Decarbonisation of Earthenware Ceramic Production Using Bivalve Shell Waste
Abstract
1. Introduction
2. Materials and Methods
2.1. Raw Materials
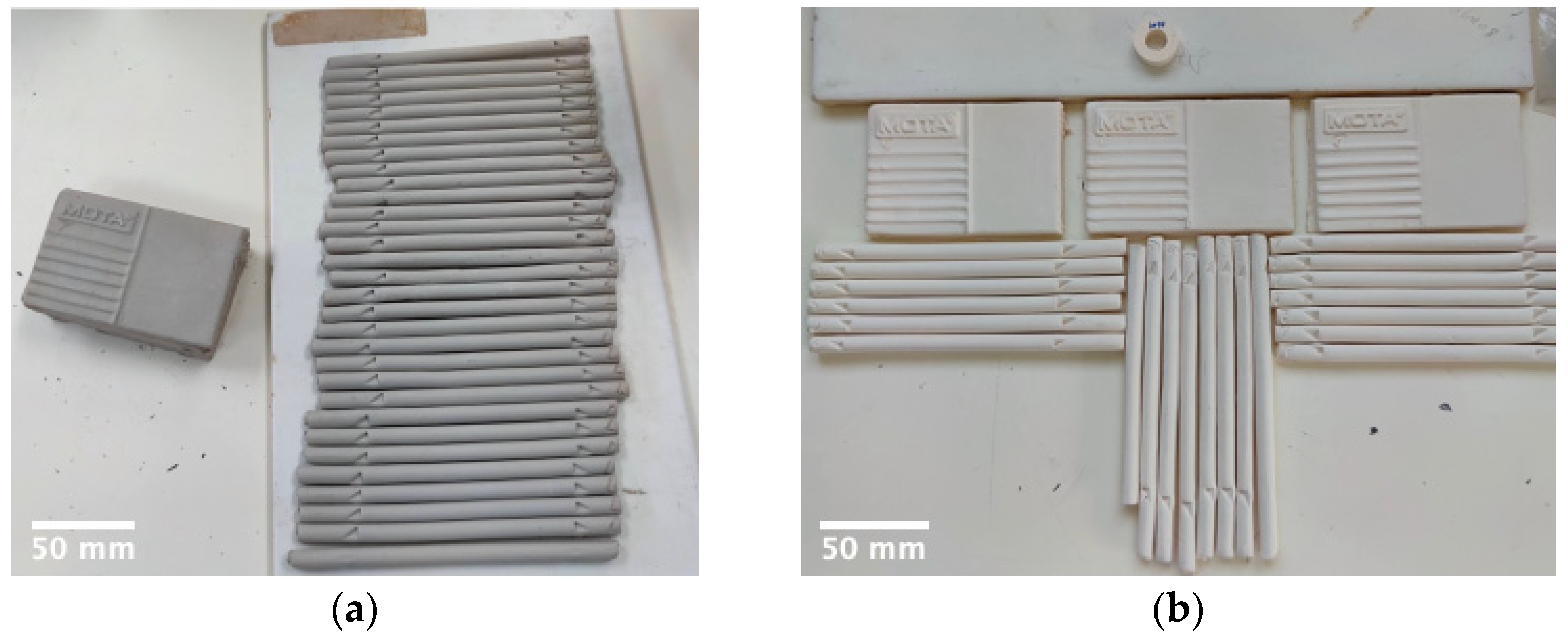
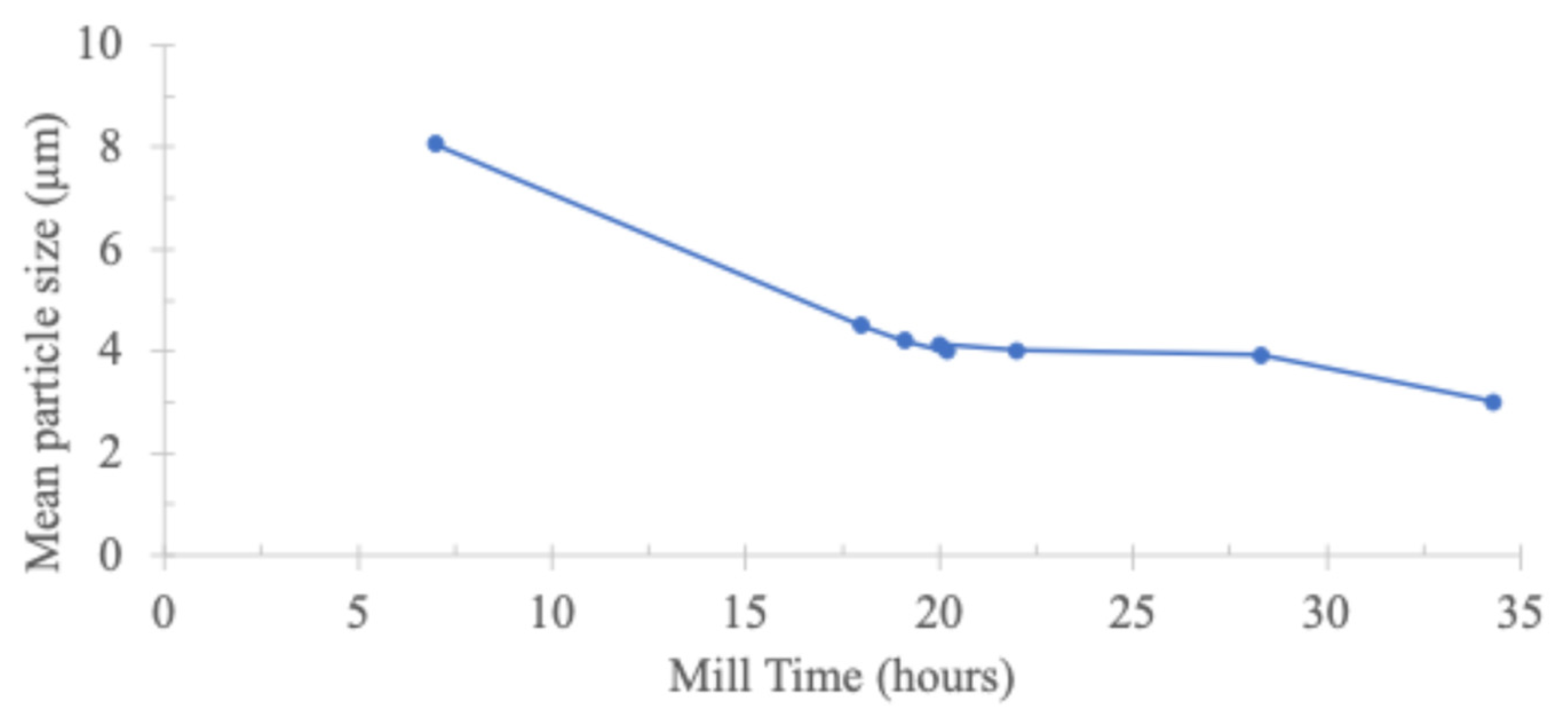
2.2. Laboratory Scale Specimen Preparation
2.3. Pilot Plant Specimen and Prototype Preparation
2.4. Characterisation Techniques
2.4.1. Calcium Carbonates and Ceramic Bodies
2.4.2. Specimens and Prototypes
3. Results and Discussion
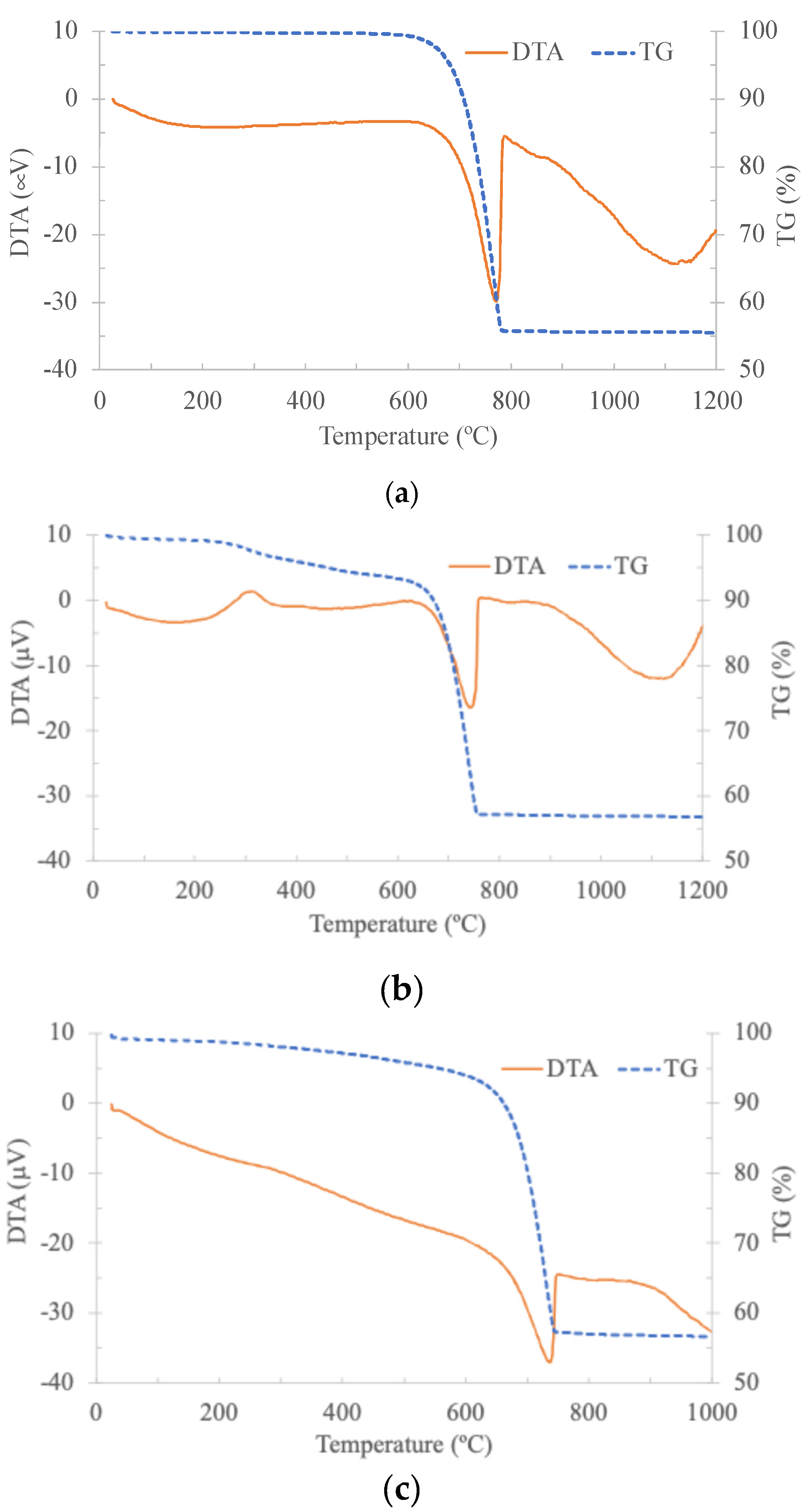
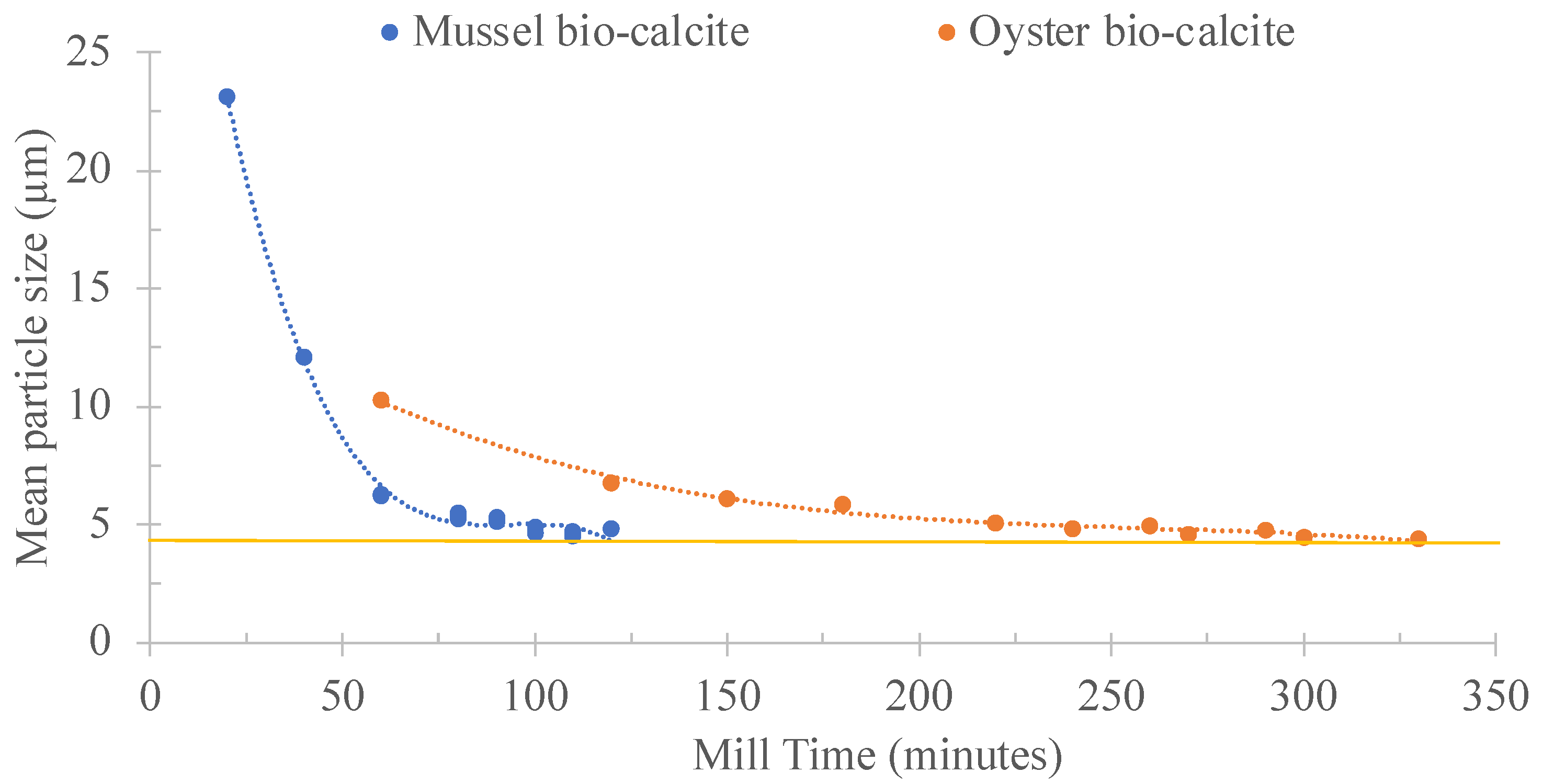
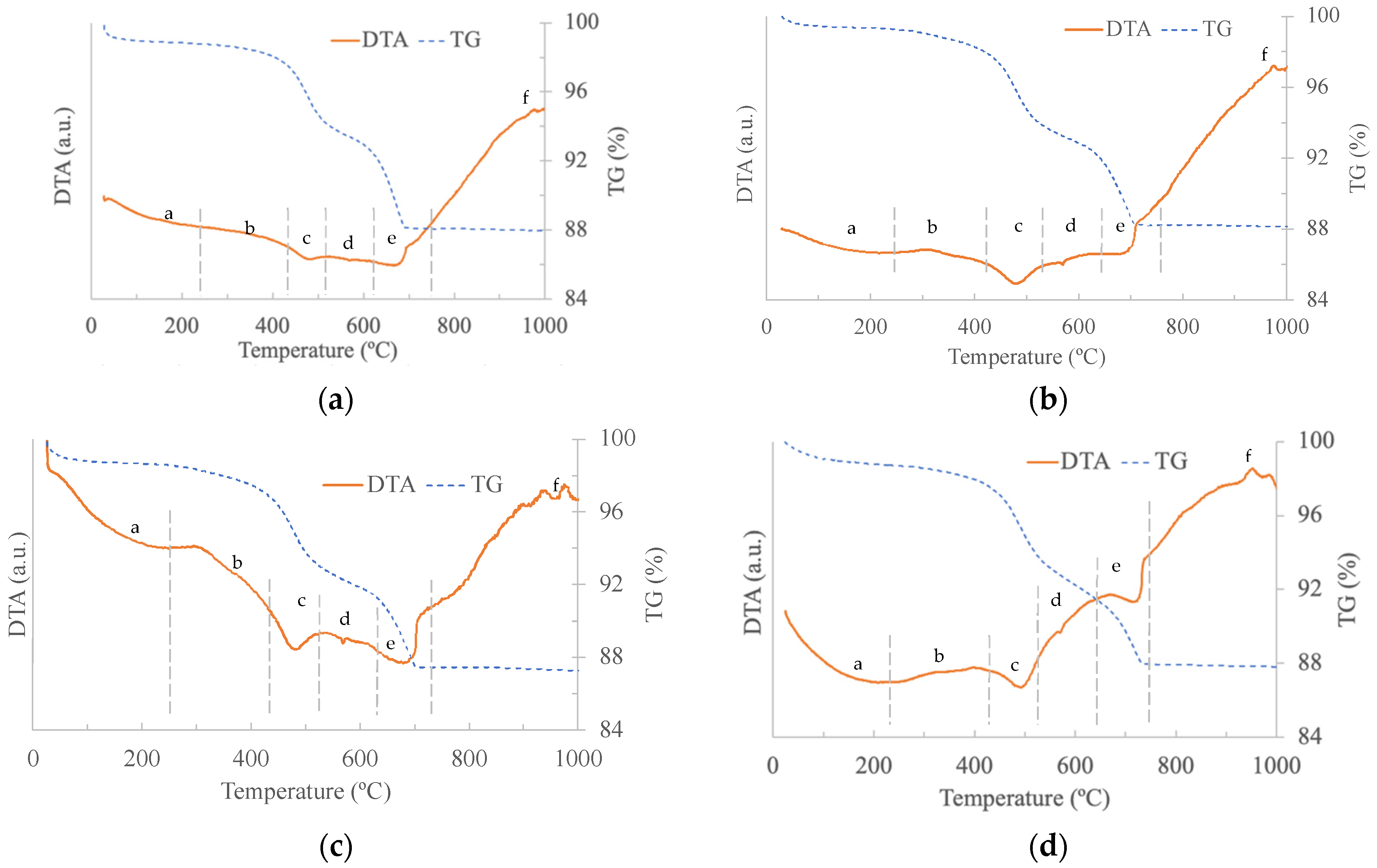
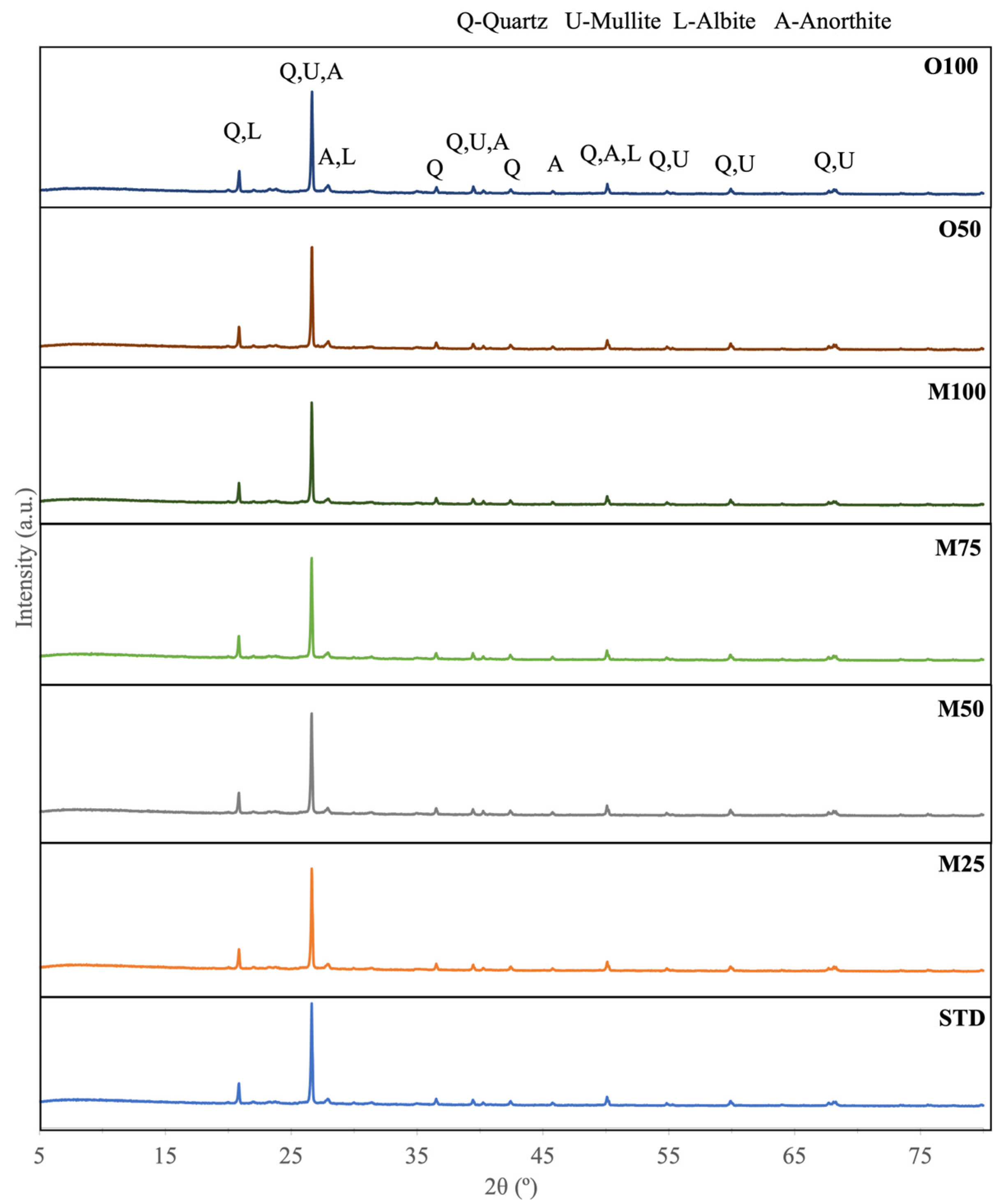
3.1. Calcium Carbonate Sources and Paste Characterisation
3.2. Laboratory Results
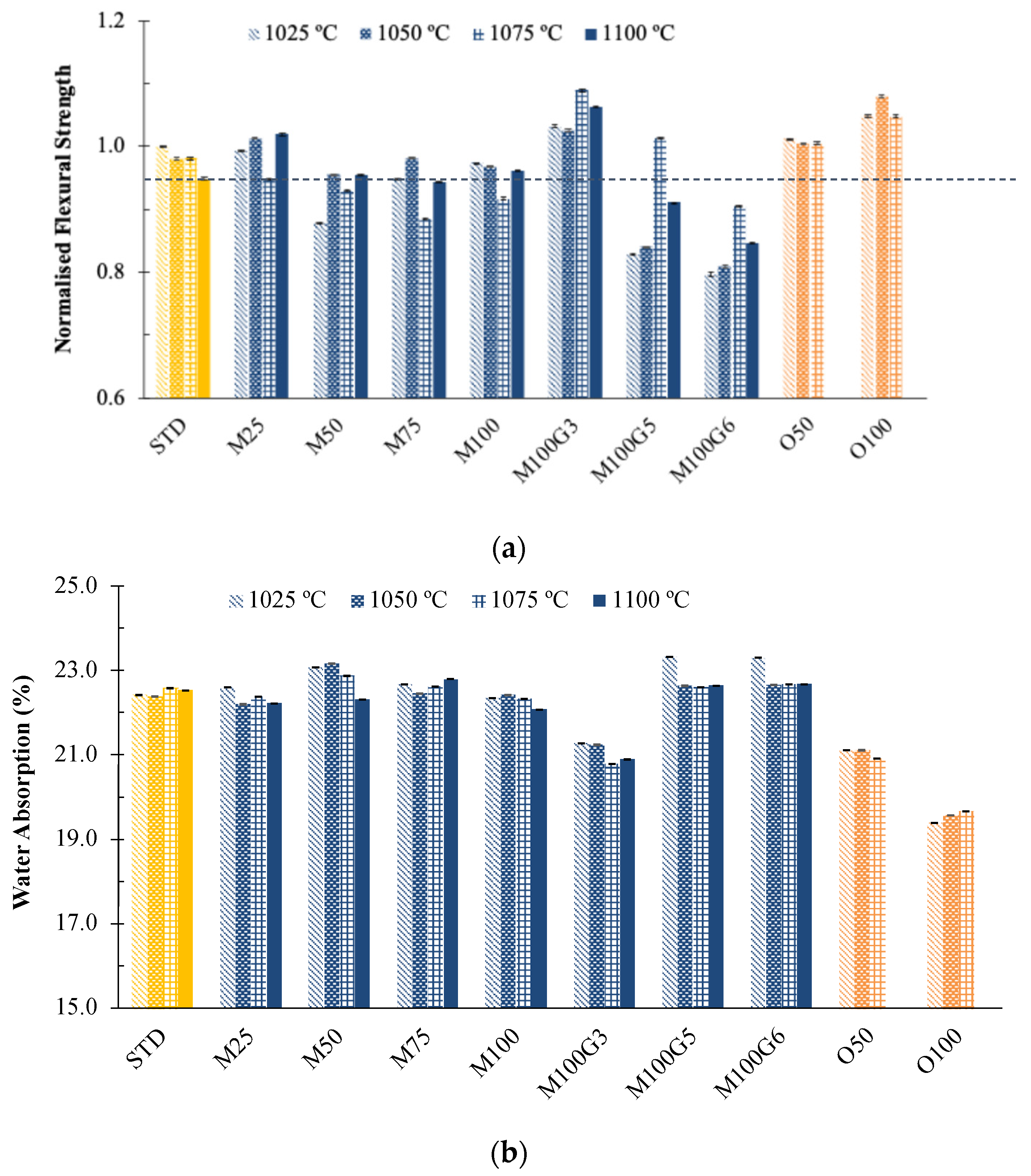
3.3. Pilot Plant Scale Results
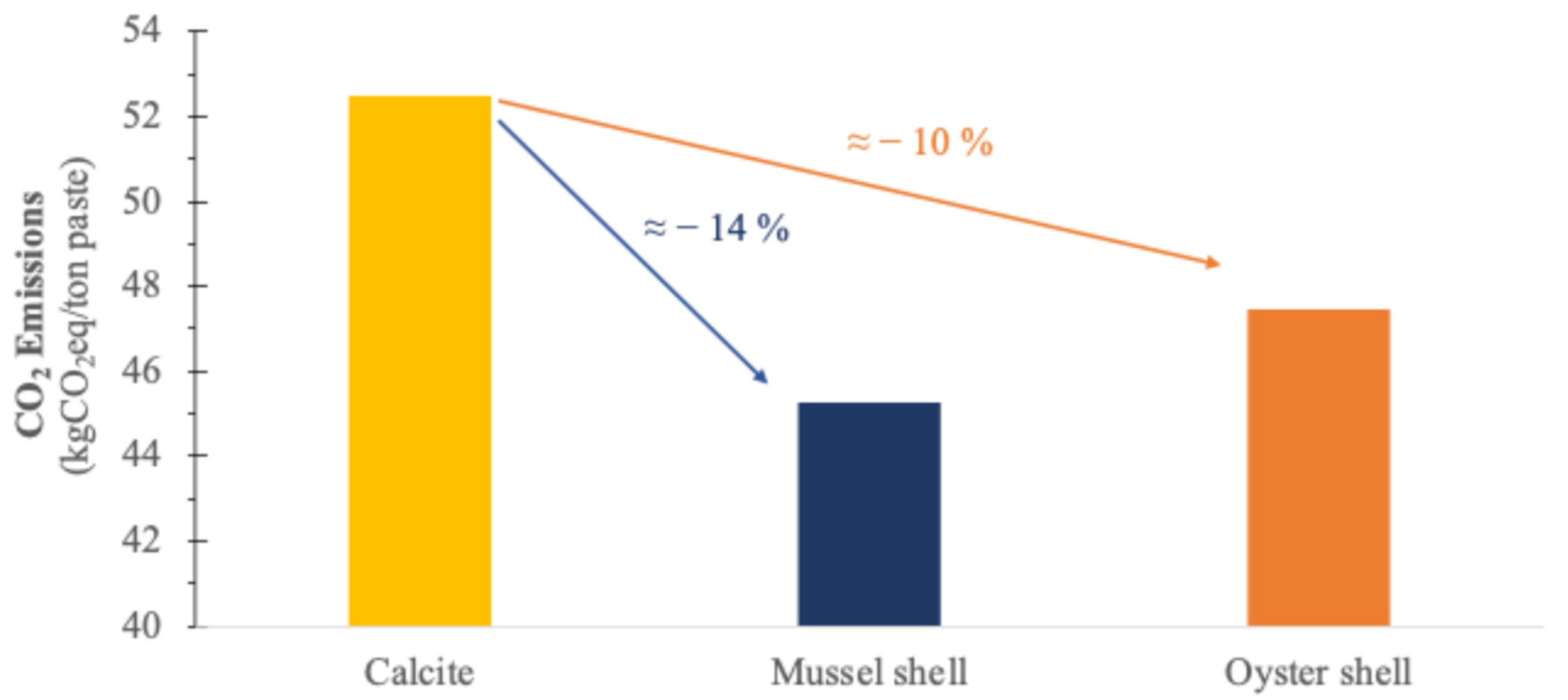
3.4. CO2 Emissions from the Different Calcium Carbonate Sources
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Cerame-Unie TECIA. Ceramic Roadmap to 2050—Continuing Our Path Towards Climate Neutrality; Cerame-Unie TECIA: Brussels, Belgium, 2021. [Google Scholar]
- Eurostat EU. EU’s Circular Material Use Rate Slightly Up in 2022. 2021. Available online: https://ec.europa.eu/eurostat/en/web/products-eurostat-news/w/ddn-20231114-2 (accessed on 5 July 2024).
- CCA-Europe CCAE Aisbl. Calcium Carbonate is a Renewable Raw Material. 2020. Available online: https://ima-europe.eu/wp-content/uploads/2021/12/Renewability-Statement.pdf (accessed on 5 July 2024).
- CCA-Europe CCAE Aisbl. Life Cycle Analysis for Calcium Carbonate. 2021. Available online: https://calcium-carbonate.org.uk/media/dcefn104/cca-lca-pcc-slurry.pdf (accessed on 5 July 2024).
- The UBA TU. Resource Use and Its Consequences. 2019. Available online: https://www.umweltbundesamt.de/en/topics/waste-resources/resource-use-its-consequences#:~:text=In%20short%2C%20raw%20material%20extraction,functions%20and%20global%20warming%20exacerbation (accessed on 5 July 2024).
- Souidi, A.; Maaloufa, Y.; Amazal, M.; Atigui, M.; Oubeddou, S.; Mounir, S.; Idoum, A.; Aharoune, A. The effect of mussel shell powder on the thermal and mechanical properties of plaster. Constr. Build. Mater. 2024, 416, 135142. [Google Scholar] [CrossRef]
- Shahroom, A.; Shapawi, R.; Mustafa, S.; Abd Halid, N.F.; Estim, A.; Tuzan, A.D. Effects of eggshell and seashell powder as natural dietary calcium supplements on growth, molting frequency, and carapace calcium composition of juvenile red claw crayfish, Cherax quadricarinatus. PeerJ 2023, 11, e15449. [Google Scholar] [CrossRef] [PubMed]
- Borciani, G.; Fischetti, T.; Ciapetti, G.; Montesissa, M.; Baldini, N.; Graziani, G. Marine biological waste as a source of hydroxyapatite for bone tissue engineering applications. Ceram. Int. 2023, 49, 1572–1584. [Google Scholar] [CrossRef]
- Peksen, C.; Koroglu, L.; Kartal, H. Utilization of seashells in matte glaze preparation. Int. J. Appl. Ceram. Technol. 2020, 17, 1940–1947. [Google Scholar] [CrossRef]
- Peceño, B.; Alonso-Fariñas, B.; Vilches, L.F.; Leiva, C. Study of seashell waste recycling in fireproofing material: Technical, environmental, and economic assessment. Sci. Total Environ. 2021, 790, 148102. [Google Scholar] [CrossRef] [PubMed]
- Baláž, M. Shells and Other Calcium Carbonate-Based Waste. In Environmental Mechanochemistry; Springer: Berlin/Heidelberg, Germany, 2021. [Google Scholar]
- Vilarinho, I.S.; Filippi, E.; Seabra, M.P. Development of eco-ceramic wall tiles with bio-CaCO3 from eggshells waste. Open Ceram. 2022, 9, 100220. [Google Scholar] [CrossRef]
- Vieira, I.; Vilarinho, I.S.; Buruberri, L.; Carneiro, J.; Seabra, M.P. Upcycling process of mill scale waste into high-value ceramic products. Ceram. Int. 2024, 50, 36800–36810. [Google Scholar] [CrossRef]
- ISO 10545-4:2019; Ceramic Tiles—Part 4: Determination of Modulus of Rupture and Breaking Strength. International Organization for Standardization: Geneva, Switzerland, 2019.
- BS EN 1217:1998; Materials and Articles in Contact With foodstuffs. Test Methods for Water Absorption of Ceramic Articles. British and European Standards: London, UK, 1998.
- ISO 10545-11:1996; Ceramic Tiles—Part 11: Determination of Crazing Resistance for Glazed Tiles. International Organization for Standardization: Geneva, Switzerland, 1996.
- Viczián, I.; Mária, F. Handbook of the thermogravimetric system of minerals and its use in geological practice. Cent. Eur. Geol. 2013, 56, 397–400. [Google Scholar] [CrossRef]
- Joaquim, M.; Ribeiro, P.M.; Abrantes, C. Moagem em moinho de bolas: Estudo de algumas variáveis e otimização energética do processo. Rev. Cerâmica Ind. 2001, 6, 7–11. [Google Scholar]
- Agrawal, P.; Misra, S.N. Irreversible Dilatometry as a Tool for Body Composition and Firing Schedule Design in Traditional Ceramics. Trans. Indian Ceram. Soc. 2014, 73, 14–21. [Google Scholar] [CrossRef]
- Zouaoui, H.; Bouaziz, J. Physical and mechanical properties improvement of a porous clay ceramic. Appl. Clay Sci. 2017, 150, 131–137. [Google Scholar] [CrossRef]
- Mücke, U. Ceramic microstructures. Property control by processing. von W. E. Lee und W. M. Rainforth, 590 Seiten, Zahlreiche Abbildungen, Chapman & Hall, London, Glasgow, Weinheim. New York, Tokio, Melbourne, Madras, 1994, £ 89.00, ISBN 0-412-43140-8. Mater. Corros. 1996, 47, 346–347. [Google Scholar] [CrossRef]
- Mokrzycki, W.S.; Tatol, M. Colour difference Delta-E—A survey. Mach. Graph. Vis. 2011, 20, 383–411. [Google Scholar]
- European Union. Commission Implementing Regulation (EU) 2018/2066 of 19 December 2018 on the Monitoring and Reporting of Greenhouse gas Emissions; Official Journal of the European Union: Luxembourg, 2018. [Google Scholar]
- EC. Regulation (EC) No 1069/2009, Animal by-Products Regulation; Official Journal of the European Union: Luxembourg, 2009; 300p. [Google Scholar]
- Statista. Daily European Union Emission Trading System (EU-ETS) Carbon Pricing from 2022 to 2024. 2024. Available online: https://www.statista.com/statistics/1322214/carbon-prices-european-union-emission-trading-scheme/#:~:text=The%20price%20of%20emissions%20allowances,reform%20of%20the%20EU%2DETS (accessed on 26 June 2024).



| Calcium Carbonate (wt.%) | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Reference | STD | M25 | M50 | M75 | M100 | M100G3 | M100G5 | M100G6 | O50 | O100 | |
| CaCO3 Source | Calcite | 100 | 75 | 50 | 25 | - | - | - | - | 50 | - |
| Mussel c | - | 25 | 50 | 75 | 100 | 100 | 100 | 100 | - | - | |
| Oyster c | - | - | - | - | - | - | - | - | 50 | 100 | |
| Components | Calcite | Mussel Shell | Oyster Shell |
|---|---|---|---|
| (wt.%) | |||
| CaO | 55.74 | 48.05 | 50.42 |
| Al2O3 | 0.25 | 0.31 | 0.54 |
| MgO | 0.32 | 0.42 | 0.52 |
| C | 8.74 | 9.52 | 8.42 |
| CaCO3 Source | Quantity of Material (kg) | Mill Time (h) | Efficiency (kg/h) |
|---|---|---|---|
| Calcite | 0.5 | 1 | 0.5 |
| Mussel bio-calcite | 1.83 | 0.27 | |
| Oyster bio-calcite | 5.25 | 0.10 |
| Component | STD | M50 | M100 | O50 | O100 |
|---|---|---|---|---|---|
| SiO2 | 60.80 | 61.08 | 60.74 | 60.44 | 60.32 |
| Al2O3 | 17.98 | 17.26 | 17.66 | 18.31 | 18.38 |
| K2O | 0.58 | 0.56 | 0.58 | 0.59 | 0.62 |
| Na2O | 0.26 | 0.16 | 0.19 | 0.29 | 0.35 |
| MgO | 0.28 | 0.18 | 0.20 | 0.29 | 0.29 |
| CaO | 6.51 | 6.41 | 6.22 | 6.46 | 6.35 |
| Fe2O3 | 0.62 | 0.62 | 0.63 | 0.61 | 0.65 |
| TiO2 | 0.24 | 0.25 | 0.25 | 0.25 | 0.25 |
| S | 0.03 | 0.03 | 0.04 | 0.03 | 0.02 |
| C | 1.51 | 1.71 | 1.69 | 1.51 | 1.49 |
| LOI | 11.18 | 11.72 | 11.80 | 11.23 | 11.29 |
| STD | M25 | M50 | M75 | M100 | M100G3 | M100G5 | M100G6 | O50 | O100 | |
|---|---|---|---|---|---|---|---|---|---|---|
| <2 μm (%) | 10.94 | 10.88 | 11.58 | 10.77 | 12.08 | 14.02 | 11.71 | 8.20 | 10.66 | 12.79 |
| <10 μm (%) | 55.60 | 55.28 | 55.45 | 54.35 | 55.64 | 57.23 | 54.22 | 41.80 | 56.34 | 55.86 |
| D50 (μm) | 8.35 | 8.42 | 8.37 | 8.68 | 8.31 | 7.80 | 8.69 | 13.80 | 8.06 | 8.25 |
| D90 (μm) | 34.10 | 34.60 | 34.20 | 34.80 | 33.60 | 33.00 | 35.40 | 47.00 | 36.10 | 33.20 |
| Firing Shrinkage (%) | ||||
|---|---|---|---|---|
| Firing Temperature (°C) | 1025 | 1050 | 1075 | 1100 |
| STD | 0.75 ± 0.06 | 0.48 ± 0.08 | 1.07 ± 0.09 | 0.45 ± 0.08 |
| M25 | 0.58 ± 0.04 | 0.58 ± 0.06 | 0.69 ± 0.04 | 0.77 ± 0.04 |
| M50 | 0.87 ± 0.08 | 0.88 ± 0.08 | 0.43 ± 0.09 | 0.70 ± 0.05 |
| M75 | 1.01 ± 0.07 | 0.81 ± 0.06 | 0.86 ± 0.07 | 0.95 ± 0.04 |
| M100 | 1.13 ± 0.09 | 0.99 ± 0.06 | 0.82 ± 0.07 | 0.75 ± 0.05 |
| M100G3 | 1.48 ± 0.08 | 1.27 ± 0.08 | 1.34 ± 0.09 | 1.06 ± 0.09 |
| M100G5 | 0.85 ± 0.06 | 0.74 ± 0.07 | 1.10 ± 0.08 | 0.74 ± 0.08 |
| M100G6 | 0.62 ± 0-05 | 0.53 ± 0.06 | 0.70 ± 0.05 | 0.83 ± 0.03 |
| O50 | 1.22 ± 0.02 | 1.16 ± 0.05 | 1.36 ± 0.03 | - |
| O100 | 2.08 ± 0.03 | 2.11 ± 0.04 | 2.12 ± 0.06 | - |
| ΔE | ||||
|---|---|---|---|---|
| Firing Temperature (°C) | 1025 | 1050 | 1075 | 1100 |
| STD | 0.47 | - | 0.79 | 1.33 |
| M25 | 1.39 | 0.75 | 0.95 | 1.37 |
| M50 | 1.71 | 0.26 | 0.71 | 1.47 |
| M75 | 1.80 | 0.83 | 0.38 | 0.73 |
| M100 | 1.17 | 0.85 | 0.78 | 0.44 |
| M100G3 | 1.49 | 1.40 | 1.01 | 0.49 |
| M100G5 | 0.96 | 0.30 | 1.25 | 1.43 |
| M100G6 | 0.43 | 0.20 | 0.84 | 1.40 |
| O50 | 1.67 | 0.90 | 0.54 | - |
| O100 | 3.22 | 3.29 | 3.27 | - |
| STD | M25 | M50 | M75 | M100 | M100G3 | M100G5 | M100G6 | O50 | O100 | |
|---|---|---|---|---|---|---|---|---|---|---|
| α × 10−6 K−1 | 8.43 | 8.16 | 8.02 | 8.19 | 8.08 | 7.86 | 8.23 | 8.15 | 8.03 | 7.92 |
| CaCO3 Source | Material Quantity (kg) | Milling Time (h) | Efficiency (kg/h) |
|---|---|---|---|
| Calcite | 50 | 12 | 4.17 |
| Mussel bio-calcite | 20 | 2.5 |
| STD | M100 | M100G3 | |
|---|---|---|---|
| L* | 92.62 | 92.55 | 92.32 |
| a* | 1.83 | 1.79 | 1.62 |
| b* | 8.08 | 8.07 | 8.31 |
| ΔE | - | 0.08 | 0.43 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vilarinho, I.S.; Ferreira, M.; Miranda, C.; Silva, J.; Batista, S.; Gonçalves, M.C.; Seabra, M.P. Decarbonisation of Earthenware Ceramic Production Using Bivalve Shell Waste. Ceramics 2025, 8, 76. https://doi.org/10.3390/ceramics8020076
Vilarinho IS, Ferreira M, Miranda C, Silva J, Batista S, Gonçalves MC, Seabra MP. Decarbonisation of Earthenware Ceramic Production Using Bivalve Shell Waste. Ceramics. 2025; 8(2):76. https://doi.org/10.3390/ceramics8020076
Chicago/Turabian StyleVilarinho, Inês Silveirinha, Miguel Ferreira, Claúdia Miranda, José Silva, Sofia Batista, Maria Clara Gonçalves, and Maria Paula Seabra. 2025. "Decarbonisation of Earthenware Ceramic Production Using Bivalve Shell Waste" Ceramics 8, no. 2: 76. https://doi.org/10.3390/ceramics8020076
APA StyleVilarinho, I. S., Ferreira, M., Miranda, C., Silva, J., Batista, S., Gonçalves, M. C., & Seabra, M. P. (2025). Decarbonisation of Earthenware Ceramic Production Using Bivalve Shell Waste. Ceramics, 8(2), 76. https://doi.org/10.3390/ceramics8020076








