First Test and Characterizations on Urban Glass Waste with Waste-Derived Carbon Fiber Treated to Realize Foam Glass for Possible Construction Applications
Abstract
1. Introduction
2. Materials and Methods
2.1. Raw Materials
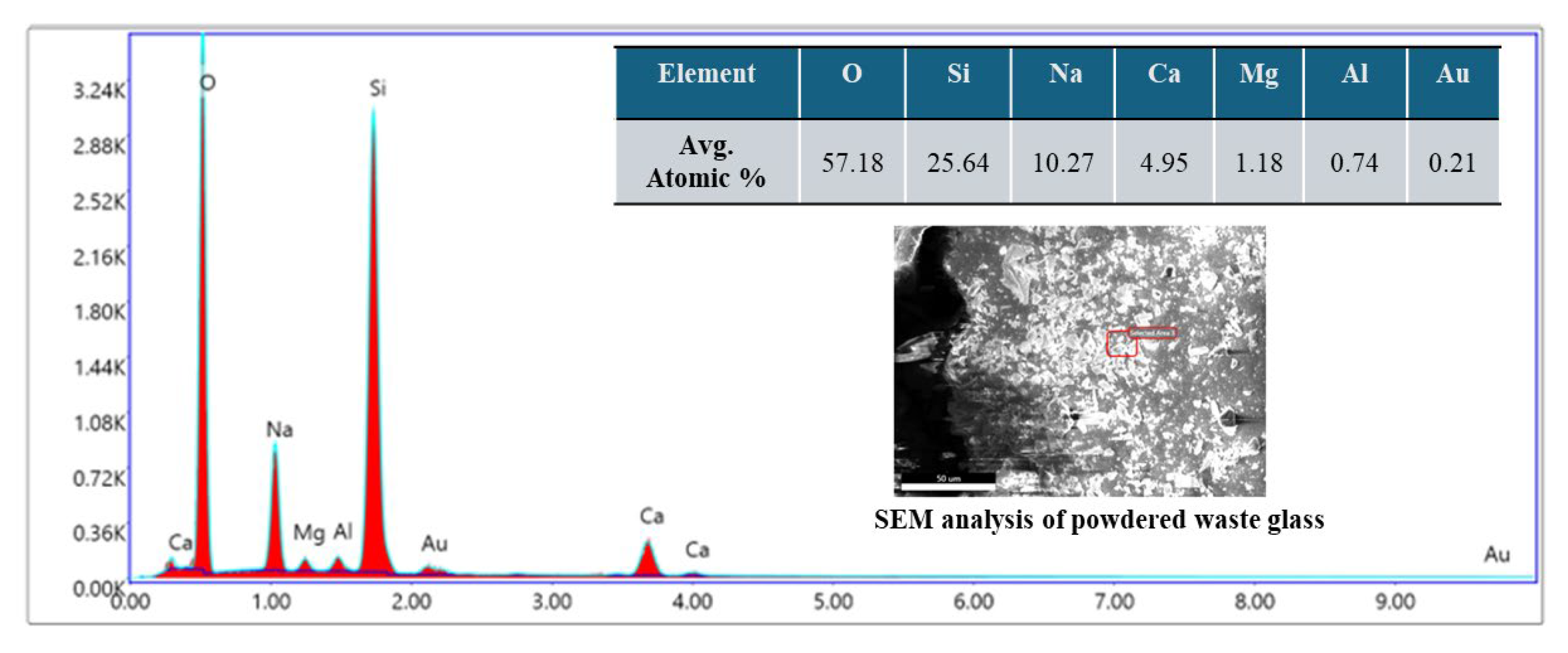
2.1.1. Powdered Waste Glass
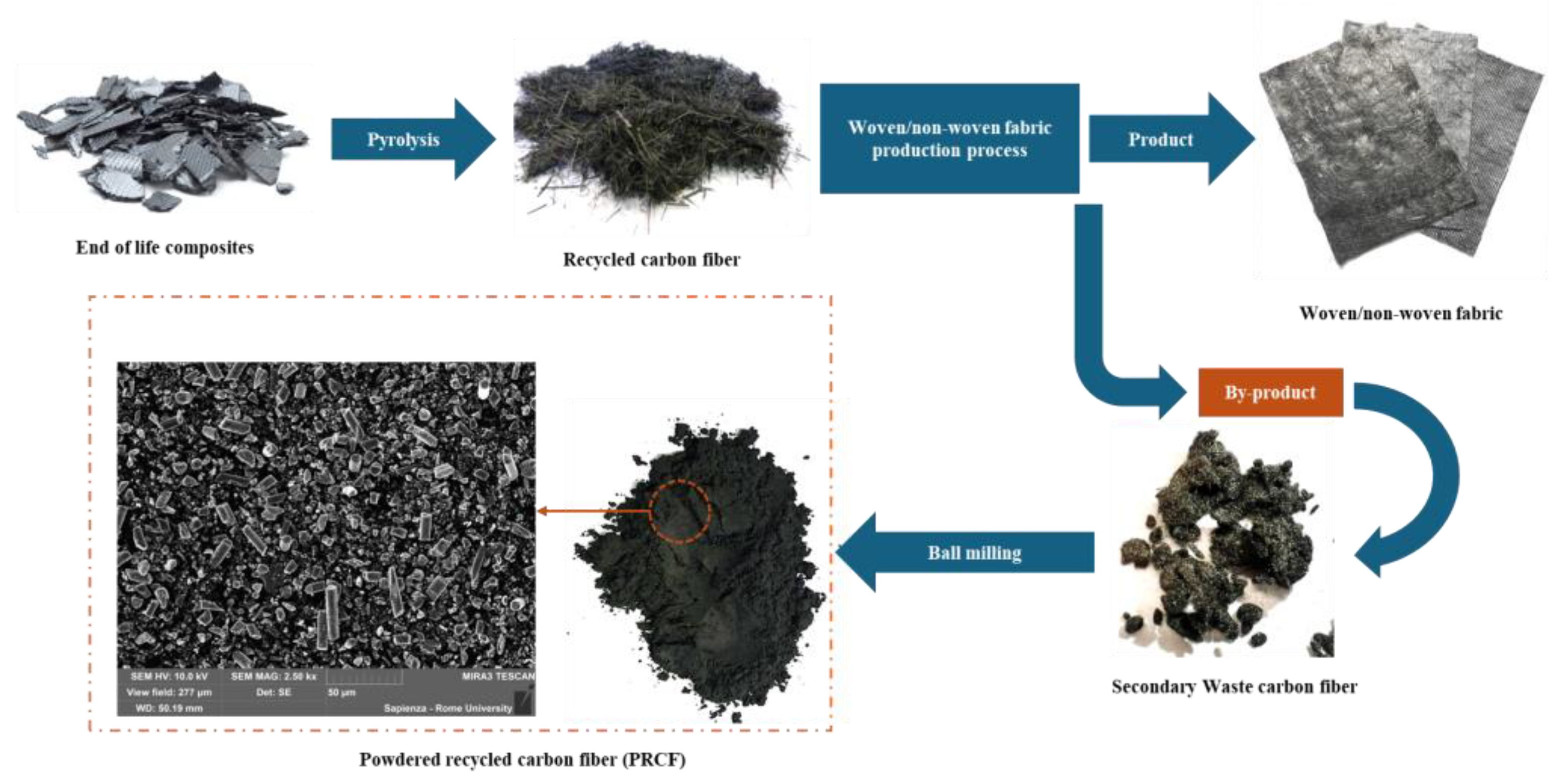
2.1.2. Powdered Recycled Carbon Fiber
2.2. FG Sample Preparation
2.3. Testing Techniques
2.3.1. Setup for Optical Microscopic Analysis of Pores
2.3.2. Density and Porosity Measurements
2.3.3. Setup for Thermal Conductivity Analysis
2.3.4. Setup for Compression Puncture Test
2.3.5. Microstructure Analysis via SEM
3. Results and Discussion
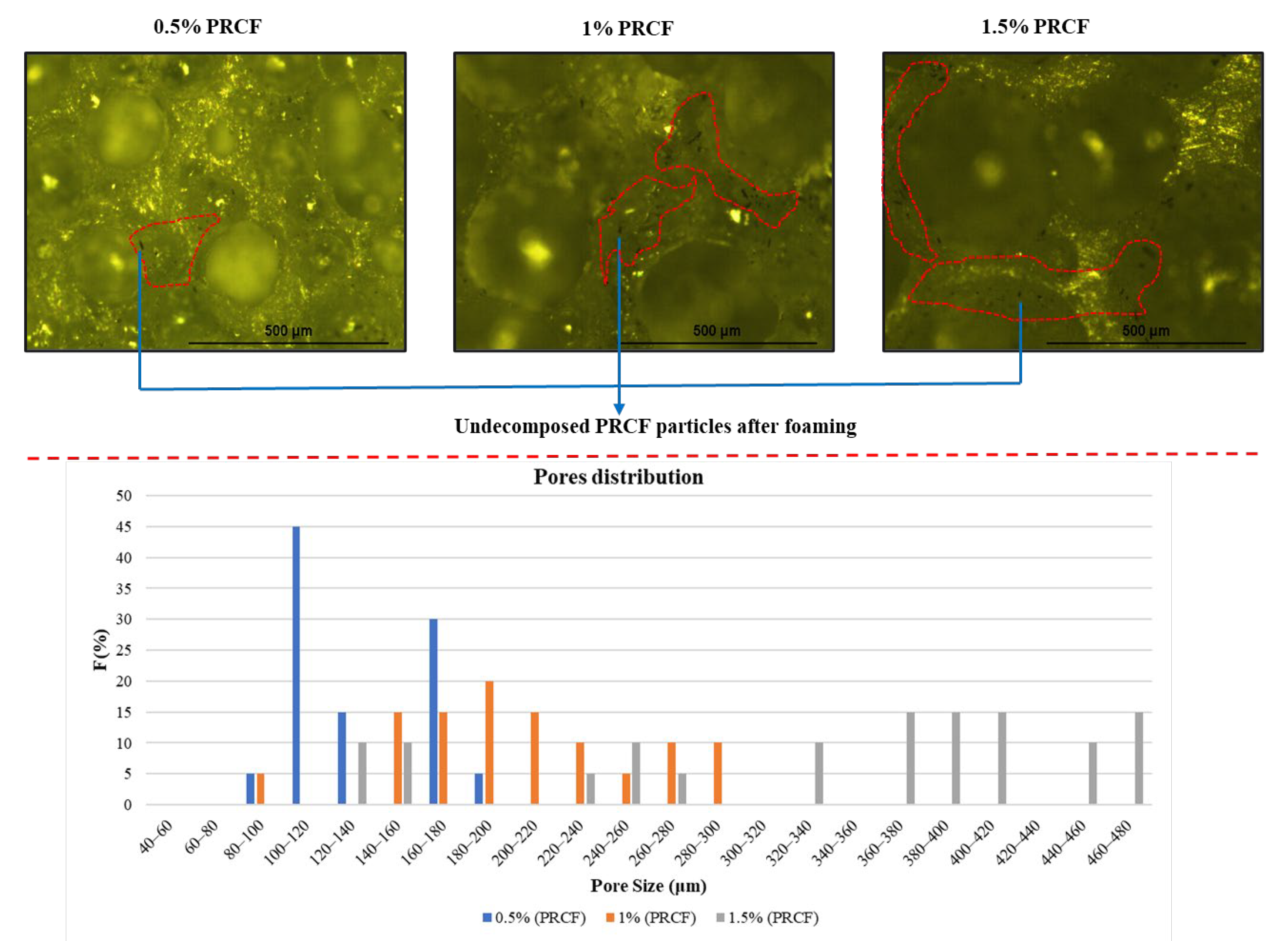
3.1. Optical Microscopic Analysis of Pores
3.2. Thermal Conductivity Analysis
3.3. SEM Analysis
3.4. Compression Puncture Test
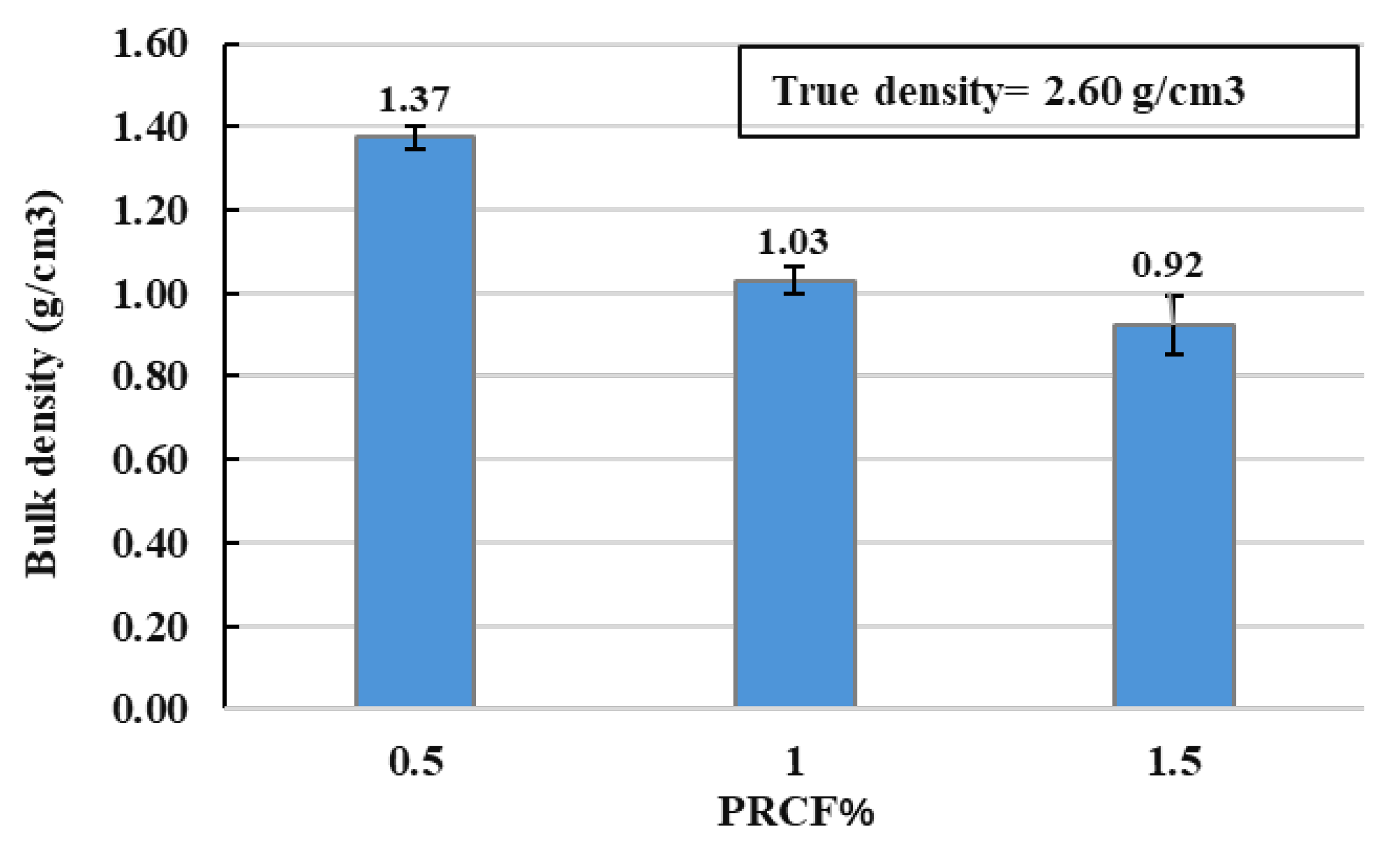
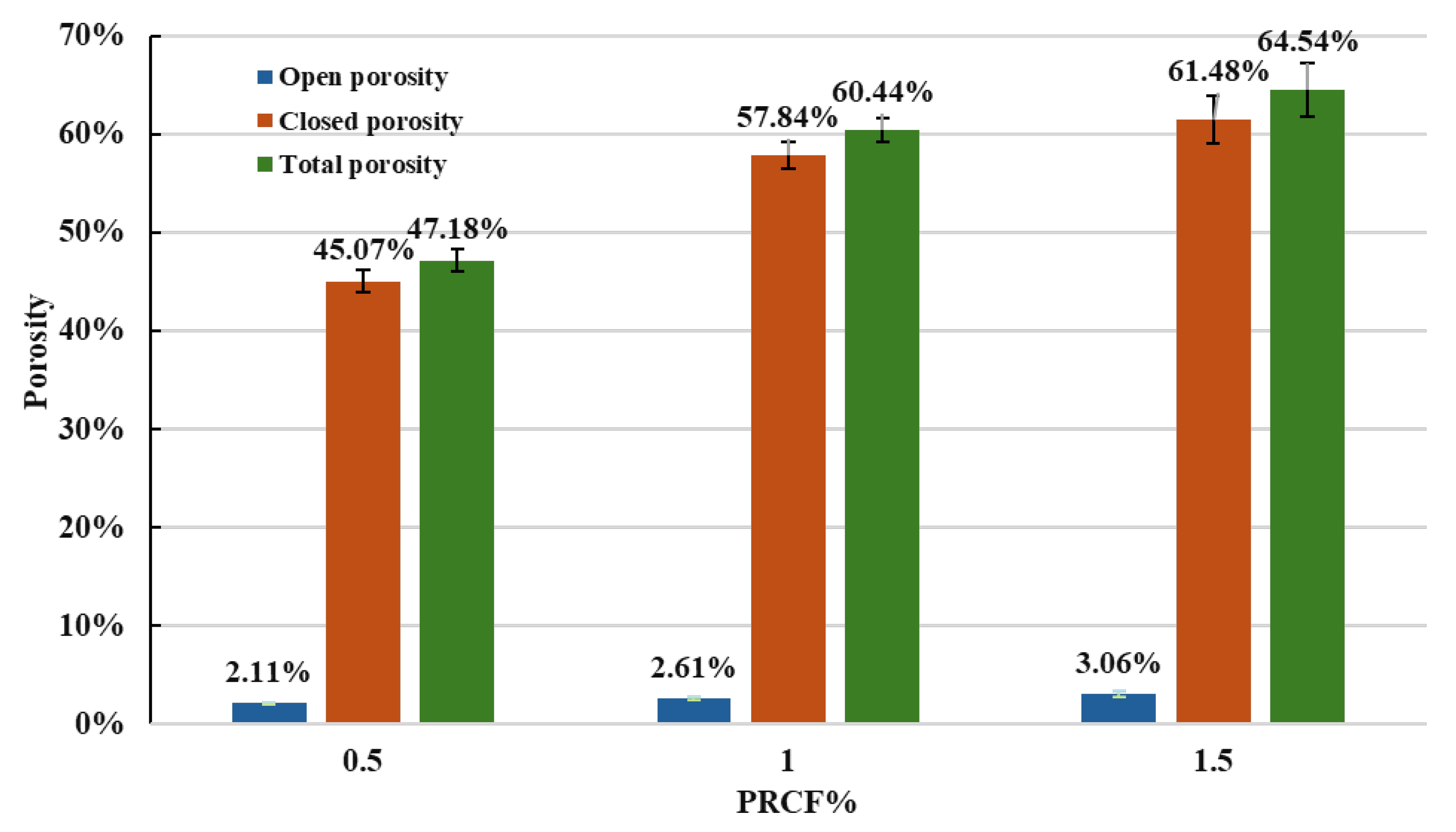
3.5. Density and Porosity
4. Conclusions
- The results revealed a direct correlation between PRCF concentration and the reduction in the density of FG samples. Density decreased from 1.37 g/cm3 at 0.5% PRCF to 0.92 g/cm3 at 1.5% PRCF, representing a 33% reduction. This trend indicates that increasing PRCF content promotes pore formation within the glass matrix, leading to a more porous structure.
- Increasing the PRCF percentage resulted in a greater degree of foaming, driven by CO2 generation from PRCF oxidation. This resulted in a significant reduction in compressive strength by 50% and thermal conductivity by 68%, as PRCF content increased from 0.5% to 1.5%.
- The findings also confirmed that PRCF effectively induces porosity in the glass matrix. Total porosity rising from 47.18% at 0.5% PRCF to 65.54% at 1.5% PRCF, which is supporting its potential application in lightweight construction materials.
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| FG | Foam glass |
| PRCF | Powdered recycled carbon fiber |
| RCF | Recycled carbon fiber |
| SEM | Scanning electron microscope |
References
- Guo, Y.; Zhang, Y.; Huang, H.; Meng, K.; Hu, K.; Hu, P.; Wang, X.; Zhang, Z.; Meng, X. Novel glass ceramic foams materials based on red mud. Ceram. Int. 2014, 40, 6677–6683. [Google Scholar] [CrossRef]
- Liu, Y.; Xie, J.; Hao, P.; Shi, Y.; Xu, Y.; Ding, X. Study on factors affecting properties of foam glass made from waste glass. J. Renew. Mater. 2020, 9, 237–253. [Google Scholar] [CrossRef]
- König, J.; Lopez-Gil, A.; Cimavilla-Roman, P.; Rodriguez-Perez, M.A.; Petersen, R.R.; Østergaard, M.B.; Iversen, N.; Yue, Y.; Spreitzer, M. Synthesis and properties of open- and closed-porous foamed glass with a low density. Constr. Build. Mater. 2020, 247, 1+18574. [Google Scholar] [CrossRef]
- Caniato, M.; Kyaw Oo D’Amore, G.; Kaspar, J.; Gasparella, A. Sound absorption performance of sustainable foam materials: Application of analytical and numerical tools for the optimization of forecasting models. Appl. Acoust. 2020, 161, 107166. [Google Scholar] [CrossRef]
- Ivanov, K.S. Preparation and Properties of Foam Glass-ceramic from Diatomite. J. Wuhan Univ. Technol. Mater. Sci. Ed. 2018, 33, 273–277. [Google Scholar] [CrossRef]
- Zhu, M.; Ji, R.; Li, Z.; Wang, H.; Liu, L.L.; Zhang, Z. Preparation of glass ceramic foams for thermal insulation applications from coal fly ash and waste glass. Constr. Build. Mater. 2016, 112, 398–405. [Google Scholar] [CrossRef]
- Scheffler, M.; Colombo, P. Cellular Ceramics: Structure, Manufacturing, Properties and Applications; Wiley-VCH: Weinheim, Germany; John Wiley: Hoboken, NJ, USA, 2005. [Google Scholar]
- Kaz’mina, O.V.; Vereshchagin, V.I.; Abiyaka, A.N. Raw Materials Prospects for Use of Finely Disperse Quartz Sands in Production of Foam-Glass Crystalline Materials. Glass Ceram. 2008, 65, 319–321. [Google Scholar] [CrossRef]
- Vancea, C.; Lazău, I. Glass foam from window panes and bottle glass wastes. Cent. Eur. J. Chem. 2014, 12, 804–811. [Google Scholar] [CrossRef]
- Zhang, J.; Liu, B.; Zhang, S. A review of glass ceramic foams prepared from solid wastes: Processing, heavy-metal solidification and volatilization, applications. Sci. Total Environ. 2021, 781, 146727. [Google Scholar] [CrossRef]
- Naser, M.Z.; Thai, S.; Thai, H.T. Evaluating structural response of concrete-filled steel tubular columns through machine learning. J. Build. Eng. 2021, 34, 101888. [Google Scholar] [CrossRef]
- Chen, N.; Zhang, S.; Pan, X.; Zhou, S.; Zhao, M. Foaming mechanism and optimal process conditions of foamed glass based on thermal analysis. J. Porous Mater. 2020, 27, 621–626. [Google Scholar] [CrossRef]
- Davraz, M.; Koru, M.; Akdağ, A.E.; Kılınçarslan Delikanlı, Y.E.; Çabuk, M. An investigation of foaming additives and usage rates in the production of ultra-light foam glass. J. Therm. Anal. Calorim. 2022, 147, 3567–3576. [Google Scholar] [CrossRef]
- Zhu, B.; Zhang, Z.; Zhang, W.; Wu, Y.; Zhang, J.; Imran, Z.; Zhang, D. Synthesis and Applications of Porous Glass. J. Shanghai Jiaotong Univ. Sci. 2019, 24, 681–698. [Google Scholar] [CrossRef]
- Harrison, E.; Berenjian, A.; Seifan, M. Recycling of waste glass as aggregate in cement-based materials. Environ. Sci. Ecotechnol. 2020, 4, 100064. [Google Scholar] [CrossRef]
- Khan, M.N.N.; Saha, A.K.; Sarker, P.K. Reuse of waste glass as a supplementary binder and aggregate for sustainable cement-based construction materials: A review. J. Build. Eng. 2020, 28, 101052. [Google Scholar] [CrossRef]
- Nistratov, A.V.; Klimenko, N.N.; Pustynnikov, I.V.; Vu, L.K. Thermal Regeneration and Reuse of Carbon and Glass Fibers from Waste Composites. Emerg. Sci. J. 2022, 6, 967–984. [Google Scholar] [CrossRef]
- Huang, D.; Sun, P.; Gao, P.; Liu, G.; Wang, Y.; Chen, X. Study on the effect and mechanism of alkali–silica reaction expansion in glass concrete. Sustainability 2021, 13, 10618. [Google Scholar] [CrossRef]
- König, J.; Nemanič, V.; Žumer, M.; Petersen, R.R.; Østergaard, M.B.; Yue, Y.; Suvorov, D. Evaluation of the contributions to the effective thermal conductivity of an open-porous-type foamed glass. Constr. Build. Mater. 2019, 214, 337–343. [Google Scholar] [CrossRef]
- Mohajerani, A.; Vajna, J.; Cheung, T.H.H.; Kurmus, H.; Arulrajah, A.; Horpibulsuk, S. Practical recycling applications of crushed waste glass in construction materials: A review. Constr. Build. Mater. 2017, 156, 443–467. [Google Scholar] [CrossRef]
- Heriyanto Pahlevani, F.; Sahajwalla, V. Synthesis of calcium silicate from selective thermal transformation of waste glass and waste shell. J. Clean. Prod. 2016, 172, 3019–3027. [Google Scholar] [CrossRef]
- Popov, M.; Zakrevskaya, L.; Vaganov, V.; Hempel, S.; Mechtcherine, V. Performance of lightweight concrete based on granulated foamglass. IOP Conf. Ser. Mater. Sci. Eng. 2015, 96, 012017. [Google Scholar] [CrossRef]
- Sambucci, M.; Nouri, S.M.; Tayebi, S.T.; Valente, M. Synergic Effect of Recycled Carbon Fibers and Microfibrillated Cellulose Gel for Enhancing the Mechanical Properties of Cement-Based Materials. Gels 2023, 9, 981. [Google Scholar] [CrossRef] [PubMed]
- Valente, M.; Sambucci, M.; Rossitti, I.; Abruzzese, S.; Sergi, C.; Sarasini, F.; Tirillò, J. Carbon-Fiber-Recycling Strategies: A Secondary Waste Stream Used for PA6,6 Thermoplastic Composite Applications. Materials 2023, 16, 5436. [Google Scholar] [CrossRef] [PubMed]
- Zhai, C.; Zhong, Y.; Liu, J.; Zhang, J.; Zhu, Y.; Wang, M.; Yeo, J. Customizing the properties of borosilicate foam glasses via additions under low sintering temperatures with insights from molecular dynamics simulations. J. Non-Cryst. Solids 2022, 576, 121273. [Google Scholar] [CrossRef]
- Adjei, E.A.; Amos-Abanyie, S.; Omer, S. Characterization of Thermo-Physical Properties of Cement-Based Blocks of Varied Sand Types Using Cost-Effective Enhancement Approach. Open J. Energy Effic. 2020, 9, 14–30. [Google Scholar] [CrossRef]
- Zhai, C.; Zhong, Y.; Li, Z.; Wang, X.; Zhao, L.; Pan, L.; Zhang, J. Preparation and characterization of mechanical properties of foam glass for artificial floating island carrier. Adv. Mech. Eng. 2015, 7, 1687814015589660. [Google Scholar] [CrossRef]
- Zhai, C.; Li, Z.; Zhu, Y.; Zhang, J.; Wang, X.; Zhao, L.; Pan, L.; Wang, P. Effects of Sb2O3 on the mechanical properties of the borosilicate foam glasses sintered at low temperature. Adv. Mater. Sci. Eng. 2014, 2014, 703194. [Google Scholar] [CrossRef]
- König, J.; Petersen, R.R.; Yue, Y.; Suvorov, D. Gas-releasing reactions in foam-glass formation using carbon and MnxOy as the foaming agents. Ceram. Int. 2017, 43, 4638–4646. [Google Scholar] [CrossRef]
- Kim, H.D.; Baek, C.R.; Jang, Y.C. Advancing glass recycling and environmental applications with porous glass: A mini-review. J. Mater. Cycles Waste Manag. 2024, 26, 2620–2633. [Google Scholar] [CrossRef]
- Qin, Z.; Li, G.; Tian, Y.; Ma, Y.; Shen, P. Numerical simulation of thermal conductivity of foam glass based on the steady-state method. Materials 2018, 12, 54. [Google Scholar] [CrossRef]
- Osfouri, M.; Simon, A. Study on the thermal conductivity and density of foam glass. Pollack Period. 2023, 18, 126–131. [Google Scholar] [CrossRef]
- Rani, M.; Choudhary, P.; Krishnan, V.; Zafar, S. A review on recycling and reuse methods for carbon fiber/glass fiber composites waste from wind turbine blades. Compos. Part B Eng. 2021, 215, 108768. [Google Scholar] [CrossRef]
- Lu, G.; Chen, Y.; Yan, Q.; Zhan, H.; Mao, P.; Liu, D. Investigation of microstructure and properties in short carbon fiber reinforced silica-based ceramic cores via atmosphere sintering. J. Eur. Ceram. Soc. 2021, 41, 7339–7347. [Google Scholar] [CrossRef]
- Yuan, M.; Li, Z.; Teng, Z. Progress and prospects of recycling technology for carbon fiber reinforced polymer. Front. Mater. 2024, 11, 1484544. [Google Scholar] [CrossRef]
- Liu, H.; Zhao, X. Thermal Conductivity Analysis of High Porosity Structures with Open and Closed Pores. Int. J. Heat Mass Transf. 2022, 183, 122089. [Google Scholar] [CrossRef]
- Lin, H.; Shen, Q.; Ma, M.; Ji, R.; Guo, H.; Qi, H.; Xing, W.; Tang, H. 3D Printing of Porous Ceramics for Enhanced Thermal Insulation Properties. Adv. Sci. 2024, 12, 2412554. [Google Scholar] [CrossRef]
- Lv, D.; Li, X.; Wang, L.; Du, J.; Zhang, J. Effect of carbon as foaming agent on pore structure of foam glass. Adv. Mater. Res. 2010, 105, 765–768. [Google Scholar] [CrossRef]
- Østergaard, M.B.; Cai, B.; Petersen, R.R.; König, J.; Lee, P.D.; Yue, Y. Impact of pore structure on the thermal conductivity of glass foams. Mater. Lett. 2019, 250, 72–74. [Google Scholar] [CrossRef]






Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hussain, Z.; Nouri, S.M.; Sambucci, M.; Valente, M. First Test and Characterizations on Urban Glass Waste with Waste-Derived Carbon Fiber Treated to Realize Foam Glass for Possible Construction Applications. Ceramics 2025, 8, 73. https://doi.org/10.3390/ceramics8020073
Hussain Z, Nouri SM, Sambucci M, Valente M. First Test and Characterizations on Urban Glass Waste with Waste-Derived Carbon Fiber Treated to Realize Foam Glass for Possible Construction Applications. Ceramics. 2025; 8(2):73. https://doi.org/10.3390/ceramics8020073
Chicago/Turabian StyleHussain, Zakim, Seyed Mostafa Nouri, Matteo Sambucci, and Marco Valente. 2025. "First Test and Characterizations on Urban Glass Waste with Waste-Derived Carbon Fiber Treated to Realize Foam Glass for Possible Construction Applications" Ceramics 8, no. 2: 73. https://doi.org/10.3390/ceramics8020073
APA StyleHussain, Z., Nouri, S. M., Sambucci, M., & Valente, M. (2025). First Test and Characterizations on Urban Glass Waste with Waste-Derived Carbon Fiber Treated to Realize Foam Glass for Possible Construction Applications. Ceramics, 8(2), 73. https://doi.org/10.3390/ceramics8020073












