The Effect of Frankincense and Myrrh on the Sealing Ability and Hardness of Glass Ionomer Cement
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of Frankincense and Myrrh Extracts
2.2. Tooth Selection and Cavity Preparation
- Group A (Control): Cavities restored with conventional GIC using a 1:1 powder-to-liquid ratio as per the manufacturer’s instructions.
- Group B: Cavities restored with 15% frankincense, using a 1:1 powder-to-modified liquid ratio (17 drops of GIC liquid mixed with 3 drops of frankincense extract = modified liquid).
- Group C: Cavities restored with 25% frankincense, using a 1:1 powder-to-modified liquid ratio (3 drops of GIC liquid mixed with 1 drop of frankincense extract = modified liquid).
- Group D: Cavities restored with 15% myrrh, using a 1:1 powder-to-modified liquid ratio (17 drops of GIC liquid mixed with 3 drops of myrrh extract = modified liquid).
- Group E: Cavities restored with 25% myrrh, using a 1:1 powder-to-modified liquid ratio (3 drops of GIC liquid mixed with 1 drop of myrrh extract = modified liquid).
2.3. SEM Specimen Preparation and Evaluation
2.4. Specimen Preparation for Microhardness Testing
- Group A: Conventional GIC (control group).
- Group B: GIC with 15% frankincense extract.
- Group C: GIC with 25% frankincense extract.
- Group D: GIC with 15% myrrh extract.
- Group E: GIC with 25% myrrh extract.
2.5. Microhardness Testing
2.6. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| GIC | Glass ionomer cement |
| g | Gram |
| mL | Milliliter |
| SEM | Scanning electron microscopy |
| µm | Micrometers |
References
- Garcia-Contreras, R.; Vilchis, R.J.; Scougall Contreras-Bulnes, R.; Sakagami, H.; Morales-Luckie, R.A.; Nakajima, H. Mechanical, antibacterial and bond strength properties of nano-titanium-enriched glass ionomer cement. J. Appl. Oral Sci. 2015, 23, 321–328. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Sidhu, S.K.; Nicholson, J.W. A Review of Glass-Ionomer Cements for Clinical Dentistry. J. Funct. Biomater. 2016, 7, 16. [Google Scholar] [CrossRef] [PubMed]
- Khadim, H.J.; Ghalib, L. The effect of frankincense chewing gum on the surface roughness and hardness of GIC. J. Eng. Sustain. Dev. 2023, 148–156. [Google Scholar] [CrossRef]
- Upadhya, N.; Kishore, G. Glass ionomer cement: The different generations. Trends Biomater. Artif. Organs. 2005, 18, 158–165. [Google Scholar]
- Prentice, L.H.; Tyas, M.J.; Burrow, M.F. The effect of ytterbium fluoride and barium sulphate nanoparticles on the reactivity and strength of a glass-ionomer cement. Dent. Mater. 2006, 22, 746–751. [Google Scholar] [CrossRef]
- Moshaverinia, A.; Ansari, S.; Moshaverinia, M.; Roohpour, N.; Darr, J.A.; Rehman, I. Effects of incorporation of hydroxyapatite and fluoroapatite nanobioceramics into conventional glass ionomer cements (GIC). Acta Biomater. 2008, 4, 432–440. [Google Scholar] [CrossRef]
- Silva, R.M.; Pereira, F.V.; Mota, F.A.; Watanabe, E.; Soares, S.M.; Santos, M.H. Dental glass ionomer cement reinforced by cellulose microfibres and cellulose nanocrystals. Mater. Sci. Eng. 2016, 58, 389–395. [Google Scholar] [CrossRef]
- Bilić-Prcić, M.; Rajić, V.B.; Ivanišević, A.; Pilipović, A.; Gurgan, S.; Miletić, I. Mechanical Properties of Glass Ionomer Cements after Incorporation of Marine Derived Hydroxyapatite. Materials 2020, 13, 3542. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Zhao, Q.; Peng, J.; Yang, X.; Yu, D.; Zhao, W. Antibacterial and mechanical properties of reduced graphene-silver nanoparticle nanocomposite modified glass ionomer cements. J. Dent. 2020, 96, 103332. [Google Scholar] [CrossRef] [PubMed]
- Rini, A.D.K.; Juwita, F.T.; Bagjana, R.W.; Octivany, S.; Purnama, R.B.; Rijal, M.S.; Anwar, A.M.; Purwasasmita, B.S.; Asri, L.A.T. Improving the mechanical properties of glass ionomer cement with nanocrystalline cellulose from rice husk. J. Biomed. Mater. Res. B Appl. Biomater. 2024, 112, e35472. [Google Scholar] [CrossRef]
- Singer, L.; Bourauel, C. Herbalism and glass-based materials in dentistry: Review of the current state of the art. J. Mater. Sci. Mater. Med. 2023, 34, 60. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, N.A.; Elsheikh, H.M.; Elhousiny, M.A. Effect of Using Boswellia Sacra Extract as Final Irrigant on Removal of Smear Layer. Al-Azhar J. Dent. 2021, 8, 55–63. [Google Scholar] [CrossRef]
- Al-Hazzaa, A.M.; Badr, A.E.; Kataia, E.M. Physical properties of root canal sealers. Mansoura J. Dent. 2020, 7, 62–70. [Google Scholar] [CrossRef]
- Ilyas, K.; Singer, L.; Akhtar, M.A.; Bourauel, C.P.; Boccaccini, A.R. Boswellia sacra extract-loaded mesoporous bioactive glass nano particles: Synthesis and biological effects. Pharmaceutics 2022, 14, 126. [Google Scholar] [CrossRef]
- Abdallah, R.M.; Abdelghany, A.M.; Aref, N.S. Myrrh Addition to a conventional Glass-Ionomer Cement: Influence on Physical and Antibacterial Properties. Egypt. Dent. J. 2016, 62, 1491. [Google Scholar] [CrossRef]
- Al-Madi, E.M.; Almohaimede, A.A.; Al-Obaida, M.I.; Awaad, A.S. Comparison of the Antibacterial Efficacy of Commiphora molmol and Sodium Hypochlorite as Root Canal Irrigants against Enterococcus faecalis and Fusobacterium nucleatum. Evid.-Based Complement. Altern. Med. 2019, 6916795. [Google Scholar] [CrossRef]
- Kim, J.W.; Park, S.; Sung, Y.W.; Song, H.J.; Yang, S.W.; Han, J.; Jo, J.W.; Lee, I.S.; Lee, S.H.; Choi, Y.K. Evaluation of antibacterial and antiviral compounds from Commiphora myrrha (T.Nees) engl. resin and their promising application with biochar. Appl. Sci. 2023, 13, 10549. [Google Scholar] [CrossRef]
- Refaey, M.S.; Abosalem, E.F.; Yasser El-Basyouni, R.; Elsheriri, S.E.; Elbehary, S.H.; Fayed, M.A. Exploring the therapeutic potential of medicinal plants and their active principles in dental care: A comprehensive review. Heliyon 2024, 10, e37641. [Google Scholar] [CrossRef]
- Coelho, A.; Amaro, I.; Rascão, B.; Marcelino, I.; Paula, A.; Saraiva, J.; Spagnuolo, G.; Ferreira, M.M.; Marto, C.M.; Carrilho, E. Effect of cavity disinfectants on dentin bond strength and clinical success of composite restorations—A systematic review of In Vitro, In Situ and Clinical Studies. Int. J. Mol. Sci. 2021, 22, 353. [Google Scholar] [CrossRef] [PubMed]
- Panahandeh, N.; Sheikholeslamian, M.; Farzaneh, H. Microleakage in Class V Cavities Restored with Sandwich Technique: Self-Etch versus Total-Etch Bonding Systems. J. Dent. Sch. 2015, 33, 74–79. [Google Scholar]
- Kharouf, N.; Reitzer, F.; Ashi, T.; Daras, N.; Haikel, Y.; Mancino, D. Effectiveness of etching with phosphoric acid when associated with rubbing technique. J. Stomatol. 2021, 74, 16–21. [Google Scholar] [CrossRef]
- Samy, F.M.; El-Kholany, N.R.; Hamama, H.H. Micromorphological Analysis of Different Bioactive Restorative Materials/Dentin interface: A Comparative In Vitro Study. Egypt. Dent. J. 2024, 70, 3909–3916. [Google Scholar] [CrossRef]
- Ibrahim, B.; Elkholany, N.; Enab, T.; Zaghloul, N. Marginal adaptation of self adhesive bulk-fill resin composite vs conventional resin composite and resin modified glass- ionomer: A comparative study. Egypt. Dent. J. 2024, 70, 3879–3886. [Google Scholar] [CrossRef]
- Elgezawi, M.; Haridy, R.; Abdalla, M.A.; Heck, K.; Draenert, M.; Kaisarly, D. Current Strategies to Control Recurrent and Residual Caries with Resin Composite Restorations: Operator- and Material-Related Factors. J. Clin. Med. 2022, 11, 6591. [Google Scholar] [CrossRef]
- Pontes, D.G.; Guedes-Neto, M.V.; Cabral, M.F.; Cohen-Carneiro, F. Microleakage evaluation of class V restorations with conventional and resin-modified glass ionomer cements. Oral Health Dent. Manag. 2014, 13, 642–646. [Google Scholar]
- Ching, H.S.; Luddin, N.; Kannan, T.P.; AbRahman, I.; Abdul Ghani, N.R.N. Modification of glass ionomer cements on their physical-mechanical and antimicrobial properties. J. Esthet. Restor. Dent. 2018, 30, 557–571. [Google Scholar] [CrossRef]
- Ruengrungsom, C.; Burrow, M.F.; Parashos, P.; Palamara, J.E.A. Comprehensive characterisation of flexural mechanical properties and a newclassification for porosity of 11 contemporary ion-leaching dental restorative materials. J. Mech. Behav. Biomed. Mater. 2021, 121, 104615. [Google Scholar] [CrossRef]
- Santos, M.J.M.C.; Leon, L.; Siddique, I.; Butler, S. Retrospective Clinical Evaluation of RMGIC/GIC Class V Restorations. Dent. J. 2023, 11, 225. [Google Scholar] [CrossRef] [PubMed]
- Baroudi, K.; Mahmoud, S. Improving Composite Resin Performance Through Decreasing its Viscosity by Different Methods. Open Dent. J. 2015, 9, 235–242. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, Y.; Imazato, S.; Kaneshiro, A.V.; Ebisu, S.; Frencken, J.E.; Tay, F.R. Antibacterial effects and physical properties of glass-ionomer cements containing chlorhexidine for the ART approach. Dent. Mater. 2006, 22, 647–652. [Google Scholar] [CrossRef]
- Nicholson, J.W.; Sidhu, S.K.; Czarnecka, B. Enhancing the mechanical properties of glass-ionomer dental cements: A review. Materials 2020, 13, 2510. [Google Scholar] [CrossRef]
- Shivanna, S.; Roshan, S.; Sameera, S.; Sivakumar, M.; Ravi, M.; Ram, L.S. Comparative evaluation of hardness in ceramic reinforced, resin modified glass ionomer cement and conventional glass ionomer cement. J. Pharm. Bioall. Sci. 2025, 17, S1916–S1919. [Google Scholar] [CrossRef]
- Saadat, M.; Moradian, M.; Mirshekari, B. Evaluation of the surface hardness and roughness of a resin-modified glass ionomer cement containing bacterial cellulose nanocrystals. Int. J. Dent. 2021, 8231473. [Google Scholar] [CrossRef] [PubMed]
- Siddiqui, A.Z.; Sultana, N.; Mirani, Z.A.; Alkhureif, A.A.; Rehan, F.; Siddiqui, I.A. Enhancing the antibacterial and surface hardness of glass ionomer cement modified with Salvadora persica and Chlorhexidine: An in vitro study. Pak. J. Med. Sci. 2024, 40, 1808–1812. [Google Scholar] [CrossRef] [PubMed]
- Xie, D.; Brantley, W.A.; Culbertson, B.M.; Wang, G. Mechanical properties and microstructures of glass-ionomer cements. Dent. Mater. 2000, 16, 129–138. [Google Scholar] [CrossRef] [PubMed]
- Sharafeddin, F.; Shirani, M.M.; Jowkar, Z. Assessing the Impact of Nano-Graphene Oxide Addition on Surface Microhardness and Roughness of Glass Ionomer Cements: A Laboratory Study. Int. J. Dent. 2024, 5597367. [Google Scholar] [CrossRef]
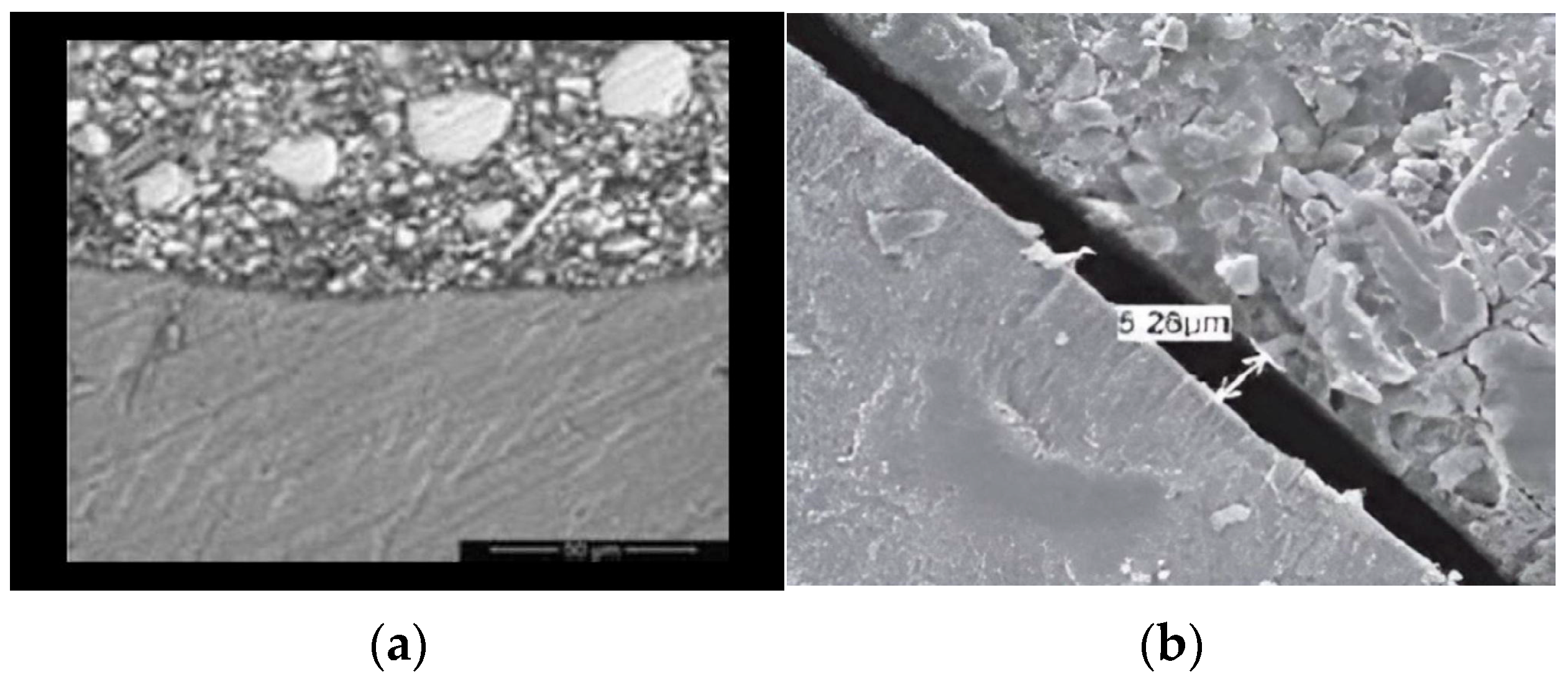
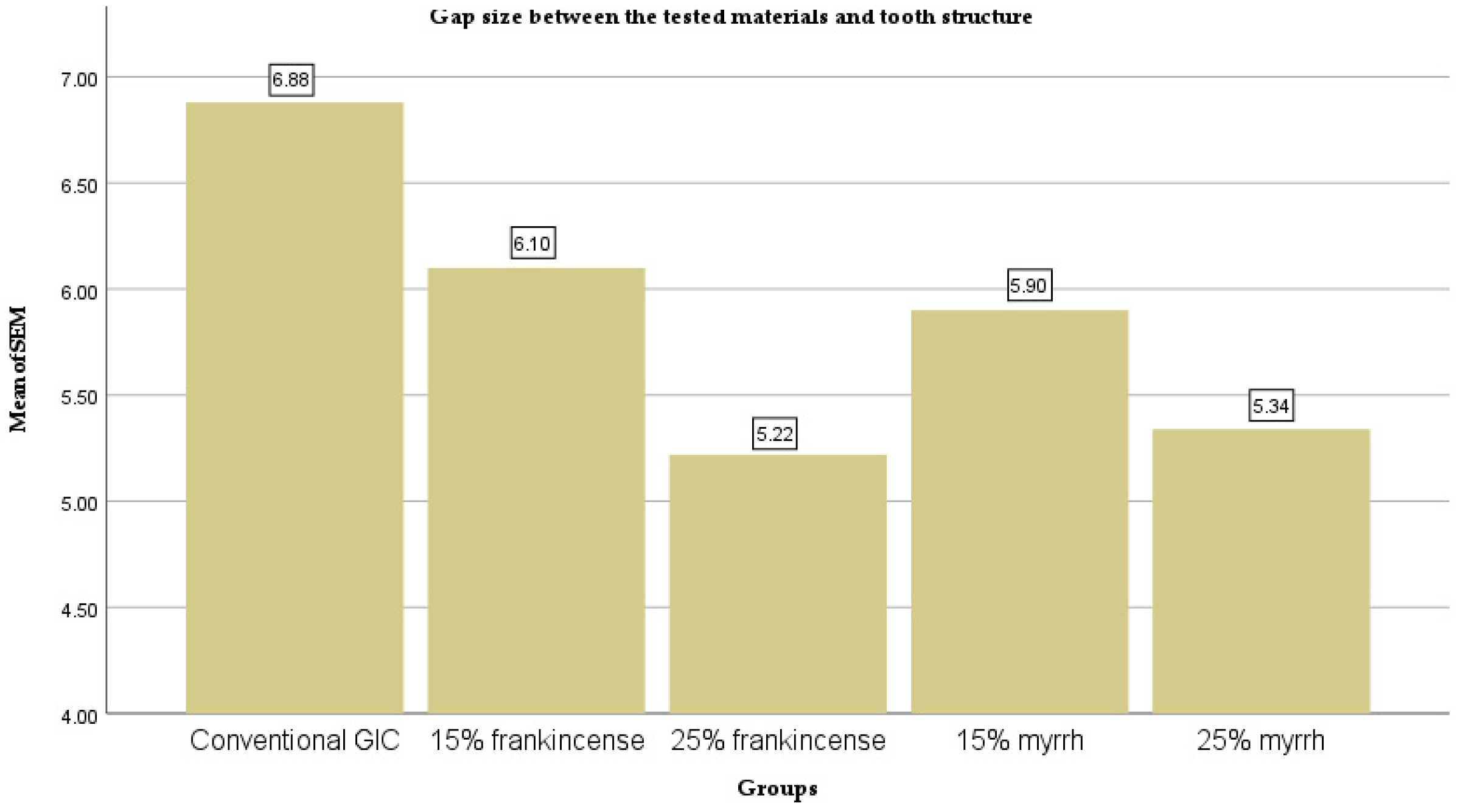
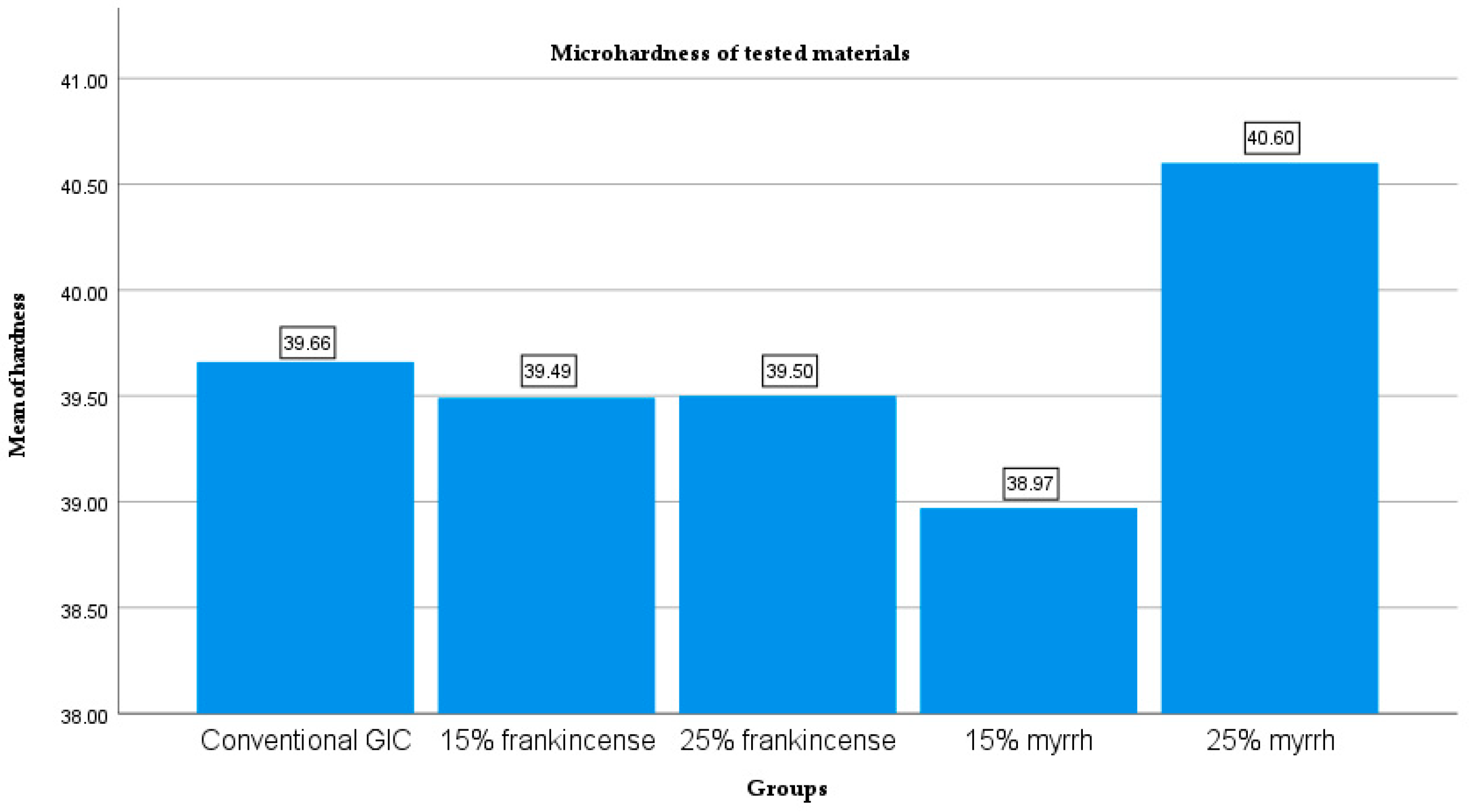
| Groups | N | Mean (µm) | Std. Deviation | Std. Error | 95% Confidence Interval for Mean | Minimum | Maximum | |
|---|---|---|---|---|---|---|---|---|
| Lower | Upper | |||||||
| Conventional GIC | 10 | 6.88 | ±0.37 | 0.12 | 6.62 | 7.14 | 6.23 | 7.44 |
| 15% frankincense | 10 | 6.1 | ±0.41 | 0.13 | 5.80 | 6.40 | 5.16 | 6.73 |
| 25% frankincense | 10 | 5.22 | ±0.29 | 0.09 | 5.01 | 5.43 | 4.91 | 5.91 |
| 15% myrrh | 10 | 5.9 | ±0.46 | 0.14 | 5.57 | 6.23 | 4.91 | 6.52 |
| 25% myrrh | 10 | 5.34 | ±0.26 | 0.08 | 5.16 | 5.52 | 4.99 | 5.81 |
| Groups | Conventional GIC | 15% Frankincense | 25% Frankincense | 15% Myrrh | 25% Myrrh |
|---|---|---|---|---|---|
| Conventional GIC | - | <0.001 | <0.001 | <0.001 | <0.001 |
| 15% frankincense | <0.001 | - | <0.001 | 0.241 | 0.001 |
| 25% frankincense | <0.001 | <0.001 | - | <0.01 | 0.241 |
| 15% myrrh | <0.001 | 0.241 | <0.01 | - | <0.01 |
| 25% myrrh | <0.001 | 0.001 | 0.241 | <0.01 | - |
| Groups | N | Mean | Std. Deviation | Std. Error | 95% Confidence Interval for Mean | Minimum | Maximum | |
|---|---|---|---|---|---|---|---|---|
| Lower Bound | Upper Bound | |||||||
| Conventional GIC | 10 | 39.66 | ±1.98 | 0.63 | 38.24 | 41.08 | 37.10 | 43.30 |
| 15% frankincense | 10 | 39.49 | ±2.95 | 0.93 | 37.38 | 41.60 | 34.10 | 44.10 |
| 25% frankincense | 10 | 39.50 | ±2.83 | 0.89 | 37.48 | 41.52 | 34.10 | 44.40 |
| 15% myrrh | 10 | 38.97 | ±1.87 | 0.59 | 37.63 | 40.31 | 36.30 | 42.10 |
| 25% myrrh | 10 | 40.60 | ±2.28 | 0.72 | 38.97 | 42.23 | 37.20 | 44.20 |
| Sum of Squares | Df | Mean Square | F | Sig. | |
|---|---|---|---|---|---|
| Between Groups | 14.129 | 4 | 3.532 | 0.603 | 0.662 |
| Within Groups | 263.494 | 45 | 5.855 | ||
| Total | 277.623 | 49 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hanna, H.; Azeez, N.; Bakr, D.K.; Saeed, M. The Effect of Frankincense and Myrrh on the Sealing Ability and Hardness of Glass Ionomer Cement. Ceramics 2025, 8, 101. https://doi.org/10.3390/ceramics8030101
Hanna H, Azeez N, Bakr DK, Saeed M. The Effect of Frankincense and Myrrh on the Sealing Ability and Hardness of Glass Ionomer Cement. Ceramics. 2025; 8(3):101. https://doi.org/10.3390/ceramics8030101
Chicago/Turabian StyleHanna, Hala, Nsar Azeez, Diyar Khalid Bakr, and Media Saeed. 2025. "The Effect of Frankincense and Myrrh on the Sealing Ability and Hardness of Glass Ionomer Cement" Ceramics 8, no. 3: 101. https://doi.org/10.3390/ceramics8030101
APA StyleHanna, H., Azeez, N., Bakr, D. K., & Saeed, M. (2025). The Effect of Frankincense and Myrrh on the Sealing Ability and Hardness of Glass Ionomer Cement. Ceramics, 8(3), 101. https://doi.org/10.3390/ceramics8030101






