Molecularly Imprinted Polymer-Supported Ceramic Catalysts for Environmental Applications: A Comprehensive Review
Abstract
1. Introduction
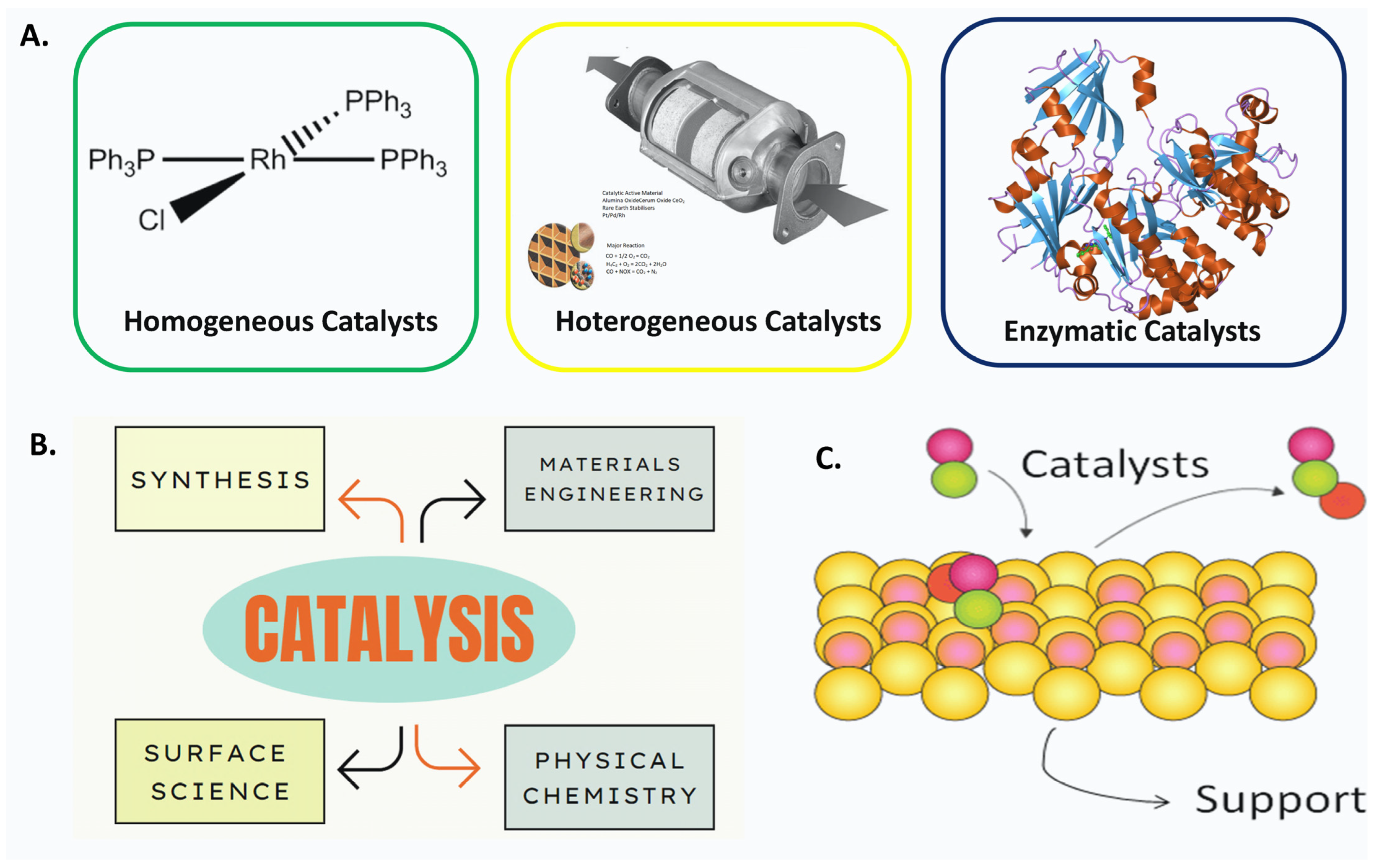
2. Fundamentals of Heterogeneous Catalysis
- ⮚
- Solid catalysts can be easily separated from the reaction products, and this is very important, allowing the recovery of the catalyst for future reuse in the reaction medium;
- ⮚
- Separation simplifies and reduces product purification washing steps;
- ⮚
- In the same way that the volume of water for washing and purifying the organic phase is reduced, it is a very important advantage from an environmental point of view due to reduced wastewater discharges;
- ⮚
- The possibility of using raw materials of lower quality and consequently lower cost.
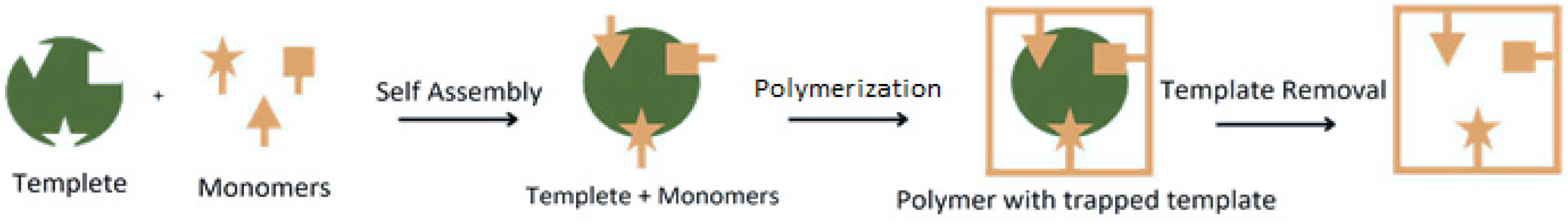
3. Overview of Molecularly Imprinted Polymers as Catalysts and Support for Catalysts
3.1. General Aspects
3.2. Types of Monomers
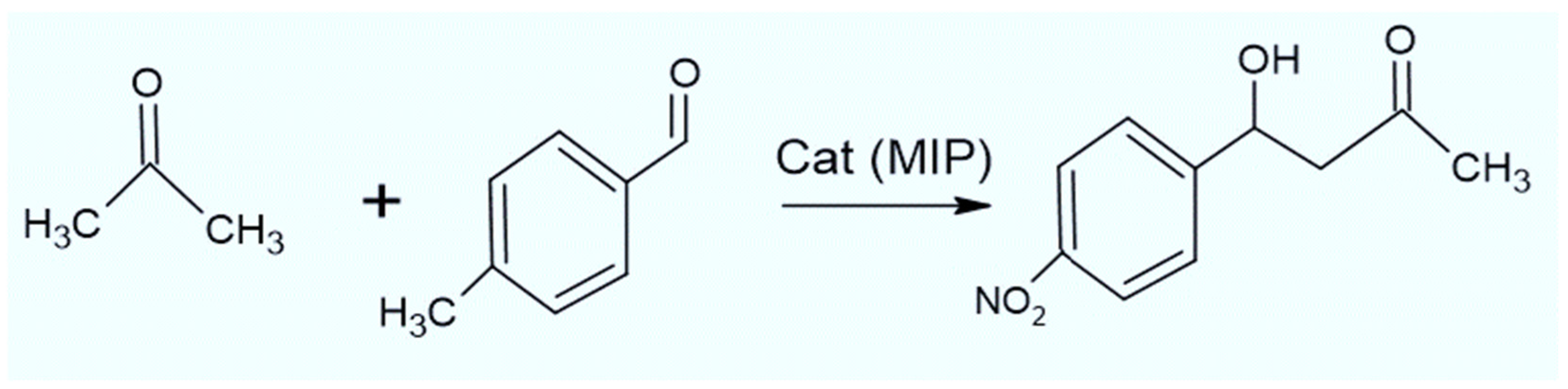
3.3. MIPs as Efficient Catalysts
3.4. MIPs as Efficient Catalytic Supports
4. Overview of Advanced Ceramic Materials as Catalysts
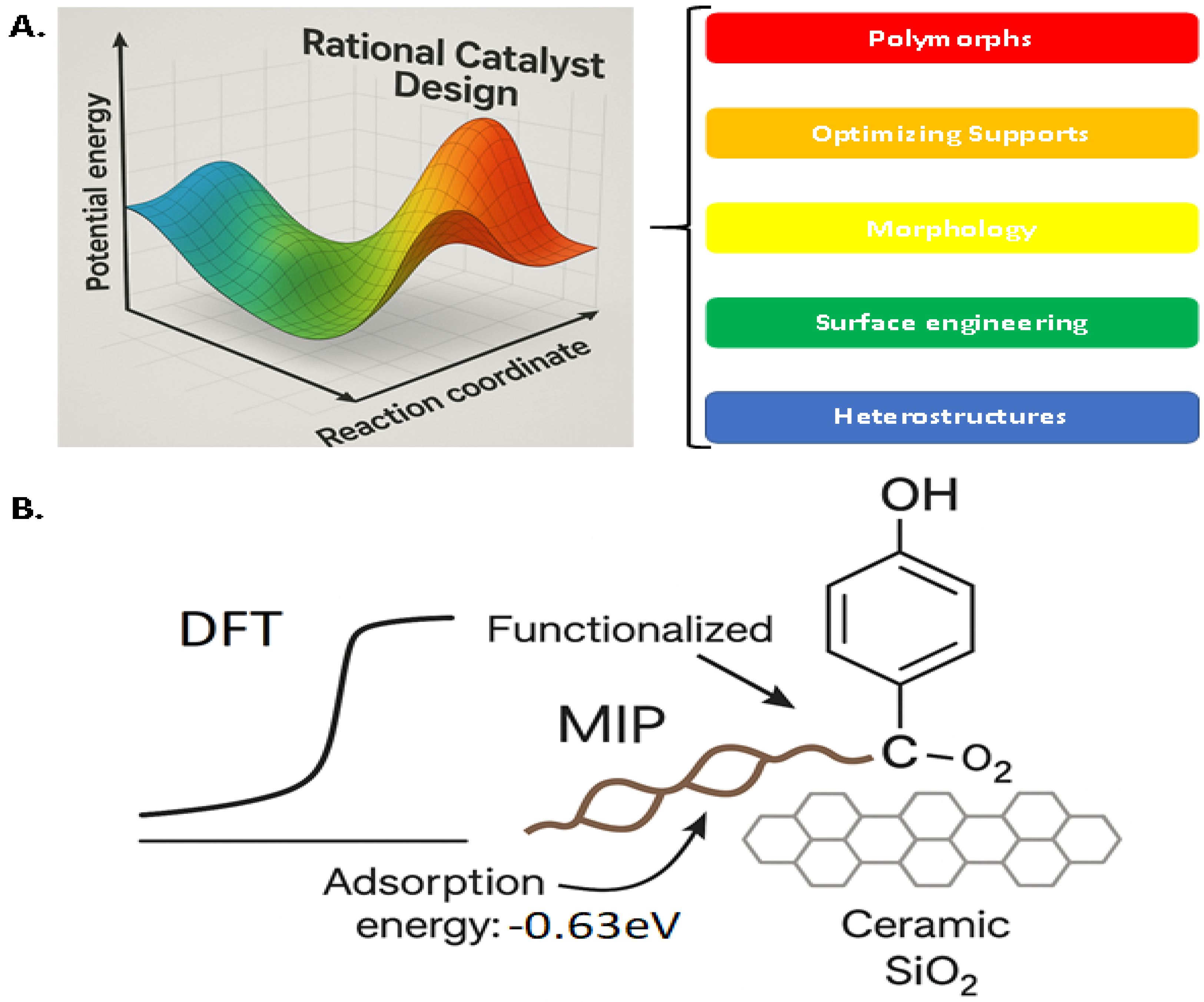
5. Computational Catalyst Design
6. MIP/Ceramic Catalysts for Environmental Applications
7. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Schlögl, R. Preparation of Solid Catalysts; Wiley-VCH: Weinheim, Germany, 1999. [Google Scholar]
- Bowker, M. The Basis and Applications of Heterogeneous Catalysis; Oxford University Press: Oxford, UK, 1998. [Google Scholar]
- Munnik, P.; de Jongh, P.E.; de Jong, K.P. Recent Developments in the Synthesis of Supported Catalysts. Chem. Rev. 2015, 115, 6687–6718. [Google Scholar] [CrossRef] [PubMed]
- Martins, A.; Nunes, N.; Carvalho, A.P.; Martins, L.M.D.R.S. Zeolites and Related Materials as Catalyst Supports for Hydrocarbon Oxidation Reactions. Catalysts 2022, 12, 154. [Google Scholar] [CrossRef]
- Luo, Z.; Wang, J.; Zhou, W.; Li, J. Catalyst-Support Interactions Promoted Acidic Electrochemical Oxygen Evolution Catalysis: A Mini Review. Molecules 2023, 28, 2262. [Google Scholar] [CrossRef] [PubMed]
- Julkapli, N.M.; Bagheri, S. Graphene Supported Heterogeneous Catalysts: An Overview. Int. J. Hydrogen Energy 2015, 40, 948–979. [Google Scholar] [CrossRef]
- Lazzarini, A.; Colaiezzi, R.; Gabriele, F.; Crucianelli, M. Support–Activity Relationship in Heterogeneous Catalysis for Biomass Valorization and Fine-Chemicals Production. Materials 2021, 14, 6796. [Google Scholar] [CrossRef]
- Vasapollo, G.; Del Sole, R.; Mergola, L.; Lazzoi, M.R.; Scardino, A.; Scorrano, S.; Mele, G. Molecularly Imprinted Polymers: Present and Future Prospective. Int. J. Mol. Sci. 2011, 12, 5908–5945. [Google Scholar] [CrossRef]
- Sooraj, M.P.; Nair, A.S. Molecularly Imprinted Polymer Composites: Introduction and Overview. In Molecularly Imprinted Polymer Composites; Elsevier: Amsterdam, The Netherlands, 2021. [Google Scholar]
- Liu, Q.; Wan, J.; Cao, X. Synthesis of Core-Shell Molecularly Imprinted Polymers (MIP) for Spiramycin I and Their Application in MIP Chromatography. Process Biochem. 2018, 70, 168–178. [Google Scholar] [CrossRef]
- Hasanova, S.; Ait Lahcen, A.; Zor, E. Recent Advances in Molecular Imprinting Techniques for the Electrochemical Analysis of Chiral Compounds. J. Pharm. Biomed. Anal. Open 2024, 4, 100046. [Google Scholar] [CrossRef]
- Liu, X.; Gao, M.; Qin, Y.; Xiong, Z.; Zheng, H.; Willner, I.; Cai, X.; Li, R. Exploring Nanozymes for Organic Substrates: Building Nano-organelles. Angew. Chem. Int. Ed. 2024, 63, e202408277. [Google Scholar] [CrossRef]
- Tonucci, M.C.; Adarme, O.F.H.; de Aquino, S.F.; Baeta, B.E.L.; Tarley, C.R.T. Synthesis of Hybrid Magnetic Molecularly Imprinted Polymers for the Selective Adsorption of Volatile Fatty Acids from Anaerobic Effluents. Polym. Int. 2020, 69, 847–857. [Google Scholar] [CrossRef]
- Hassan, R.A.; Abu Hanifah, S.; Heng, L.Y. Advancements and Prospects of Molecularly Imprinted Polymers as Chemical Sensors: A Comprehensive Review. Talanta 2025, 287, 127592. [Google Scholar] [CrossRef]
- Tonucci, M.C.; Santos, X.L.P.; da Silva, A.C.; de Aquino, S.F.; Baeta, B.E.L. Removal of Estradiol from Water with a Hybrid MIP-TiO2 Catalytic Adsorbent. Water Air Soil Pollut. 2020, 231, 215. [Google Scholar] [CrossRef]
- Zhang, Z.; Luo, E.; Wang, W.; Huang, D.; Liu, J.; Du, Z. Molecularly Imprinted Nanozymes with Substrate Specificity: Current Strategies and Future Direction. Small 2025, 21, 2408343. [Google Scholar] [CrossRef]
- Xavier, L.P.S.; Dias, A.C.; Baeta, B.E.L.; Santos, L.A.; Ramalho, T.C.; de Aquino, S.F.; Silva, A.C. Experimental and theoretical studies of solvent polarity influence on the preparation of molecularly imprinted polymers for the removal of estradiol from water. NJC 2019, 43, 1775–1784. [Google Scholar] [CrossRef]
- Gkika, D.A.; Tolkou, A.K.; Lambropoulou, D.A.; Bikiaris, D.N.; Kokkinos, P.; Kalavrouziotis, I.K.; Kyzas, G.Z. Application of molecularly imprinted polymers (MIPs) as environmental separation tools. RSC Appl. Polym. 2024, 2, 127–148. [Google Scholar] [CrossRef]
- Ndunda, E.N.; Mizaikoff, B. Molecularly Imprinted Polymers for the Analysis and Removal of Polychlorinated Aromatic Compounds in the Environment: A Review. Anal. 2016, 141, 3141–3156. [Google Scholar] [CrossRef]
- Liu, Z.; Xu, Z.; Wang, D.; Yang, Y.; Duan, Y.; Ma, L.; Lin, T.; Liu, H. A Review on Molecularly Imprinted Polymers Preparation by Computational Simulation-Aided Methods. Polymers 2021, 13, 2657. [Google Scholar] [CrossRef] [PubMed]
- Yin, Y.; Dong, Z.; Luo, Q.; Liu, J. Biomimetic Catalysts Designed on Macromolecular Scaffolds. Prog. Polym. Sci. 2012, 37, 1476–1509. [Google Scholar] [CrossRef]
- Adeel, M.; Bilal, M.; Rasheed, T.; Sharma, A.; Iqbal, H.M.N. Graphene and Graphene Oxide: Functionalization and Nano-Bio-Catalytic System for Enzyme Immobilization and Biotechnological Perspective. Int. J. Biol. Macromol. 2018, 120, 1430–1440. [Google Scholar] [CrossRef]
- Dupuis, R.; Benoit, M.; Tuckerman, M.E.; Méheut, M. Importance of a Fully Anharmonic Treatment of Equilibrium Isotope Fractionation Properties of Dissolved Ionic Species As Evidenced by Li+(aq). Acc. Chem. Res. 2017, 50, 1597–1605. [Google Scholar] [CrossRef]
- Sapre, A. Role of chemical reaction engineering for sustainable growth: One industrial perspective from India. AIChE J. 2023, 69, e17685. [Google Scholar] [CrossRef]
- Armor, J.N. A History of Industrial Catalysis. Catal. Today 2011, 163, 3–9. [Google Scholar] [CrossRef]
- Gonçalves, C.S.; Marsaioli, A.J. Fatos e Tendências da Biocatálise. Quim. Nova 2013, 36, 1587–1590. [Google Scholar] [CrossRef]
- Conti, M.C.M.D.; Camargo, L.P.; Fabris, G.S.; Silva, P.R.C.D.; Santana, H.; Fernandes, R.V.; Dall’Antonia, L.H.; Vandresen, F.; Sambrano, J.R.; La Porta, F.A. Hydrothermal Growth of Zn2GeO4 Nanorods for Optical and (Photo)Catalytic Applications: An Experimental and Theoretical Study. Mater. Today Chem. 2024, 41, 102313. [Google Scholar] [CrossRef]
- Swiegers, G.F. (Ed.) Mechanical Catalysis; Wiley-VCH: Weinheim, Germany, 2008. [Google Scholar]
- Suzuki, V.Y.; Amorin, L.H.C.; Fabris, G.S.L.; Dey, S.; Sambrano, J.R.; Cohen, H.; Oron, D.; La Porta, F.A. Enhanced Photocatalytic and Photoluminescence Properties Resulting from Type-I Band Alignment in the Zn2GeO4/g-C3N4 Nanocomposites. Catalysts 2022, 12, 692. [Google Scholar] [CrossRef]
- Wang, Y.; Tian, Y.; Pan, S.; Snyder, S.W. Catalytic Processes to Accelerate Decarbonization in a Net-Zero Carbon World. ChemSusChem 2022, 15, e202201290. [Google Scholar] [CrossRef]
- Seh, Z.W.; Kibsgaard, J.; Dickens, C.F.; Chorkendorff, I.; Nørskov, J.K.; Jaramillo, T.F. Combining Theory and Experiment in Electrocatalysis: Insights into Materials Design. Science 2017, 355, 1462–1466. [Google Scholar] [CrossRef]
- Brown, T.L.; LeMay, H.E.; Bursten, B.E.; Murphy, C.J.; Woodward, P.M.; Stoltzfus, M.W. Chemistry: The Central Science, 14th ed.; Pearson: London, UK, 2018; pp. 102–201. [Google Scholar]
- Ferreira, L.E.M.; Ribeiro, R.S.A.; Madriaga, V.G.C.; Vasconcelos, S.C.; Shimabukuro, E.T.T.; Rossa, V.; Vieira, S.S.; Passos, F.B.; Lima, T.M. Uma breve revisão sobre a catálise por átomos isolados: Conceitos e aplicações. Quím. Nova 2021, 45, 194–206. [Google Scholar] [CrossRef]
- Somorjai, A.; Yimin, L. Introduction to Surface Chemistry and Catalysis; Wiley: Hoboken, NJ, USA, 2010. [Google Scholar]
- Taseska, T.; Yu, W.; Wilsey, M.K.; Cox, C.P.; Meng, Z.; Ngarnim, S.S.; Müller, A.M. Analysis of the Scale of Global Human Needs and Opportunities for Sustainable Catalytic Technologies. Top. Catal. 2023, 66, 338–374. [Google Scholar] [CrossRef]
- Russel, J.B. Química Geral; McGraw-Hill: São Paulo, Brazil, 1982; pp. 115–350. [Google Scholar]
- Bartholomew, C.H. Mechanisms of Catalyst Deactivation. Appl. Catal. A Gen. 2001, 212, 17–60. [Google Scholar] [CrossRef]
- Friend, C.M.; Xu, B. Heterogeneous Catalysis: A Central Science for a Sustainable Future. Acc. Chem. Res. 2017, 50, 517–521. [Google Scholar] [CrossRef] [PubMed]
- Sivasamy, A.; Cheah, K.Y.; Fornasiero, P.; Kemausuor, F.; Zinoviev, S.; Miertus, S. Catalytic Applications in the Production of Biodiesel from Vegetable Oils. ChemSusChem 2009, 2, 278–300. [Google Scholar] [CrossRef]
- Thomas, J.M.T.; Thomas, W.J. Principle and Practice of Heterogeneous Catalysis; VCH Publishers: New York, NY, USA, 1997. [Google Scholar]
- Roque-Malherbe, R. Physical Chemistry of Materials. Energy and Environmental Applications; CRC Press: Boca Raton, FL, USA, 2009. [Google Scholar]
- Liu, M.; Ye, Y.; Ye, J.; Gao, T.; Wang, D.; Chen, G.; Song, Z. Recent Advances of Magnetite (Fe3O4)-Based Magnetic Materials in Catalytic Applications. Magnetochemistry 2023, 9, 110. [Google Scholar] [CrossRef]
- Oliveira, L.C.A.; Fabris, J.D.; Pereira, M.C. Óxidos de Ferro e Suas Aplicações em Processos Catalíticos: Uma Revisão. Quim. Nova 2013, 36, 123–130. [Google Scholar] [CrossRef]
- Kadhim, S.H.; Mgheer, T.H.; Ismael, H.I.; Kadem, K.J.; Abbas, S.A.; Atiyah, A.J.; Mohamad, I.J. Synthesis, Characterization and Catalytic Activity of NiO-CoO-MgO Nano-Composite Catalyst. Indones. J. Chem. 2019, 19, 675. [Google Scholar] [CrossRef]
- Argyle, M.; Bartholomew, C.H. Heterogeneous Catalyst Deactivation and Regeneration: A Review. Catalysts 2015, 5, 145–269. [Google Scholar] [CrossRef]
- Lott, P.; Deutschmann, O. Heterogeneous Chemical Reactions—A Cornerstone in Emission Reduction of Local Pollutants and Greenhouse Gases. Proc. Combust. Inst. 2023, 39, 3183–3215. [Google Scholar] [CrossRef]
- Cardoso, T.S.; Santos, R.A.; Costa, R.T.; Aviz, E.O.; Araújo, J.F.; Silva, A.P.; Freitas, M.C.C.; Correia, L.M. Uma revisão da utilização de catalisadores heterogêneos para a produção de biodiesel. BASR 2020, 4, 240–276. [Google Scholar] [CrossRef]
- Cintra, J.S.A.; Portela, M.N.; Silvany, T.C.; Pedroza, G.A.G.; Santos, L.C.L.; Lobato, A.K. de C.L. Influência do Tempo de Reação na Produção de Biodiesel via Catálise Heterogênea. Holos 2017, 1, 195–204. [Google Scholar] [CrossRef]
- Nath, I.; Chakraborty, J.; Verpoort, F. Degradation of Environmental Contaminants by Topical Heterogeneous Photocatalysts. In Handbook of Smart Photocatalytic Materials; Elsevier: Amsterdam, The Netherlands, 2020; pp. 151–182. [Google Scholar]
- Czulak, J.; Jakubiak-Marcinkowska, A.; Trochimczuk, A. Polymer Catalysts Imprinted with Metal Ions as Biomimics of Metalloenzymes. Adv. Mater. Sci. Eng. 2013, 1, 464265. [Google Scholar] [CrossRef]
- Ma, Z.; Zaera, F. Heterogeneous Catalysis by Metals. In Encyclopedia of Inorganic Chemistry; Wiley: Chichester, UK, 2005. [Google Scholar]
- Bailie, J.E.; Hutchings, G.J.; O’Leary, S. Supported Catalysts. In Encyclopedia of Materials: Science and Technology; Elsevier: Oxford, UK, 2001. [Google Scholar]
- Zhang, H.; Liu, G.; Shi, L.; Ye, J. Single-Atom Catalysts: Emerging Multifunctional Materials in Heterogeneous Catalysis. Adv. Energy Mater. 2018, 8, 1701343. [Google Scholar] [CrossRef]
- Liu, H.; Han, L.; Duan, X.; Sun, H.; Wang, S.; Zhang, J. Photothermal Catalytic C1 Conversion on Supported Catalysts. Energy Adv. 2023, 2, 1541–1564. [Google Scholar] [CrossRef]
- Aboelfetoh, E.F.; Fechtelkord, M.; Pietschnig, R. Structure and Catalytic Properties of MgO-Supported Vanadium Oxide in the Selective Oxidation of Cyclohexane. J. Mol. Catal. A Chem. 2010, 318, 51–59. [Google Scholar] [CrossRef]
- Hutchings, G.J.; Védrine, J.C. Heterogeneous Catalyst Preparation; Springer: Berlin, Germany, 2004; pp. 215–258. [Google Scholar]
- Rioux, R.M.; Song, H.; Hoefelmeyer, J.D.; Yang, P.; Somorjai, G.A. High-Surface-Area Catalyst Design: Synthesis, Characterization, and Reaction Studies of Platinum Nanoparticles in Mesoporous SBA-15 Silica. J. Phys. Chem. B 2005, 109, 2192–2202. [Google Scholar] [CrossRef] [PubMed]
- Ro, I.; Resasco, J.; Christopher, P. Approaches for Understanding and Controlling Interfacial Effects in Oxide-Supported Metal Catalysts. ACS Catal. 2018, 8, 7368–7387. [Google Scholar] [CrossRef]
- Tauster, S.J.; Fung, S.C.; Garten, R.L. Strong Metal-Support Interactions. Group 8 Noble Metals Supported on Titanium Dioxide. J. Am. Chem. Soc. 1978, 100, 170–175. [Google Scholar] [CrossRef]
- Tang, H.; Su, Y.; Zhang, B.; Lee, A.F.; Isaacs, M.A.; Wilson, K.; Li, L.; Ren, Y.; Huang, J.; Haruta, M.; et al. Classical strong metal–support interactions between gold nanoparticles and titanium dioxide. Sci. Adv. 2017, 3, e1700231. [Google Scholar] [CrossRef]
- Kalan, R.E.; Yaparatne, S.; Amirbahman, A.; Tripp, C.P. P25 Titanium Dioxide Coated Magnetic Particles: Preparation, Characterization and Photocatalytic Activity. Appl. Catal. B Environ. 2016, 187, 249–258. [Google Scholar] [CrossRef]
- Zhang, H.; Ye, L.; Mosbach, K. Non-Covalent Molecular Imprinting with Emphasis on Its Application in Separation and Drug Development. J. Mol. Recognit. 2006, 19, 248–259. [Google Scholar] [CrossRef]
- Hasanah, A.N.; Susanti, I. Molecularly Imprinted Polymers for Pharmaceutical Impurities: Design and Synthesis Methods. Polymers 2023, 15, 3401. [Google Scholar] [CrossRef]
- Ferreira, J.P.; Viveiros, R.; Lourenço, A.; Silva, M.S.; Rosatella, A.; Casimiro, T.; Afonso, C.A.M. Integrated desulfurization of diesel by combination of metal-free oxidation and product removal by molecularly imprinted polymers. RSC Adv. 2014, 4, 54948–54952. [Google Scholar] [CrossRef]
- Sales, T.A.; Ramalho, T.C. Computational Design of Synthetic Receptors for Drug Detection: Interaction between Molecularly Imprinted Polymers and MDMA (3,4-Methylenedioxymethamphetamine). Theor. Chem. Acc. 2020, 139, 31. [Google Scholar] [CrossRef]
- BelBruno, J.J. Molecularly Imprinted Polymers. Chem. Rev. 2019, 119, 94–119. [Google Scholar] [CrossRef] [PubMed]
- Yuliani, F.; Fauzia, S.; Saefumillah, A. Molecularly Imprinted Polymers (MIPs): Bibliometric Analysis. E3S Web Conf. 2024, 503, 08005. [Google Scholar] [CrossRef]
- Scorrano, S.; Mergola, L.; Del Sole, R.; Vasapollo, G. Synthesis of Molecularly Imprinted Polymers for Amino Acid Derivates by Using Different Functiona Monomers. Int. J. Mol. Sci. 2011, 12, 1735–1743. [Google Scholar] [CrossRef]
- Ashkani, M.; Bouhendi, H.; Kabiri, K.; Rostami, M.R. Synthesis of Poly(2-acrylamido-2-methylpropane Sulfonic Acid) with High Water Absorbency and Absorption under Load (AUL) as Concrete Grade Superabsorbent and Its Performance. Constr. Build. Mater. 2019, 206, 540–551. [Google Scholar] [CrossRef]
- Barral, S.; Guerreiro, A.; Villa-García, M.; Rendueles, M.; Díaz, M.; Piletsky, S. Synthesis of 2-(Diethylamino)ethyl Methacrylate-Based Polymers: Effect of Crosslinking Degree, Porogen and Solvent on the Textural Properties and Protein Adsorption Performance. React. Funct. Polym. 2010, 70, 890–899. [Google Scholar] [CrossRef]
- Wang, X.; Jing, S.; Qiu, X.; Zhao, S.; Liu, Y.; Tan, Y. Novel Bile Acid Sequestrant: A Biodegradable Hydrogel Based on Amphiphilic Allylamine Copolymer. Chem. Eng. J. 2016, 304, 493–502. [Google Scholar] [CrossRef]
- Tarley, C.R.T.; Santos, A.L.; Oliveira, A.L.M.; Souza, A.L.S.; Silva, A.A. Polímeros Biomiméticos em Química Analítica. Parte 1: Preparo e Aplicações de MIP (“Molecularly Imprinted Polymers”) em Técnicas de Extração e Separação. Quím. Nova 2005, 28, 1055–1064. [Google Scholar] [CrossRef]
- Garcia-Diego, C.; Cuellar, J. Design of Polymeric Microparticles with Improved Structural Properties: Influence of Ethylstyrene Monomer and of High Proportions of Crosslinker. Eur. Polym. J. 2008, 44, 1487–1500. [Google Scholar] [CrossRef]
- Soykan, C.; Ergül, E. Novel Methacrylamide Polymers Based on Thiazole and Styrene: A Study on Synthesis, Characterization, and Determination of Monomer Reactivity Ratios. J. Res. Updates Polym. Sci. 2022, 11, 9–15. [Google Scholar] [CrossRef]
- Leenaraj, D.R.; Manimaran, D.; Joe, I.H. Molecular Docking and Structural Analysis of Non-Opioid Analgesic Drug Acemetacin with Halogen Substitution: A DFT Approach. J. Mol. Struct. 2016, 1123, 180–190. [Google Scholar] [CrossRef]
- Favetta, P.; Ayari, M.G.; Agrofoglio, L.A. Molecularly Imprinted Polymers-Based Separation and Sensing of Nucleobases, Nucleosides, Nucleotides, and Oligonucleotides. In Molecularly Imprinted Polymers: Synthesis and Applications; Sellergren, B., Ed.; Polymer Chemistry Series; Royal Society of Chemistry: Cambridge, UK, 2005. [Google Scholar]
- Rechichi, A.; Cristallini, C.; Vitale, U.; Ciardelli, G.; Barbani, N.; Vozzi, G.; Giusti, P. New Biomedical Devices with Selective Peptide Recognition Properties. Part 1: Characterization and Cytotoxicity of Molecularly Imprinted Polymers. J. Cell Mol. Med. 2007, 11, 1367–1376. [Google Scholar] [CrossRef] [PubMed]
- Vlatakis, G.; Andersson, L.I.; Müller, R.; Mosbach, K. Drug Assay Using Antibody Mimics Made by Molecular Imprinting. Nature 1993, 361, 645–647. [Google Scholar] [CrossRef]
- Ulubayram, K. Molecularly Imprinted Polymers; Springer: Boston, MA, USA, 2004. [Google Scholar]
- Sharma, P.S.; D’Souza, F.; Kutner, W. Molecular Imprinting for Selective Chemical Sensing of Hazardous Compounds and Drugs of Abuse. TrAC Trends Anal. Chem. 2012, 34, 59–77. [Google Scholar] [CrossRef]
- Andre, R.S.; Facure, M.H.M.; Pereira, T.S.; Migliorini, F.L.; Mercante, L.A.; Correa, D.S. Sensores Químicos Baseados em Fibras Eletrofiadas. In Eletrofiação e Nanofibras: Fundamentos e Aplicações; Atena Editora: São Paulo, Brazil, 2023. [Google Scholar]
- Jensen, F. Introduction to Computational Chemistry; Wiley: Chichester, UK, 2007; pp. 120–250. [Google Scholar]
- Lewars, E.G. Computational Chemistry: Introduction to the Theory and Applications of Molecular and Quantum Mechanics; Springer: Berlin, Germany, 2011; pp. 55–110. [Google Scholar]
- Vargas-Berrones, K.; Ocampo-Perez, R.; Rodríguez-Torres, I.; Medellín-Castillo, N.A.; Flores-Ramírez, R. Molecularly Imprinted Polymers (MIPs) as Efficient Catalytic Tools for the Oxidative Degradation of 4-Nonylphenol and Its By-products. Environ. Sci. Pollut. Res. 2023, 30, 90741–90756. [Google Scholar] [CrossRef]
- Chen, Z.; Huang, S.; Zhao, M. Molecularly Imprinted Polymers for Biomimetic Catalysts. In Molecularly Imprinted Catalysts; Elsevier: Amsterdam, The Netherlands, 2016; pp. 229–239. [Google Scholar]
- Zhang, J.; Zhang, M.; Tang, K.; Verpoort, F.; Sun, T. Polymer-Based Stimuli-Responsive Recyclable Catalytic Systems for Organic Synthesis. Small 2014, 10, 32–46. [Google Scholar] [CrossRef]
- Carboni, D.; Flavin, K.; Servant, A.; Gouverneur, V.; Resmini, M. The First Example of Molecularly Imprinted Nanogels with Aldolase Type I Activity. Chem. Eur. J. 2008, 14, 7059–7065. [Google Scholar] [CrossRef]
- Li, S. Molecularly Imprinted Catalysts: Principles, Syntheses, and Applications; Elsevier: Amsterdam, The Netherlands, 2016; pp. 35–88. [Google Scholar]
- Wagner, J.; Lerner, R.A.; Barbas, C.F. Efficient Aldolase Catalytic Antibodies That Use the Enamine Mechanism of Natural Enzymes. Science 1995, 270, 1797–1800. [Google Scholar] [CrossRef]
- Mukhopadhyay, S.; Trivedi, P. Recent Advances in Nanogels in Drug Delivery Systems. Int. J. Pharm. Sci. Nanotech. 2021, 14, 5278–5286. [Google Scholar] [CrossRef]
- Silva, A.T.; Figueiredo, R.; Azenha, M.; Jorge, P.A.S.; Pereira, C.M.; Ribeiro, J.A. Imprinted Hydrogel Nanoparticles for Protein Biosensing: A Review. ACS Sens. 2023, 8, 2898–2920. [Google Scholar] [CrossRef] [PubMed]
- Cardoso, A.R.; Frasco, M.F.; Serrano, V.; Fortunato, E.; Sales, M.G.F. Molecular Imprinting on Nanozymes for Sensing Applications. Biosensors 2021, 11, 152. [Google Scholar] [CrossRef]
- Guo, M.; Hu, Y.; Wang, R.; Yu, H.; Sun, L. Molecularly Imprinted Polymer-Based Photocatalyst for Highly Selective Degradation of Methylene Blue. Environ. Res. 2021, 194, 110684. [Google Scholar] [CrossRef]
- Chen, L.; Xu, S.; Li, J. Recent Advances in Molecular Imprinting Technology: Current Status, Challenges, and Highlighted Applications. Chem. Soc. Rev. 2011, 40, 2922. [Google Scholar] [CrossRef] [PubMed]
- Moein, M.M. Advancements of Chiral Molecularly Imprinted Polymers in Separation and Sensor Fields: A Review of the Last Decade. Talanta 2021, 224, 121794. [Google Scholar] [CrossRef]
- Martins, R.O.; Batista Junior, A.C.; Brito, C.C.S.M.; Rocha, Y.A.; Chaves, A.R. Greener molecularly imprinted polymers: Strategies and applications in separation and mass spectrometry methods. Trends Anal. Chem. 2023, 168, 117285. [Google Scholar] [CrossRef]
- Ali, A.E.; Hejji, L.; Lahcen, A.A.; Pérez-Villarejo, L.; Azzouz, A.; Kim, K.-H. Progress and Prospects in the Green Synthesis of Molecularly Imprinted Polymers for Sorptive Extraction and Sensing Applications Toward Emerging Contaminants in Various Sample Matrices. TrAC Trends Anal. Chem. 2024, 170, 117466. [Google Scholar]
- Rahman, M.; Khan, M.A.; Rub, M.A.; Hoque, M.A. Effect of Temperature and Salts on the Interaction of Cetyltrimethylammonium Bromide with Ceftriaxone Sodium Trihydrate Drug. J. Mol. Liq. 2016, 223, 716–724. [Google Scholar] [CrossRef]
- Kirk, O.; Borchert, T.V.; Fuglsang, C.C. Industrial Enzyme Applications. Curr. Opin. Biotechnol. 2002, 13, 345–351. [Google Scholar] [CrossRef]
- Lai, C.; Wang, M.M.; Zeng, G.M.; Liu, Y.G.; Huang, D.L.; Zhang, C.; Wang, R.Z.; Xu, P.; Cheng, M.; Huang, C.; et al. Synthesis of surface molecular imprinted TiO2/graphene photocatalyst and its highly efficient photocatalytic degradation of target pollutant under visible light irradiation. Appl. Surf. Sci. 2016, 390, 368–376. [Google Scholar] [CrossRef]
- Ding, Q.; Hu, X. Mesoporous Materials as Catalyst Support for Wastewater Treatment. Madridge J. Nanotechnol. Nanosc. 2019, 4, 160–167. [Google Scholar] [CrossRef]
- Murray, A.; Örmeci, B. Application of Molecularly Imprinted and Non-Imprinted Polymers for Removal of Emerging Contaminants in Water and Wastewater Treatment: A Review. Environ. Sci. Pollut. Res. 2012, 19, 3820–3830. [Google Scholar] [CrossRef]
- Safari, M.; Nobakht, V. Encapsulation of Metal Nanoparticles (MNPs) as Catalyst. In Nanocomposite Materials for Biomedical and Energy Storage Applications; IntechOpen: London, UK, 2022. [Google Scholar]
- Aoulad, E.; Hadj Ali, Y.; Azzouz, A.; Ahrouch, M.; Lamaoui, A.; Raza, N.; Ait Lahcen, A. Molecular Imprinting Technology for Next-Generation Water Treatment via Photocatalysis and Selective Pollutant Adsorption. J. Environ. Chem. Eng. 2024, 12, 112768. [Google Scholar] [CrossRef]
- Lim, K.F.; Zin, A.M.; Romano, E.; Wanless, E.J.; Holdsworth, C.I. Advances and Challenges in the Design and Synthesis of Molecularly Imprinted Microspheres. In Molecularly Imprinted Catalysts; Elsevier: Amsterdam, The Netherlands, 2016. [Google Scholar]
- Turiel, E.; Esteban, A.M. Molecularly Imprinted Polymers. In Solid-Phase Extraction; Elsevier: Amsterdam, The Netherlands, 2020. [Google Scholar]
- Lee, S.; Doong, R. Adsorption and Selective Recognition of 17ß-Estradiol by Molecularly Imprinted Polymers. J. Polym. Res. 2012, 19, 9939. [Google Scholar] [CrossRef]
- Ruela, A.L.M.; Figueiredo, E.C.; Pereira, G.R. Molecularly Imprinted Polymers as Nicotine Transdermal Delivery Systems. Chem. Eng. J. 2014, 248, 1–8. [Google Scholar] [CrossRef]
- Niu, D.; Zhou, Z.; Yang, W.; Li, Y.; Xia, L.; Jiang, B.; Xu, W.; Huang, W.; Zhu, T. Preparation and Characterization of Magnetic Molecularly Imprinted Polymers for Selective Recognition of 3-Methylindole. J. Appl. Polym. Sci. 2013, 130, 2859–2866. [Google Scholar] [CrossRef]
- Chen, L.; Liu, J.; Zeng, Q.; Wang, H.; Yu, A.; Zhang, H.; Ding, L. Preparation of magnetic molecularly imprinted polymer for the separation of tetracycline antibiotics from egg and tissue samples. J. Chromatogr. A 2009, 1216, 3710–3719. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, Y.; Masumoto, S.; Matsunaga, H.; Haginaka, H. Molecularly Imprinted Polymer for Glutathione by Modified Precipitation Polymerization and Its Application to Determination of Glutathione in Supplements. J. Pharm. Biomed. Anal. 2017, 144, 230–235. [Google Scholar] [CrossRef]
- He, X.-P.; Lian, Z.-R.; Tan, L.-J.; Wang, J.-T. Preparation and Characterization of Magnetic Molecularly Imprinted Polymers for Selective Trace Extraction of Dienestrol in Seawater. J. Chromatogr. A 2016, 1469, 8–16. [Google Scholar] [CrossRef]
- Kyzas, G.Z.; Bikiaris, D.N.; Lazaridis, N.K. Selective Separation of Basic and Reactive Dyes by Molecularly Imprinted Polymers (MIPs). Chem. Eng. J. 2009, 149, 263–272. [Google Scholar] [CrossRef]
- Zhao, Y.; Wang, H.; Wang, T. Adsorption of NO from Flue Gas by Molecularly Imprinted Adsorbents. Chem. Eng. J. 2016, 306, 832–839. [Google Scholar] [CrossRef]
- Xiao, Y.; Xiao, R.; Tang, J.; Zhu, Q.; Li, X.; Xiong, Y.; Wu, X. Preparation and adsorption properties of molecularly imprinted polymer via RAFT precipitation polymerization for selective removal of aristolochic acid I. Talanta 2017, 162, 415–422. [Google Scholar] [CrossRef] [PubMed]
- Madikizela, L.M.; Chimuka, L. Synthesis, Adsorption and Selectivity Studies of a Polymer Imprinted with Naproxen, Ibuprofen and Diclofenac. J. Environ. Chem. Eng. 2016, 4, 4029–4037. [Google Scholar] [CrossRef]
- Fan, D.; Jia, L.; Xiang, H.; Peng, M.; Li, H.; Shi, S. Synthesis and Characterization of Hollow Porous Molecular Imprinted Polymers for the Selective Extraction and Determination of Caffeic Acid in Fruit Samples. Food Chem. 2017, 224, 32–36. [Google Scholar] [CrossRef]
- Gholivand, M.B.; Khodadadian, M. Rationally Designed Molecularly Imprinted Polymers for Selective Extraction of Methocarbamol from Human Plasma. Talanta 2011, 85, 1680–1688. [Google Scholar] [CrossRef]
- Masqué, N.; Marcé, R.M.; Borrull, F.; Cormack, P.A.G.; Sherrington, D.C. Synthesis and Evaluation of a Molecularly Imprinted Polymer for Selective On-Line Solid-Phase Extraction of 4-Nitrophenol from Environmental Water. Anal. Chem. 2000, 72, 4122–4126. [Google Scholar] [CrossRef] [PubMed]
- Otitoju, T.A.; Okoye, P.U.; Chen, G.; Li, Y.; Okoye, M.O.; Li, S. Advanced Ceramic Components: Materials, Fabrication, and Applications. J. Ind. Eng. Chem. 2020, 85, 34–65. [Google Scholar] [CrossRef]
- Shvydyuk, K.O.; Nunes-Pereira, J.; Rodrigues, F.F.; Silva, A.P. Review of Ceramic Composites in Aeronautics and Aerospace: A Multifunctional Approach for TPS, TBC and DBD Applications. Ceramics 2023, 6, 195–230. [Google Scholar] [CrossRef]
- Pinto, F.M.; Suzuki, V.Y.; Silva, R.C.; La Porta, F.A. Oxygen Defects and Surface Chemistry of Reducible Oxides. Front. Mater. 2019, 6, 260. [Google Scholar] [CrossRef]
- de Jesus, J.P.A.; Santos, A.C.L.; Pinto, F.M.; Taft, C.A.; La Porta, F.A. Review: Theoretical and Experimental Investigation of the Intrinsic Properties of Zn2GeO4 Nanocrystals. J. Mater. Sci. 2021, 56, 4552–4568. [Google Scholar] [CrossRef]
- Silva Junior, E.; La Porta, F.A.; Liu, M.S.; Andrés, J.; Varela, J.A.; Longo, E. A Relationship Between Structural and Electronic Order–Disorder Effects and Optical Properties in Crystalline TiO2 Nanomaterials. Nanoscale 2016, 8, 7559–7571. [Google Scholar]
- Burda, C.; Chen, X.; Narayanan, R.; El-Sayed, M.A. Chemistry and Properties of Nanocrystals of Different Shapes. Chem. Rev. 2005, 105, 1025–1102. [Google Scholar] [CrossRef]
- Danks, A.E.; Hall, S.R.; Schnepp, Z. The Evolution of ‘Sol–Gel’ Chemistry as a Technique for Materials Synthesis. Mater. Horiz. 2016, 3, 91–112. [Google Scholar] [CrossRef]
- Bokov, D.; Jalil, A.T.; Chupradit, S.; Suksatan, W.; Ansari, M.J.; Shewael, I.H.; Valiev, G.H.; Kianfar, E. Nanomaterial by Sol-Gel Method: Synthesis and Application. Adv. Mater. Sci. Eng. 2021, 2021, 5102014. [Google Scholar] [CrossRef]
- de Conti, M.C.M.D.; Dey, S.; Pottker, W.E.; La Porta, F.A. An Overview into Advantages and Applications of Conventional and Unconventional Hydro(Solvo)thermal Approaches for Novel Advanced Materials Design. Mater. Today Sustain. 2023, 23, 100458. [Google Scholar]
- Fajardo, H.V.; Taylor, J.G.; Teixeira, M.P.; Chagas, P.; Oliveira, L.C.A.; Gonçalves, M.A.; Ramalho, T.C.; Silva, A.C. Oxidation of Thioanisole Using Niobium–Silica Catalysts: Theoretical and Experimental Studies. Catal. Lett. 2024, 154, 2903–2918. [Google Scholar] [CrossRef]
- Batista, F.M.C.; La Porta, F.A.; Gracia, L.; Cerdeiras, E.; Mestres, L.; Siu Li, M.; Batista, N.C.; Andrés, J.; Longo, E.; Cavalcante, L.S. A Joint Experimental and Theoretical Study on the Electronic Structure and Photoluminescence Properties of Al2(WO4)3 Powders. J. Mol. Struct. 2015, 1081, 381–388. [Google Scholar] [CrossRef]
- Mesquita, A.M.; Guimarães, I.R.; de Castro, G.M.M.; Gonçalves, M.A.; Ramalho, T.C.; Guerreiro, M.C. Boron as a Promoter in the Goethite (α-FeOOH) Phase: Organic Compound Degradation by Fenton Reaction. Appl. Catal. B Environ. 2016, 192, 286–295. [Google Scholar] [CrossRef]
- Labhsetwar, N.; Doggali, P.; Rayalu, S.; Yadav, R.; Mistuhashi, T.; Haneda, H. Ceramics in Environmental Catalysis: Applications and Possibilities. Chin. J. Catal. 2012, 33, 1611–1621. [Google Scholar] [CrossRef]
- Keane, M.A. Ceramics for Catalysis. J. Mater. Sci. 2003, 38, 4661–4675. [Google Scholar] [CrossRef]
- Kurian, M.; Thankachan, S.; Nair, S.S. Ceramic Catalysts: Materials, Synthesis, and Applications. In Elsevier Series in Advanced Ceramic Materials; Elsevier: Oxford, UK, 2021. [Google Scholar]
- Alexander, C.; Andersson, H.S.; Andersson, L.I.; Ansell, R.J.; Kirsch, N.; Nicholls, I.A.; O’Mahony, J.; Whitcombe, M.J. Molecular imprinting science and technology: A survey of the literature for the years up to and including 2003. J. Mol. Recognit. 2006, 19, 106–180. [Google Scholar]
- Komiyama, M.; Takeuchi, T.; Mukawa, T.; Asanuma, H. Molecular Imprinting: From Fundamentals to Applications; Wiley-VCH: Weinheim, Germany, 2003. [Google Scholar]
- Takeuchi, T.; Haginaka, J. Separation and Sensing Based on Molecular Recognition Using Molecularly Imprinted Polymers. J. Chromatogr. B Biomed. Sci. Appl. 1999, 728, 1–20. [Google Scholar] [CrossRef]
- Washington, J.W.; Henderson, W.M.; Ellington, J.J.; Jenkins, T.M.; Evans, J.J. Analysis of Perfluorinated Carboxylic Acids in Soils II: Optimization of Chromatography and Extraction. J. Chromatogr. A 2008, 1181, 21–32. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Wang, X.; Lu, W.; Wu, X.; Li, J. Molecular Imprinting: Perspectives and Applications. Chem. Soc. Rev. 2016, 45, 2137–2211. [Google Scholar] [CrossRef]
- Sales, T.A.; Ferreira, L.V.F.; Nogueira, A.G.; Ramalho, T.C. A Theoretical Protocol for the Rational Design of the Bioinspired Multifunctional Hybrid Material MIP@Cercosporin. J. Mol. Model. 2023, 29, 321. [Google Scholar] [CrossRef] [PubMed]
- Nicholls, I.A.; Golker, K.; Olsson, G.D.; Suriyanarayanan, S.; Wiklander, J.G. The Use of Computational Methods for the Development of Molecularly Imprinted Polymers. Polymers 2021, 13, 2841. [Google Scholar] [CrossRef]
- Kearley, G.J.; Gray, V.; Riley, D.P.; Kirstein, O.; Kutteh, R.; Kisi, E.H. Inelastic Neutron Scattering and Density Functional Theory–Molecular Dynamics Study of Si Dynamics in Ti3SiC2. J. Am. Ceram. Soc. 2014, 97, 916–922. [Google Scholar] [CrossRef]
- Narang, K. Glass-Ceramics: Properties, Applications and Technology; Elsevier: Amsterdam, The Netherlands, 2019. [Google Scholar]
- Poma, A.; Turner, A.P.F.; Piletsky, S.A. Advances in the Manufacture of MIP Nanoparticles. Trends Biotechnol. 2010, 28, 629–637. [Google Scholar] [CrossRef]
- Brovini, E.M.; Martucci, M.E.P.; de Oliveira, M.; Oliveira, A.H.S.; de Aquino, S.F. A faster and more accessible method for the analysis of acephate and methamidophos in water samples by LC-MS/MS. Int. J. Environ. Anal. Chem. 2024, 8, 1–16. [Google Scholar] [CrossRef]
- Brovini, E.M.; Moreira, F.D.; Martucci, M.E.P.; Aquino, S.F. Water Treatment Technologies for Removing Priority Pesticides. J. Water Process Eng. 2023, 53, 103730. [Google Scholar] [CrossRef]
- Barros, L.A.; Custodio, R.; Rath, S. Design of a New Molecularly Imprinted Polymer Selective for Hydrochlorothiazide Based on Theoretical Predictions Using Gibbs Free Energy. J. Braz. Chem. Soc. 2016, 27, 2301–2311. [Google Scholar] [CrossRef]
- Lee, H.B.; Peart, T.E.; Svoboda, M.L. Determination of Endocrine-Disrupting Phenols, Acidic Pharmaceuticals, and Personal-Care Products in Sewage by Solid-Phase Extraction and Gas Chromatography–Mass Spectrometry. J. Chromatogr. A 2005, 1094, 122–129. [Google Scholar] [CrossRef] [PubMed]
- Kümmerer, K. The Presence of Pharmaceuticals in the Environment Due to Human Use—Present Knowledge and Future Challenges. J. Environ. Manag. 2009, 90, 2354–2366. [Google Scholar] [CrossRef] [PubMed]
- Martínez, S.L.N.; Penido, R.G.; Santos, L.A.; Ramalho, T.C.; Baeta, B.E.L.; Pereira, M.C.; Silva, A.C. Molecularly imprinted polymers for selective adsorption of quinoline: Theoretical and experimental studies. RSC Adv. 2018, 8, 28775–28786. [Google Scholar] [CrossRef] [PubMed]
- Pichon, V.; Chapuis-Hugon, F. Role of Molecularly Imprinted Polymers for Selective Determination of Environmental Pollutants—A Review. Anal. Chim. Acta 2008, 622, 48–61. [Google Scholar] [CrossRef]
- Yu, L.; Chu, K.; Ye, H.; Liu, X.; Yu, L.; Xu, X.; Chen, G. Recent advances in microemulsion electrokinetic chromatography. Trends Anal. Chem. 2012, 34, 140–151. [Google Scholar] [CrossRef]
- Xiao, X.; Zhang, Y.; Wu, J.; Jia, L. Poly(norepinephrine)-Coated Open Tubular Column for the Separation of Proteins and Recombinant Human Erythropoietin by Capillary Electrochromatography. J. Sep. Sci. 2017, 40, 4636–4644. [Google Scholar] [CrossRef]
- Cavalera, S.; Anfossi, L.; Di Nardo, F.; Baggiani, C. Mycotoxins-Imprinted Polymers: A State-of-the-Art Review. Toxins 2024, 16, 47. [Google Scholar] [CrossRef]
- Kubiak, A.; Stachowiak, M.; Cegłowski, M. Unveiling the Latest Developments in Molecularly Imprinted Photocatalysts: A State-of-the-Art Review. Polymers 2023, 15, 4152. [Google Scholar] [CrossRef]
- Satchanska, G.; Davidova, S.; Petrov, P.D. Natural and Synthetic Polymers for Biomedical and Environmental Applications. Polymers 2024, 16, 1159. [Google Scholar] [CrossRef]
- Thakur, V.K.; Thakur, M.K. Processing and Characterization of Natural Cellulose Fibers/Thermoset Polymer Composites. Carbohydr. Polym. 2014, 109, 102–117. [Google Scholar] [CrossRef] [PubMed]
- Gyanjyoti, G.A.; Guleria, P.; Awasthi, A.; Singh, K.; Kumar, V. Recent Advancement in Fluorescent Materials for Optical Sensing of Pesticides. Mater. Today Commun. 2023, 34, 105193. [Google Scholar] [CrossRef]
- Villarreal-Lucio, D.S.; Vargas-Berrones, K.X.; Díaz de León-Martínez, L.; Flores-Ramíez, R. Molecularly Imprinted Polymers for Environmental Adsorption Applications. Environ. Sci. Pollut. Res. 2022, 29, 89923–89942. [Google Scholar] [CrossRef] [PubMed]
- Jamieson, O.; Liu, X.; Read, C.; Wang, Y.; Brown, P.; Zhang, Q. Molecularly Imprinted Polymer-Based Sensors for the Monitoring of Antibiotic Traces and Microorganisms in Water Samples to Combat Antimicrobial Resistance. In Molecularly Imprinted Polymers as Artificial Antibodies for the Environmental Health; Springer Nature Switzerland: Cham, Switzerland, 2024; pp. 143–165. [Google Scholar]
- Lin, H.; Li, B.; Bai, Y.; Chen, X.; Zhang, M. Development of Magnetic Molecularly Imprinted Polymers for Selective Extraction of Benzoxazolinone-Type Alkaloids from Acanthus Plants. J. Chromatogr. A 2024, 1713, 464542. [Google Scholar] [CrossRef]
- Metwally, M.; Benhawy, A.; Khalifa, R.; El Nashar, R.; Trojanowicz, M. Application of Molecularly Imprinted Polymers in the Analysis of Waters and Wastewaters. Molecules 2021, 26, 6515. [Google Scholar] [CrossRef] [PubMed]
- Lehmann, E.L.; Arruda, M.A.Z. Minimalist Strategies Applied to Analysis of Forensic Samples Using Elemental and Molecular Analytical Techniques—A Review. Anal. Chim. Acta 2019, 1063, 9–17. [Google Scholar] [CrossRef]
- Luo, Y.; Feng, X.; Chen, Z.; Shen, X. Molecularly Imprinted Photocatalysts: Fabrication, Application and Challenges. Mater. Adv. 2022, 3, 8830–8847. [Google Scholar] [CrossRef]
- El-Schich, Z.; Zhang, Y.; Feith, M.; Belloni, A.; Louka, A. Molecularly Imprinted Polymers in Biological Applications. BioTechniques 2020, 69, 407–420. [Google Scholar] [CrossRef]
- Tahir, N.; Krishnaraj, C.; Leus, K.; Van Der Voort, P. Development of Covalent Triazine Frameworks as Heterogeneous Catalytic Supports. Polymers 2019, 11, 1326. [Google Scholar] [CrossRef]
- Salvo, A.; Giacalone, F.; Gruttadauria, M. Advances in Organic and Organic-Inorganic Hybrid Polymeric Supports for Catalytic Applications. Molecules 2016, 21, 1288. [Google Scholar] [CrossRef]
- Keçili, R.; Hussain, C.M. Recent Progress of Imprinted Nanomaterials in Analytical Chemistry. Int. J. Anal. Chem. 2018, 2018, 8503853. [Google Scholar] [CrossRef] [PubMed]
- Okutucu, B. Wastewater Treatment Using Imprinted Polymeric Adsorbents. In Waste in Textile and Leather Sectors; IntechOpen: London, UK, 2020. [Google Scholar]
- Ye, L.; Haupt, K. Molecularly Imprinted Polymers as Antibody and Receptor Mimics for Assays, Sensors and Drug Discovery. Anal. Bioanal. Chem. 2004, 378, 1887–1897. [Google Scholar] [CrossRef] [PubMed]
- Hu, R.; Qin, A.; Tang, B.Z. AIE Polymers: Synthesis and Applications. Prog. Polym. Sci. 2020, 100, 101176. [Google Scholar] [CrossRef]
- Bu, Z.; Huang, L.; Li, S.; Tian, Q.; Tang, Z.; Diao, Q.; Chen, X.; Liu, J.; Niu, X. Introducing molecular imprinting onto nanozymes: Toward selective catalytic analysis. Anal. Bioanal. Chem. 2024, 416, 5859–5870. [Google Scholar] [CrossRef]
| Characteristics | Monomer Chemical Structure | Name | Advantages/ Disadvantages | Reference |
|---|---|---|---|---|
| Acidic |  | acrylic acid (AA) | Strong hydrogen bonding capacity; may be unstable under extreme pH conditions. | [68] |
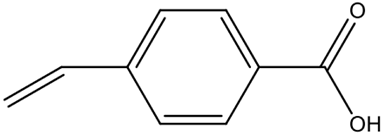 | p-vinylbenzoic acid (VBA) | High selectivity; limited solubility may restrict applications. | [68] | |
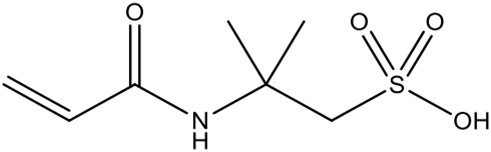 | 2-acrylamido-2-methyl-1-propanesulfonic acid (AMPSA) | Excellent hydrophilicity and thermal stability; excess may interfere with polymerization. | [69] | |
| Basic | 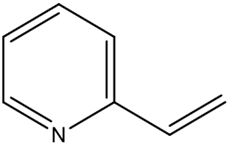 | 2-vinylpyridine (2-VP) | Strong interaction with acidic templates; strong odor and toxicity are drawbacks. | [68] |
 | N,N-diethylaminoethyl methacrylate (DEAEM) | Good structural flexibility; sensitive to oxidation. | [70] | |
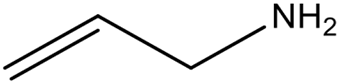 | Allylamine | High reactivity and affinity for acidic groups; unstable under light and oxygen exposure. | [71] | |
| Neutral | 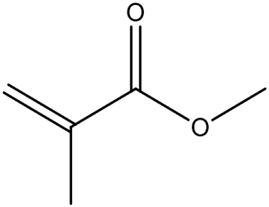 | methyl methacrylate (MMA) | High stability and easy polymerization; low affinity for specific targets. | [72] |
 | 4-ethylstyrene | Stable and non-polar, suitable for hydrophobic targets; limited interaction functionality | [73] | |
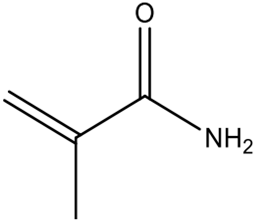 | methacrylamide | Good solvent compatibility; lower reactivity compared to other monomers. | [74] | |
| Cross-linking | 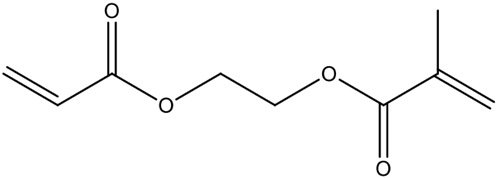 | ethylene glycol dimethacrylate (EGDMA) | Widely used, ensures rigidity and structural stability; may limit analyte diffusion. | [75] |
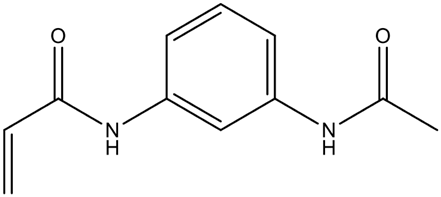 | N,N′ -1,3-phenylenebis(2- methyl-2-propenamide) (PDBMP) | Rigid structure, ideal for selective recognition; more complex synthesis. | [76] | |
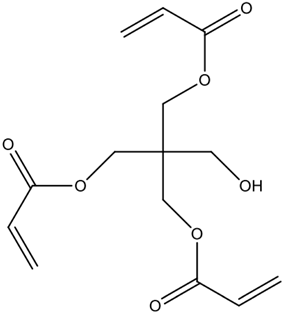 | pentaerythritol triacrylate (PETRA) | High cross-linking density and stability; may reduce accessibility to active sites. | [77] |
| MIP | Key Features | Application | References |
|---|---|---|---|
| Nanogels with Proline Derivatives | -High catalytic activity -High enantioselectivity -Enamine-based mechanism typical of aldolase I enzymes | Cross-aldol reaction between acetone and 4-nitrobenzaldehyde | [87,88] |
| Acrylamide-Based MIPs | -Covalent method with reversible enaminones -High stability and catalytic activity | Catalysis of organic reactions with high selectivity | [89] |
| Enzyme-Mimicking MIPs | -“Nanozymes” mimicking natural enzymes -High efficiency in biochemical reactions | Biosensors, environmental applications, peroxidase for pollutant degradation | [90,91] |
| MIP Photocatalysts | -Molecular recognition under light irradiation -High reaction rates and specificity | Pollutant degradation and fine chemical synthesis | [92] |
| MIPs for Asymmetric Catalysis | -Chiral cavities in the polymer matrix -Synthesis of chiral compounds with high selectivity | Pharmaceutical compound production | [93,94] |
| MIPs in Green Chemistry | -Facilitation of reactions under mild conditions -Reduction in the use of harsh reagents | Sustainable and environmentally friendly synthesis | [95] |
| MIPs in Industrial Applications | -Sustainable methods -High efficiency and reusability | Large-scale catalysis and environmentally friendly synthesis pathways | [96,97] |
| Nanogels for Drug Delivery | -High versatility -Biomedical compatibility | Controlled drug delivery systems | [98] |
| Functional Monomer | Molecule Template | Porogenic Solvent | Catalyst | BET (m2/g) | Qmax (mg/g) | Ref. |
|---|---|---|---|---|---|---|
| Methacrylate Acid (MMA) | 17-β-estradiol | Acetonitrile | --- | 128 | 42 | [107] |
| Nicotine | Methylene chloride | --- | 97 | 55 | [108] | |
| 3-Methylindole | Toluene | Fe3O4 | 112 | 64 | [109] | |
| Oxytetracycline | Water | Fe3O4 | 85 | 37 | [110] | |
| Glutathione | Acetonitrile/toluene | --- | 143 | 58 | [111] | |
| Dienoestrol | Acetonitrile | Fe3O4@SiO2 | 134 | 49 | [112] | |
| Acrylic acid | Red Remazol | Dimetilformamida | Fe3O4 | 120 | 63 | [113] |
| Methanoic acid | Acetonitrile/toluene | Fe3O4@SiO2 | 110 | 51 | [114] | |
| Aristolochic acid | Dimethylformamide | --- | 115 | 56 | [115] | |
| 4-VP | Ibuprofen | Acetonitrile | --- | 120 | 50 | [116] |
| Caffeic acid | Dimethylformide | --- | 130 | 60 | [117] | |
| methocarbamol | Tetrahydrofuran | --- | 110 | 47 | [118] | |
| 4-nitrophenol | Acetonitrile | Fe3O4 | 140 | 55 | [119] |
| Aspects | Details |
|---|---|
| Properties of MIPs | High specificity, stability under extreme conditions (pH, temperature), reusability, and robustness for various applications. |
| Advantages over Enzymes | Replace natural enzymes (expensive and unstable), mimicking their catalytic function with high regioselectivity and stereoselectivity. |
| Practical Example | Aldol reaction between acetone and 4-nitrobenzaldehyde catalyzed by nanogels containing proline derivatives, with high catalytic activity and enantioselectivity. |
| Recent Innovations | -Active-site titration method for precise calculation of catalytic parameters. -Molecular imprinting based on acrylamide for nanogels with superior catalytic activity. |
| Emerging Applications | -Enzymatic mimicking (“nanozymes”). -Photocatalysis for pollutant degradation. -Asymmetric catalysis for the synthesis of chiral compounds. |
| Impact on Green Chemistry | Reactions under milder conditions, reduction in harsh chemicals, and more sustainable syntheses for industrial and pharmaceutical applications. |
| Future Perspectives | Integration into biosensors, environmental catalysis, and the development of efficient drug delivery systems. |
| Title | Short Summary | Years |
|---|---|---|
| Natural and Synthetic Polymers for Biomedical and Environmental Applications [156,157]. | Use of MIPs to remove heavy metals and other pollutants. | 2024 |
| Recent advancement in fluorescent materials for optical sensing of pesticides [158]. | addresses the use of luminescent MIPs for pesticide detection, emphasizing their high selectivity and sensitivity. | 2023 |
| MIPs for environmental adsorption applications [159]. | Study highlights the development of MIPs for the selective capture of anti-inflammatory drugs in river water samples | 2022 |
| Molecularly Imprinted Polymer-Based Sensors for the Monitoring of Antibiotic Traces and Microorganisms in Water Samples to Combat Antimicrobial Resistance [160]. | This study develops MIPs for the selective detection of antibiotics in environmental water samples, demonstrating high specificity and sensitivity in detecting contaminants at very low concentrations. | 2024 |
| Development of magnetic MIPs for selective extraction of Benzoxazolinone-type alkaloids from acanthus plants [161]. | The article addresses the synthesis of magnetic MIPs for the efficient extraction of pesticides from soil samples, highlighting the use of advanced characterization techniques to optimize the performance of MIPs. | 2024 |
| Application of MIPs in the Analysis of Waters and Wastewaters [160]. | This research explores the use of MIPs for the adsorption and degradation of heavy metals in wastewater, showing promising results in the effective removal of metal contaminants. | 2021 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gonçalves, M.A.; la Porta, F.d.A.; da Silva, A.C.; Ramalho, T.C.; Aquino, S.F.d. Molecularly Imprinted Polymer-Supported Ceramic Catalysts for Environmental Applications: A Comprehensive Review. Ceramics 2025, 8, 53. https://doi.org/10.3390/ceramics8020053
Gonçalves MA, la Porta FdA, da Silva AC, Ramalho TC, Aquino SFd. Molecularly Imprinted Polymer-Supported Ceramic Catalysts for Environmental Applications: A Comprehensive Review. Ceramics. 2025; 8(2):53. https://doi.org/10.3390/ceramics8020053
Chicago/Turabian StyleGonçalves, Mateus Aquino, Felipe de Almeida la Porta, Adilson Candido da Silva, Teodorico Castro Ramalho, and Sérgio Francisco de Aquino. 2025. "Molecularly Imprinted Polymer-Supported Ceramic Catalysts for Environmental Applications: A Comprehensive Review" Ceramics 8, no. 2: 53. https://doi.org/10.3390/ceramics8020053
APA StyleGonçalves, M. A., la Porta, F. d. A., da Silva, A. C., Ramalho, T. C., & Aquino, S. F. d. (2025). Molecularly Imprinted Polymer-Supported Ceramic Catalysts for Environmental Applications: A Comprehensive Review. Ceramics, 8(2), 53. https://doi.org/10.3390/ceramics8020053









