Fayalite-Based Geopolymer Foam
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.3. Geopolymer Foam Synthesis
3. Results
3.1. Physical and Mechanical Properties
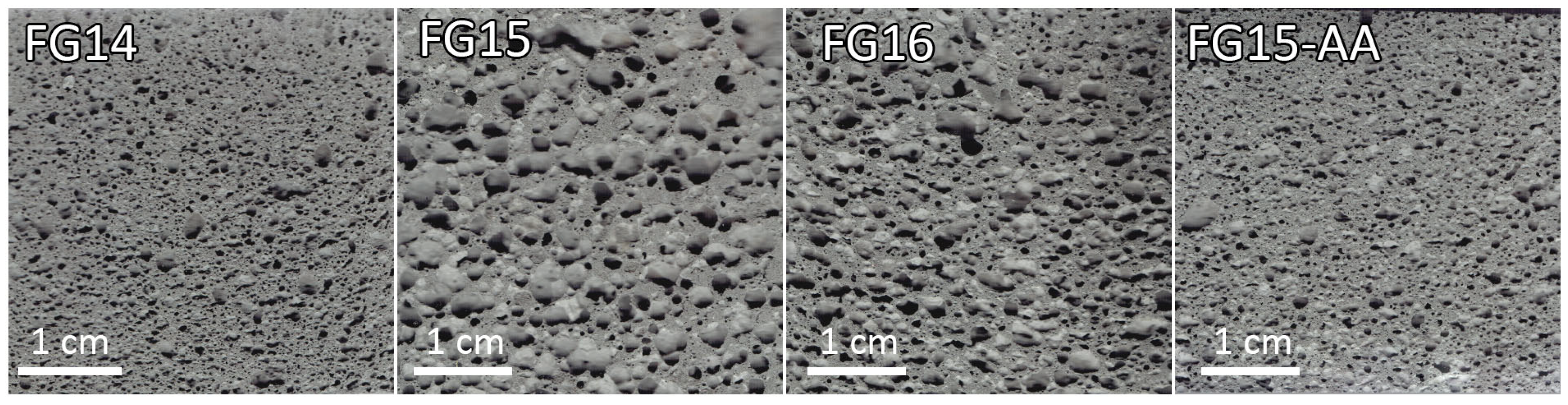
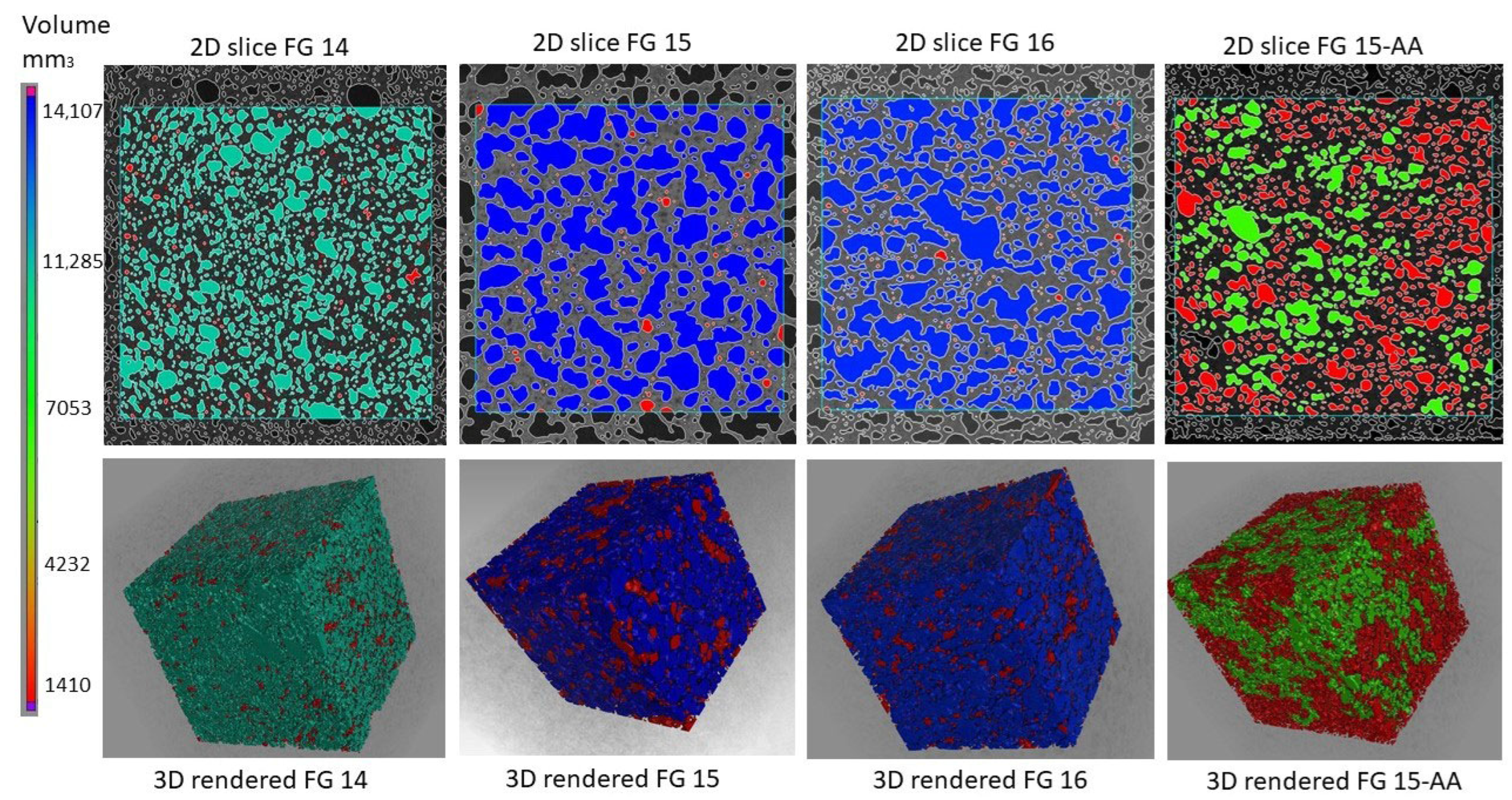
3.2. X-Ray Computed Tomography (Micro-CT)
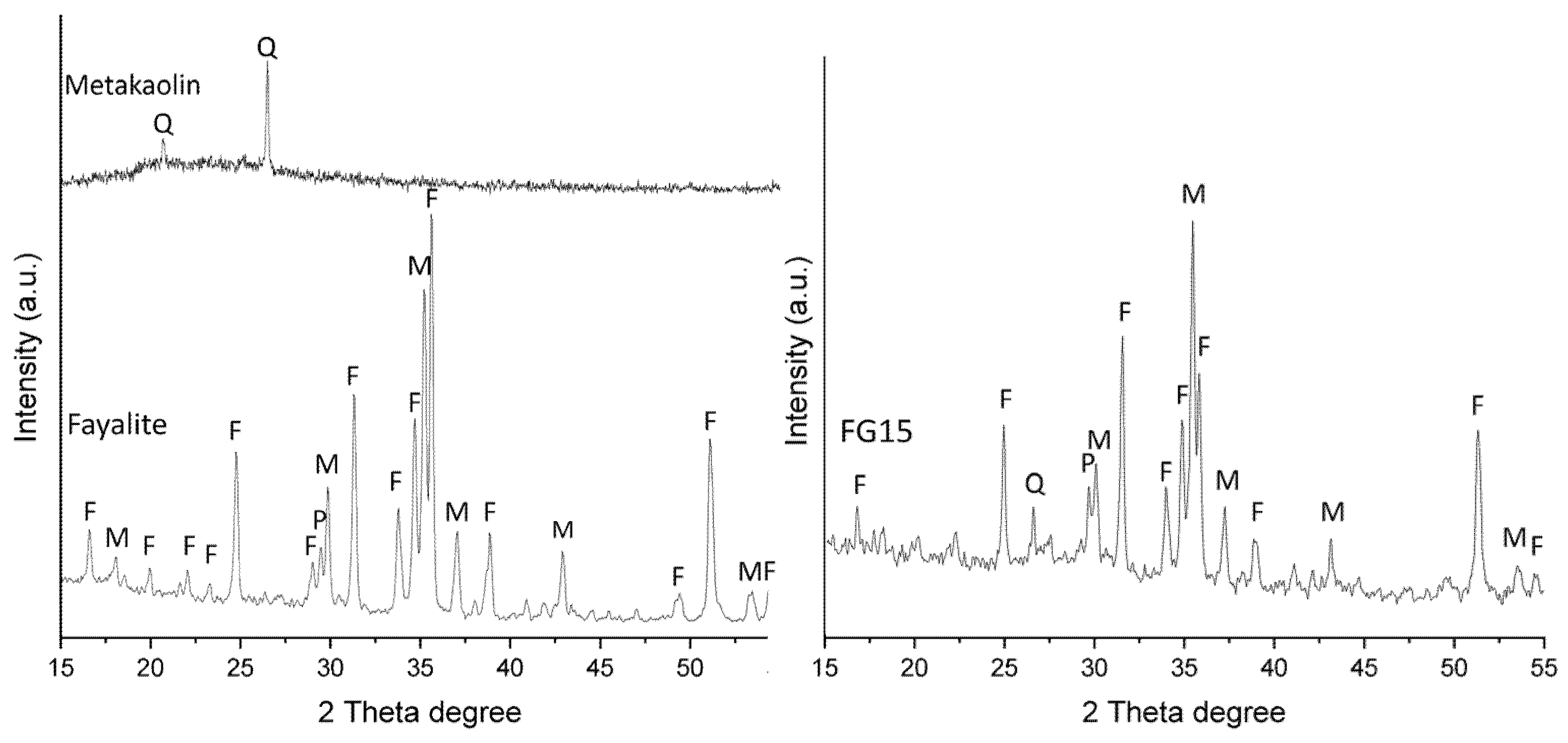
3.3. Powder XRD
3.4. SEM
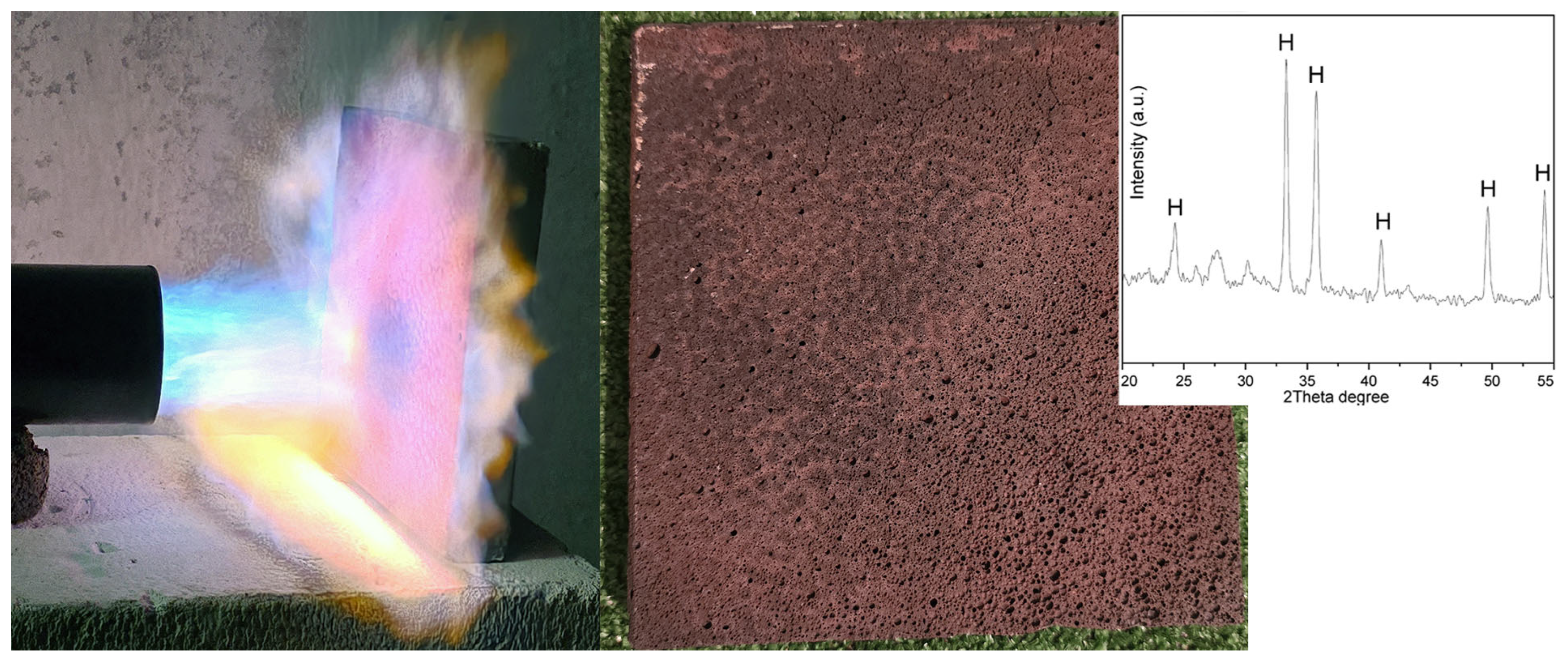
3.5. Real-Size Experiments—Thermal Conductivity and Preliminary Fire Resistance Test
4. Conclusions
- Fayalite slag, despite its high density (3.80 g/cm3), can be effectively utilized to produce lightweight geopolymer materials through the direct foaming method. This approach offers a practical solution for mitigating the heavy nature of fayalite slag, enabling the production of normal to lightweight components.
- Geopolymer foams with a water-to-solid ratio of 0.15 (series FG15) demonstrated optimal characteristics, achieving the highest relative porosity (73.2%) and the lowest measured density (0.92 g/cm3). The absolute density was measured to 3.43 g/cm3, which is comparatively high for geopolymers due to the presence of dense mineral phases in the fayalite slag, such as fayalite and magnetite. As a result, the foams combine the lightweight nature of porous materials with a geopolymer gel matrix composed of inherently heavier components.
- The addition of an air-entraining admixture resulted in a geopolymer foam with more pore counts, uniform pore distribution, decreased pore size, reduced coalescence, and improved mechanical properties. This modification increased compressive strength to 2.8 MPa, with a decrease in relative porosity (64.5%).
- Microcomputed tomography revealed that the pore network consisted of interconnected pores. The pore structure was greatly influenced by the water-to-solid ratio. The FG15 series exhibited the highest relative porosity and interconnected pore networks, whereas FG15-AA demonstrated a higher pore count with smaller, more evenly distributed pores.
- Powder XRD analysis and SEM study indicated the main phases in fayalite slag—fayalite and magnetite remained inert during geopolymerization, with partial reactivity observed in the amorphous phases. The metakaolin and probably ferro-aluminosilicate glass in the fayalite slag contributed to the formation of geopolymer gel, evidenced by the amorphous hump in the XRD pattern, while the crystalline phases such as quartz, fayalite, magnetite, and pyroxene remained unreacted, acting as a filler in the geopolymer matrix.
- Real-size specimens (300 × 300 × 30 mm) prepared using recipe FG15-AA showed slightly higher values of density (1.29 g/cm3) but lower water absorption (15.35%) compared to the initial sample FG15-AA (1.22 g/cm3, 20.4%, respectively) due to the size effect and scaling the technology of preparation. The geopolymer foam blocks were characterized by a thermal conductivity coefficient of 0.243 W/mK. The geopolymer foam resisted direct flame exposure without disintegration, highlighting its potential as a fire-resistant material.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| Micro-CT | X-ray computed tomography |
| ROI | Region of interest |
| SEM | Scanning electron microscopy |
| XRD | X-ray diffraction |
References
- Korniejenko, K.; Pławecka, K.; Bazan, P.; Figiela, B.; Kozub, B.; Mróz, K.; Łach, M. Green building materials for circular economy—Geopolymer foams. Proc. Eng. Technol. Innov. 2023, 25, 26–34. [Google Scholar] [CrossRef]
- Davidovits, J. Geopolymers: Ceramic-like inorganic polymers. J. Ceram. Sci. Technol. 2017, 8, 335–350. [Google Scholar] [CrossRef]
- Davidovits, J.; Davidovits, R. Ferro-Sialate Geopolymers (-Fe-O-Si-O-Al-O-); Geopolymer Institute Library: Saint-Quentin, France, 2020. [Google Scholar]
- Lemougna, P.N.; MacKenzie, K.J.; Jameson, G.N.; Rahier, H.; Chinje Melo, U. The role of iron in the formation of inorganic polymers (geopolymers) from volcanic ash: A 57 Fe Mössbauer spectroscopy study. J. Mater. Sci. 2013, 48, 5280–5286. [Google Scholar] [CrossRef]
- Onisei, S.; Lesage, K.; Blanpain, B.; Pontikes, Y. Early age microstructural transformations of an inorganic polymer made of fayalite slag. J. Am. Ceram. Soc. 2015, 98, 2269–2277. [Google Scholar] [CrossRef]
- Komnitsas, K.; Zaharaki, D.; Perdikatsis, V. Geopolymerisation of low calcium ferronickel slags. J. Mater. Sci. 2007, 42, 3073–3082. [Google Scholar] [CrossRef]
- Adediran, A. Alkali Activation of Fayalite Slag. Master’s Thesis, University of Oulu, Oulu, Finland, 2017. [Google Scholar]
- Mihailova, I.; Uzunov, I.; Mehandjiev, D. Waste Copper Slag/Aluminium Dross-Based Geopolymer. J. Chem. Technol. Metall. 2021, 56, 653–659. [Google Scholar]
- Nikolov, A. Alkali-activated geopolymers based on iron-rich slag from copper industry. In Proceedings of the 11th International Conference on Civil Engineering Design and Construction, Varna, Bulgaria, 10–12 September 2020; p. 012006. [Google Scholar] [CrossRef]
- Nikolov, A. Characterization of geopolymer based on fayalite waste and metakaolin with standard consistence. Comptes Rendus L’Académie Bulg. Sci. 2021, 74, 1461–1498. [Google Scholar] [CrossRef]
- Nikolov, A.; Karamanov, A. Thermal properties of geopolymer based on fayalite waste from copper production and metakaolin. Materials 2022, 15, 2666. [Google Scholar] [CrossRef]
- Kočí, V.; Černý, R. Directly foamed geopolymers: A review of recent studies. Cem. Concr. Compos. 2022, 130, 104530. [Google Scholar] [CrossRef]
- Hajimohammadi, A.; Ngo, T.; Mendis, P.; Sanjayan, J. Regulating the chemical foaming reaction to control the porosity of geopolymer foams. Mater. Des. 2017, 120, 255–265. [Google Scholar] [CrossRef]
- Xu, F.; Gu, G.; Zhang, W.; Wang, H.; Huang, X.; Zhu, J. Pore structure analysis and properties evaluations of fly ash-based geopolymer foams by chemical foaming method. Ceram. Int. 2018, 44, 19989–19997. [Google Scholar] [CrossRef]
- Zhang, Z.; Provis, J.L.; Reid, A.; Wang, H. Geopolymer foam concrete: An emerging material for sustainable construction. Constr. Build. Mater. 2014, 56, 113–127. [Google Scholar] [CrossRef]
- Nikolov, A.; Barbov, B. Lightweight geopolymer based on fly ash. Rev. Bulg. Geol. Soc. 2018, 79, 23–24. [Google Scholar]
- František, Š.; Rostislav, Š.; Zdeněk, T.; Petr, S.; Vít, Š.; Zuzana, Z.C. Preparation and properties of fly ashbased geopolymer foams. Ceram.-Silikáty 2014, 58, 188–197. [Google Scholar]
- Suksiripattanapong, C.; Krosoongnern, K.; Thumrongvut, J.; Sukontasukkul, P.; Horpibulsuk, S.; Chindaprasirt, P. Properties of cellular lightweight high calcium bottom ash-portland cement geopolymer mortar. Case Stud. Constr. Mater. 2020, 12, e00337. [Google Scholar] [CrossRef]
- Vaou, V.; Panias, D. Thermal insulating foamy geopolymers from perlite. Miner. Eng. 2010, 23, 1146–1151. [Google Scholar] [CrossRef]
- Shakouri, S.; Bayer, Ö.; Erdoğan, S.T. Development of silica fume-based geopolymer foams. Constr. Build. Mater. 2020, 260, 120442. [Google Scholar] [CrossRef]
- Bai, C.; Zheng, K.; Sun, F.; Wang, X.; Zhang, L.; Zheng, T.; Colombo, P.; Wang, B. A review on metakaolin-based porous geopolymers. Appl. Clay Sci. 2024, 258, 107490. [Google Scholar] [CrossRef]
- Novais, R.M.; Pullar, R.C.; Labrincha, J.A. Geopolymer foams: An overview of recent advancements. Prog. Mater. Sci. 2020, 109, 100621. [Google Scholar] [CrossRef]
- Shen, S.; Tian, J.; Zhu, Y.; Zhang, X.; Hu, P. Synthesis of industrial solid wastes based geopolymer foams for building energy conservation: Effects of metallic aluminium and reclaimed materials. Constr. Build. Mater. 2022, 328, 127083. [Google Scholar] [CrossRef]
- Manolova, E. Aurubis iron-silicate fines: Universal sustainable construction material: A state-of-the-art review. In Proceedings of the 11th International Conference on Civil Engineering Design and Construction, Varna, Bulgaria, 10–12 September 2020; p. 012005. [Google Scholar] [CrossRef]
- Pham, L.T.; Cramer, S.M. Effects of air-entraining admixtures on stability of air bubbles in concrete. J. Mater. Civ. Eng. 2021, 33, 04021018. [Google Scholar] [CrossRef]
- Nikolov, A.T.L.; Barbov, B. Lightweight heavy geopolymer foam based on fayalite slag: Influence of alkali concentration on cellular structure. Mach. Technol. Mater. 2025, 19, 79–82. [Google Scholar]
- Ducman, V.; Korat, L. Characterization of geopolymer fly-ash based foams obtained with the addition of Al powder or H2O2 as foaming agents. Mater. Charact. 2016, 113, 207–213. [Google Scholar] [CrossRef]
- Karamanov, A.; Colombini, E.; Ferrante, D.; Georgiev, I.; Raykovska, M.; Karamanova, E.; Atanasova, S.; Veronesi, P.; Leonelli, C. Benefits of Microwave-Assisted Heat Treatment for Sintered Diopside Glass-Ceramics. Materials 2025, 18, 421. [Google Scholar] [CrossRef]
- Yatsenko, E.A.; Goltsman, B.M.; Izvarin, A.I.; Kurdashov, V.M.; Smoliy, V.A.; Ryabova, A.V.; Klimova, L.V. Recycling ash and slag waste from thermal power plants to produce foamed geopolymers. Energies 2023, 16, 7535. [Google Scholar] [CrossRef]
- Prałat, K.; Ciemnicka, J.; Koper, A.; Buczkowska, K.E.; Łoś, P. Comparison of the thermal properties of geopolymer and modified gypsum. Polymers 2021, 13, 1220. [Google Scholar] [CrossRef]


| Precursor | FeO | SiO2 | Al2O3 | CaO | ZnO | MgO | K2O | Na2O | CuO | PbO | TiO2 | MoO3 | SO3 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Fayalite | 55.83 | 31.16 | 4.67 | 2.82 | 1.40 | 0.95 | 0.75 | 0.62 | 0.52 | 0.39 | 0.32 | 0.29 | 0.28 |
| Metakaolin | 1.03 | 54.00 | 43.25 | 0.15 | n.d. | 0.09 | 0.62 | 0.11 | n.d. | n.d. | 0.74 | n.d. | 0.01 |
| Series | Water-to Solid-Ratio | Density, g/cm3 | Absolute Density, g/cm3 | Relative Porosity, % | Water Absorption, % | Compressive Strength, MPa | Specific Strength, kN/m·kg |
|---|---|---|---|---|---|---|---|
| FG14 | 0.14 | 1.25 | 3.44 | 63.7 | 19.3 ± 0.6 | 2.4 ± 0.2 | 1.96 |
| FG15 | 0.15 | 0.92 | 3.43 | 73.2 | 30.9 ± 0.3 | 1.3 ± 0.1 | 1.46 |
| FG16 | 0.16 | 1.08 | 3.42 | 68.4 | 23.8 ± 1.1 | 1.5 ± 0.1 | 1.39 |
| FG15-AA 1 | 0.15 | 1.22 | 3.44 | 64.5 | 20.4 ± 0.2 | 2.8 ± 0.1 | 2.3 |
| Series | FG14 | FG15 | FG16 | FG15-AA |
|---|---|---|---|---|
| Pore count | 20,575 | 23,549 | 33,726 | 43,293 |
| Relative porosity, % | 38.58 | 54.21 | 50.84 | 33.50 |
| Total volume pores, mm3 | 10,405 | 14,630 | 13,789 | 8657 |
| Sample | Density | Water Absorption | Thermal Conductivity Coefficient |
|---|---|---|---|
| Real-size specimen based on FG15-AA (300 × 300 × 30 mm) | 1.29 g/cm3 | 15.35% | 0.243 W/mK |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nikolov, A.; Tarassov, M.; Rostovsky, I.; Raykovska, M.; Georgiev, I.; Korniejenko, K. Fayalite-Based Geopolymer Foam. Ceramics 2025, 8, 77. https://doi.org/10.3390/ceramics8020077
Nikolov A, Tarassov M, Rostovsky I, Raykovska M, Georgiev I, Korniejenko K. Fayalite-Based Geopolymer Foam. Ceramics. 2025; 8(2):77. https://doi.org/10.3390/ceramics8020077
Chicago/Turabian StyleNikolov, Aleksandar, Mihail Tarassov, Ivan Rostovsky, Miryana Raykovska, Ivan Georgiev, and Kinga Korniejenko. 2025. "Fayalite-Based Geopolymer Foam" Ceramics 8, no. 2: 77. https://doi.org/10.3390/ceramics8020077
APA StyleNikolov, A., Tarassov, M., Rostovsky, I., Raykovska, M., Georgiev, I., & Korniejenko, K. (2025). Fayalite-Based Geopolymer Foam. Ceramics, 8(2), 77. https://doi.org/10.3390/ceramics8020077










