Clarification of Clove Basil Extract Using Spinel Hollow Fiber Membranes
Abstract
1. Introduction
2. Materials and Methods
2.1. Material
2.2. Fabrication of Spinel-Based Hollow Fiber Membranes
2.3. Membrane Filtration of the Clove Basil Extract
2.4. Physicochemical Analyses
3. Results and Discussion
3.1. Characteristics of the Produced Spinel Hollow Fibers
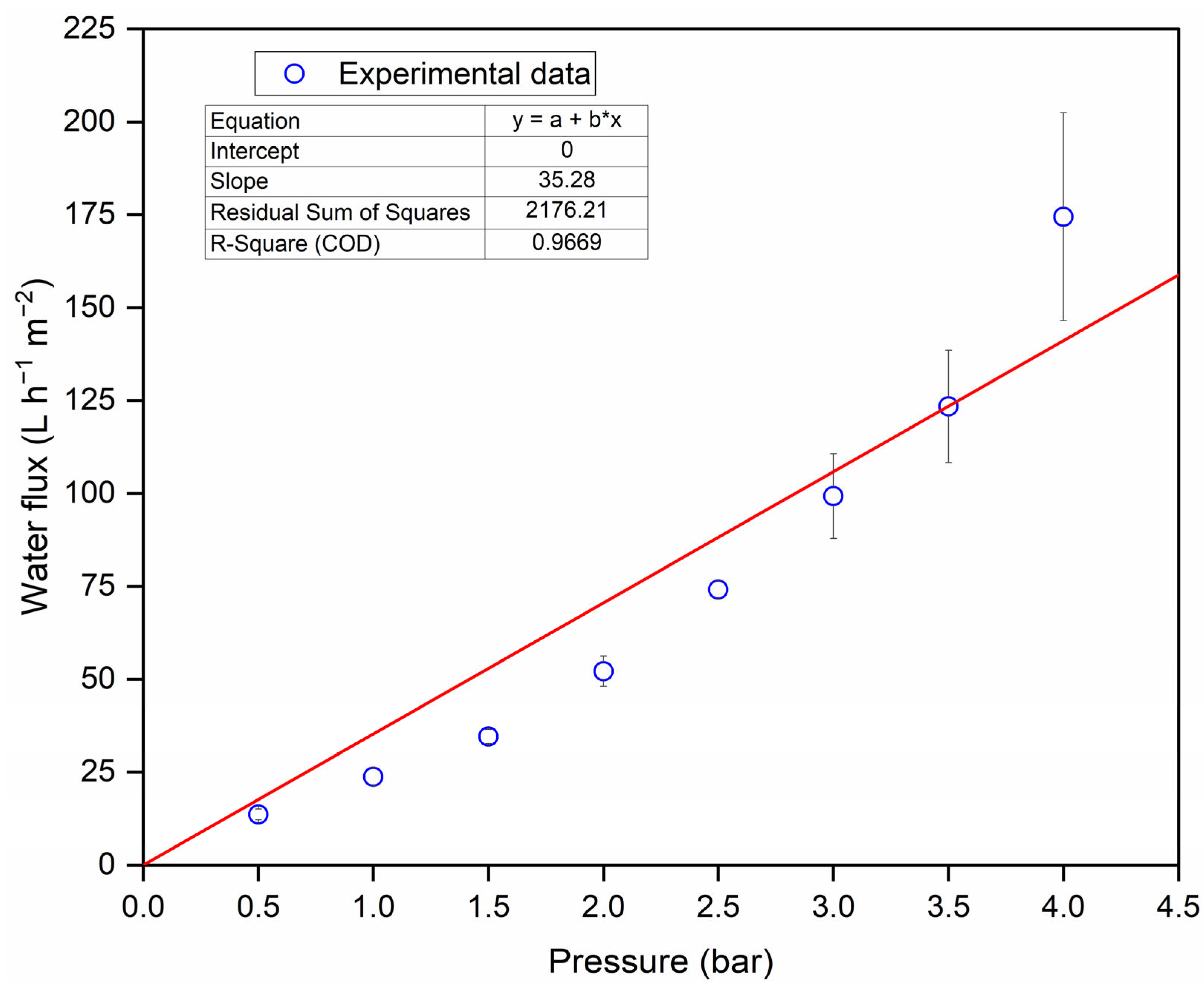
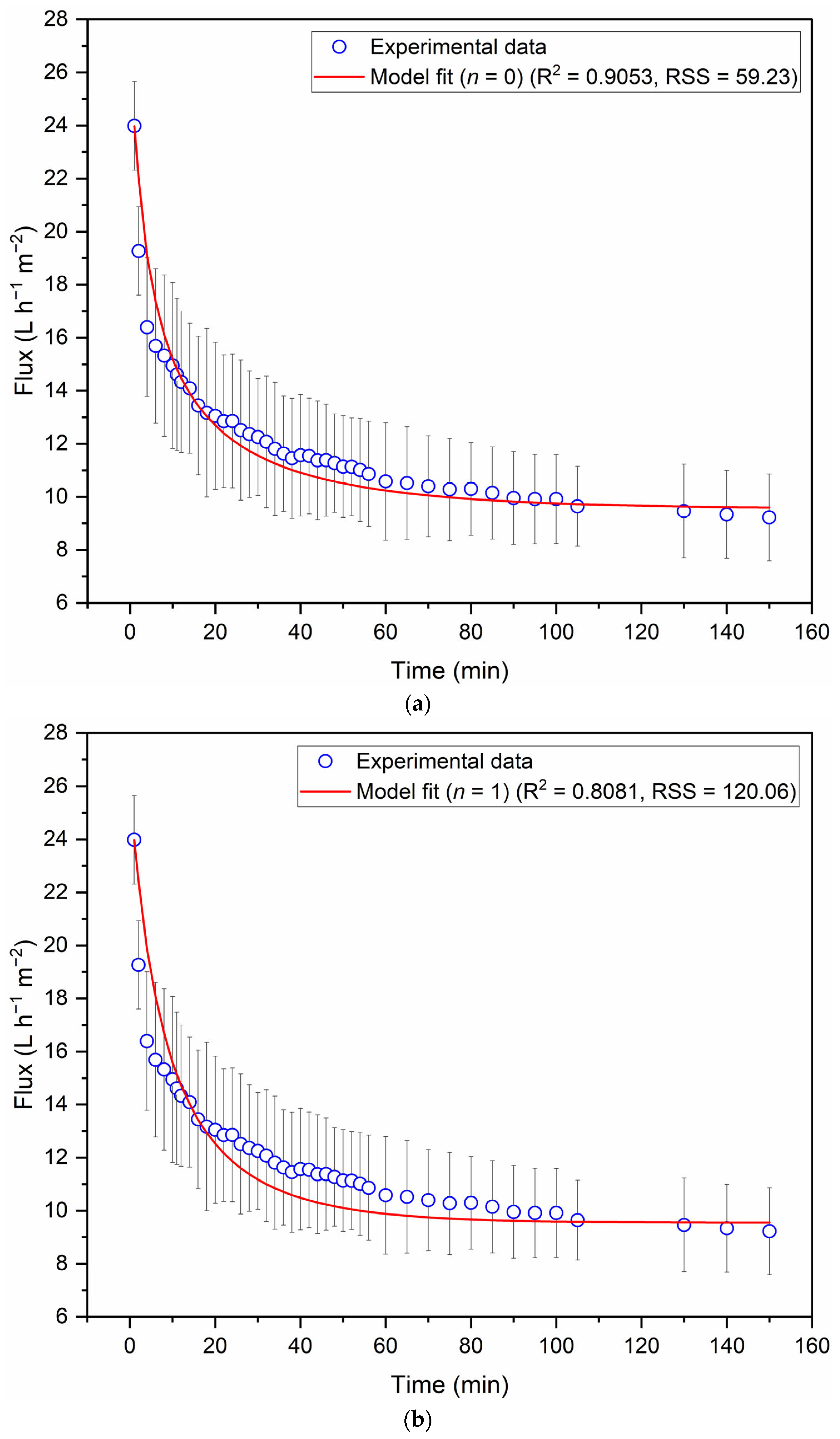
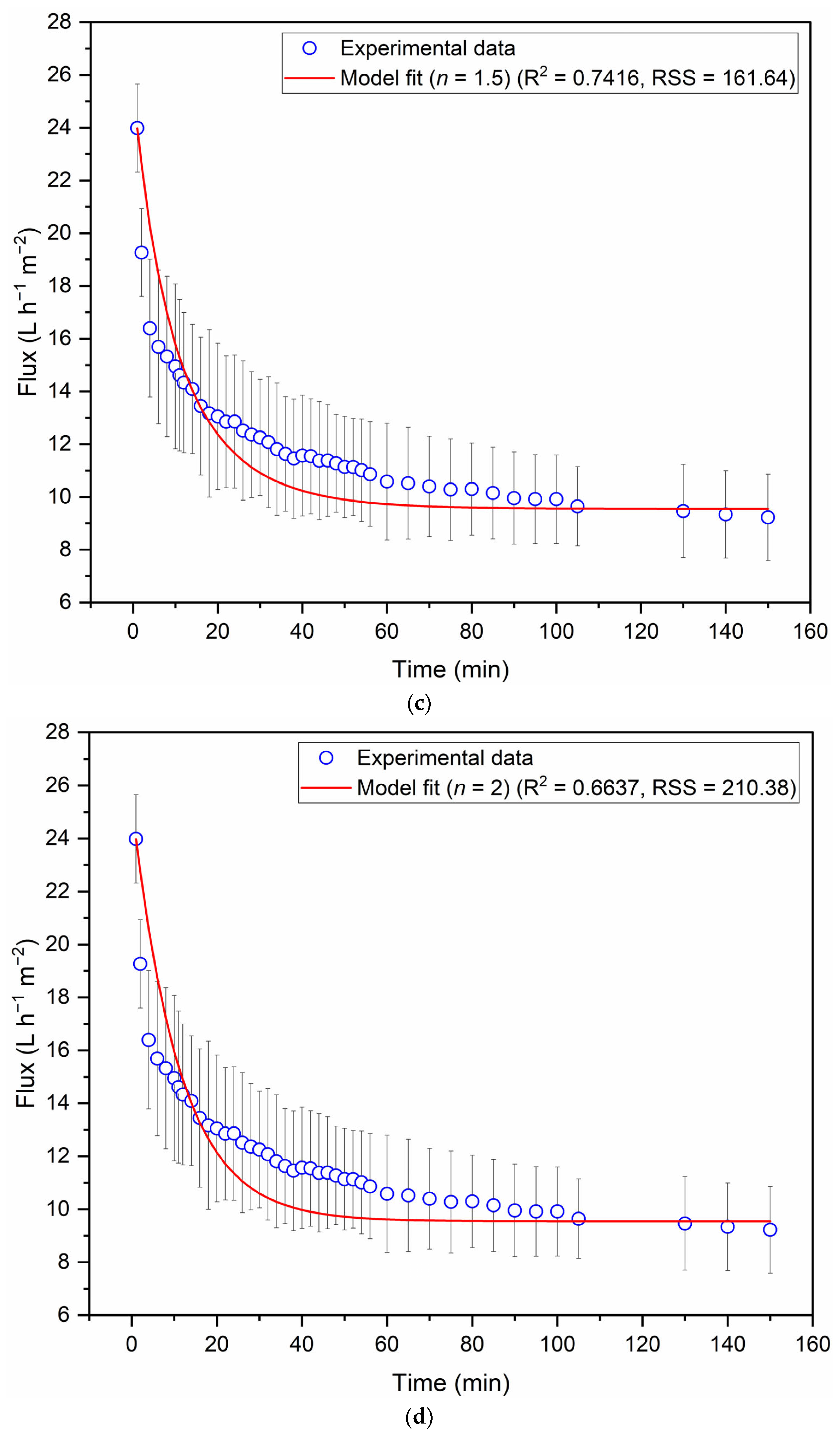
3.2. Filtration of Clove Basil Extract Through the Spinel Hollow Fibers
3.3. Physicochemical Characteristics of Feed and Permeate
4. Conclusion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Paraíso, C.M.; Santos, S.S.; Pereira Bessa, L.; Lopes, A.P.; Ogawa, C.Y.L.; Costa, S.C.; Reis, M.H.M.; Filho, U.C.; Sato, F.; Visentainer, J.V.; et al. Performance of Asymmetric Spinel Hollow Fiber Membranes for Hibiscus (Hibiscus sabdariffa L.) Extract Clarification: Flux Modeling and Extract Stability. J. Food Process Preserv. 2020, 44, e14948. [Google Scholar] [CrossRef]
- Fung, Y.L.E.; Wang, H. Nickel Aluminate Spinel Reinforced Ceramic Hollow Fibre Membrane. J. Memb. Sci. 2014, 450, 418–424. [Google Scholar] [CrossRef]
- Mohamad Esham, M.I.; Ahmad, A.L.; Othman, M.H.D.; Adam, M.R. A Comprehensive Review on Low-Cost & Eco-Friendly Ceramic Hollow Fiber Membranes for Oilfield-Produced Water Treatment: Innovation, Efficiency, Challenges and Future Prospects. J. Water Process Eng. 2024, 68, 106591. [Google Scholar] [CrossRef]
- Ramanamane, N.; Pita, M.; Sob, B. Advanced Low-Cost Natural Materials for High-Performance Oil-Water Filtration Membranes: Achievements, Challenges, and Future Directions. Membranes 2024, 14, 264. [Google Scholar] [CrossRef]
- Di Quarto, F.; Zaffora, A.; Di Franco, F.; Santamaria, M. Modeling of Optical Band-Gap Values of Mixed Oxides Having Spinel Structure AB2O4 (A = Mg, Zn and B = Al, Ga) by a Semiempirical Approach. ACS Org. Inorg. Au 2024, 4, 120–134. [Google Scholar] [CrossRef]
- Chen, M.; Zhu, L.; Chen, J.; Yang, F.; Tang, C.Y.; Guiver, M.D.; Dong, Y. Spinel-Based Ceramic Membranes Coupling Solid Sludge Recycling with Oily Wastewater Treatment. Water Res. 2020, 169, 115180. [Google Scholar] [CrossRef] [PubMed]
- Aziz, M.H.A.; Othman, M.H.D.; Hashim, N.A.; Rahman, M.A.; Jaafar, J.; Hubadillah, S.K.; Tai, Z.S. Pretreated Aluminium Dross Waste as a Source of Inexpensive Alumina-Spinel Composite Ceramic Hollow Fibre Membrane for Pretreatment of Oily Saline Produced Water. Ceram. Int. 2019, 45, 2069–2078. [Google Scholar] [CrossRef]
- Jiang, D.; Gao, C.; Liu, L.; Yu, T.; Li, Y.; Wang, H. Customized Copper/Cobalt-Rich Ferrite Spinel-Based Construction Ceramic Membrane Incorporating Gold Tailings for Enhanced Treatment of Industrial Oily Emulsion Wastewater. Sep. Purif. Technol. 2023, 320, 124131. [Google Scholar] [CrossRef]
- Bessa, L.P.; de Paulo Ferreira, E.; de Santana Magalhães, F.; Ferreira, F.B.; Cardoso, V.L.; Reis, M.H.M. Micro-Structured and Reinforced Spinel Hollow Fiber Membranes: Influence of Sintering Temperature and Ceramic Powder Composition. Ceram. Int. 2019, 45, 23632–23642. [Google Scholar] [CrossRef]
- Qi, T.; Chen, X.; Fan, Y.; Zhong, J. Ceramic Membrane Technology for the Separation and Purification of Bioactive Compounds: A Critical Review of Applications, Diafiltration Modeling, and Fouling Prevention. Sep. Purif. Technol. 2025, 361, 131301. [Google Scholar] [CrossRef]
- Dorneles, K.R.; da Rocha, A.C.; Cardoso, V.L.; Reis, M.H.M. Recovery of Health-Promoting Phenolic Compounds from Clove Basil (Ocimum Gratissimum L.) by Aqueous Extraction and Ultrafiltration. Food Bioprod. Process. 2025, 149, 392–400. [Google Scholar] [CrossRef]
- Field, R.W.; Wu, J.J. Modelling of Permeability Loss in Membrane Filtration: Re-Examination of Fundamental Fouling Equations and Their Link to Critical Flux. Desalination 2011, 283, 68–74. [Google Scholar] [CrossRef]
- Mahmoud, S.A.; Elsisi, M.E.; Mansour, A.F. Synthesis and Electrochemical Performance of α-Al2O3 and M-Al2O4 Spinel Nanocomposites in Hybrid Quantum Dot-Sensitized Solar Cells. Sci. Rep. 2022, 12, 17009. [Google Scholar] [CrossRef]
- Chen, Y.; Feng, Q.; Zhang, G.; Liu, D.; Liu, R. Effect of Sodium Pyrophosphate on the Reverse Flotation of Dolomite from Apatite. Minerals 2018, 8, 278. [Google Scholar] [CrossRef]
- Dias, W.A.; Ascendino, G.G.; Gelamo, R.V.; de Souza Ferreira, J.; Reis, M.H.M. Composite Graphene Oxide Membranes Produced from Recycled Graphite with Tunable Molecular Selectivity for Wastewater Treatment. J. Water Process Eng. 2025, 70, 106998. [Google Scholar] [CrossRef]
- Zhou, Q.; Chang, Q.; Lu, Y.; Sun, J. Mussel-Inspired Construction of Silica-Decorated Ceramic Membranes for Oil–Water Separation. Ceramics 2024, 7, 250–263. [Google Scholar] [CrossRef]
- Wang, W.; Shen, Y.; Shen, J.; Yan, P.; Kang, J.; Cheng, Y.; Shen, L.; Wu, X.; Zhao, S.; Liu, Y.; et al. Preparation of Low-Cost Silicate-Based Microfiltration Membrane: Characterization, Membrane Fouling Mechanism and Antifouling Performance. Chem. Eng. Res. Des. 2022, 185, 344–355. [Google Scholar] [CrossRef]
- Castro-Muñoz, R.; Fíla, V.; Barragán-Huerta, B.E.; Yáñez-Fernández, J.; Piña-Rosas, J.A.; Arboleda-Mejía, J. Processing of Xoconostle Fruit (Opuntia Joconostle) Juice for Improving Its Commercialization Using Membrane Filtration. J. Food Process Preserv. 2018, 42, e13394. [Google Scholar] [CrossRef]
- Anusmitha, K.M.; Aruna, M.; Job, J.T.; Narayanankutty, A.; PB, B.; Rajagopal, R.; Alfarhan, A.; Barcelo, D. Phytochemical Analysis, Antioxidant, Anti-Inflammatory, Anti-Genotoxic, and Anticancer Activities of Different Ocimum Plant Extracts Prepared by Ultrasound-Assisted Method. Physiol. Mol. Plant Pathol. 2022, 117, 101746. [Google Scholar] [CrossRef]
- Ibrahim, R.Y.M.; Mansour, S.M.; Elkady, W.M. Phytochemical Profile and Protective Effect of Ocimum Basilicum Aqueous Extract in Doxorubicin/Irradiation-Induced Testicular Injury. J. Pharm. Pharmacol. 2020, 72, 101–110. [Google Scholar] [CrossRef]
- Castro-Muñoz, R.; Yáñez-Fernández, J.; Fíla, V. Phenolic Compounds Recovered from Agro-Food by-Products Using Membrane Technologies: An Overview. Food Chem. 2016, 213, 753–762. [Google Scholar] [CrossRef] [PubMed]
- Reig-Valor, M.-J.; Rozas-Martínez, J.; López-Borrell, A.; Lora-García, J.; López-Pérez, M.-F. Experimental Study of a Sequential Membrane Process of Ultrafiltration and Nanofiltration for Efficient Polyphenol Extraction from Wine Lees. Membranes 2024, 14, 82. [Google Scholar] [CrossRef] [PubMed]
- Cimini, A.; Moresi, M. Pale Lager Clarification Using Novel Ceramic Hollow-Fiber Membranes and CO2 Backflush Program. Food Bioproc Tech. 2015, 8, 2212–2224. [Google Scholar] [CrossRef]
- Mora, F.; Pérez, K.; Quezada, C.; Herrera, C.; Cassano, A.; Ruby-Figueroa, R. Impact of Membrane Pore Size on the Clarification Performance of Grape Marc Extract by Microfiltration. Membranes 2019, 9, 146. [Google Scholar] [CrossRef]
- Tomczak, W.; Gryta, M. Clarification of 1,3-Propanediol Fermentation Broths by Using a Ceramic Fine UF Membrane. Membranes 2020, 10, 319. [Google Scholar] [CrossRef]
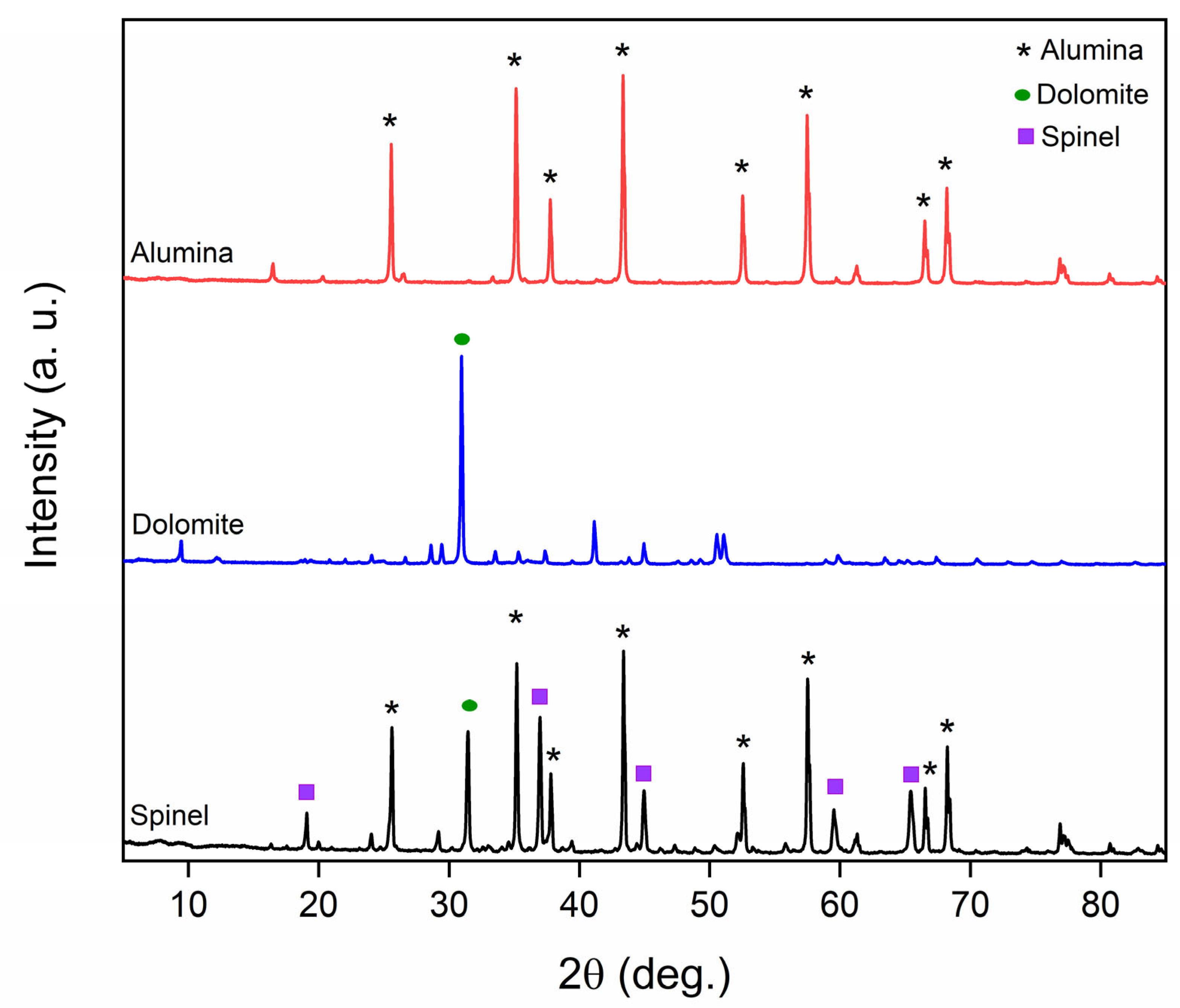
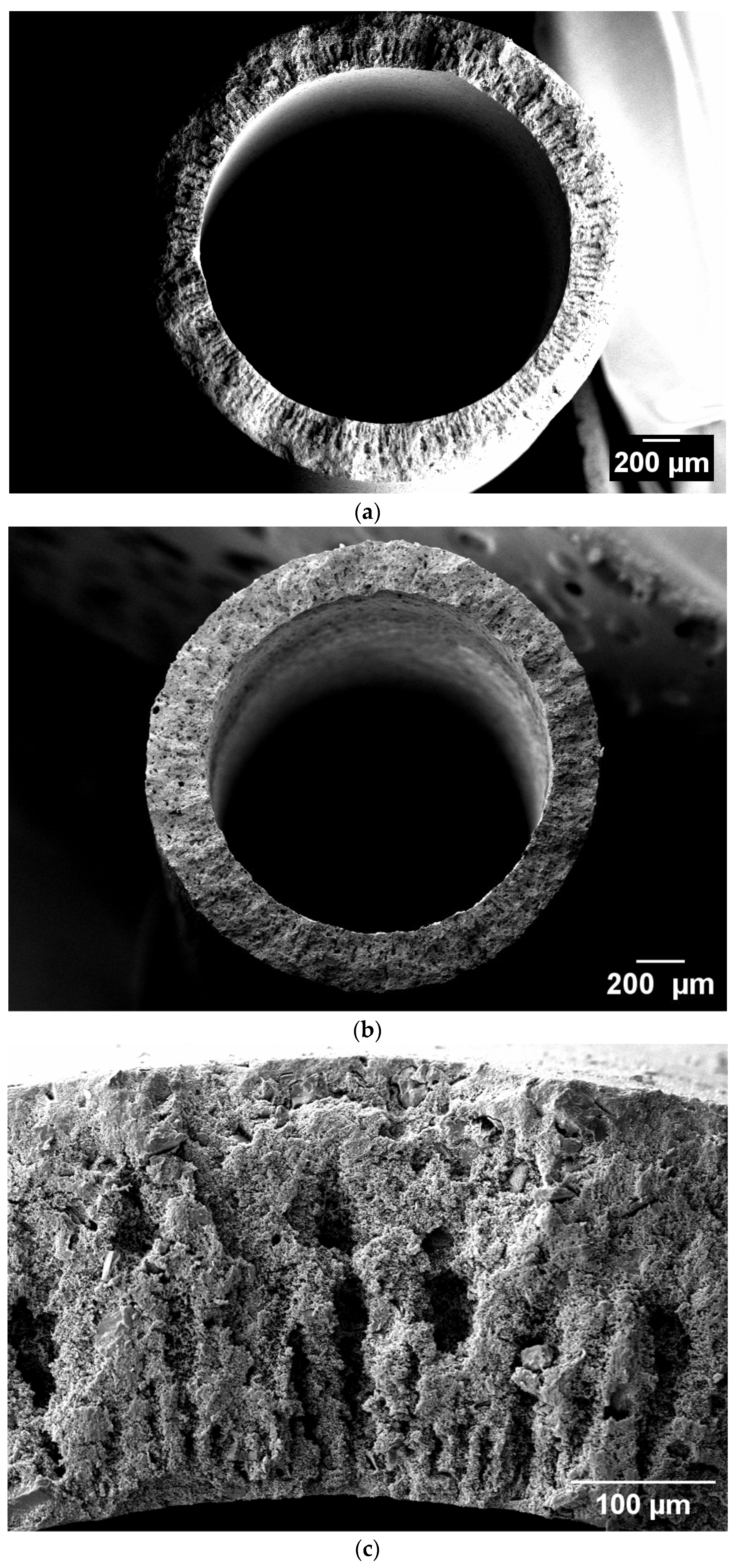

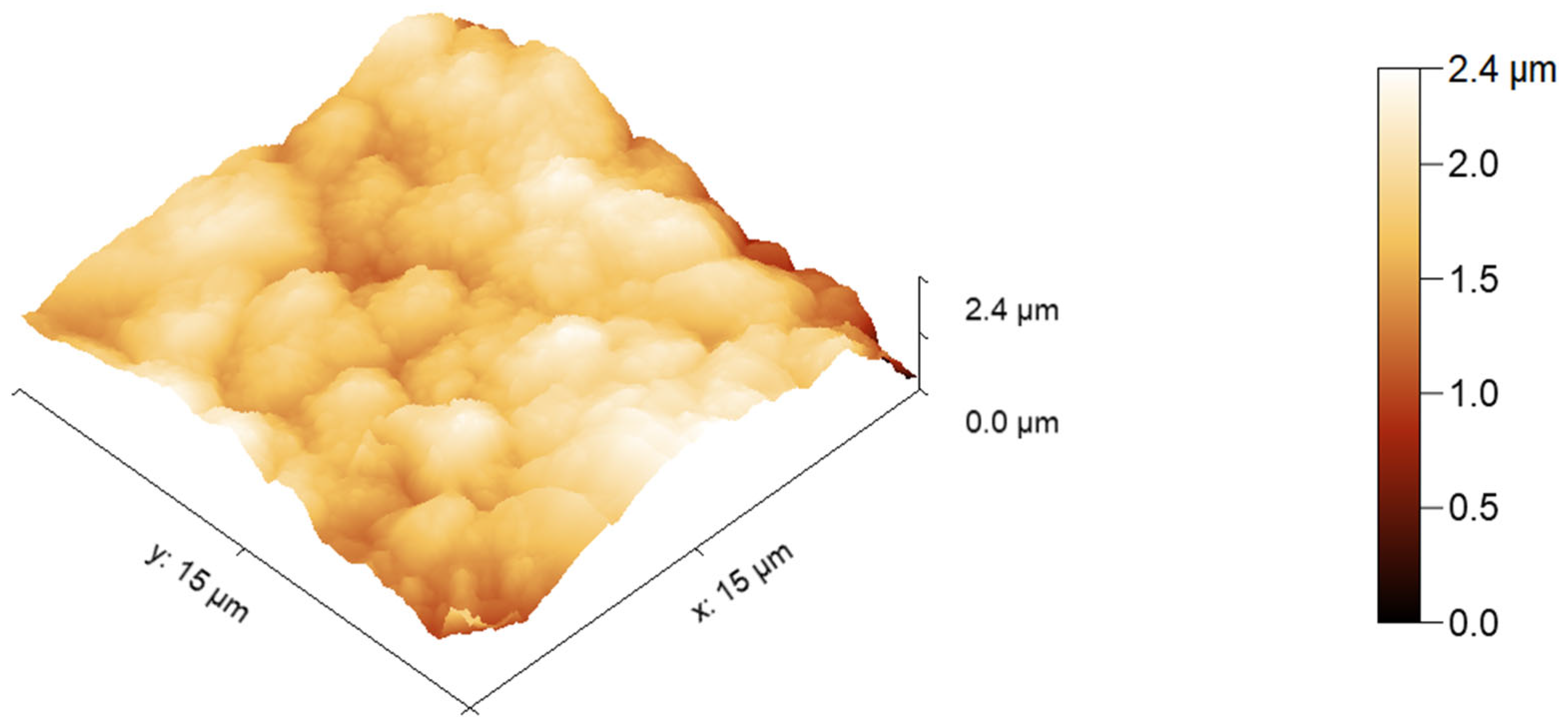

| Property | Value | Implications | Reference |
|---|---|---|---|
| Average roughness of the outer surface | 144.31 ± 12.93 nm | Moderate roughness enhances permeability while maintaining fouling resistance | This work |
| Water contact angle | 74° | The hydrophilic nature improves water permeability and reduces organic fouling | This work |
| Pore size in the sponge-like layer | 0.16 µm | Provides mechanical support while contributing to selective separation | [9] |
| Pore size of the microchannels | 5.29 µm | Facilitates high permeate flux by reducing hydraulic resistance | [9] |
| Porosity | 43% | Enhances permeability for filtration | [9] |
| Bending Strength | 54.88 ± 4.25 MPa | Ensures durability under operational pressures (critical for industrial use) | [9] |
| Parameter | Clove Basil Extracts | ||
|---|---|---|---|
| Crude | Centrifuged | Permeate | |
| TPC (mgGAE g−1) | 232.95 a ± 5.86 | 229.29 a ± 1.21 | 128.59 b ± 0.34 |
| Antioxidant capacity (DPPH, µmolTE g−1) | 1923.37 a ± 16.50 | 1024.72 b ± 3.91 | 510.94 c ± 1.05 |
| Turbidity (NTU) | 58.3 a ± 1.5 | 16.2 b ± 0.3 | 6.5 c ± 0.3 |
| Soluble solids (%Bx) | 0.40 a ± 0.00 | 0.35 a,b ± 0.07 | 0.20 b ± 0.00 |
| Color coordinate L* | 88.47 c ± 0.01 | 90.24 b ± 0.08 | 92.06 a ± 0.01 |
| Color coordinate a* | −1.00 a ± 0.01 | −1.22 c ± 0.01 | −1.17 b ± 0.00 |
| Color coordinate b* | 16.16 a ± 0.01 | 12.27 b ± 0.01 | 9.38 c ± 0.01 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dorneles, K.R.; Ascendino, G.G.; Cardoso, V.L.; Reis, M.H.M. Clarification of Clove Basil Extract Using Spinel Hollow Fiber Membranes. Ceramics 2025, 8, 57. https://doi.org/10.3390/ceramics8020057
Dorneles KR, Ascendino GG, Cardoso VL, Reis MHM. Clarification of Clove Basil Extract Using Spinel Hollow Fiber Membranes. Ceramics. 2025; 8(2):57. https://doi.org/10.3390/ceramics8020057
Chicago/Turabian StyleDorneles, Kristopher Rodrigues, Guilherme Guimarães Ascendino, Vicelma Luiz Cardoso, and Miria Hespanhol Miranda Reis. 2025. "Clarification of Clove Basil Extract Using Spinel Hollow Fiber Membranes" Ceramics 8, no. 2: 57. https://doi.org/10.3390/ceramics8020057
APA StyleDorneles, K. R., Ascendino, G. G., Cardoso, V. L., & Reis, M. H. M. (2025). Clarification of Clove Basil Extract Using Spinel Hollow Fiber Membranes. Ceramics, 8(2), 57. https://doi.org/10.3390/ceramics8020057








