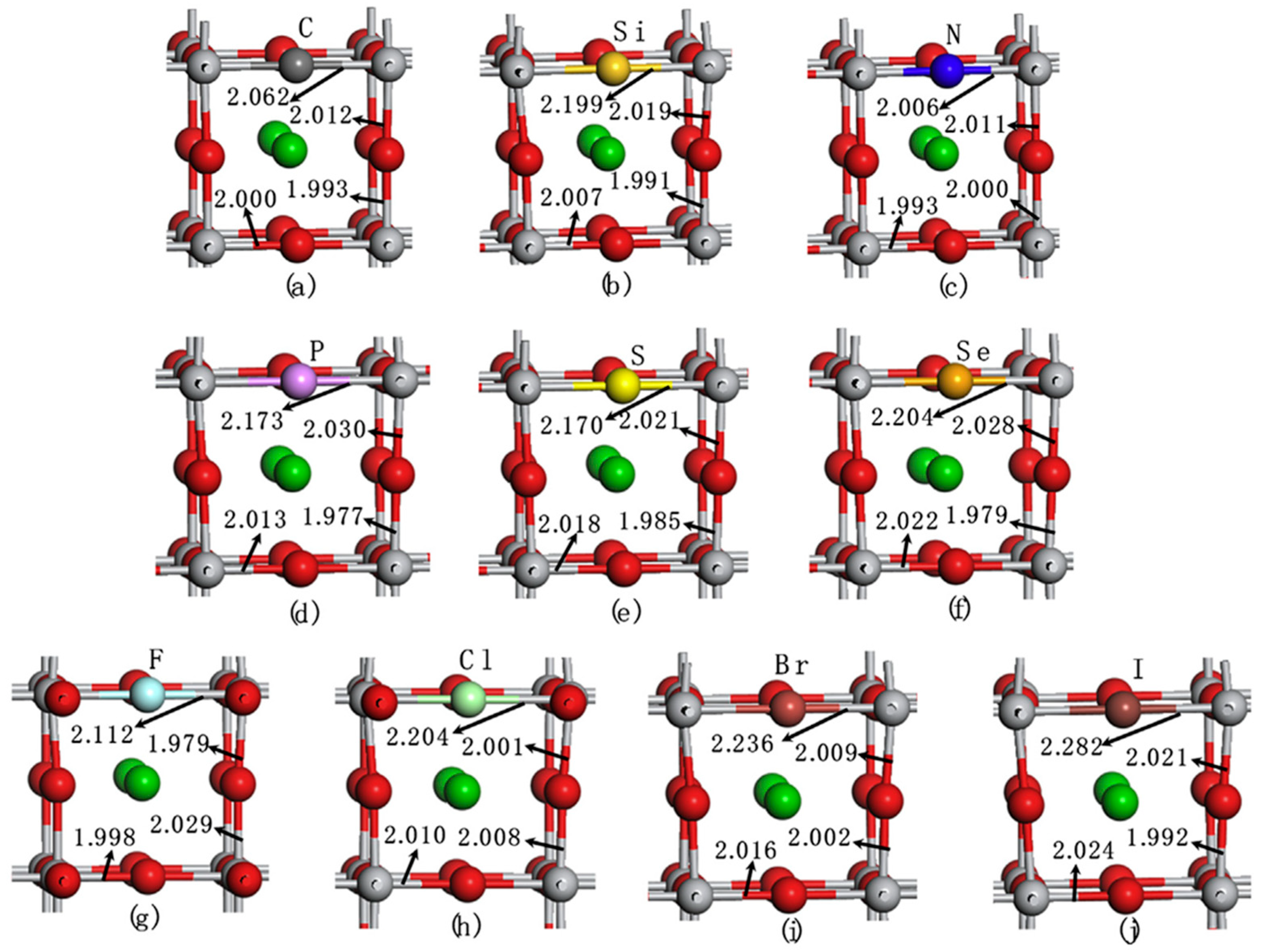
| Pure BaTiO3, non-metal-doped BaTiO3 (X@O or X@Ti, X = C, Si, N, P, S, Se, F, Cl, Br, I) [141] |
Spin-polarized DFT calculations using VASP with GGA-PBE functional. PAW method for core electrons. Plane-wave cutoff energy: 400 eV. 9 × 9 × 9 Monkhorst–Pack k-point mesh. Fully optimized cubic BaTiO3 unit cell with a lattice parameter of 4.004 Å. Geometry convergence criterion: forces < 0.01 eV/Å. Hybrid functional (HSE06) for electronic structure calculations with Hartree–Fock (HF) exchange fraction (α) = 0.32. Substituting O or Ti with non-metal dopants at a doping concentration of 2.5 at.%. |
Structural and electronic properties of BaTiO3 were well reproduced. Bandgap improved with HSE06 functional, aligning with experimental values. Basis for further doping studies to enhance photocatalytic properties. F- and N-doped BaTiO3 (X@O) and Si-doped BaTiO3 (X@Ti) showed negative formation energy, indicating thermodynamic stability. Stability of doping systems depends on ionic radius and electronegativity of dopants relative to O or Ti. C-, S-, Se-, and I-doped BaTiO3 (X@O) extended the absorption edge into the visible light region, enhancing photocatalytic water splitting capabilities. S- and Se-doped BaTiO3 (X@Ti) exhibited potential for water splitting under visible light. Doping-induced modifications improved both photo-oxidation and photo-reduction properties of BaTiO3. |
| Pure BaTiO3, La-doped BaTiO3 [142] |
Materials Studio DFT with GGA-PBE functional. Birch–Murnaghan equation of state for lattice optimization. Cut-off energy: 340 eV. |
BaTiO3 exists in a cubic structure (Pm3m) with Ba at corners, Ti at the body center, and O at face centers. The calculated lattice constant is 4.034 Å, closely matching experimental values. Optical properties such as dielectric function, absorption, and refractive index are analyzed. La doping at Ba sites reduces the lattice parameter (a = 3.971 Å) and unit cell volume. Reduced bandgap enhances conductivity by facilitating electron–hole recombination. The La-5d states contribute significantly to the conduction band. Optical properties, including dielectric function, absorption, and refractive index, are modified. |
| BaTiO3 with Ba and Ti vacancy [143] |
Modeled using Materials Studio. Optimized structure using VASP. First-principles calculations based on DFT framework. 2 × 2 × 2 crystal structure containing 8 Ba, 24 O, and 8 Ti atoms. PAW and PBE methods used for structure optimization and charge density calculations. |
Lattice distortion occurs due to Ba and Ti vacancies, affecting oxygen coordination and Coulomb repulsion. Oxygen vacancies are necessary for charge conservation in the system. Lattice expansion and distortion due to Ti and O vacancies are significantly higher than those caused by Ba and O vacancies. Charge density changes:
|
| Pure BaTiO3, Mo-doped BaTiO3 (2.5 at%) [144] | First-principles calculations using DFT with the supercell approach, performed using VASP. Functional: GGA for the PAW method. Structural model: cubic 1×1×1 BaTiO3 unit cell. Plane-wave energy cutoff: 500 eV. K-point sampling: Monkhorst–Pack grid of 7 × 7 × 7. | The calculated bandgap of pure BaTiO3 is 1.56 eV, which is underestimated due to DFT limitations. Charge–density analysis confirms covalent Ti–O bonding. Mo doping narrows the bandgap to 1.27 eV due to impurity levels formed by Ti 3d and Mo 3d interactions, and DFT limitations. Mo–O bonding results in a more uniform charge distribution than pure BaTiO3. |
| Pure BaTiO3, Cs-doped BaTiO3 (0.13%, 0.26%, 0.39%) [145] | Geometry optimization and property investigation with GGA-PBE exchange correlation functional with DFT+U correction (U = 4 for Ti-d orbital). Vanderbilt-type ultrasoft pseudopotentials for electron–ion interactions. Pulay density mixing scheme applied. Monkhorst–Pack method for k-point sampling (6 × 6 × 6 k-points mesh). Energy cutoff = 630 eV. Total energy difference per atom: 2 × 105 eV. Max ionic displacement: 2 × 103 Å. Cubic phase (Pm3m, 221) chosen. |
For pure BaTiO3. Total density of state maximum peak at 4.29 eV (6.58 value), with other peaks at 1.79 eV and 0.95 eV. Phonon spectra show no imaginary frequencies, confirming stability. For Cs-doped BaTiO3 (0.13%, 0.26%, 0.39%). Bandgap converts from indirect to direct upon Cs doping. Total density of state of 0.13% Cs-doped BaTiO3 shows enhanced peaks, with a maximum peak at 0.77 eV (57.46 value). New peaks in total density of state appear at 3.43, 2.37, 2.40, 3.36, and 4.47 eV. Phonon spectra confirm stability for 0.13% Cs-doped BaTiO3 (no imaginary frequencies detected). |
| BaTiO3 (111) surfaces with different terminations [146] | DFT calculations using VASP. PAW method for core electrons. Plane-wave basis with 400 eV cutoff. DFT+U approach with PBE functional (Ueff = 4.0 eV for Ti 3d). Considered stoichiometric (BaO3, Ti) and non-stoichiometric (BaO2, BaO, Ba, O3, O2, O) terminations. |
Surface energy and stability. BaO2 and O terminations have the lowest cleavage energies, making them the most thermodynamically stable. Removal of oxygen, Ti, or Ba reduces cleavage energy, stabilizing polar surfaces. Excess Ba (BaO + O2) or oxygen (Ba + O3) leads to instability with higher cleavage energies. Phase diagram analysis. BaO2 and O terminations dominate under wide O- and Ba-rich conditions. Stoichiometric BaO3 and Ti terminations are stable only in limited conditions. Results from O-Ti phase diagram match O-Ba phase diagram, confirming BaO2 and O as the most stable. Charge compensation mechanism. Bader charge analysis shows charge redistribution in surface layers to compensate dipole moments. |
| BaTiO3 doped with chalcogens (S, Se, Te) at different concentrations [147] |
DFT calculations using WIEN2K package with FP-LAPW method and LDA+mBJ exchange-correlation potential. Calculation of ε(ω) = ε1(ω) + iε2(ω). |
BaTiO3 has a cubic Pm3m structure. Lattice constant (a0 = 3.9412 Å) agrees with experimental (4.0000 Å) and theoretical values. The forbidden bandgap decreases with increasing chalcogen concentration due to electronegativity differences. Doping reduces the bandgap significantly. Strong hybridization occurs between O 2p and chalcogen p orbitals. |
| Pressed BaTiO3 (2.3% axial compressive strain), BaTiO3 under triaxial compressive strain [148] |
Ab initio calculations based on DFT. Exchange correlation potential: local density approximation (LDA). Brillouin zone integration: 6 × 6 × 6 k-points for electronic and optical properties, 10 × 10 × 10 for thermoelectric properties. Structural optimization: comparison with experimental and theoretical results. | Lattice constant reduced to ap = 3.8505 Å. Pressed BaTiO3 exhibits a direct bandgap at the Γ point, unlike pure BaTiO3, which has an indirect bandgap. Further bandgap reduction compared to non-pressed doped structures. Pressed BaTiO3exhibits slightly higher optical property peaks in ε1(ω) and ε2(ω) compared to pure BaTiO3. Electronic properties: Pure BaTiO3is a semiconductor with an indirect bandgap. Under ξ = 2.3% compressive strain, BaTiO3 transitions to a direct bandgap semiconductor, improving potential for photovoltaic applications. Density of states analysis confirms VB is mainly O 2p, while CB is Ti 3d. Bandgap increases with strain, indicating possible piezoelectric properties. |
| BaTiO3 (001) surfaces doped with metal and non-metal elements [149] |
DFT calculations using VASP, PBE functional under GGA, and HSE06 hybrid functional. Plane-wave cutoff energy: 400 eV. K-point mesh: 9 × 9 × 9 for bulk optimization and 3 × 3 × 1 for surface calculations. |
The tetragonal BaTiO3 unit cell was fully optimized, with lattice parameters a = b = 3.992 Å, c = 4.056 Å, matching experimental and theoretical results. BaTiO3 (001) surface modeled with TiO2- and BaO- terminations. Symmetric slabs (odd atomic layers) were adopted due to the absence of macroscopic dipole moments. Co-doped systems (M+X) are more stable when M and X are adjacent due to M-X bond formation. Formation energies indicate that O substitution by C or N is easier under Ti-rich conditions, while Ti substitution by metal dopants is favored under O-rich conditions. Binding energy calculations show that co-doped systems are more stable than mono-doped systems. The computed bandgap of bulk BaTiO3 is 3.03 eV, while the pure BaTiO3 (001) surface has a bandgap of 1.42 eV. Passivated co-doping (e.g., V+N, Nb+N, Ta+N) introduces charge compensation, eliminating mid-gap states. The Ta+N co-doping system leads to the most significant bandgap narrowing (1.09 eV) due to the upshift of the valence band maximum. |
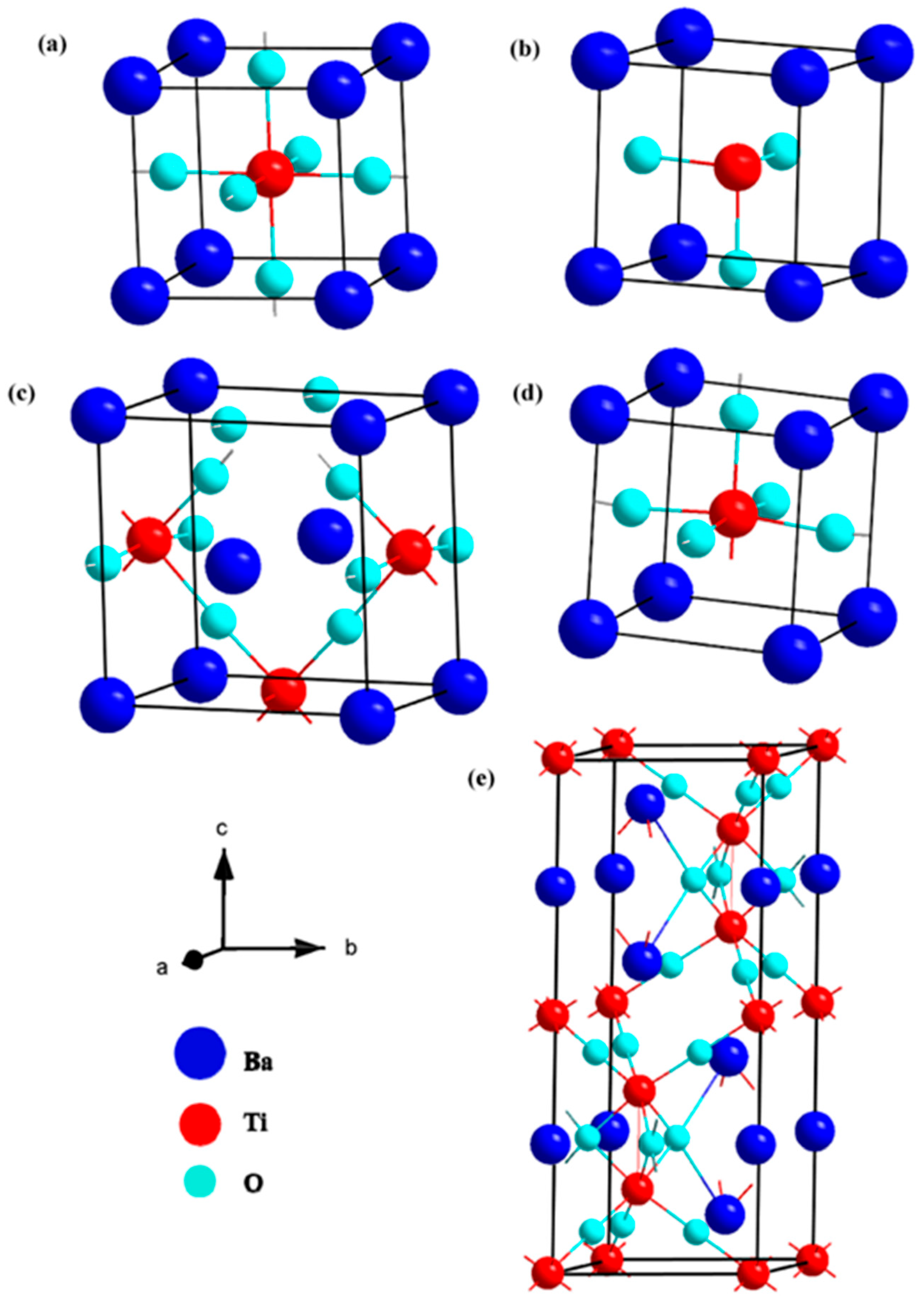
| BaTiO3 polymorphs (cubic, rhombohedral, orthorhombic, tetragonal, hexagonal) [150] | First-principles calculations using DFT framework (GGA-PBE, LDA, and HSE06 functionals). |
Optimized lattice parameters are consistent with theoretical and experimental results. Formation enthalpies indicate all phases are energetically stable, with the cubic phase being the most stable. Band structure analysis shows indirect bandgaps for four phases and a direct bandgap for the hexagonal phase. GGA-PBE and LDA underestimate bandgaps, while HSE06 gives values closer to experimental data. Higher electron mobility and conductivity inferred from band structure analysis. Density of states analysis confirms structural stability and electrical conductivity. |
| Porous graphene with BaTiO3, [151] |
Electronic structure and density of states calculations using Quantum Espresso with PBE pseudopotentials. k-mesh: 9 × 9 × 1 for self-consistent field (scf) and 18 × 18 × 1 for non-self-consistent field calculations. Energy cutoff: 90 Ry for wavefunctions, 740 Ry for charge density. | Redshift in absorption edges of porous graphene with BaTiO3 compared to pure BaTiO3. Lower fluorescence intensity indicates reduced charge carrier recombination, enhancing photocatalytic efficiency. Electron migration from BaTiO3 to porous graphene via Ba–C bond supports charge separation. Fully relaxed 5 × 5 × 1 supercell of porous graphene with BaTiO3 with a 12 Å vacuum to prevent interaction between composites. Estimated bandgap of 1.74 eV (indirect, R to Γ), lower due to DFT underestimation. Additional bandgaps observed: direct at Γ, indirect from M to Γ. BaTiO3: VB primarily from O ‘p’ states; CB dominated by Ti ‘p’ states with minor O ‘p’ contributions. |
| Ba1−xGaxTiO3 (x = 50%) [152] |
DFT calculations. Tetra-elastic package for elastic properties. Ba1−xGaxTiO3 was studied using full-potential linearized augmented plane wave method. A 2000 k-point mesh was used for Brillouin zone integration. Band structure and density of states were analyzed for electronic properties. Elastic coefficients were calculated using a Eulerian strain approach. The unit cell structure was modeled with tetragonal symmetry. |
Pristine BaTiO3 exhibits an indirect bandgap of 2.65 eV. Partial density of states analysis shows significant contributions from O p, Ti d, and Ga p states. Dielectric constant (ε1 (0)) increased from 8.8 (pure) to 100 (Ga-doped). A peak in the imaginary dielectric function ε2(ω) at 3.9 eV corresponds to O p electron transitions to the conduction band. Ga doping shifts absorption peaks towards the visible and infrared regions, enhancing optical activity. |
| BaTiO3/NiFe heterojunctions [153] |
First-principles DFT calculations within GGA using a PBE functional. PAW potentials for ionic cores. Plane-wave basis set with a 450 eV cutoff. | Formation of BaTiO3/NiFe heterojunctions increased Ni3⁺ content (45% → 68% for NiFe, 61% → 83% for BaTiO3/NiFe) after oxygen evolution reaction test. Fe3⁺/Fe2⁺ ratio increased slightly after oxygen evolution reaction test, improving oxygen evolution reaction electrocatalytic activity. Free energy calculation showed a lower rate-determining step energy for heterojunction. Charge density difference analysis showed electron transfer from NiFe to BaTiO3, improving oxygen evolution reaction activity. |
| BaTiO3 [154] |
DFT using VASP. PBE exchange-correlation function. PAW pseudopotentials. Cutoff energy: 520 eV. Monkhorst–Pack 2 × 2 × 1 k-points for Brillouin zone sampling. |
The bandgaps of synthesized materials (3.24 eV, 3.20 eV, and 3.13 eV) are close to theoretical values, confirming minimal influence from PtOx loading. Pt-O-Ti3⁺ sites act as defect energy levels and oxidation sites. Charge density analysis revealed electron accumulation around PtOx and depletion around Ti atoms. Polarization studies showed improved current response for PtOx-loaded samples, confirming enhanced photocatalytic activity. Pt serves as an electron aggregation center, accelerating proton reduction for hydrogen production. Oxygen vacancies facilitate charge aggregation, and Ti3⁺ defects enhance rapid electron transfer. |
| BaTiO3/SrTiO3 [155] | First-principles calculations using DFT, VASP. GGA with PBE functional. Kinetic cutoff energy: 520 eV. Brillouin zone sampling: 5 × 5 × 1. External electrostatic field along [001] direction (E = 0.1 eV/Å). |
The BaTiO3/SrTiO3 heterojunction has a lower bandgap compared to individual SrTiO3 and BaTiO3, promoting photocatalytic efficiency. Differential charge density analysis reveals efficient electron transfer from BaTiO3 to SrTiO3 at the heterostructure interface. Hydrogen adsorption Gibbs free energy shows SrTiO3 (0.57 eV), BaTiO3 (−1.01 eV), and BaTiO3/SrTiO3 (−0.42 eV), indicating BaTiO3/SrTiO3 has optimized adsorption–desorption balance. |
| Zr+X co-doped BaTiO3 systems [156] | DFT calculations. Full-potential linearized augmented plane-wave. 2 × 2 × 2 supercell approach for constructing doped and co-doped systems. K-mesh: 12 × 12 × 12 for bulk, 6×6×6 for supercell. | Structural and thermodynamic properties: The computed cohesive energies of S, Se, and Te match well with previous studies. Electronic properties: X-doped systems have valence band edges composed of O 2p states with contributions from X p states. Zr-doped system shows conduction band modifications due to Zr 4d states. Zr+X co-doping leads to a reduced bandgap, making it promising for visible light applications. |
| Metal oxide/BaTiO3 [157] | DFT using Quantum Espresso. GGA for exchange-correlation functional. Plane wave basis (320 Ry cut-off) k-point meshes: 6 × 6 × 1 for integration, 12 × 12 × 1 for density of states. Marzari–Vanderbilt cold smearing (0.05 Ry). Charge carrier effective masses calculated from Bloch band curvature. | Structural properties: ZnO/BaTiO3 shows a decrease in BaTiO3 lattice vector c due to interface-induced tetragonality enhancement. Interface distances: ZnO/BaTiO3 (2 Å), TiO2/BaTiO3 and SnO2/BaTiO3 (4 Å). ZnO mid-slab oxygen layers exhibit large displacements due to interface interactions. Lattice mismatch effects cause strain in BaTiO3, compressing c in ZnO/BaTiO3. Electronic properties: Bandgaps in bulk: BaTiO3 (3.28 eV), ZnO (3.41 eV), TiO2 (3.17 eV), SnO2 (3.52 eV). Interface effects modify band structures, introducing metal-induced gap states in ZnO/BaTiO3. |
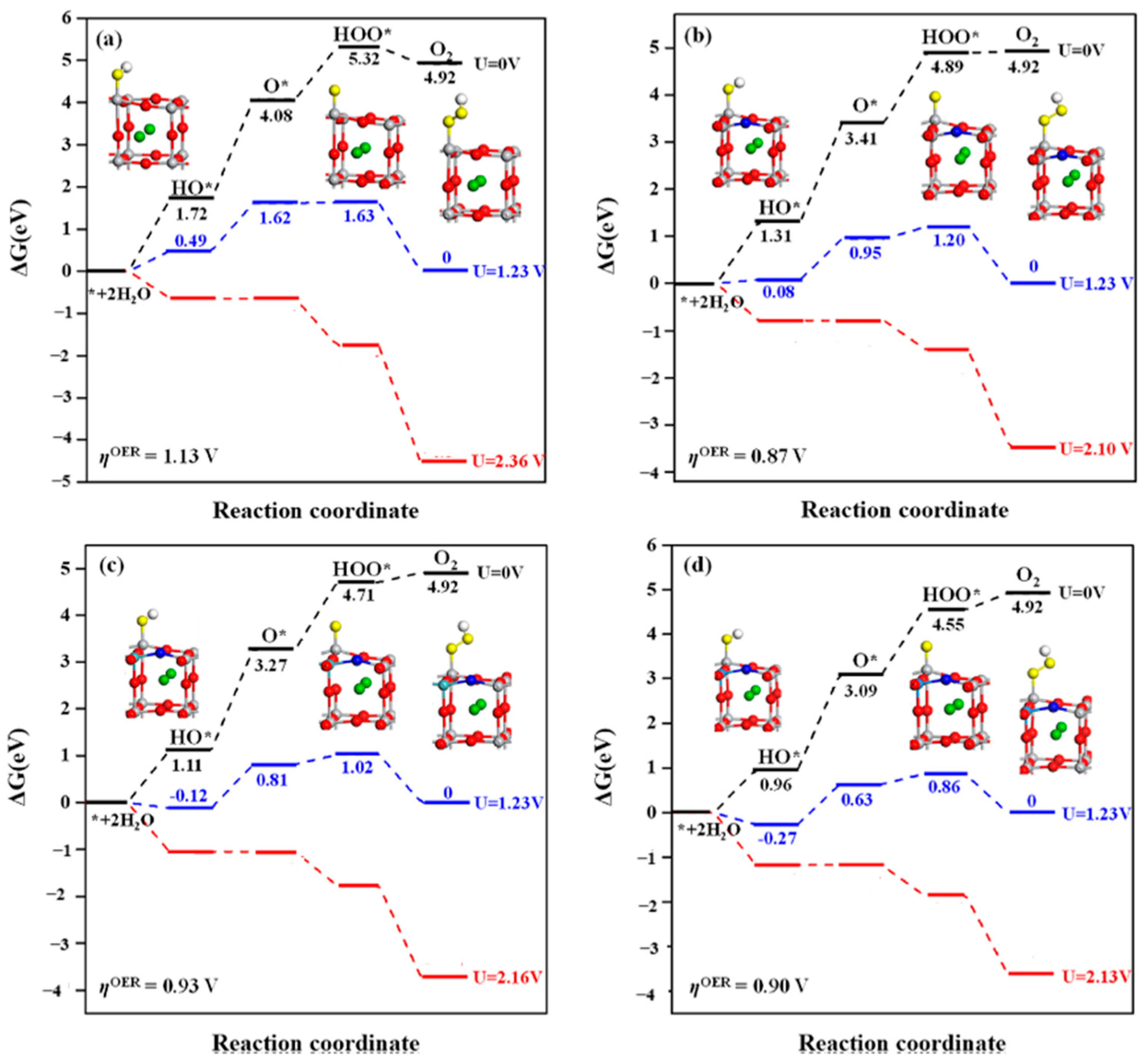
| Rhombohedral BaTiO3 surface, pure and Rh-doped [158] | Ab initio plane-wave calculations using VASP with PAW formalism and PBE-GGA exchange-correlation functional. Monkhorst–Pack grid: 2 × 2 × 2 for bulk, 2 × 2 × 1 for slab. Cutoff energy: 520 eV. Convergence tolerance: 10−6 eV. Slab models with seven alternating TiO2- and BaO-planes and 13 Å vacuum gap. Rh doping effects analyzed by replacing Ti with Rh and re-optimizing structures. | Rhombohedral BaTiO3 is ferroelectric and stable below 90 °C. Structural calculations show good agreement with experimental and previous theoretical studies. Ti displacement (−0.0137 Å) and O displacement (0.0232 Å) along [111] in rhombohedral BaTiO3. Calculated Ba–O (2.87 Å) and Ti–O (1.89 Å) bond lengths match experimental data. Direct bandgap of 2.25 eV is consistent with previous theoretical studies, though underestimated by GGA-PBE. BaTiO3 (001) surface (TiO2-terminated) is nonpolar with a vacuum gap of 13 Å in slab models. Rh doping (substituting Ti with Rh) slightly affects lattice structure; minimal bond length change observed. Effective charge of Rh (1.66 e) is lower than Ba (2.55 e). Rh doping reduces the bandgap from 1.45 eV to 0.67 eV and introduces an in-bandgap acceptor level (0.115 eV above Fermi level). Rh and O hybridized orbitals create defect states in the bandgap, influencing photocatalytic performance. |
| BaTiO3/LaAlO3 heterostructures [159] | DFT calculations using Quantum Espresso. Norm-conserving pseudopotentials GGA-PBE functional for exchange-correlation. Monkhorst–Pack k-point grid (10 × 10 × 1 for heterostructure, 12 × 12 × 1 for bulk). 30 Å vacuum space with dipole correction DFT-D3(BJ) for van der Waals interactions. Plane-wave cutoff energy: 45 Ry. Slab model for surface and interface calculations. | Optimized lattice parameters of bulk LaAlO3 (3.83 Å) and BaTiO3 (3.97 Å) agree with experimental values. Small lattice mismatch (−3.16%) heterostructure allows epitaxial growth. Ab initio MD and phonon dispersion results confirm dynamic and thermal stability of BaTiO3/LaAlO3(001) heterostructures at 300 K. BaTiO3(001) surface has the lowest bandgap (3.44 eV), favoring higher photocatalytic performance. BaTiO3(011) and (111) surfaces show direct bandgap behavior (4.05 eV, 3.75 eV). Partial density of states analysis reveals that charge carrier separation efficiency is influenced by surface composition. |
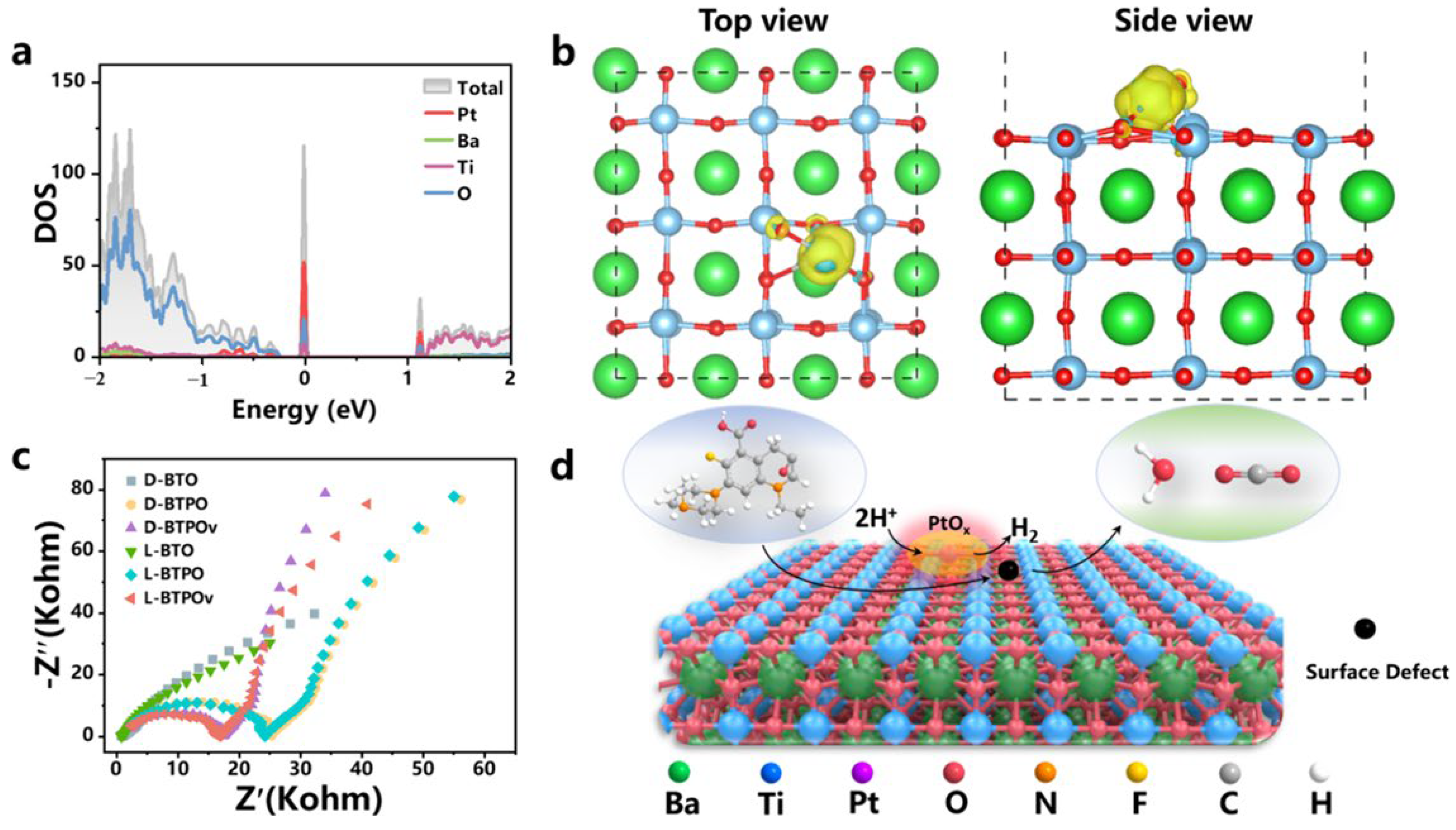
| BaTiO3 thin films with TiO2- and BaO-terminated slabs for electrocatalysis [160] | Ab initio periodic DFT+U calculations using the Quantum Espresso package, with GGA+U approximation and ultrasoft pseudopotentials. U = 4 eV for Ti d states. Kinetic energy cutoff: 320 eV. K-point grids: 4 × 4 × 1. Slabs modeled with four BaO and four TiO2 layers on Pt as an electron reservoir. | Polarization direction affects electronic structure: Upward polarization → Electron-rich surface (downward band bending, Ti d states near Fermi level). Downward polarization → Hole-doped surface (upward band bending, O p states near Fermi level). Surface energy calculations: TiO2-terminated slabs are the most stable. Hydrogen evolution reaction activity trends: Poled-up surfaces show smaller reaction barriers for hydrogen evolution reaction, making them more favorable. Only H adsorption on O site of poled-down surface is optimal. |
| Up-poled and down-poled BiFeO3/BiVO4 heterostructures [161] | DFT calculations using CRYSTAL23 code with B3LYP functional, D3 dispersion corrections, and spin polarization. Slabs modeled in R3c space group with (110) surface exposed. | Up-poled BiFeO3 surface: spontaneously dissociates water molecules, converting surface O to OH. Oxygen vacancies migrate to the surface under upward polarization, enhancing OH adsorption. Stronger interaction with water compared to down-poled BiFeO3, enhancing OW-C and OW-P peaks. Binds molecular oxygen more strongly, which may slow reaction rate. Down-poled BiFeO3 surface: H+ adsorption promotes surface OH− formation, enhancing OL-H peak. OL and OL-H peaks shift to higher binding energies due to ferroelectric polarization effects. Weaker interaction with water, dominated by physisorption, leading to weaker OW-C peak and stronger OW-P peak. More fluid interaction with water and easier oxygen desorption, improving reaction rate. pH significantly affects BiFeO3-water interactions due to availability of H+/OH−. |
| Anionic mono- and co-doped BaTiO3 [162] | QuantumATK software package DFT with PBE-GGA. Norm-conserving PseudoDojo pseudopotential. Self-consistent field simulations, 10−8 Ha tolerance. HSE06 hybrid density functional for electronic calculations. 2 × 2 × 2 supercell approach with periodic boundary conditions. | Lattice constants of mono-doped and co-doped BaTiO3 structures decrease due to incorporation of anionic elements. Formation energy calculations indicate anionic co-doping is more stable than mono-doping, especially in O-poor conditions. N-doping introduces asymmetrical density of state, leading to magnetic behavior (+1.0 μB). P-doping also induces magnetism (+1.0 μB) and localized states near the Fermi level. C-doping introduces two acceptor levels, with a strong magnetic moment (+2.002 μB). S-doping maintains valence electron count, interacting with Ti 3d states and resulting in a favorable bandgap (2.24 eV) for visible light absorption. Co-doped systems (e.g., N-N, C-S, N-P) exhibit lower formation energies than their mono-doped counterparts, making them more thermodynamically favorable. N–N co-doping is the most stable due to similar atomic radii and strong anionic interactions. |
| Ir-doped BaTiO3 [163] | DFT calculations using VASP. PAW method, GGA with PBE functional. GGA+U method (U values: Ti = 4 eV, O = 8 eV, Ir = 2 eV). Self-consistent and non-self-consistent field calculations with Monkhorst−Pack k-point grids (3 × 3 × 3 and 7 × 7 × 7). Cutoff energy: 500 eV. | Ir doping at the Ti site in BaTiO3 induces a transition from n-type to p-type conductivity. Density of state calculations reveal a substantial downward shift in the Fermi level (from 4.36 eV to 3.18 eV), confirming p-type behavior. Ir doping at the Ba site does not induce a similar Fermi-level shift. Density of states analysis indicates partially and fully occupied Ir 5d orbitals below and above the Fermi level. Charge neutrality is maintained by Ir3⁺ to Ir4⁺ transitions, contributing to hole formation and p-type behavior. Findings align with previous studies on Rh-doped SrTiO3. Ir-doped BaTiO3 exhibits visible-light absorption, making it a promising material for optoelectronic and photocatalytic applications. Further investigations of solar hydrogen evolution activity are in progress. |
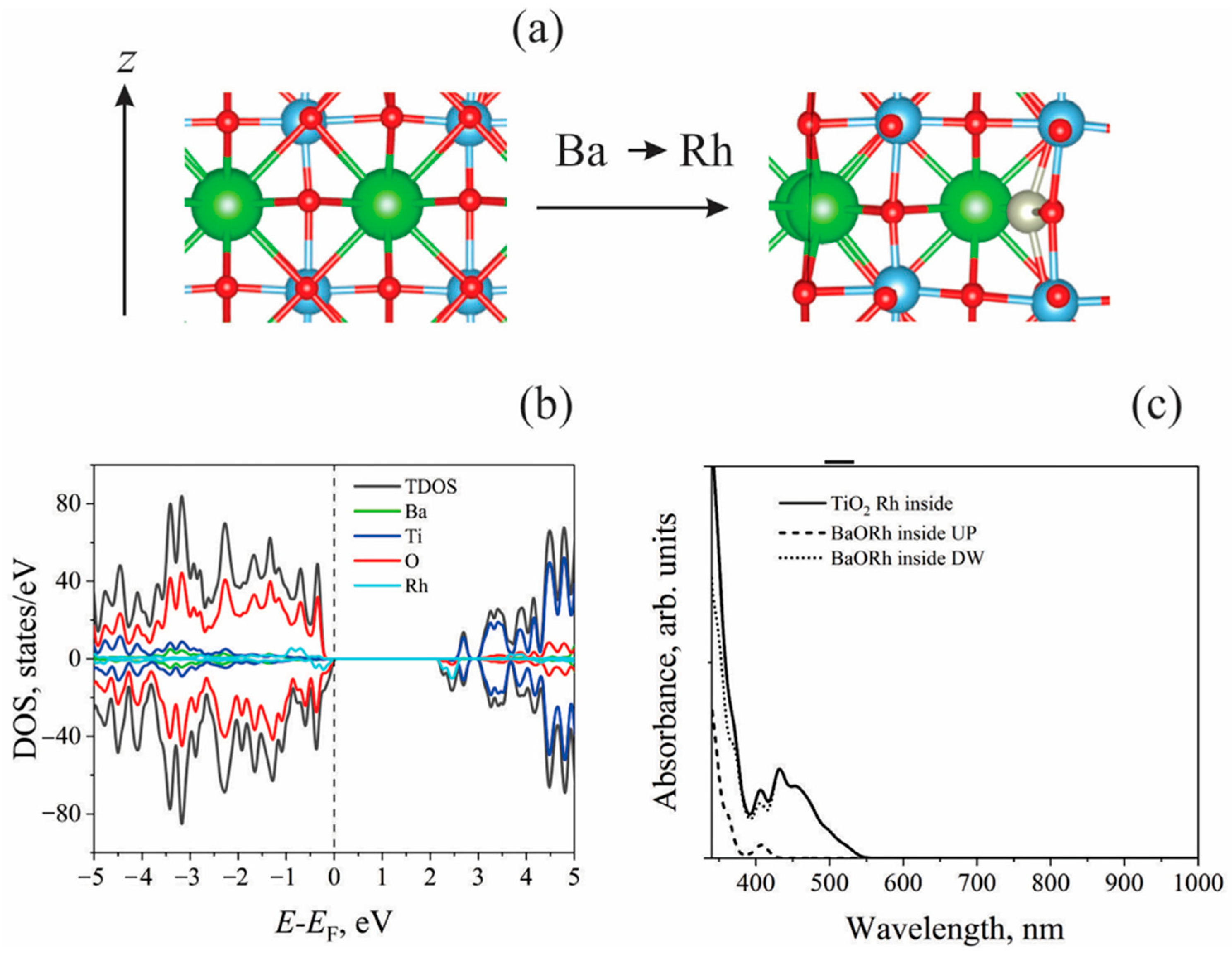
| Rh-doped BaTiO3 (Case A: Rh at Ba and Ti sites) [164] | First-principles DFT calculations using Quantum Espresso. LDA pseudopotential. Norm-conserving pseudopotential with valence electrons: 6s2 (Ba), 3d24s2 (Ti), 2s22p4 (O). Plane wave cutoff: 120 Ry, charge density cutoff: 480 Ry. K-point mesh: 4 × 4 × 4, 8 × 8 × 8. Electronic structure along G-X-M-G-R-X path. | BaTiO3 has a cubic perovskite structure. Direct bandgap of 1.929 eV at G point due to folding of R point onto G point in 2 × 2 × 2 supercell. Additional indirect bandgap transitions (R → G and M → G). Underestimation of bandgap in DFT due to derivative discontinuities. Valence band formed by O p-orbitals, conduction band formed by Ti d-orbitals. Ba atoms have an ionic nature and do not contribute significantly to partial density of states. Rh-doped BaTiO3 (Case A: Rh at Ba and Ti sites). Acceptor level formed due to hybridization of Rh (Ba site) d-orbitals and O p-orbitals. Deep defect states observed in wavefunction analysis. Direct bandgap: 2.028 eV at G point. Indirect bandgap: 1.796 eV (X → G) due to defect band overlapping with valence band edge. Hybridization of O p-orbitals and Rh d-orbitals at defect band region. Rh-doped BaTiO3 (Case C: Rh at Ba sites only). Valence band mainly from O p-orbitals, with hybridization with Rh d-orbitals. Minor Rh d-orbital contributions in conduction band. Single occupancy ensures continuous band structure, facilitating charge carrier migration. |
| BaTiO3 surfaces with different polarization states for hydrogen evolution reaction [165] | First-principles calculations using VASP 5.4.4 with GGA-PBE functional and DFT-D3 dispersion correction. | The tetragonal phase of BaTiO3 was used, as it is stable at room temperature where hydrogen evolution reaction occurs. GGA was chosen due to limitations of LDA for hydrogen-bonded ferroelectrics. Lattice constants were fixed to experimental values. Surface structure relaxation leads to rumpling, affecting adsorption behavior. For out-of-plane polarized BaTiO3, the most stable hydrogen adsorption site is the surface oxygen site. The surface titanium site is inactive for hydrogen evolution reaction. In-plane polarization states can be modulated via thin-film growth techniques and electrochemical poling. |
| La-N/B co-doped BaTiO3 [166] | DFT computations using Material Studio. PBE exchange-correlation functional with GGA + U (U = 4.3 eV for Ti-3d, 8.1 eV for La-4f). Energy cutoff: 500 eV. K-point grid: 3 × 3 × 3. Ultra-soft pseudopotentials. Energy convergence: 1.0 × 10−5 eV/atom. | La and N mono-doping effects: La substitution at the Ba site reduced the bandgap to 1.55 eV. La substitution at the Ti site caused a slight bandgap increase (+0.10 eV). Co-doping impact (La-N@B, 25%): Band edge positions were more favorable for photocatalytic water decomposition. Modulated electronic structure and optimized bandgap for improved absorption properties. Partial density of state and total density of state analysis revealed Ti-3d and O-2p as dominant contributors to the CB minimum and VB maximum. The cubic BaTiO3 phase (Pm3m) was used as a structural model despite its high-temperature stability for computational feasibility. |
| Pt-doped BaTiO3 [167] | First-principles calculations using the supercell method, DFT with GGA-PW91, PAW approach. Energy cutoff: 300 eV, Monkhorst–Pack k-mesh (4 × 4 × 4), Scissor operator (0.75 eV) applied | Optimized BaTiO3 unit cell and constructed 2 × 2 × 2 supercell (40 atoms). Pt doping at Ba and Ti sites (0.125 ratio) slightly reduces stability but remains thermodynamically favorable. Strong hybridization between Pt–5d and O–2p states. Mulliken charge analysis shows increased charge redistribution around O atoms. Pt doping introduces ferromagnetism in BaTiO3. Charge density analysis confirms the ionic-covalent bonding nature. |
| BaTiO3/Cu2O heterojunction [168] | Quantum Espresso package DFT, GGA using PBE functional. Ultrasoft pseudopotentials. Plane-wave basis set (30 Ry energy cutoff, 180 Ry charge density cutoff). Monkhorst–Pack mesh for Brillouin zone sampling. Structural optimization via Hellman–Feynman forces. | Band alignment and offsets were calculated using supercell periodic slab models. BaTiO3/Cu2O interface shows a staggered (Type-II) band alignment, which favors charge separation and enhances photoelectrochemical activity. Band offset values were obtained by considering VB and CB discontinuities. Effective mass of electrons and holes was calculated, revealing that Cu2O has a lower electron effective mass, indicating higher carrier mobility. The interface has a built-in dipole due to electronic charge transfer, influencing potential shifts across the heterojunction. |
| Tetragonal BaTiO3 with (001) TiO2- and BaO-terminated surfaces [58,136,169] | DFT calculations using HSE06 functional. Geometry optimization and substitution energy calculations. Density of states and optical absorption analysis. | Modeled BaTiO3 (001) surfaces with TiO2- and BaO-terminated slabs. Rh doping of Ba/Ti sites prevents dipole moments due to symmetry preservation. BaO-terminated surfaces found to be unstable under operating conditions. Substitution of Ti4+ with Rh4+ slightly distorts the lattice, while Ba2+ → Rh3+ + OH− substitution leads to significant structural changes. Doping the TiO2-terminated surface with Rh4+ introduces Rh-4d states in the bandgap, reducing its value. Optical absorption threshold shifts due to Rh4+ doping, with density of state analysis confirming bandgap modifications. |
| BaTiO3 (001) surfaces, including perfect and oxygen-deficient (TiO2-terminated) surfaces [170] | DFT with DFT+U using the VASP. | PBE+U(Ti,O) approach improves the accuracy of bandgap calculations and bond energy predictions compared to standard PBE and PBE+U(Ti). Oxygen vacancies introduce in-gap states with Ti 3d character, positioned ~1.0 eV above the VB maximum and ~0.8 eV below the CB minimum. The stability of BaO- and TiO2-terminated surfaces depends on temperature: BaO is more stable at 0 K, but TiO2 dominates at high temperatures (>1000 K). Formation of O vacancy is energetically more favorable on TiO2-terminated surfaces than on BaO-terminated surfaces. |
| Designed System | Methods | Main Findings |
|---|---|---|
| BaTiO3 surface [60] | Spin-polarized DFT calculations (VASP) with PAW pseudopotentials. High plane-wave cutoff (520 eV). Dataset of 16,162 configurations, trained with a 95:5 train–validation split, utilizing a multi-layer perceptron (tanh activation). MD simulations were conducted at 300 K, 500 K, and 700 K for 50 ps. Production MD simulations: accelerated with MLP models and run for 500 ps at 300 K with a timestep of 0.25 fs. Metadynamics simulations: explored oxygen evolution reaction mechanisms using coordination number as collective variables and studied oxygen desorption by tracking Ti-O2/Ni-O2 distances. | The energy barrier for oxygen desorption is lower than for oxygen evolution reaction, leading to the choice of specific metadynamics parameters (Gaussian height = 0.01 eV, width = 0.05, deposition rate = 6.25 fs). Water dissociation on the surface forms OH* intermediates with a free energy barrier (∆G‡ H2O→OH) of 0.06 eV for BaTiO3. Oxygen evolution reaction steps analyzed using coordination number as collective variables. Formation of OOH* species occurs when coordination number (Os-Oaw) ≈ 0.3. Transition from OOH* to O2* is barrierless with rapid proton abstraction. The calculated free energy barrier for the O→O2 transition (∆G‡ O→O2) is 1.57 eV for BaTiO3 and 1.20 eV for Ni/BaTiO3. The oxygen desorption step is endothermic, with ∆GO→O2 values of 1.37 eV for BaTiO3 and 0.97 eV for Ni/BaTiO3. MLP models enable longer simulation times with DFT-level accuracy, improving efficiency compared to ab initio MD. |
| Covalent triazine frameworks (CTF)/BaTiO3 photoanodes [186] | DFT calculations using VASP 6.3.0. PBE functional within GGA. Plane-wave energy cutoff: 500 eV. K-mesh: 8 × 8 × 8 for bulk and 3 × 2 × 1 for supercell BaTiO3-x. BaTiO3-x slab modeled with (001) surface and (3 × 3 × 1) supercell with 30 Å vacuum. CTF/BaTiO3-x model constructed by depositing CTF on BaTiO3-x slab. | Introduction of CTF reduces the rate-determining step energy barrier from 1.03 eV to 0.84 eV, enhancing oxygen evolution reaction kinetics. The CTF/BaTiO3−x photoanode achieves a high photocurrent density of 0.83 mA/cm2 at 1.23 V and a low onset potential of 0.23 V. CTF acts as a protective layer, improving stability for real water redox reactions. Provides a universal strategy for organic/inorganic hybrid photoanodes with high photoconversion efficiency. |
| Designed Systems | Methods | Main Findings |
|---|---|---|
| BaTiO3 surface and its interaction with OH− ions in an electrolyte [59] | DFT calculation. Materials Studio. Classical all-atom MD Simulations. Forcite module in Materials Studio. COMPASSIII force field. Electric field of 0.01 eV/Å applied to study positive polarization effects. | Higher OH− concentration leads to increased adsorption on the BaTiO surface. At a 10:1 (H2O:OH−) ratio, adsorption is significantly higher compared to a 50:1 ratio. At a 50:1 (H2O:OH−) ratio, polarization significantly impacts OH− adsorption, but at higher OH− concentrations, the effect diminishes. Polarization field enhances photoanode performance in near-neutral conditions by improving surface states and hole collection efficiency. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abuova, A.U.; Tolegen, U.Z.; Inerbaev, T.M.; Karibayev, M.; Satanova, B.M.; Abuova, F.U.; Popov, A.I. A Brief Review of Atomistic Studies on BaTiO3 as a Photocatalyst for Solar Water Splitting. Ceramics 2025, 8, 100. https://doi.org/10.3390/ceramics8030100
Abuova AU, Tolegen UZ, Inerbaev TM, Karibayev M, Satanova BM, Abuova FU, Popov AI. A Brief Review of Atomistic Studies on BaTiO3 as a Photocatalyst for Solar Water Splitting. Ceramics. 2025; 8(3):100. https://doi.org/10.3390/ceramics8030100
Chicago/Turabian StyleAbuova, Aisulu U., Ulzhan Zh. Tolegen, Talgat M. Inerbaev, Mirat Karibayev, Balzhan M. Satanova, Fatima U. Abuova, and Anatoli I. Popov. 2025. "A Brief Review of Atomistic Studies on BaTiO3 as a Photocatalyst for Solar Water Splitting" Ceramics 8, no. 3: 100. https://doi.org/10.3390/ceramics8030100
APA StyleAbuova, A. U., Tolegen, U. Z., Inerbaev, T. M., Karibayev, M., Satanova, B. M., Abuova, F. U., & Popov, A. I. (2025). A Brief Review of Atomistic Studies on BaTiO3 as a Photocatalyst for Solar Water Splitting. Ceramics, 8(3), 100. https://doi.org/10.3390/ceramics8030100









