Zirconium Nanostructures Obtained from Anodic Synthesis By-Products and Their Potential Use in PVA-Based Coatings
Abstract
1. Introduction
2. Materials and Methods
2.1. Synthesis and Zr Nanostructure Recovery
2.2. Coating Preparation
2.3. Elemental Composition of ZrO2 Nanostructure Analysis
2.4. Morphological Characterization and Chemical Composition
2.5. Morphological Characterization and Crystallinity
2.6. Transparency Evaluation
2.7. ZrO2 Incorporation in the Polymeric Matrix
2.8. Quality and Surface Roughness of the Coating
2.9. Thermic Resistance
3. Results and Discussion
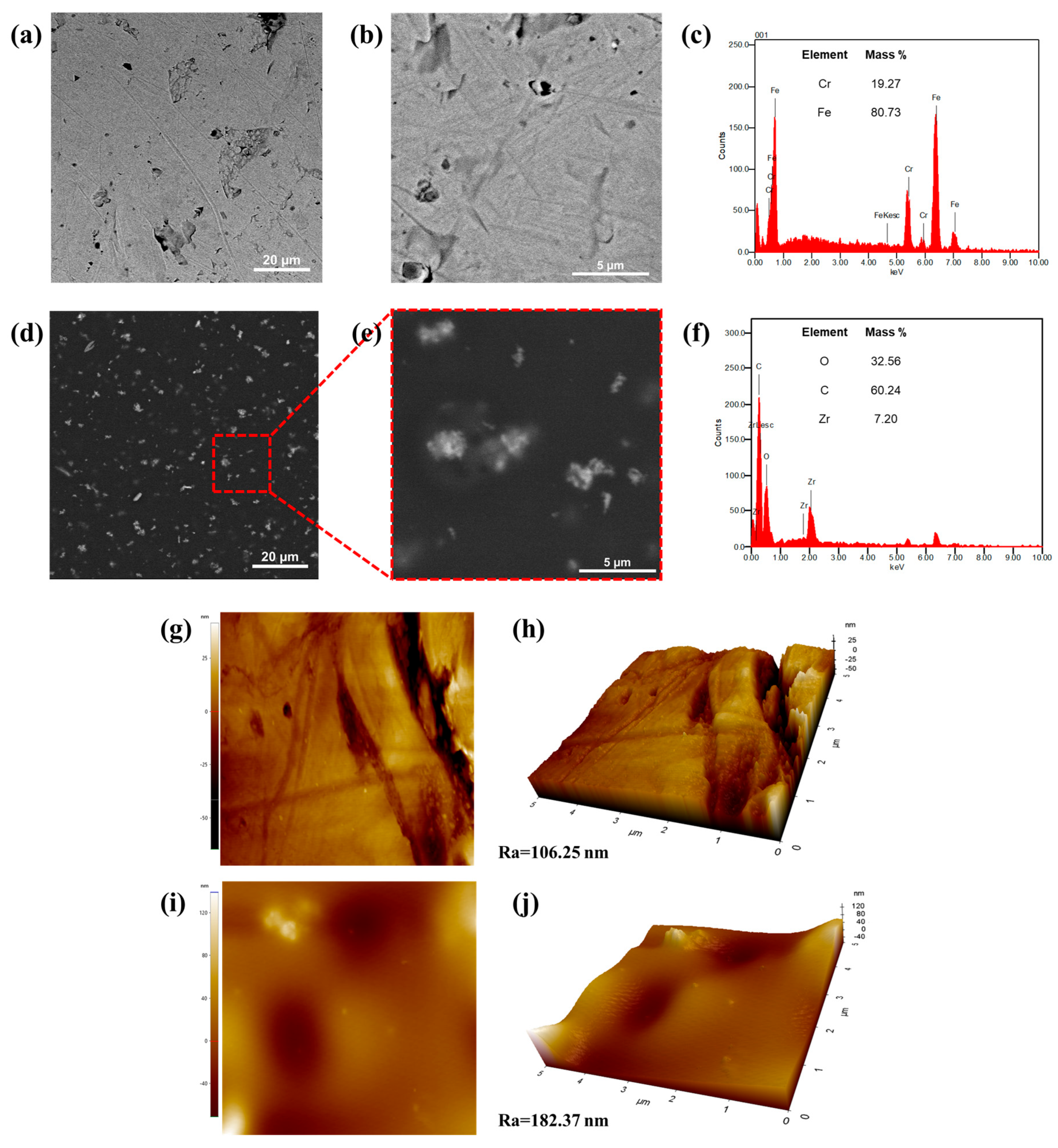
3.1. Characterization of the ZrO2 Nanostructures
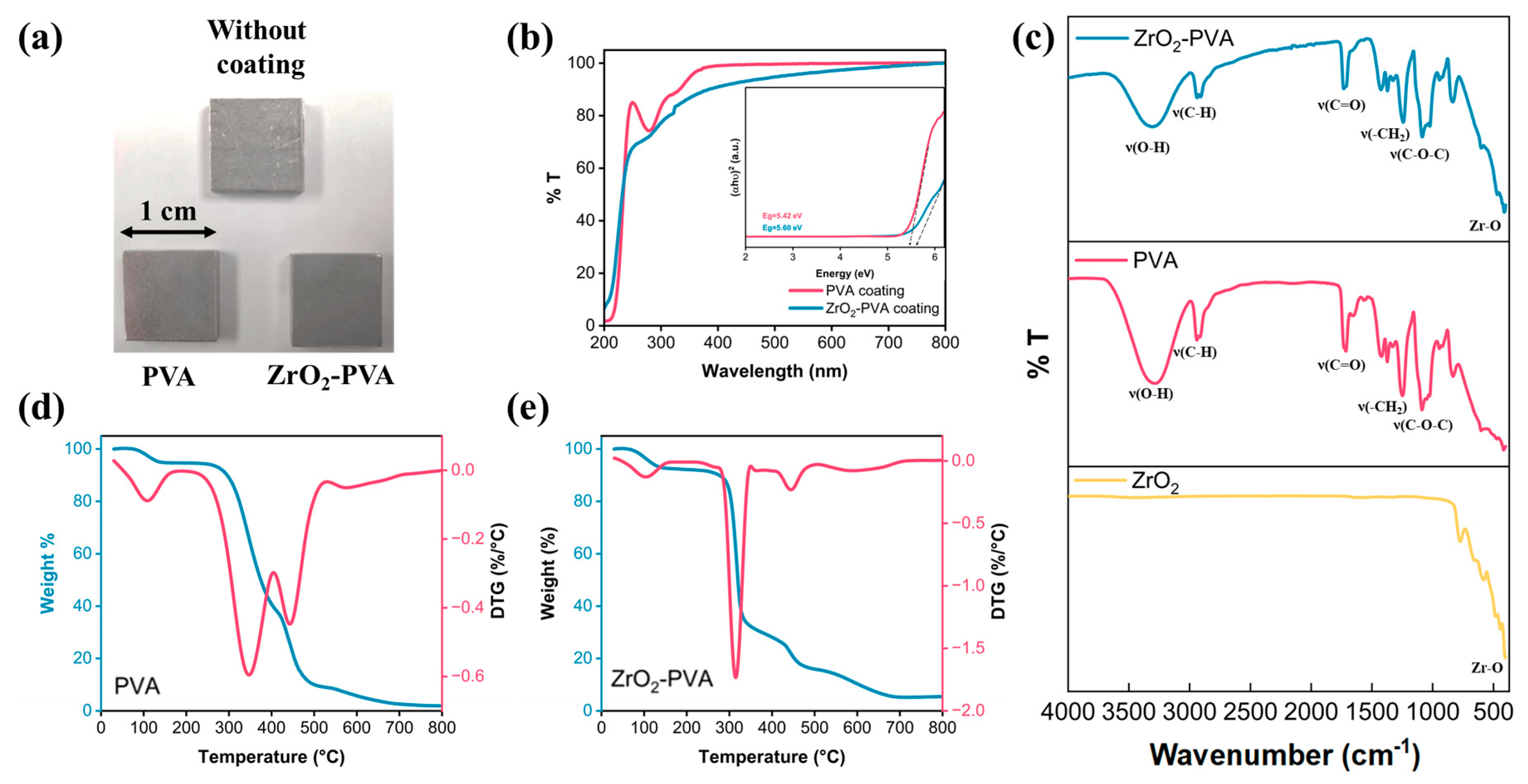
3.2. Coating Characterization
3.2.1. Distribution and Topography
3.2.2. Coating Properties
3.2.3. Thermal Stability
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Fang, D.; Liu, S.; Luo, Z.; Xiong, C.; Xu, W. Facile Fabrication of Freestanding Through-Hole ZrO2 Nanotube Membranes via Two-Step Anodization Methods. Appl. Surf. Sci. 2012, 258, 6217–6223. [Google Scholar] [CrossRef]
- Li, L.; Yan, D.; Lei, J.; He, Y.; Xu, J.; Li, N.; Zhang, S. In Situ Investigation of Initial Stage Growth of Anodic ZrO2 Nanotubes by Spectroscopic Ellipsometry. Electrochem. Commun. 2014, 42, 13–16. [Google Scholar] [CrossRef]
- Salomón-Carlos, J.; Valdez-Salas, B.; Castillo-Saenz, J.; Beltrán-Partida, E. One-Step Synthesis of Freestanding and Translucent ZrO2 Nanotube Membranes by Direct Electrochemical Anodization. Mater. Lett. 2024, 368, 136635. [Google Scholar] [CrossRef]
- Catauro, M.; Barrino, F.; Bononi, M.; Colombini, E.; Giovanardi, R.; Veronesi, P.; Tranquillo, E. Coating of Titanium Substrates with ZrO2 and ZrO2-SiO2 Composites by Sol-Gel Synthesis for Biomedical Applications: Structural Characterization, Mechanical and Corrosive Behavior. Coatings 2019, 9, 200. [Google Scholar] [CrossRef]
- Sinitsyn, D.Y.; Anikin, V.N.; Eremin, S.A.; Yudin, A.G. Protective Coatings Based on ZrO2–Y2O3 and Al2O3–TiO2 Systems with Modifying Additives on CCCM. Refract. Ind. Ceram. 2017, 58, 194–201. [Google Scholar] [CrossRef]
- Song, X.; Ding, Y.; Zhang, J.; Jiang, C.; Liu, Z.; Lin, C.; Zheng, W.; Zeng, Y. Thermophysical and Mechanical Properties of Cubic, Tetragonal and Monoclinic ZrO2. J. Mater. Res. Technol. 2023, 23, 648–655. [Google Scholar] [CrossRef]
- Hallensleben, M.L. Polyvinyl Compounds, Others. In Ullmann’s Encyclopedia of Industrial Chemistry; Wiley-VCH, Ed.; Wiley: Hoboken, NJ, USA, 2000. [Google Scholar] [CrossRef]
- Zeng, S.; Li, L.; Wang, Q. Structure-Property Correlation of Polyvinyl Alcohol Films Fabricated by Different Processing Methods. Polym. Test. 2023, 126, 108143. [Google Scholar] [CrossRef]
- Kwon, H.-J.; Hong, S.-M.; Park, S.-M.; Lee, C.W. Characterization of Acid-Modified Polyvinyl Alcohol and Its Application to Barrier-Coated Paper for Eco-Friendly Food Packaging. Food Packag. Shelf Life 2024, 43, 101271. [Google Scholar] [CrossRef]
- Suleiman, G.S.A.; Zeng, X.; Chakma, R.; Wakai, I.Y.; Feng, Y. Recent Advances and Challenges in Thermal Stability of PVA -based Film: A Review. Polym. Adv. Technol. 2024, 35, e6327. [Google Scholar] [CrossRef]
- Mutlu, I.H.; Emre, M.C.; Kaya, A.O. A Comparison of the Corrosion Resistance of Galvanized Low Steel with Solgel Method Coated ZrO2, ZrO2+Polymer Coating. Kuwait J. Sci. 2023, 50, 524–538. [Google Scholar] [CrossRef]
- Petousis, M.; Moutsopoulou, A.; Korlos, A.; Papadakis, V.; Mountakis, N.; Tsikritzis, D.; Ntintakis, I.; Vidakis, N. The Effect of Nano Zirconium Dioxide (ZrO2)-Optimized Content in Polyamide 12 (PA12) and Polylactic Acid (PLA) Matrices on Their Thermomechanical Response in 3D Printing. Nanomaterials 2023, 13, 1906. [Google Scholar] [CrossRef]
- Simon, S.M.; Chandran, A.; George, G.; Sajna, M.S.; Valparambil, P.; Kumi-Barmiah, E.; Jose, G.; Biju, P.R.; Joseph, C.; Unnikrishnan, N.V. Development of Thick Superhydrophilic TiO2–ZrO2 Transparent Coatings Realized through the Inclusion of Poly(Methyl Methacrylate) and Pluronic-F127. ACS Omega 2018, 3, 14924–14932. [Google Scholar] [CrossRef] [PubMed]
- Alshammari, K.; Alashgai, T.; Alshammari, A.H.; Abdelhamied, M.M.; Alotibi, S.; Atta, A. Effects of Nd2O3 Nanoparticles on the Structural Characteristics and Dielectric Properties of PVA Polymeric Films. Polymers 2023, 15, 4084. [Google Scholar] [CrossRef] [PubMed]
- Hou, Z.; Chavez, S.E.; LaChance, A.M.; Jones, M.D.; French, C.D.; Walsh, A.M.; Shaw, M.T.; Sun, L. Polyvinyl Alcohol (PVA)/Montmorillonite (MMT) Nanocomposite Coatings via a Rotational Coating Method. Adv. Compos. Hybrid. Mater. 2024, 7, 150. [Google Scholar] [CrossRef]
- Wong-Miramontes, I.M.; Valdez-Salas, B.; Beltrán-Partida, E.; Salvador-Carlos, J.; Guillén-Carvajal, K.; Castillo-Saenz, J.; Moe, P.; Cheng, N. Development and Evaluation of Chitosan Active Films for Food Packaging Materials: A New Exoskeleton Source. ChemistrySelect 2025, 10, e202402708. [Google Scholar] [CrossRef]
- Saei, E.; Ramezanzadeh, B.; Amini, R.; Kalajahi, M.S. Effects of Combined Organic and Inorganic Corrosion Inhibitors on the Nanostructure Cerium Based Conversion Coating Performance on AZ31 Magnesium Alloy: Morphological and Corrosion Studies. Corros. Sci. 2017, 127, 186–200. [Google Scholar] [CrossRef]
- Valdez, B.; Kiyota, S.; Stoytcheva, M.; Zlatev, R.; Bastidas, J.M. Cerium-Based Conversion Coatings to Improve the Corrosion Resistance of Aluminium Alloy 6061-T6. Corros. Sci. 2014, 87, 141–149. [Google Scholar] [CrossRef]
- Zhao, Y.; Chen, X.-H.; Hu, J.-M. A Novel and Facile Method for Constructing Micro-Nano Porous Phytic Acid Pretreatment Layer on Metal Surface. Corros. Sci. 2021, 186, 109464. [Google Scholar] [CrossRef]
- Wu, C.-H.; Chen, S.-Y.; Shen, P. On the Densification of Cubic ZrO2 Nanocondensates by Capillarity Force and Turbostratic C–Si–H Multiple Shell. J. Solid. State Chem. 2013, 200, 170–178. [Google Scholar] [CrossRef]
- Tsuchiya, H.; Macak, J.M.; Taveira, L.; Schmuki, P. Fabrication and Characterization of Smooth High Aspect Ratio Zirconia Nanotubes. Chem. Phys. Lett. 2005, 410, 188–191. [Google Scholar] [CrossRef]
- Rani, G.E.; Murugeswari, R.; Siengchin, S.; Rajini, N.; Kumar, M.A. Quantitative Assessment of Particle Dispersion in Polymeric Composites and Its Effect on Mechanical Properties. J. Mater. Res. Technol. 2022, 19, 1836–1845. [Google Scholar] [CrossRef]
- Wu, L.; Cai, Z.; Li, L.; Wang, X. Breakdown Strength and Energy Density Enhancement in Polymer-Ceramic Nanocomposites: Role of Particle Size Distribution. Compos. Sci. Technol. 2021, 212, 108868. [Google Scholar] [CrossRef]
- Ruzova, T.A.; Haddadi, B. Surface Roughness and Its Measurement Methods—Analytical Review. Results Surf. Interfaces 2025, 19, 100441. [Google Scholar] [CrossRef]
- Kaya, S.; Ozturk, O.; Arda, L. Roughness and Bearing Analysis of ZnO Nanorods. Ceram. Int. 2020, 46, 15183–15196. [Google Scholar] [CrossRef]
- Bashir, A.; Farooq, M.; Malik, A.; Naseem, S.; Bhatti, A.S. UV-A Treatment of ZrO2 Thin Films Fabricated by Environmental Friendlier Water-Based Solution Processing: Structural and Optical Studies. Coatings 2021, 11, 821. [Google Scholar] [CrossRef]
- De Sá, R.G.; Arantes, T.M.; De Macedo, E.F.; Dona’, L.M.; Pereira, J.C.F.; Hurtado, C.R.; Varghese, R.J.; Oluwafemi, O.S.; Tada, D.B. Photoprotective Activity of Zirconia Nanoparticles. Colloids Surf. B Biointerfaces 2021, 202, 111636. [Google Scholar] [CrossRef]
- Alshammari, K. Influence of ZrO2 Nanoparticles on the Structural and Photocatalytic Properties of Three-Dimensional PVA/g-C3N4 Polymer Films. J. Alloys Compd. 2024, 1003, 175622. [Google Scholar] [CrossRef]
- Nohynek, G.J.; Lademann, J.; Ribaud, C.; Roberts, M.S. Grey Goo on the Skin? Nanotechnology, Cosmetic and Sunscreen Safety. Crit. Rev. Toxicol. 2007, 37, 251–277. [Google Scholar] [CrossRef] [PubMed]
- Aegerter, M.A.; Al-Dahoudi, N.; Solieman, A.; Kavak, H.; Oliveira, P. Transparent Conducting Coatings Made by Chemical Nanotechnology Processes. Mol. Cryst. Liq. Cryst. 2004, 417, 105–114. [Google Scholar] [CrossRef]
- Szyszka, B.; Dewald, W.; Gurram, S.K.; Pflug, A.; Schulz, C.; Siemers, M.; Sittinger, V.; Ulrich, S. Recent Developments in the Field of Transparent Conductive Oxide Films for Spectral Selective Coatings, Electronics and Photovoltaics. Curr. Appl. Phys. 2012, 12, S2–S11. [Google Scholar] [CrossRef]
- Horti, N.C.; Kamatagi, M.D.; Nataraj, S.K.; Wari, M.N.; Inamdar, S.R. Structural and Optical Properties of Zirconium Oxide (ZrO2) Nanoparticles: Effect of Calcination Temperature. Nano Ex. 2020, 1, 010022. [Google Scholar] [CrossRef]
- Mansur, H.S.; Sadahira, C.M.; Souza, A.N.; Mansur, A.A.P. FTIR Spectroscopy Characterization of Poly (Vinyl Alcohol) Hydrogel with Different Hydrolysis Degree and Chemically Crosslinked with Glutaraldehyde. Mater. Sci. Eng. C 2008, 28, 539–548. [Google Scholar] [CrossRef]
- Mohsin, M.; Hossin, A.; Haik, Y. Thermal and Mechanical Properties of Poly(Vinyl Alcohol) Plasticized with Glycerol. J. Appl. Polym. Sci. 2011, 122, 3102–3109. [Google Scholar] [CrossRef]
- Tsioptsias, C.; Fardis, D.; Ntampou, X.; Tsivintzelis, I.; Panayiotou, C. Thermal Behavior of Poly(Vinyl Alcohol) in the Form of Physically Crosslinked Film. Polymers 2023, 15, 1843. [Google Scholar] [CrossRef]
- Channe, S.S.; Singh, R.; Kulkarni, S.G. Effect of Metal Oxide Nanoparticles on Thermal Behavior of Polyvinyl Alcohol. Polym. Bull. 2024, 81, 3403–3438. [Google Scholar] [CrossRef]
- Rezaei, A.; Katoueizadeh, E.; Zebarjad, S.M. Investigating of the Influence of Zinc Oxide Nanoparticles Morphology on the Properties of Electrospun Polyvinyl Alcohol/Chitosan (PVA/CS) Nanofibers. J. Drug Deliv. Sci. Technol. 2023, 86, 104712. [Google Scholar] [CrossRef]
- Davar, F.; Majedi, A.; Mirzaei, A. Polyvinyl Alcohol Thin Film Reinforced by Green Synthesized Zirconia Nanoparticles. Ceram. Int. 2018, 44, 19377–19382. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Valdez-Salas, B.; Salvador-Carlos, J.; Beltrán-Partida, E.A.; Castillo-Sáenz, J.; Chairez-González, J.; Curiel-Álvarez, M. Zirconium Nanostructures Obtained from Anodic Synthesis By-Products and Their Potential Use in PVA-Based Coatings. Ceramics 2025, 8, 74. https://doi.org/10.3390/ceramics8020074
Valdez-Salas B, Salvador-Carlos J, Beltrán-Partida EA, Castillo-Sáenz J, Chairez-González J, Curiel-Álvarez M. Zirconium Nanostructures Obtained from Anodic Synthesis By-Products and Their Potential Use in PVA-Based Coatings. Ceramics. 2025; 8(2):74. https://doi.org/10.3390/ceramics8020074
Chicago/Turabian StyleValdez-Salas, Benjamín, Jorge Salvador-Carlos, Ernesto Alonso Beltrán-Partida, Jhonathan Castillo-Sáenz, Jimena Chairez-González, and Mario Curiel-Álvarez. 2025. "Zirconium Nanostructures Obtained from Anodic Synthesis By-Products and Their Potential Use in PVA-Based Coatings" Ceramics 8, no. 2: 74. https://doi.org/10.3390/ceramics8020074
APA StyleValdez-Salas, B., Salvador-Carlos, J., Beltrán-Partida, E. A., Castillo-Sáenz, J., Chairez-González, J., & Curiel-Álvarez, M. (2025). Zirconium Nanostructures Obtained from Anodic Synthesis By-Products and Their Potential Use in PVA-Based Coatings. Ceramics, 8(2), 74. https://doi.org/10.3390/ceramics8020074






