Physical and Mechanical Properties of Fired Bricks from Amazon Bauxite Tailings with Granite Powder
Abstract
1. Introduction
2. Materials and Methods
2.1. Raw Materials
2.2. The Characterization of the Samples
2.2.1. Mineralogical Composition
2.2.2. Chemical Composition with X-Ray Fluorescence Spectrometry
2.2.3. Thermogravimetric Analysis (TGA) and Differential Scanning Calorimetry (DSC)
2.3. Formulation and Characterization of Fired Brick
2.3.1. Water Absorption
2.3.2. Apparent Porosity
2.3.3. Compressive Strength
3. Results
3.1. Raw Materials
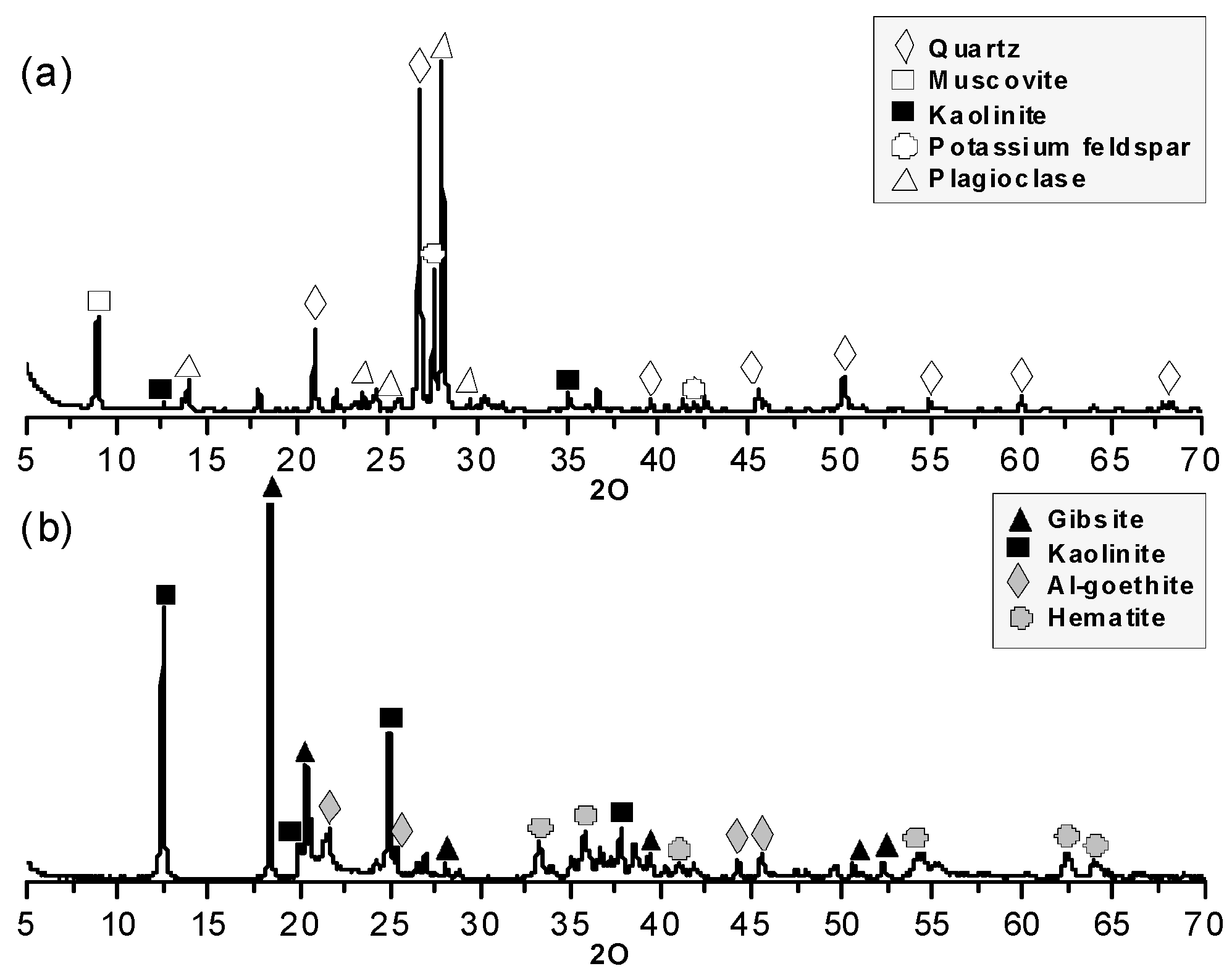
3.1.1. Mineralogical Composition
3.1.2. Chemical Composition
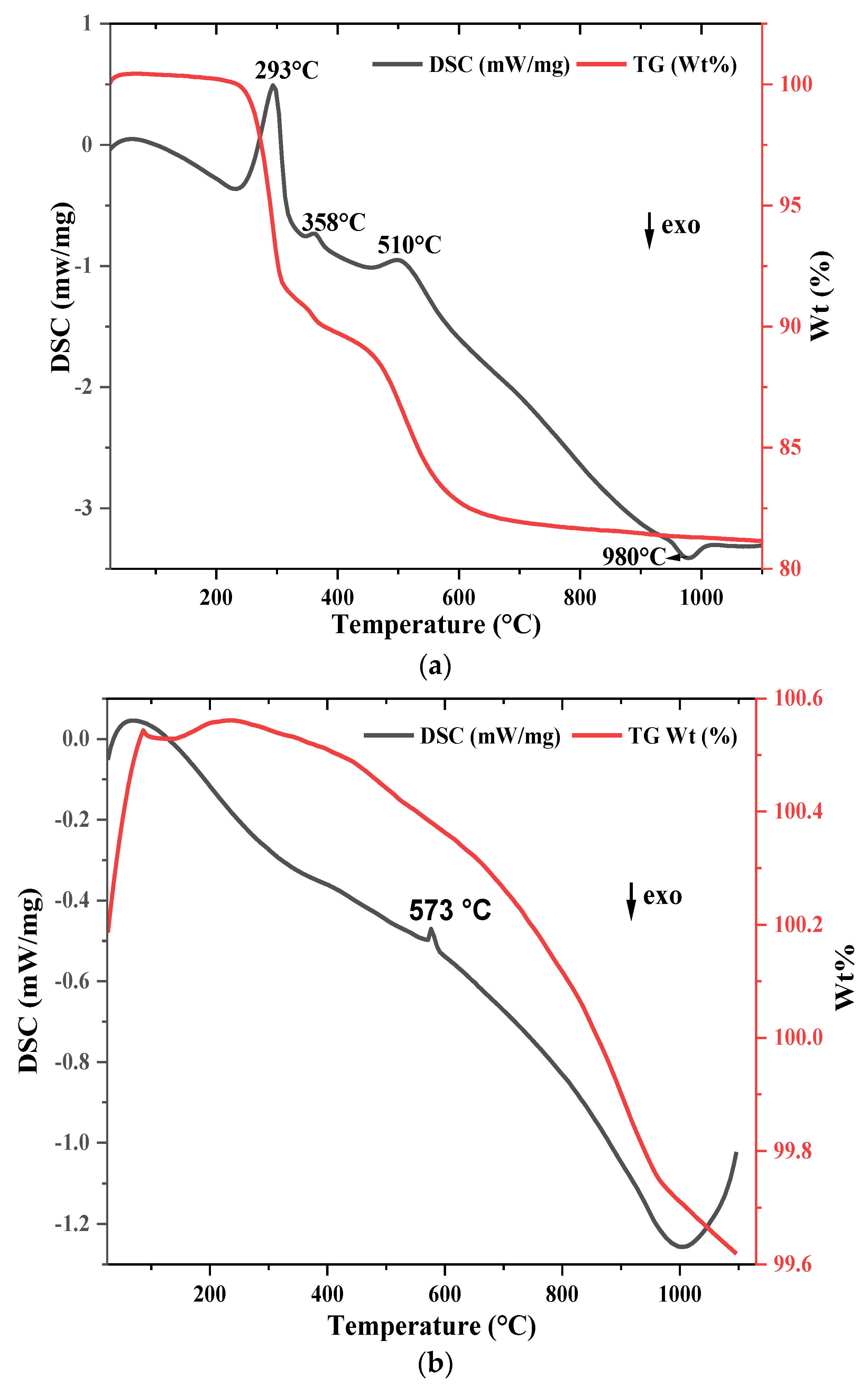
3.1.3. Thermal Behavior
3.2. Fired Bricks (Specimens)
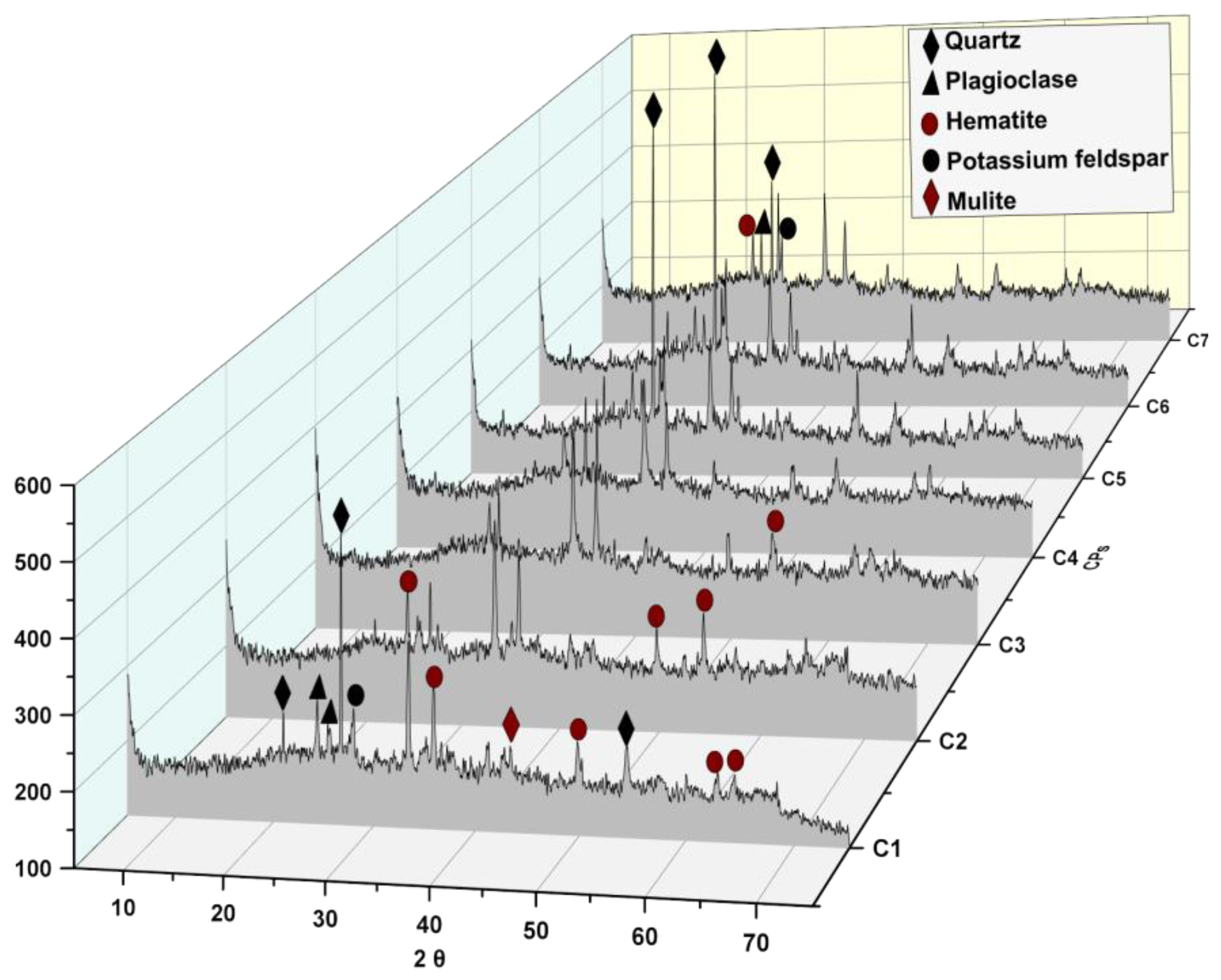
3.2.1. Mineralogical Composition
3.2.2. Linear Firing Shrinkage (RLq)
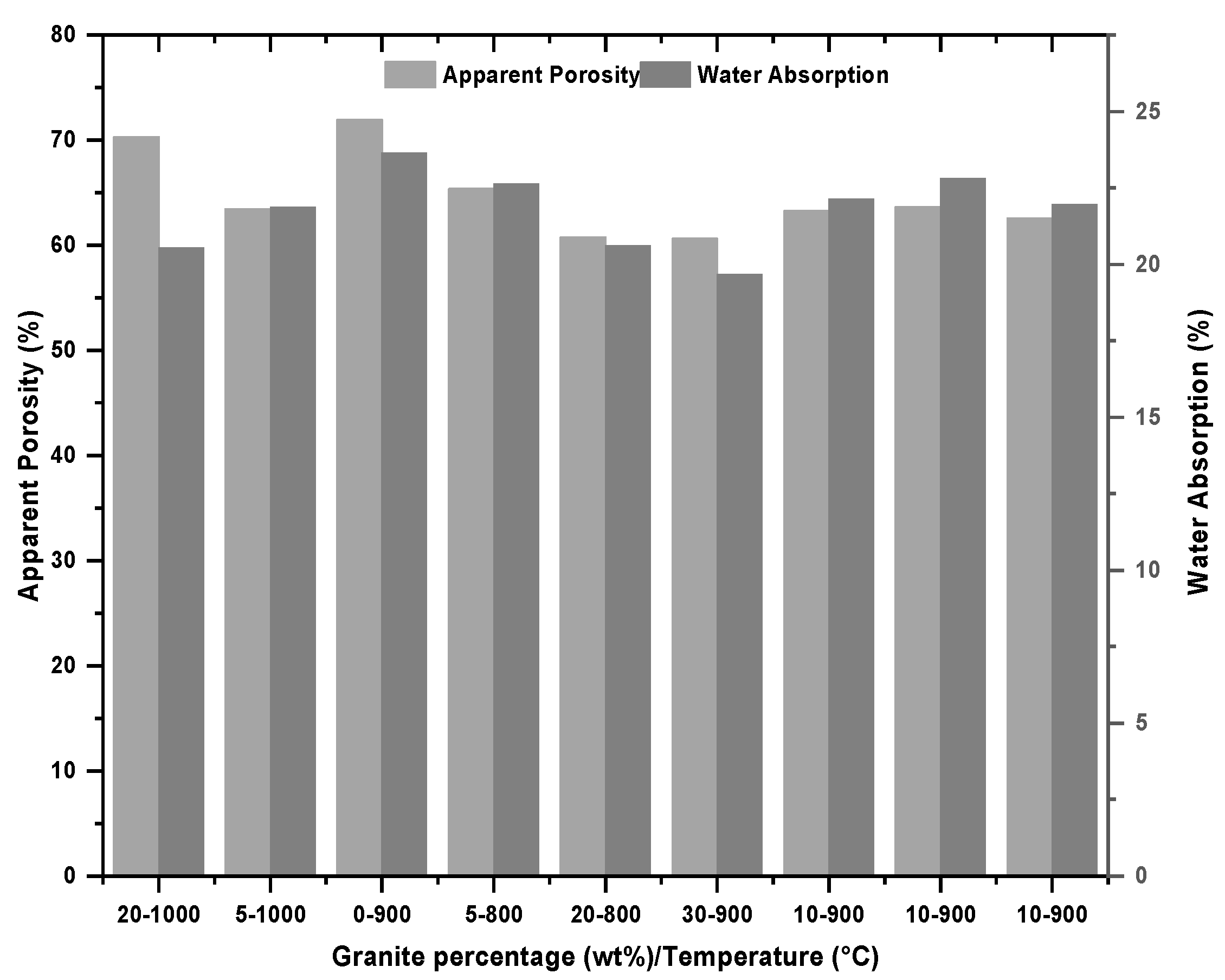
3.2.3. Water Absorption and Apparent Porosity
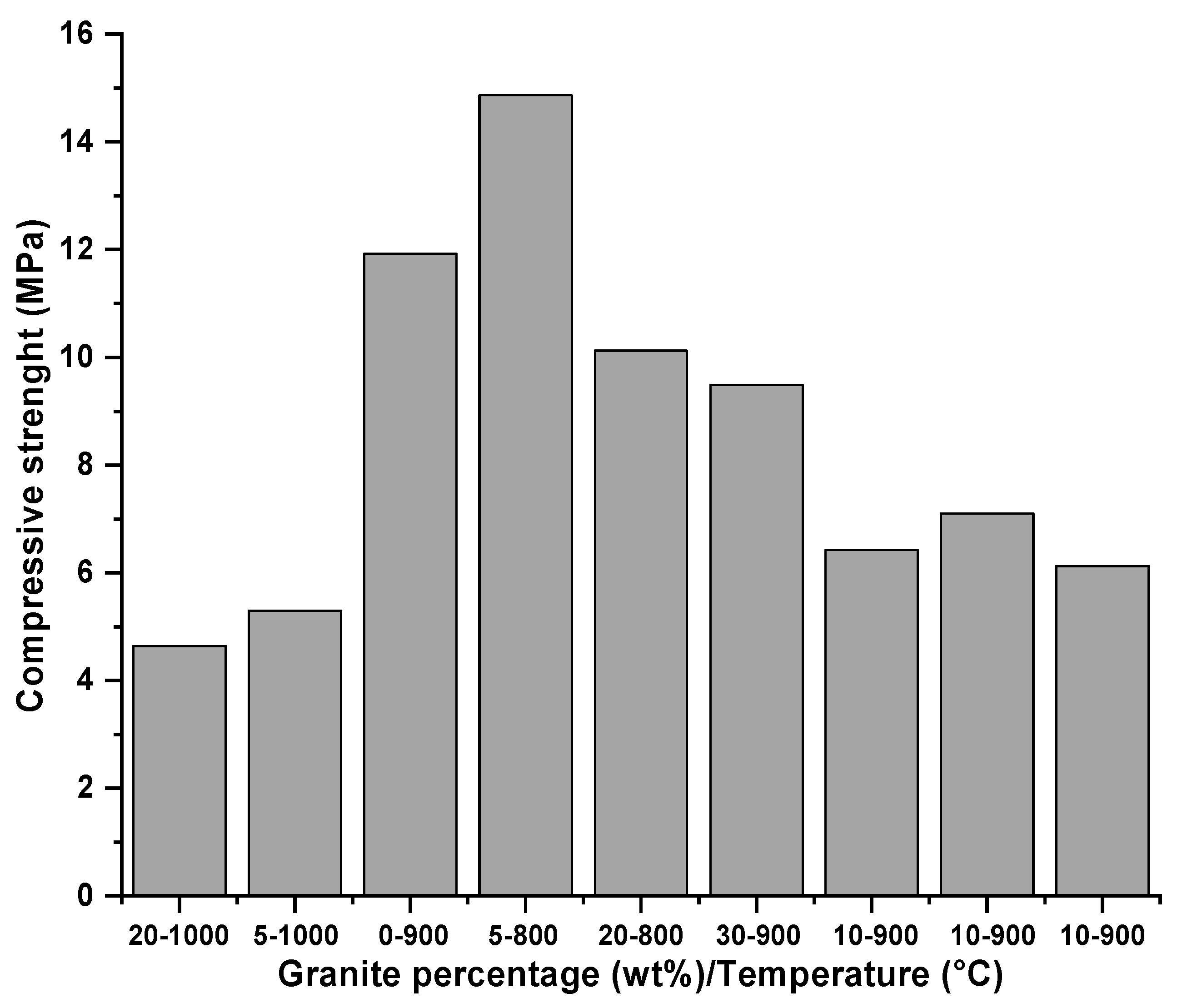
3.2.4. Compressive Strength
4. Discussion
4.1. Raw Materials
4.1.1. Mineralogical Composition and Chemical Composition
4.1.2. Thermal Behavior
4.2. Specimens
4.2.1. Mineralogical Composition
4.2.2. Linear Firing Shrinkage (RLq)
4.2.3. Water Absorption and Apparent Porosity
4.2.4. Compressive Strength
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bai, M.; Xiao, J.; Gao, Q.; Shen, J. Utilization of Construction Spoil and Recycled Powder in Fired Bricks. Case Stud. Constr. Mater. 2023, 18, e02024. [Google Scholar] [CrossRef]
- Sarani, N.A.; Kadir, A.A.; Din, M.F.M.; Amiza Hashim, A.; Hassan, M.I.H.; Hamid, N.J.A.; Hashar, N.N.H.; Hissham, N.F.N.; Johan, S.F.S.M. Physical-Mechanical Properties and Thermogravimetric Analysis of Fired Clay Brick Incorporating Palm Kernel Shell for Alternative Raw Materials. Constr. Build. Mater. 2023, 376, 131032. [Google Scholar] [CrossRef]
- Wang, S.; Gainey, L.; Mackinnon, I.D.R.; Allen, C.; Gu, Y.; Xi, Y. Thermal Behaviors of Clay Minerals as Key Components and Additives for Fired Brick Properties: A Review. J. Build. Eng. 2023, 66, 105802. [Google Scholar] [CrossRef]
- Adazabra, A.N.; Viruthagiri, G.; Yirijor, J. Combined Effects of Biomass Bottom Ashes and Spent Charcoal on Characteristics of Fired Clay Bricks. Constr. Build. Mater. 2023, 399, 132570. [Google Scholar] [CrossRef]
- Makrygiannis, I.; Karalis, K. Optimizing Building Thermal Insulation: The Impact of Brick Geometry and Thermal Coefficient on Energy Efficiency and Comfort. Ceramics 2023, 6, 1449–1466. [Google Scholar] [CrossRef]
- Makrygiannis, I.; Tsetsekou, A.; Papastratis, O.; Karalis, K. Assessing the Effects of Refuse-Derived Fuel (RDF) Incorporation on the Extrusion and Drying Behavior of Brick Mixtures. Ceramics 2023, 6, 2367–2385. [Google Scholar] [CrossRef]
- Gadioli, M.C.B.; de Aguiar, M.C.; Vidal, F.W.H.; Sant’Ana, M.A.K.; de Almeida, K.M.; Giori, A.J.N. Incorporation of Ornamental Stone Waste in the Manufacturing of Red Ceramics. Materials 2022, 15, 5635. [Google Scholar] [CrossRef] [PubMed]
- Kedziora, S.; Decker, T.; Museyibov, E.; Morbach, J.; Hohmann, S.; Huwer, A.; Wahl, M. Strength Properties of 316L and 17-4 PH Stainless Steel Produced with Additive Manufacturing. Materials 2022, 15, 6278. [Google Scholar] [CrossRef]
- Gonçalves, R.M.; Martinho, A.; Oliveira, J.P. Recycling of Reinforced Glass Fibers Waste: Current Status. Materials 2022, 15, 1596. [Google Scholar] [CrossRef]
- Badini, S.; Regondi, S.; Pugliese, R. Unleashing the Power of Artificial Intelligence in Materials Design. Materials 2023, 16, 5927. [Google Scholar] [CrossRef]
- Freeland, B.; McCarthy, E.; Balakrishnan, R.; Fahy, S.; Boland, A.; Rochfort, K.D.; Dabros, M.; Marti, R.; Kelleher, S.M.; Gaughran, J. A Review of Polylactic Acid as a Replacement Material for Single-Use Laboratory Components. Materials 2022, 15, 2989. [Google Scholar] [CrossRef] [PubMed]
- Bellucci, D.; Cannillo, V. Low-Temperature Sintering of a New Bioactive Glass Enriched with Magnesium Oxide and Strontium Oxide. Materials 2022, 15, 6263. [Google Scholar] [CrossRef]
- Balti, S.; Boudenne, A.; Yahya, K.; Hamdi, N. Advancing Reinforcement of Sustainable Gypsum Composites: High-Performance Design by Reusing Waste Materials. Mater. Today Sustain. 2024, 27, 100946. [Google Scholar] [CrossRef]
- Negrão, L.B.A.; da Costa, M.L.; Pöllmann, H. Waste Clay from Bauxite Beneficiation to Produce Calcium Sulphoaluminate Eco-Cements. Constr. Build. Mater. 2022, 340, 127703. [Google Scholar] [CrossRef]
- Dong, Y.; Guo, W.; Jiang, C.; Shao, Y.; Zhang, L.; Wang, D.; Lu, X.; Huang, S.; Cheng, X. Using CaO as a Modifier Agent to Optimize the Pore Structure of Foamed Ceramics from Granite Scrap. Ceram. Int. 2023, 49, 13443–13451. [Google Scholar] [CrossRef]
- Adazabra, A.N.; Viruthagiri, G.; Atingabono, J. Developing Fired Clay Bricks by Incorporating Scrap Incinerated Waste and River Dredged Sediment. Process Saf. Environ. Prot. 2023, 179, 108–123. [Google Scholar] [CrossRef]
- Adazabra, A.N.; Viruthagiri, G. Effect of Variegated Biosolids Incorporation on the Technological Properties of Fired Clay Bricks Using Taguchi Method. Case Stud. Constr. Mater. 2023, 19, e02314. [Google Scholar] [CrossRef]
- Wahab, R.A.A.; Mohammad, M.; Mazlan, M.; Yaki, A.N.A.; Bahari, N.S.S.; Fadzli, S.N.A.M.; Zahanis, Z.H.B.; Zaid, M.H.M. Study on the Physical and Mechanical Properties of Low Energy Consumption Fired Industrial Waste Clay Bricks from Eggshells and Rice Husks. Mater. Today Proc. 2023, 75, 79–83. [Google Scholar] [CrossRef]
- Mohammad, M.; Abdul Wahab, R.A.; Mazlan, M.; Nisa Syuhaidah Mohamad Fazil, N.; Suraya Hanim Ibrahim, N.; Najiha Muhamad Nizam, U.; Humaidi Abu Hanifah, A.; Hafiz Mohd Zaid, M. Physical and Mechanical Properties of Fired Industrial Waste-Clay Bricks from Clam Shells and Soda Lime Silica Glass. In Materials Today: Proceedings; Elsevier Ltd.: Amsterdam, The Netherlands, 2022; Volume 75, pp. 151–155. [Google Scholar]
- ABNT—Associação Brasileira de Normas Técnicas. Alvenaria Estrutural—Blocos Cerâmicos Parte 3: Métodos de Ensaio; Associação Brasileira de Normas Técnicas: Rio de Janeiro, RJ, Brazil, 2017; Volume 34. [Google Scholar]
- ABNT—Associação Brasileira de Normas Técnicas. NBR 15270-1: Componentes Cerâmicos. Parte 1: Blocos Cerâmicos Para Alvenaria de Vedação—Terminologia e Requisitos; Associação Brasileira de Normas Técnicas: Rio de Janeiro, RJ, Brazil, 2005; Volume 15. [Google Scholar]
- ABNT—Associação Brasileira de Normas Técnicas. NBR 15270-2: Componentes Cerâmicos Parte 2: Blocos Cerâmicos Para Alvenaria Estrutural—Terminologia e Requisitos; Associação Brasileira de Normas Técnicas: Rio de Janeiro, RJ, Brazil, 2017; Volume 15. [Google Scholar]
- ASTM C20-00; Standard Test Methods for Apparent Porosity, Water Absorption, Apparent Specific Gravity, and Bulk Density of Burned Refractory Brick and Shapes by Boiling Water. American Society for Testing and Materials: West Conshohocken, PA, USA, 2015; pp. 1–3. [CrossRef]
- Adazabra, A.N.; Viruthagiri, G.; Foli, B.Y. Evaluating the Technological Properties of Fired Clay Bricks Incorporated with Palm Kernel Shell. J. Build. Eng. 2023, 72, 106673. [Google Scholar] [CrossRef]
- Zhao, J.; Wang, W.; Luan, Z.; He, M. First-Principles Analysis on Phase Transition, Atomic, Electronic, and Mechanical Properties of Kaolinite under Pressures. Phys. B Condens. Matter. 2024, 674, 415554. [Google Scholar] [CrossRef]
- Pacheco, G.R.C.; Gonçalves, G.E.; Lins, V. de F.C. Design of Magnesia–Spinel Bricks for Improved Coating Adherence in Cement Rotary Kilns. Ceramics 2021, 4, 652–666. [Google Scholar] [CrossRef]
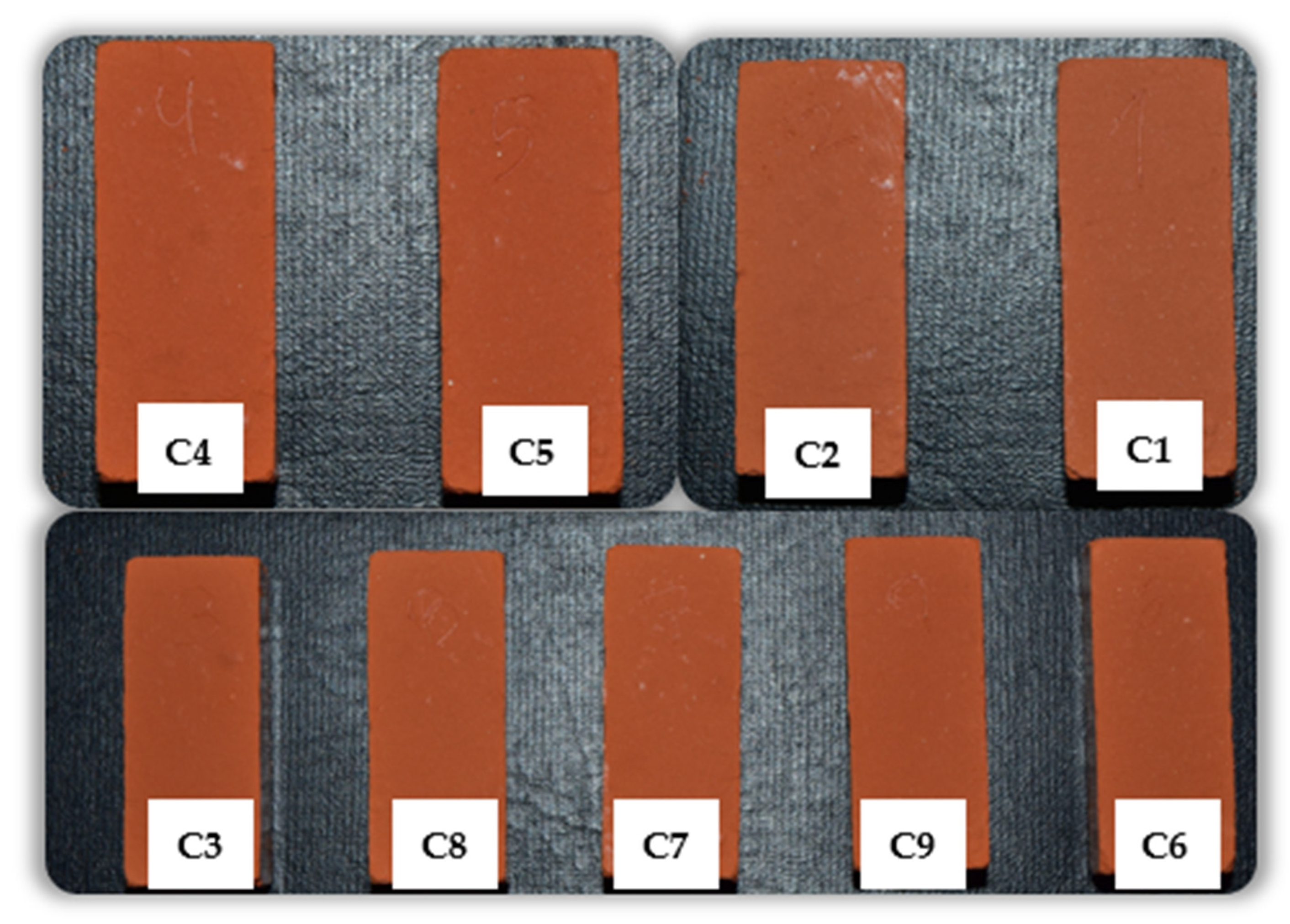
| Experiment | Temperature (°C) | Percentage of Granite Powder (wt/wt) |
|---|---|---|
| C1 | 1000 | 20 |
| C2 | 1000 | 5 |
| C3 | 900 | 0 |
| C4 | 800 | 5 |
| C5 | 800 | 20 |
| C6 | 900 | 30 |
| C7 | 900 | 10 |
| C8 | 900 | 10 |
| C9 | 900 | 10 |
| Sample | SiO2 | Al2O3 | Fe2O3 | TiO2 | K2O | Na2O | LOI | Other | Total |
|---|---|---|---|---|---|---|---|---|---|
| ALB-1 | 23 | 41.12 | 15.35 | 2.15 | 0.02 | 0.01 | 19.18 | 0.37 | 101.2 |
| GRT-1 | 74.4 | 14.42 | 1.05 | 0.11 | 4.45 | 3.86 | 0.87 | 1.09 | 100.25 |
| Experiment | Dry Length | Length After Calcination | Linear Shrinkage (%) |
|---|---|---|---|
| C1 | 51.82 | 50.55 | 2.51 |
| C2 | 51.81 | 50.24 | 3.13 |
| C3 | 51.93 | 50.07 | 3.71 |
| C4 | 51.79 | 50.96 | 1.63 |
| C5 | 51.79 | 51.18 | 1.19 |
| C6 | 51.93 | 51.23 | 1.37 |
| C7 | 51.78 | 50.68 | 2.15 ± 0.25 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Barreto, I.A.R.; da Costa, M.L. Physical and Mechanical Properties of Fired Bricks from Amazon Bauxite Tailings with Granite Powder. Ceramics 2025, 8, 37. https://doi.org/10.3390/ceramics8020037
Barreto IAR, da Costa ML. Physical and Mechanical Properties of Fired Bricks from Amazon Bauxite Tailings with Granite Powder. Ceramics. 2025; 8(2):37. https://doi.org/10.3390/ceramics8020037
Chicago/Turabian StyleBarreto, Igor A. R., and Marcondes L. da Costa. 2025. "Physical and Mechanical Properties of Fired Bricks from Amazon Bauxite Tailings with Granite Powder" Ceramics 8, no. 2: 37. https://doi.org/10.3390/ceramics8020037
APA StyleBarreto, I. A. R., & da Costa, M. L. (2025). Physical and Mechanical Properties of Fired Bricks from Amazon Bauxite Tailings with Granite Powder. Ceramics, 8(2), 37. https://doi.org/10.3390/ceramics8020037











