Eco-Fired Bricks from Phosphate Mine Waste Rocks: The Effects of Marble Waste Powder on the Physical and Microstructural Properties
Abstract
1. Introduction
2. Materials and Methods
Characterization Techniques
3. Results and Discussion
3.1. Characterization of Raw Materials
3.2. Thermal Analysis (TG-DTG)
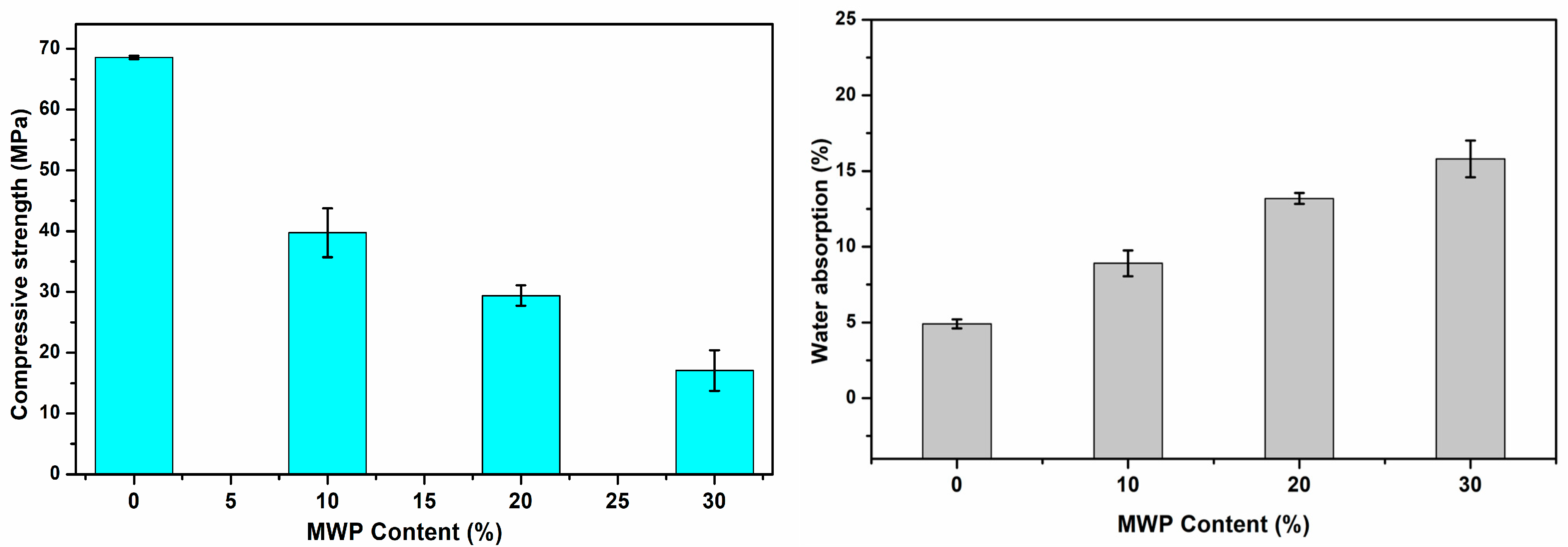
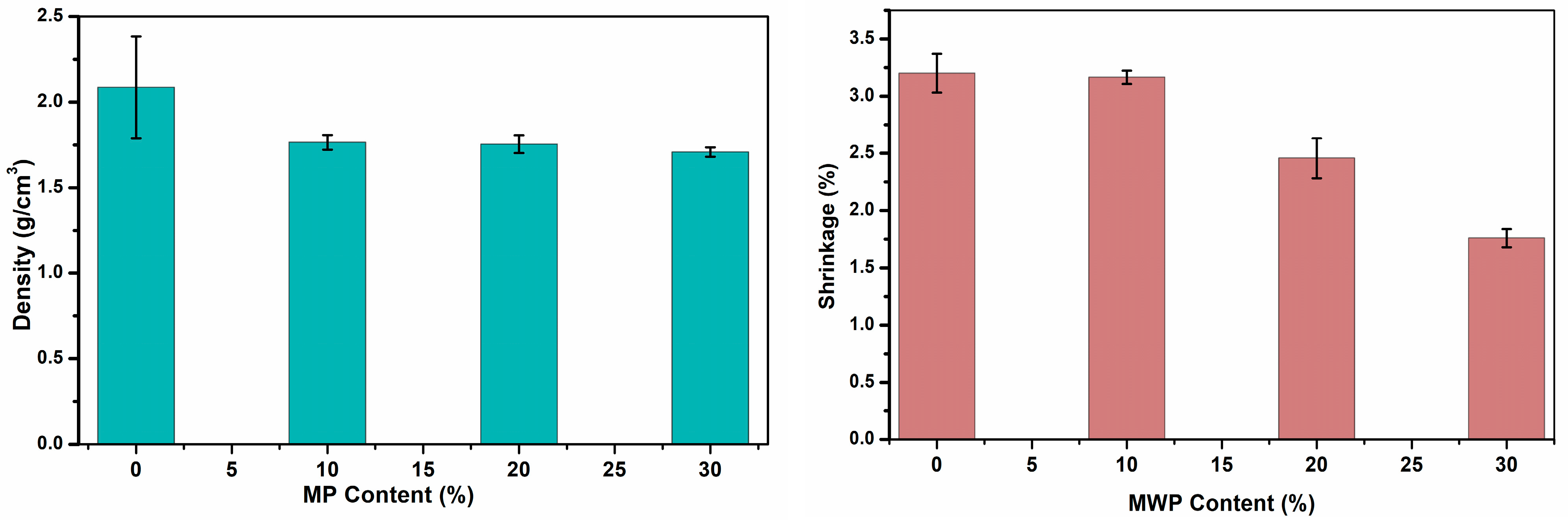
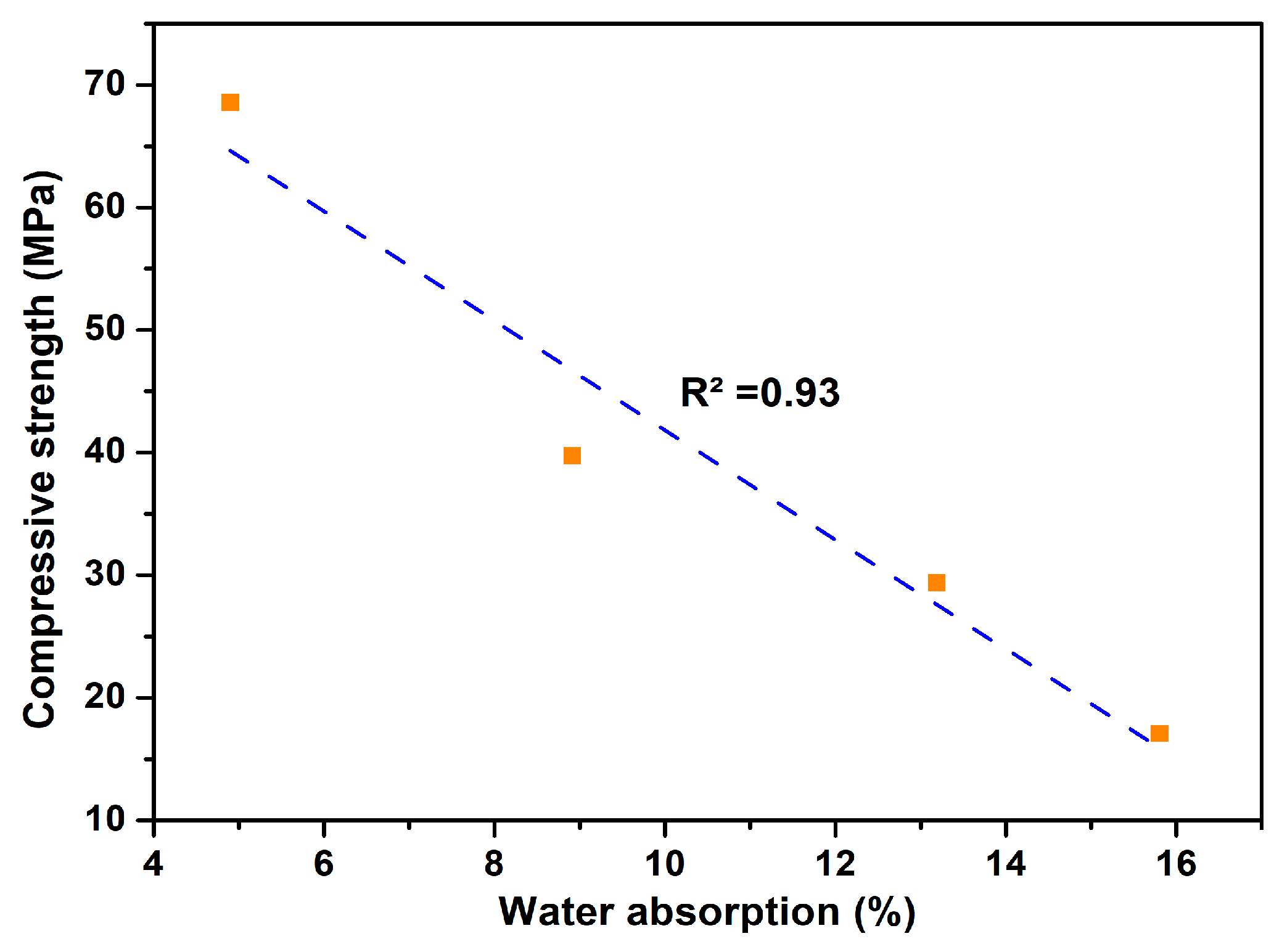
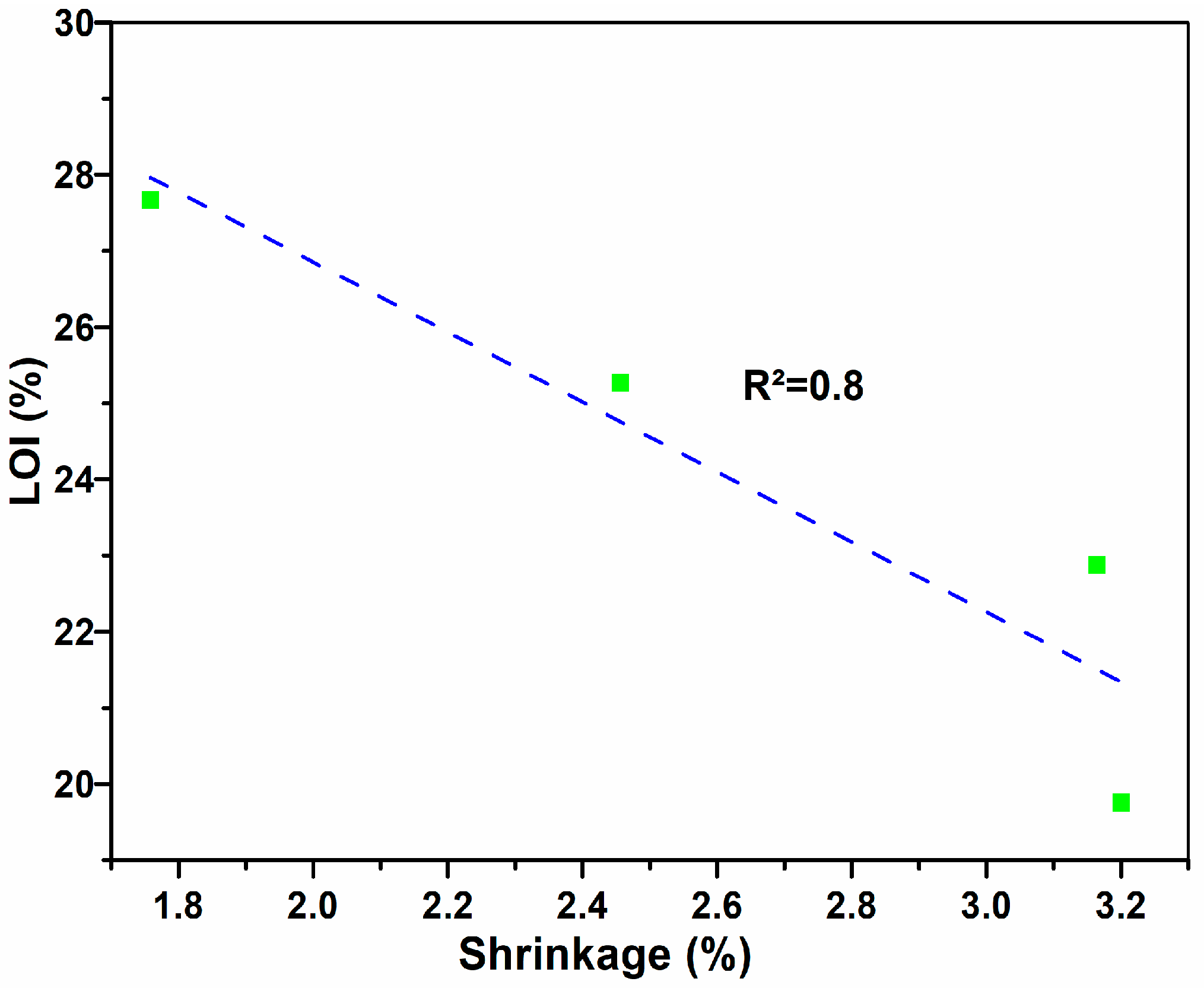
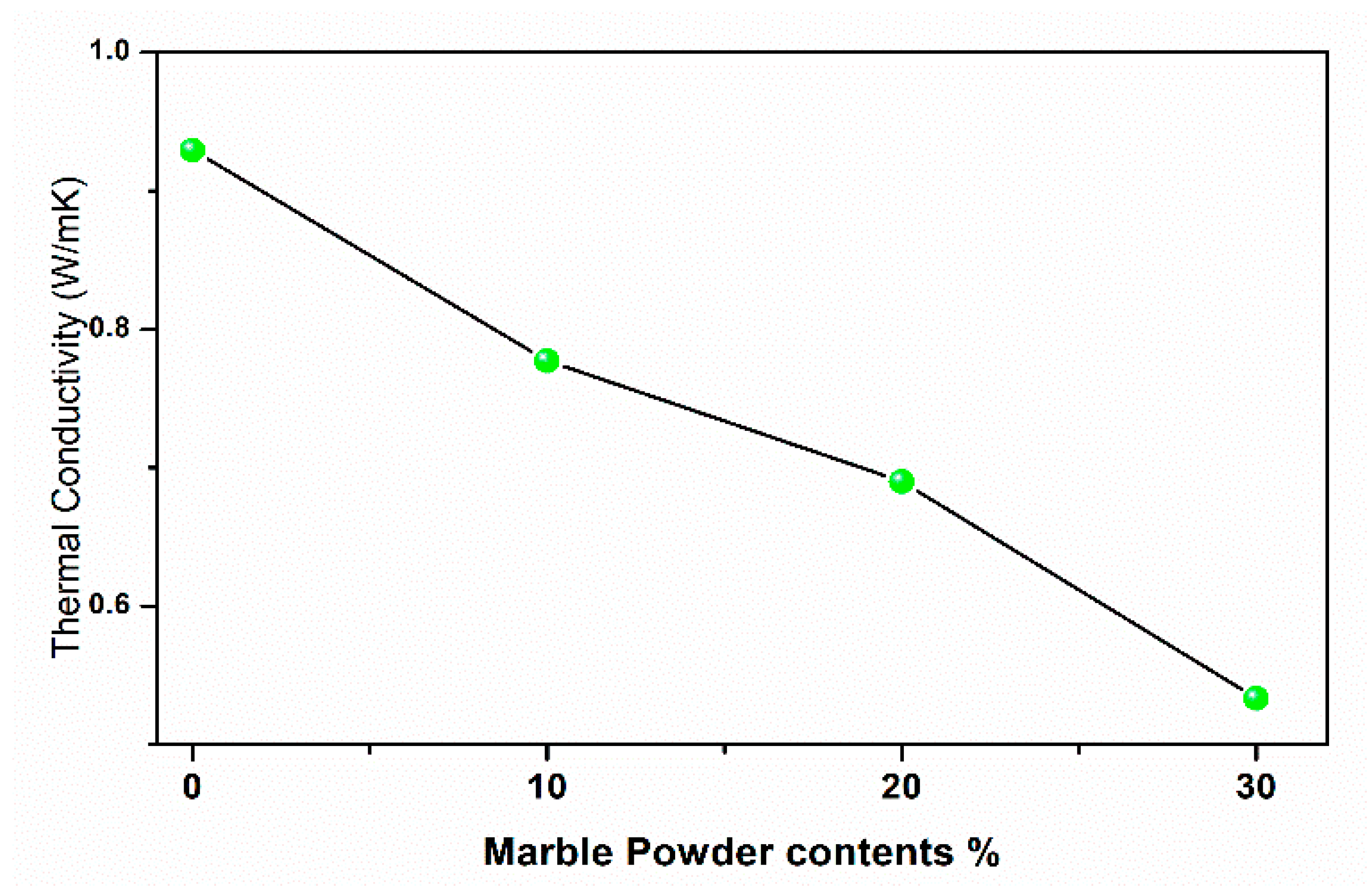
3.3. Physical Properties of Fired Bricks
3.4. Microstructural Analysis of Fired Bricks
4. Conclusions
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| RM | Red Marl |
| MWP | Marble Waste Powder |
References
- Beniddar, H.; El Machi, A.; El Abbassi, F.-E.; Taha, Y.; Benzaazoua, M.; Hakkou, R. Sustainable utilization of phosphate mine waste rocks as sand substitutes in cement mortar production. Constr. Build Mater. 2024, 438, 136949. [Google Scholar] [CrossRef]
- Hu, S.; Wang, J.; Xia, X. Study on development of circular economy in Hefei. In Proceedings of the 3rd International Conference on Environmental Technology and Knowledge Transfer, Hefei, China, 13–14 May 2010. [Google Scholar]
- Yuan, H.; Wang, J. A system dynamics model for determining the waste disposal charging fee in construction. Eur. J. Oper. Res. 2014, 237, 988–996. [Google Scholar] [CrossRef]
- Olejarczyk, M.; Rykowska, I.; Urbaniak, W. Management of Solid Waste Containing Fluoride—A Review. Materials 2022, 15, 3461. [Google Scholar] [CrossRef] [PubMed]
- Pandey, V.; Panda, S.K.; Singh, V.K. Preparation and characterization of high-strength insulating porous bricks by reusing coal mine overburden waste, red mud and rice husk. J. Clean. Prod. 2024, 469, 143134. [Google Scholar] [CrossRef]
- Moukannaa, S.; Bagheri, A.; Benzaazoua, M.; Sanjayan, J.G.; Pownceby, M.I.; Hakkou, R. Elaboration of alkali activated materials using a non-calcined red clay from phosphate mines amended with fly ash or slag: A structural study. Mater. Chem. Phys. 2020, 256, 123678. [Google Scholar] [CrossRef]
- Oubaha, S.; El Machi, A.; Mabroum, S.; Taha, Y.; Benzaazoua, M.; Hakkou, R. Recycling of phosphogypsum and clay by-products from phosphate mines for sustainable alkali-activated construction materials. Constr. Build. Mater. 2024, 411, 134262. [Google Scholar] [CrossRef]
- Mouih, K.; Hakkou, R.; Taha, Y.; Benzaazoua, M. Performances of compressed stabilized bricks using phosphate waste rock for sustainable construction. Constr. Build. Mater. 2023, 388, 131577. [Google Scholar] [CrossRef]
- Bayoussef, A.; Oubani, M.; Loutou, M.; Taha, Y.; Benzaazoua, M.; Manoun, B.; Hakkou, R. Manufacturing of high-performance ceramics using clays by-product from phosphate mines. Mater. Today Proc. 2021, 37, 3994–4000. [Google Scholar] [CrossRef]
- Gavali, H.R.; Ralegaonkar, R.V. Design development of sustainable alkali-activated bricks. J. Build. Eng. 2020, 30, 101302. [Google Scholar] [CrossRef]
- Boughriet, A.; Allahdin, O.; Poumaye, N.; Doyemet, G.; Tricot, G.; Revel, B.; Ouddane, B.; Wartel, M. Alkali-Activated Brick Aggregates as Industrial Valorized Wastes: Synthesis and Properties. Ceramics 2023, 6, 108. [Google Scholar] [CrossRef]
- Bahhou, A.; Taha, Y.; El Khessaimi, Y.; Idrissi, H.; Hakkou, R.; Amalik, J.; Benzaazoua, M. Use of phosphate mine by-products as supplementary cementitious materials. Mater. Today Proc. 2021, 37, 3781–3788. [Google Scholar] [CrossRef]
- Loutou, M.; Hajjaji, M.; Mansori, M.; Favotto, C.; Hakkou, R. Phosphate sludge: Thermal transformation and use as lightweight aggregate material. J. Environ. Manag. 2013, 130, 354–360. [Google Scholar] [CrossRef]
- Loutou, M.; Taha, Y.; Benzaazoua, M.; Daafi, Y.; Hakkou, R. Valorization of clay by-product from moroccan phosphate mines for the production of fired bricks. J. Clean Prod. 2019, 229, 169–179. [Google Scholar] [CrossRef]
- Ahmad, S.; Shah, M.U.H.; Ullah, A.; Shah, S.N.; Rehan, M.S.; Khan, I.A.; Ahmad, M.I. Sustainable Use of Marble Waste in Industrial Production of Fired Clay Bricks and Its Employment for Treatment of Flue Gases. ACS Omega 2021, 6, 22559–22569. [Google Scholar] [CrossRef]
- Galetakis, M.; Soultana, A. A review on the utilisation of quarry and ornamental stone industry fine by-products in the construction sector. Constr. Build Mater. 2016, 102, 769–781. [Google Scholar] [CrossRef]
- Tunc, E.T. Recycling of marble waste: A review based on strength of concrete containing marble waste. J. Environ. Manag. 2019, 231, 86–97. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.; Khan, B.; Shahzada, K.; Khan, S.; Wahab, N.; Ahmad, M.I. Conversion of Waste Marble Powder into a Binding Material. Civ. Eng. J. 2020, 6, 431–445. [Google Scholar] [CrossRef]
- Das, D.; Gołąbiewska, A.; Rout, P.K. Geopolymer bricks: The next generation of construction materials for sustainable environment. Constr. Build Mater. 2024, 445, 137876. [Google Scholar] [CrossRef]
- Wang, Y.; Xiao, J.; Zhang, J.; Duan, Z. Mechanical Behavior of Concrete Prepared with Waste Marble Powder. Sustainability 2022, 14, 4170. [Google Scholar] [CrossRef]
- Vardhan, K.; Goyal, S.; Siddique, R.; Singh, M. Mechanical properties and microstructural analysis of cement mortar incorporating marble powder as partial replacement of cement. Constr. Build. Mater. 2015, 96, 615–621. [Google Scholar] [CrossRef]
- Rehman, M.U.; Ahmad, M.; Rashid, K. Influence of fluxing oxides from waste on the production and physico-mechanical properties of fired clay brick: A review. J. Build. Eng. 2020, 27, 100965. [Google Scholar] [CrossRef]
- Eliche-Quesada, D.; Leite-Costa, J. Use of bottom ash from olive pomace combustion in the production of eco-friendly fired clay bricks. Waste Manag. 2016, 48, 323–333. [Google Scholar] [CrossRef] [PubMed]
- Kazmi, S.M.S.; Munir, M.J.; Patnaikuni, I.; Wu, Y.F.; Fawad, U. Thermal performance enhancement of eco-friendly bricks incorporating agro-wastes. Energy Build 2018, 158, 1117–1129. [Google Scholar] [CrossRef]
- Dai, Z.; Wu, Y.; Hu, L.; Zhang, W.; Mao, L. Evaluating physical-mechanical properties and long periods environmental risk of fired clay bricks incorporated with electroplating sludge. Constr. Build. Mater. 2019, 227, 116716. [Google Scholar] [CrossRef]
- Hofmann, E.; Németh, T.; Bidló, A. Thermal analysis of soils formed on limestone in the Bükk Mountains, North Hungary. J. Agrokémia És Talajt 2018, 67, 5–22. [Google Scholar] [CrossRef]
- Wang, S.; Gainey, L.; Wang, X.; Mackinnon, I.D.R.; Xi, Y. Influence of palygorskite on in-situ thermal behaviours of clay mixtures and properties of fired bricks. Appl. Clay Sci. 2022, 216, 106384. [Google Scholar] [CrossRef]
- Collard, F.-X.; Blin, J. A review on pyrolysis of biomass constituents: Mechanisms and composition of the products obtained from the conversion of cellulose, hemicelluloses and lignin. J. Renew. Sustain. Energy Rev. 2014, 38, 594–608. [Google Scholar] [CrossRef]
- Carazo, E.; Borrego-Sánchez, A.; García-Villén, F.; Sánchez-Espejo, R.; Viseras, C.; Cerezo, P.; Aguzzi, C. Adsorption and characterization of palygorskite-isoniazid nanohybrids. Appl. Clay Sci. 2018, 160, 180–185. [Google Scholar] [CrossRef]
- Kuang, W.; Facey, G.A.; Detellier, C. Dehydration and rehydration of palygorskite and the influence of water on the nanopores. Clays Clay Miner. 2004, 52, 635–642. [Google Scholar] [CrossRef]
- Dong, W.; Lu, Y.; Wang, W.; Zong, L.; Zhu, Y.; Kang, Y.; Wang, A. A new route to fabricate high-efficient porous silicate adsorbents by simultaneous inorganic-organic functionalization of low-grade palygorskite clay for removal of Congo red. Microporous Mesoporous Mater. 2019, 277, 267–276. [Google Scholar] [CrossRef]
- Harrati, A.; Arkame, Y.; Manni, A.; Aqdim, S.; Zmemla, R.; Chari, A.; El Bouari, A.; Hassani, I.-E.E.A.E.; Sdiri, A.; Hassani, F.O.; et al. Akermanite-based ceramics from Moroccan dolomite and perlite: Characterization and in vitro bioactivity assessment. Open Ceram. 2022, 10, 100276. [Google Scholar] [CrossRef]
- Pramono, E.; Pratiwi, W.; Wahyuningrum, D.; Radiman, C.L. Thermal properties of Bentonite Modified with 3-aminopropyltrimethoxysilane. IOP Conf. Ser. Mater. Sci. Eng. 2018, 333, 12084. [Google Scholar] [CrossRef]
- Qian, H.; Kai, W.; Hongde, X. A novel perspective of dolomite decomposition: Elementary reactions analysis by thermogravimetric mass spectrometry. J. Thermochim. Acta 2019, 676, 47–51. [Google Scholar] [CrossRef]
- Scott, S.A.; Dennis, J.S.; Davidson, J.F.; Hayhurst, A.N. Thermogravimetric measurements of the kinetics of pyrolysis of dried sewage sludge. J. Fuel 2006, 85, 1248–1253. [Google Scholar] [CrossRef]
- Zhuginissov, M.T.; Nurlybayev, R.E.; Orynbekov, Y.S.; Zhumadilova, Z.O.; Khamza, Y.Y.; Bulenbayev, M.Z. The Influence of the Burning Environment on the Properties of Ceramic Products Based on Fusible Raw Materials. Ceramics 2023, 6, 872–885. [Google Scholar] [CrossRef]
- Ozturk, S.; Sutcu, M.; Erdogmus, E.; Gencel, O. Influence of tea waste concentration in the physical, mechanical and thermal properties of brick clay mixtures. Constr. Build. Mater. 2019, 217, 592–599. [Google Scholar] [CrossRef]
- Singh, D.; Kumar, R.; Nighot, N.S.; Rajput, A.; Prajapati, A.; Singh, B.K.; Kirgiz, M.S.; Srinivasaraonaik, B.; Mishra, R.K.; Khan, S.; et al. A comprehensive review on valorisation of octal by-product as supplementary admixtures in the production of fired and unfired bricks. Constr. Build. Mater. 2023, 408, 133641. [Google Scholar] [CrossRef]
- Munir, M.J.; Abbas, S.; Nehdi, M.L.; Kazmi, S.M.S.; Khitab, A. Development of Eco-Friendly Fired Clay Bricks Incorporating Recycled Marble Powder. J. Mater. Civ. Eng. 2018, 30, 04018069. [Google Scholar] [CrossRef]
- Kadir, A.A.; Mohajerani, A. Effect of heating rate on gas emissions and properties of fired clay bricks and fired clay bricks incorporated with cigarette butts. Appl. Clay Sci. 2015, 104, 269–276. [Google Scholar] [CrossRef]
- Wang, C.; Wang, J.; Li, Q.; Xu, S.; Yang, J. A review on recent development of foam Ceramics prepared by particle-stabilized foaming technique. Adv. Colloid Interface Sci. 2024, 330, 103198. [Google Scholar] [CrossRef]
- Evcin, A.; Ersoy, B.; Çiftçi, H. Utilization of Marble and Boron Waste in Brick Products. Int. J. Comput. Exp. Sci. Eng. 2019, 5, 19–22. [Google Scholar] [CrossRef]
- Makrygiannis, I.; Karalis, K. Optimizing Building Thermal Insulation: The Impact of Brick Geometry and Thermal Coefficient on Energy Efficiency and Comfort. Ceramics 2023, 6, 1449–1466. [Google Scholar] [CrossRef]
- Gunasekaran, S.; Anbalagan, G. Thermal decomposition of natural dolomite. Bull. Mater. Sci. 2007, 30, 339–344. [Google Scholar] [CrossRef]
- Bozkaya, Ö.; Yalçın, H. Mineral Chemistry of Low-Temperature Phyllosilicates in Early Paleozoic Metaclastic Rocks, Eastern Tauride Belt, Türkiye. Minerals 2022, 12, 1088. [Google Scholar] [CrossRef]
- Meunier, A.; Velde, B. Illite: Origins, Evolution and Metamorphism; Springer: Berlin/Heidelberg, Germany, 2010. [Google Scholar]
- Li, M.; Su, P.; Guo, Y.; Zhang, W.; Mao, L. Effects of SiO2, Al2O3 and Fe2O3 on leachability of Zn, Cu and Cr in ceramics incorporated with electroplating sludge. J. Environ. Chem. Eng. 2017, 5, 3143–3150. [Google Scholar] [CrossRef]
- Souza, A.E.; Teixeira, S.R.; Santos, G.T.A.; Costa, F.B.; Longo, E. Reuse of sugarcane bagasse ash (SCBA) to produce ceramic materials. J. Environ. Manag. 2011, 92, 2774–2780. [Google Scholar] [CrossRef]
- Aouba, L.; Bories, C.; Coutand, M.; Perrin, B.; Lemercier, H. Properties of fired clay bricks with incorporated biomasses: Cases of Olive Stone Flour and Wheat Straw residues. Constr. Build. Mater. 2016, 102, 7–13. [Google Scholar] [CrossRef]
- Menezes, R.R.; Ferreira, H.S.; Neves, G.A.; de L. Lira, H.; Ferreira, H.C. Use of granite sawing wastes in the production of ceramic bricks and tiles. J. Eur. Ceram. Soc. 2005, 25, 1149–1158. [Google Scholar] [CrossRef]
- Subari, F.E. Utilization of Marble and Limestone Wastes for Making the Eksposed Bricks Based Synthetic Wollastonite. J. Teknol. Miner. Dan Batubara 2016, 12, 171–178. [Google Scholar]
- Cultrone, G.; Rodriguez-Navarro, C.; Sebastian, E.; Cazalla, O.; De La Torre, M.J. Carbonate and silicate phase reactions during ceramic firing. J. Eur. J. Mineral. 2001, 13, 621–634. [Google Scholar] [CrossRef]
- Karaman, S.; Ersahin, S.; Gunal, H. Firing temperature and firing time influence on mechanical and physical properties of clay bricks. J. Sci. Ind. Res. 2006, 65, 153–159. [Google Scholar]
- Tsega, E. Effects of Firing Time and Temperature on Physical Properties of Fired Clay Bricks. Am. J. Civ. Eng. 2017, 5, 21. [Google Scholar] [CrossRef]
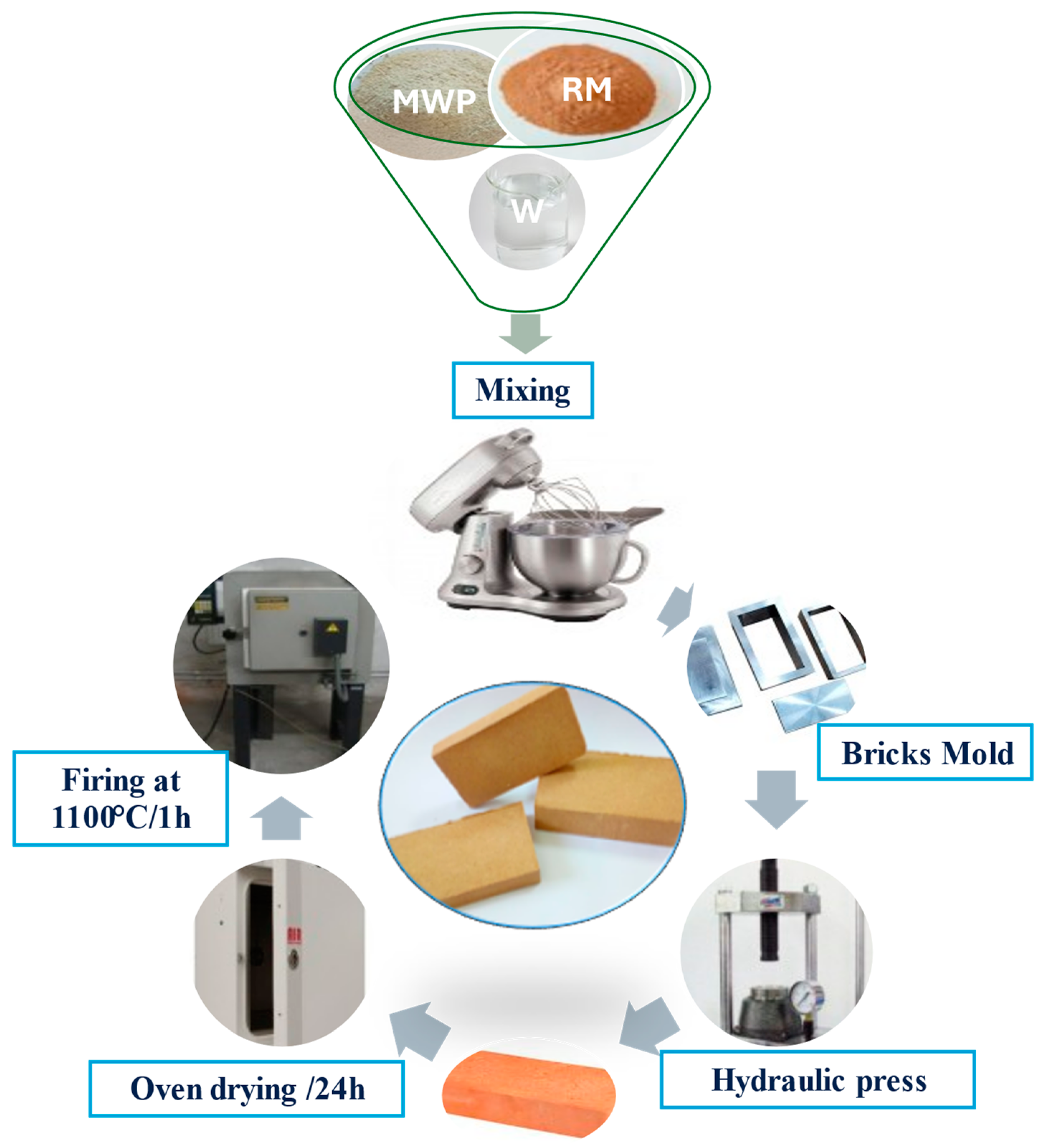
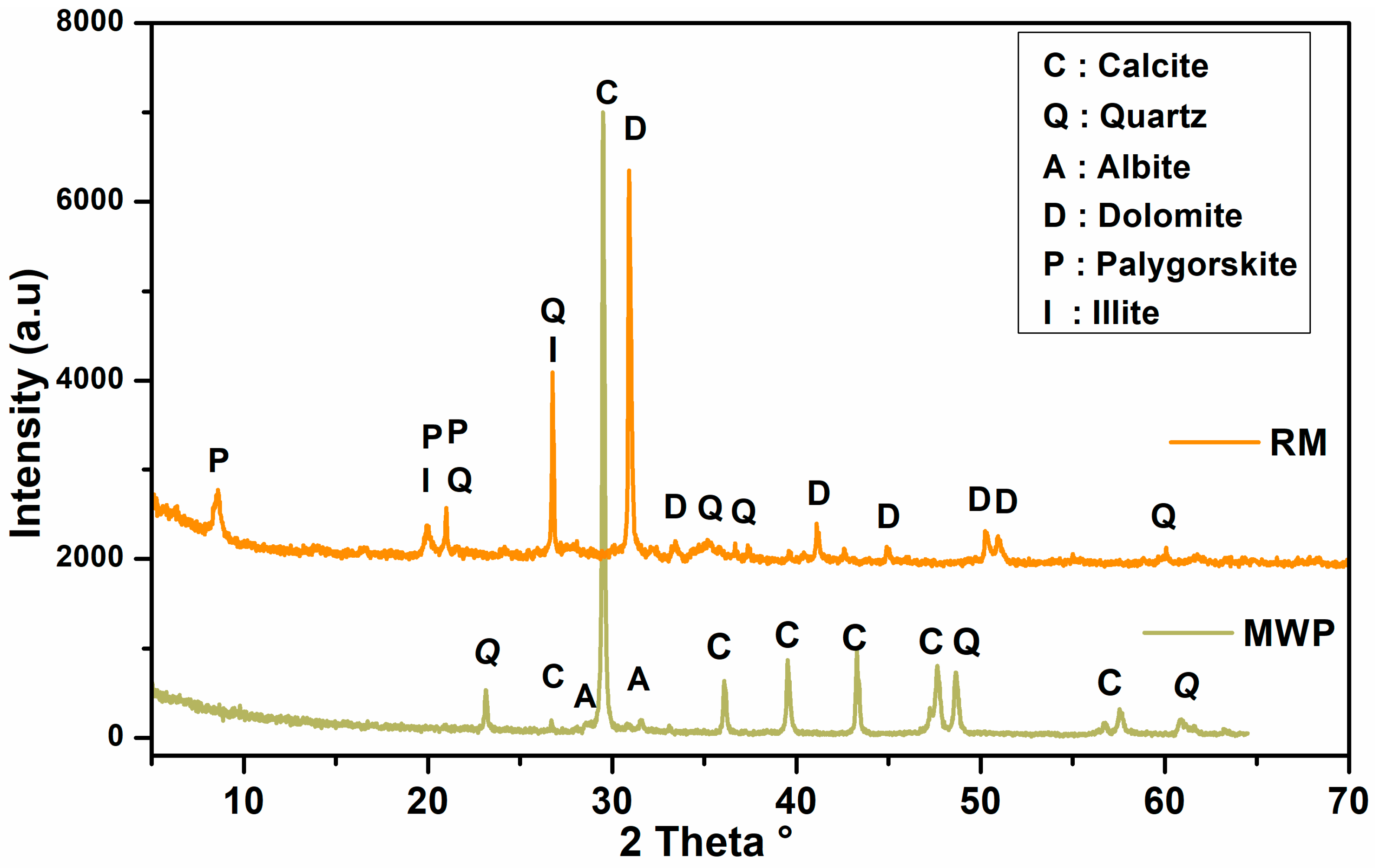





| Sample ID | Red Marl (wt %) | Marble Waste Powder (wt %) | Water Content (wt%) |
|---|---|---|---|
| MP 0 | 100 | 0 | 10 |
| MP 10 | 90 | 10 | 10 |
| MP 20 | 80 | 20 | 10 |
| MP 30 | 70 | 30 | 10 |
| Element (%) | SiO2 | Al2O3 | Fe2O3 | CaO | MgO | K2O | TiO2 | P2O5 | S | Na2O |
|---|---|---|---|---|---|---|---|---|---|---|
| RM | 38.16 | 8.57 | 3.54 | 14.97 | 11.64 | 1.78 | 0.45 | 1.96 | 0.32 | 0.2 |
| MWP | 1.38 | 0.76 | 0.81 | 54.49 | 1.46 | 0.49 | 0.02 | <0.01 | 0.57 | 0.16 |
| MWP Content (%) | Water Absorption (%) | Density (g/cm3) | Shrinkage (%) | Compressive Strength (MPa) | LOI (%) |
|---|---|---|---|---|---|
| 10.00 | 8.91 | 1.77 | 3.16 | 39.74 | 22.88 |
| 20.00 | 13.19 | 1.75 | 2.46 | 29.40 | 25.26 |
| 30.00 | 15.80 | 1.71 | 1.76 | 17.09 | 27.67 |
| Element (g/t) | As | Ba | Cd | Co | Cr | Cu | Ni | Pb | Zn |
|---|---|---|---|---|---|---|---|---|---|
| RM | 24 | 133 | <2 | <8 | 718 | 114 | 172 | <26 | 2270 |
| MWP | 90 | 21 | <2 | <8 | 111 | 81 | 101 | <26 | 1009 |
| Oxygen | Magnesium | Aluminium | Silicon | Calcium | Phosphorus | Carbon | Sum | |
|---|---|---|---|---|---|---|---|---|
| P1 | 35.49 | 2.17 | 4.96 | 14.19 | 3.26 | 0 | 0 | 60.06 |
| P2 | 62.67 | 1.79 | 1.13 | 3.35 | 23.84 | 4.31 | 0 | 97.10 |
| P3 | 59.4 | 0 | 0 | 0 | 35.4 | 0 | 14.87 | 110.24 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bayoussef, A.; Moukannaa, S.; Loutou, M.; Taha, Y.; Benzaazoua, M.; Hakkou, R. Eco-Fired Bricks from Phosphate Mine Waste Rocks: The Effects of Marble Waste Powder on the Physical and Microstructural Properties. Ceramics 2025, 8, 48. https://doi.org/10.3390/ceramics8020048
Bayoussef A, Moukannaa S, Loutou M, Taha Y, Benzaazoua M, Hakkou R. Eco-Fired Bricks from Phosphate Mine Waste Rocks: The Effects of Marble Waste Powder on the Physical and Microstructural Properties. Ceramics. 2025; 8(2):48. https://doi.org/10.3390/ceramics8020048
Chicago/Turabian StyleBayoussef, Ayoub, Samira Moukannaa, Mohamed Loutou, Yassine Taha, Mostafa Benzaazoua, and Rachid Hakkou. 2025. "Eco-Fired Bricks from Phosphate Mine Waste Rocks: The Effects of Marble Waste Powder on the Physical and Microstructural Properties" Ceramics 8, no. 2: 48. https://doi.org/10.3390/ceramics8020048
APA StyleBayoussef, A., Moukannaa, S., Loutou, M., Taha, Y., Benzaazoua, M., & Hakkou, R. (2025). Eco-Fired Bricks from Phosphate Mine Waste Rocks: The Effects of Marble Waste Powder on the Physical and Microstructural Properties. Ceramics, 8(2), 48. https://doi.org/10.3390/ceramics8020048













