Comparing the Efficacies of Electrospun ZnO and TiO2 Nanofibrous Interlayers for Electron Transport in Perovskite Solar Cells
Abstract
1. Introduction
2. Materials and Methodology
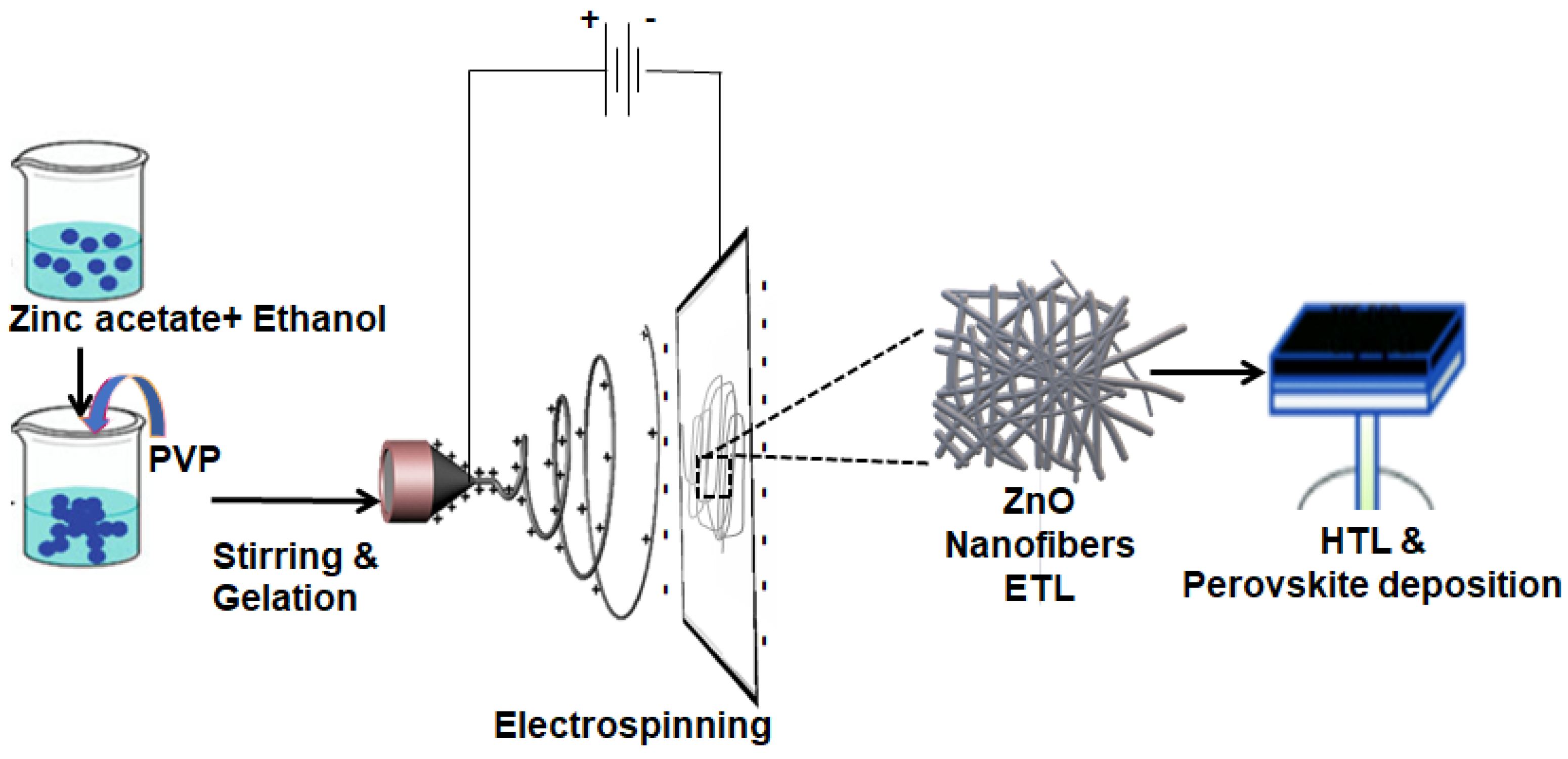
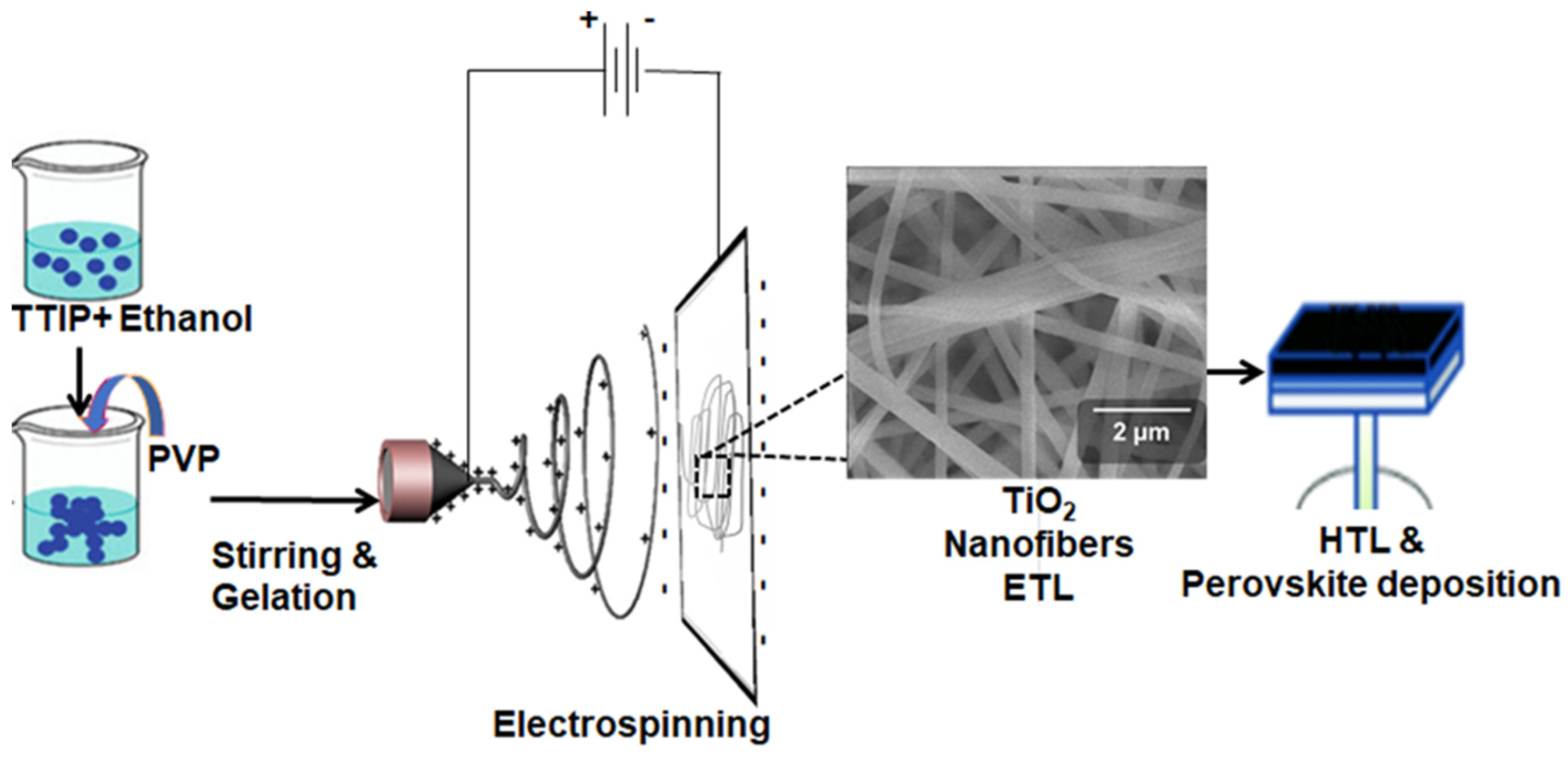
2.1. ZnO and TiO2 Nanofiber Synthesis
2.2. Solar Cell Fabrication
2.3. Characterizations
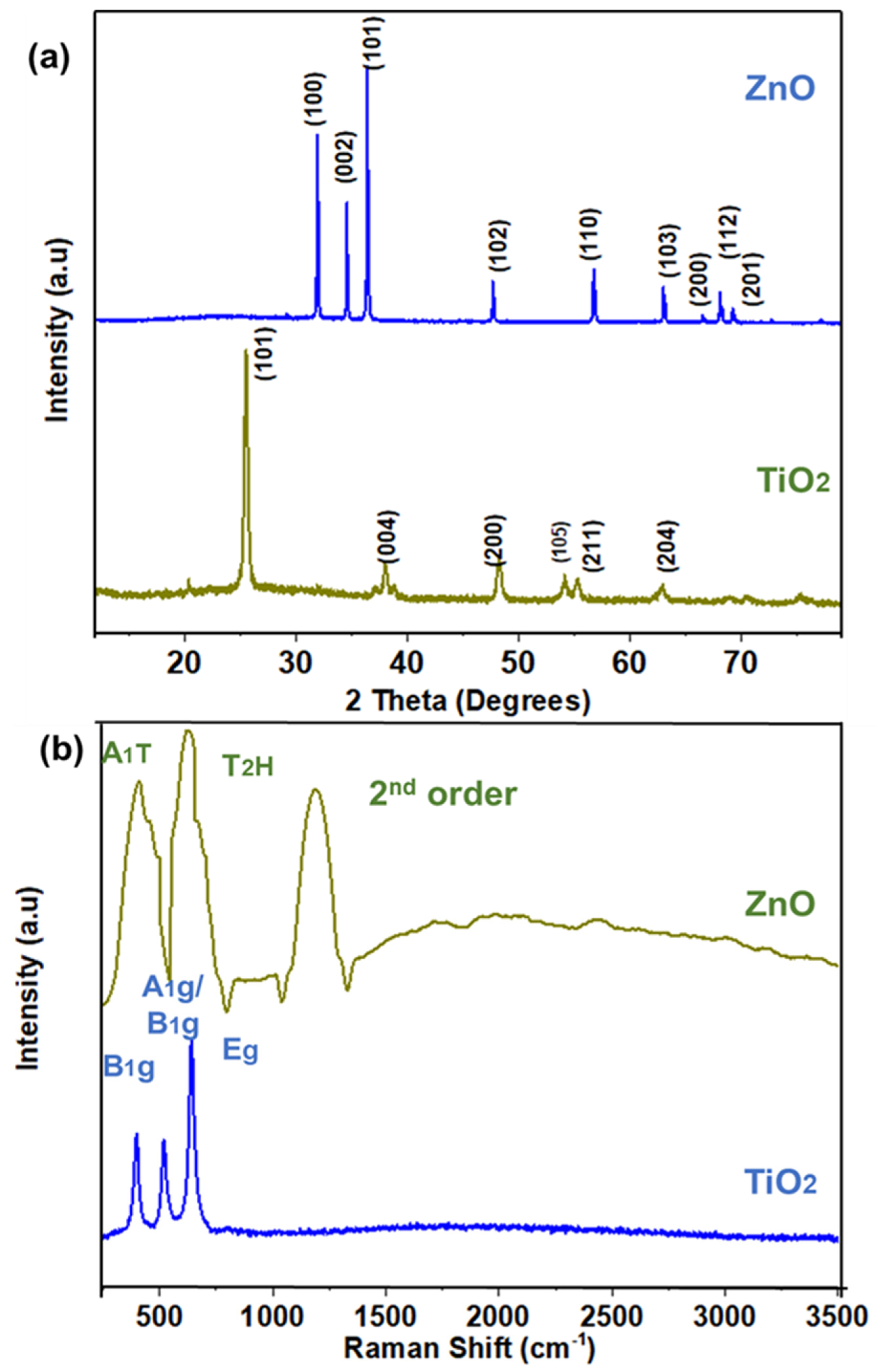
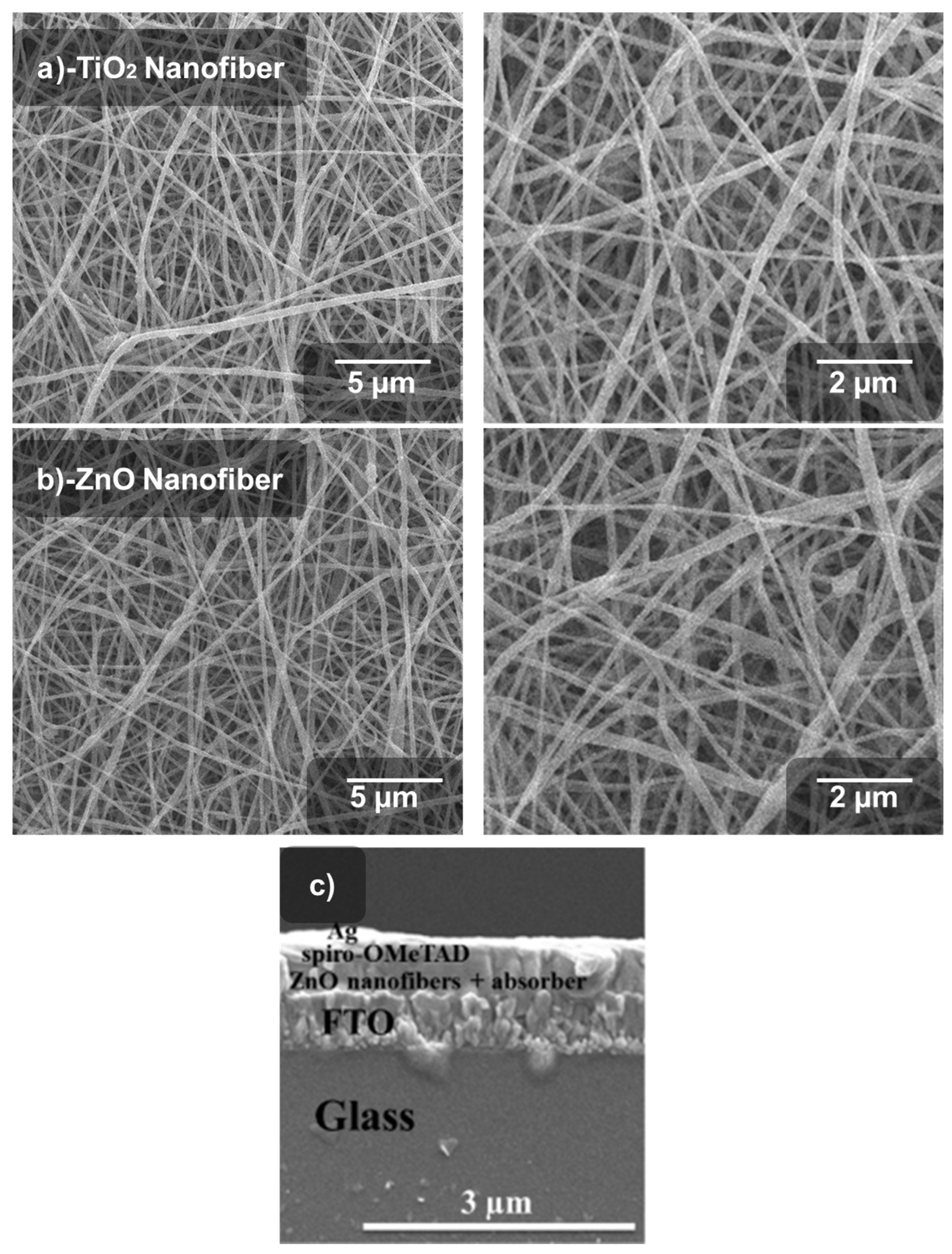
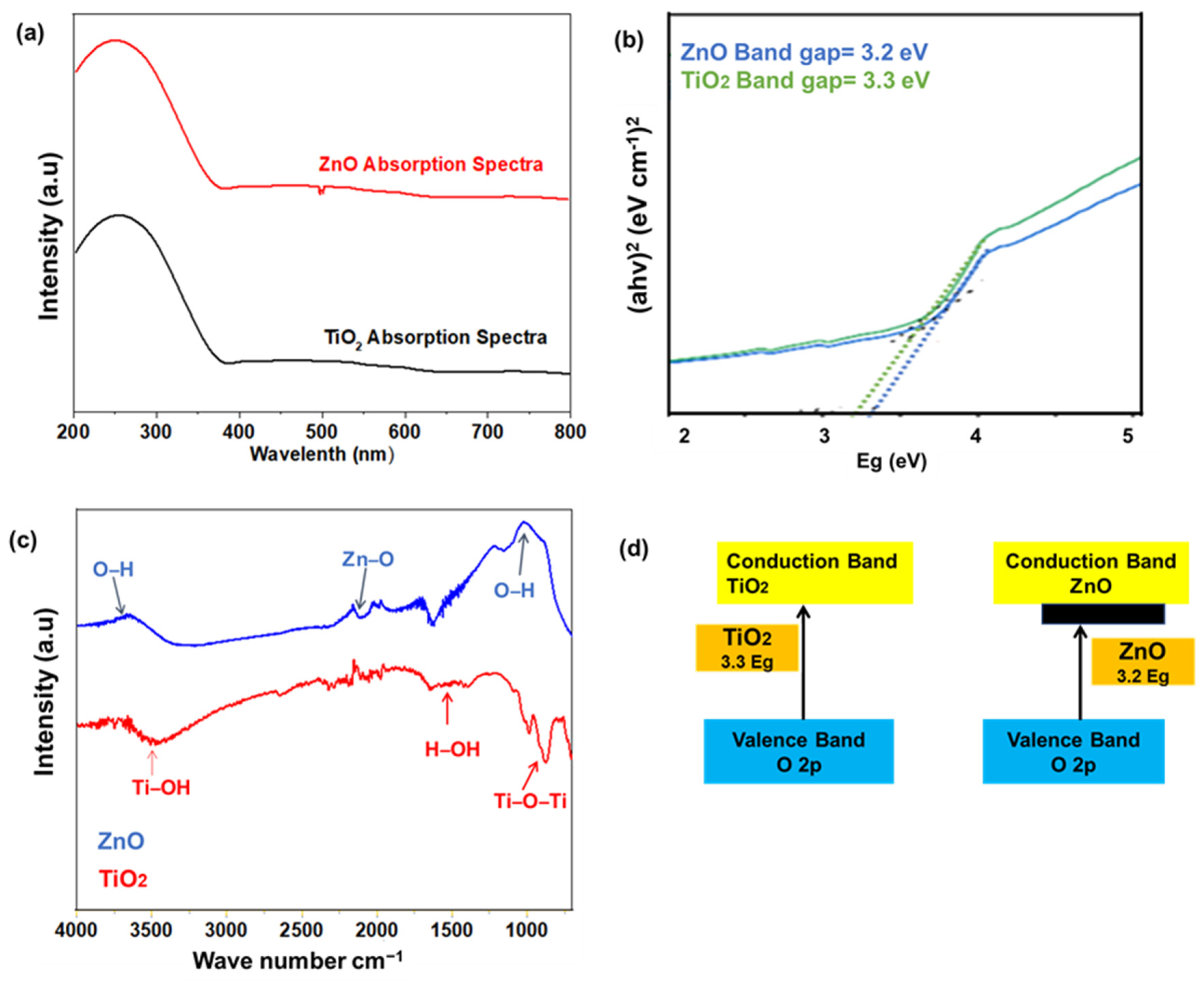
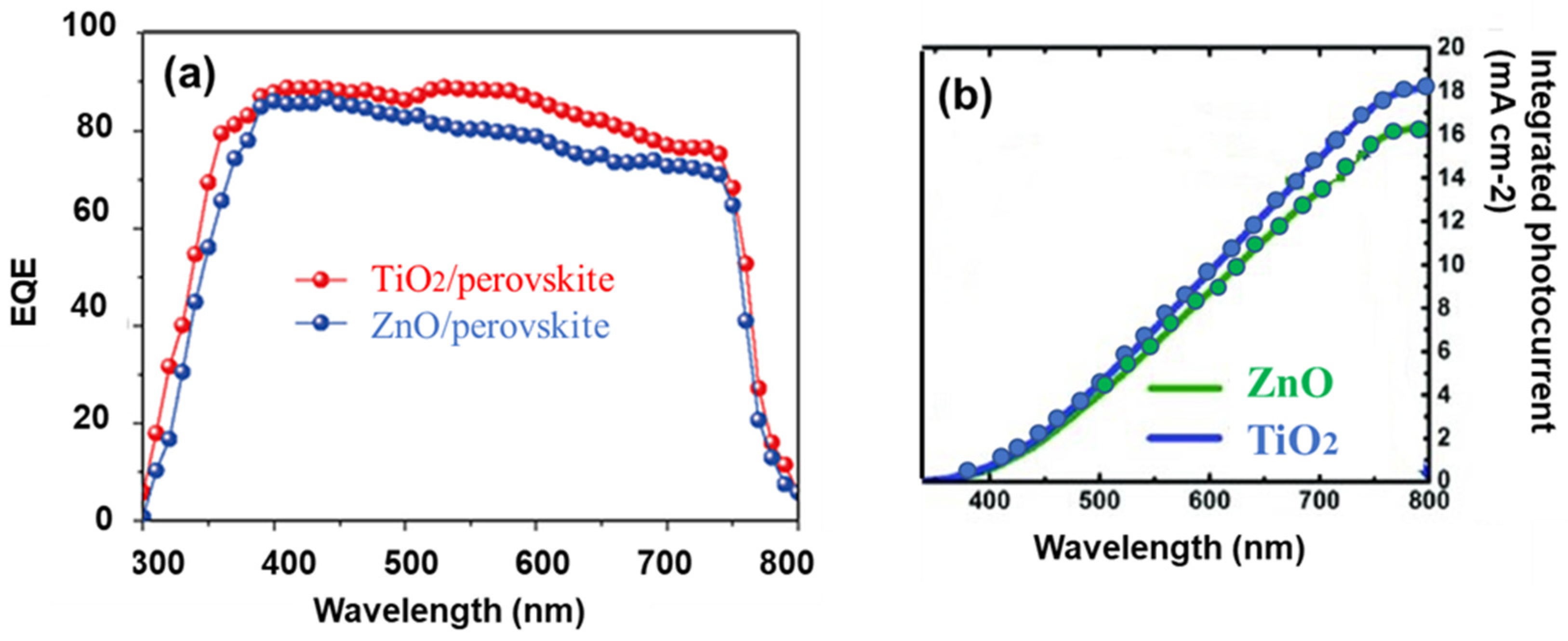
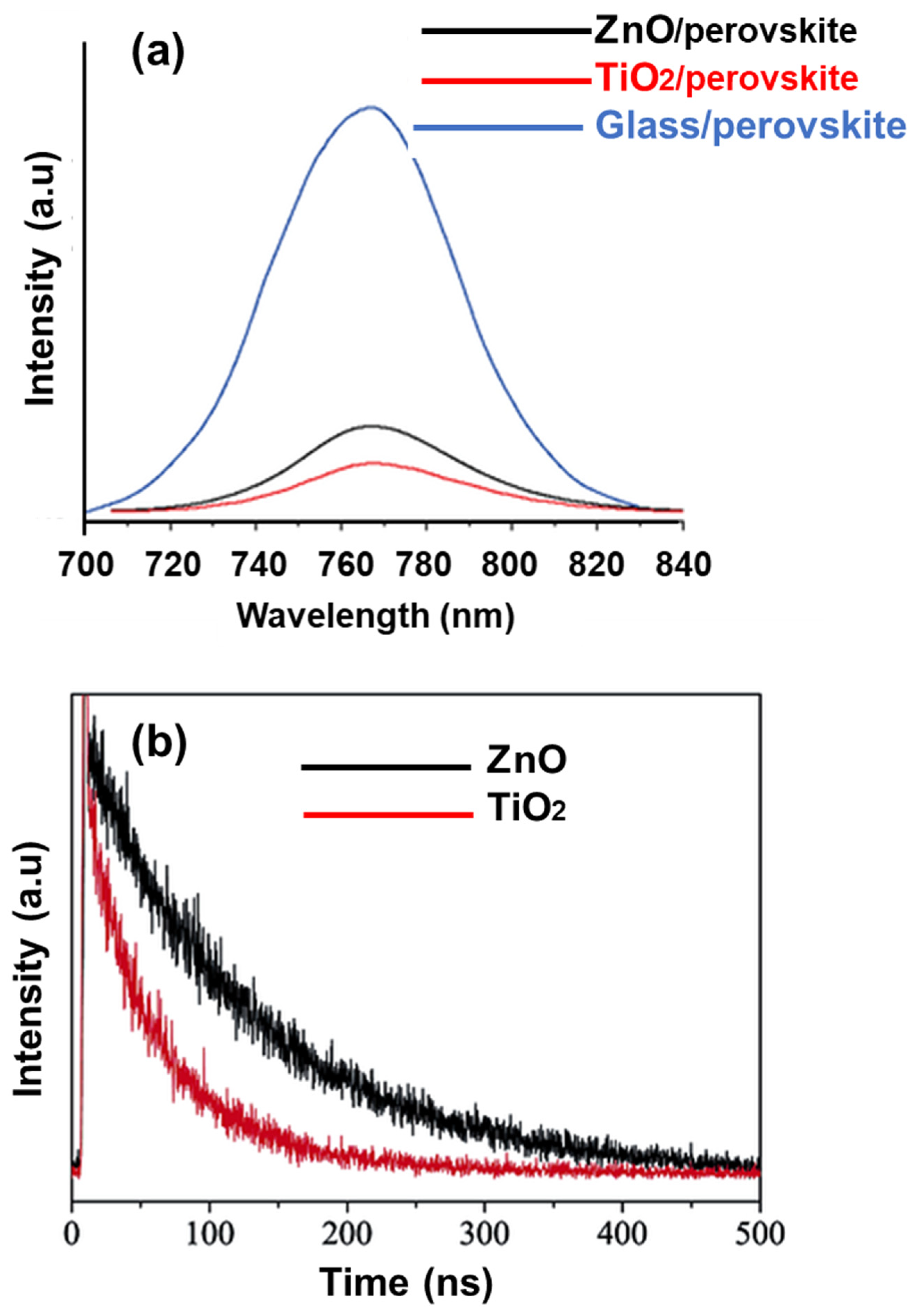
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lotus, A.; Kang, Y.; Ramsier, R.; Chase, G.G. Investigation of the physical and electronic properties of indium doped zinc oxide nanofibers synthesized by electrospinning. J. Vac. Sci. Technol. B 2009, 27, 2331–2336. [Google Scholar] [CrossRef]
- Wang, D.; Wright, M.; Elumalai, N.K.; Uddin, A. Stability of perovskite solar cells. Sol. Energy Mater. Sol. Cells 2016, 147, 255–275. [Google Scholar] [CrossRef]
- Patil, J.V.; Mali, S.S.; Patil, A.P.; Patil, P.S.; Hong, C.K. Highly efficient mixed-halide mixed-cation perovskite solar cells based on rGO-TiO2 composite nanofibers. Energy 2019, 189, 116396. [Google Scholar] [CrossRef]
- Park, N.-G. Perovskite solar cells: An emerging photovoltaic technology. Mater. Today 2015, 18, 65–72. [Google Scholar] [CrossRef]
- Mali, S.S.; Shim, C.S.; Kim, H.; Patil, P.S.; Hong, C.K. In situ processed gold nanoparticle-embedded TiO2 nanofibers enabling plasmonic perovskite solar cells to exceed 14% conversion efficiency. Nanoscale 2016, 8, 2664–2677. [Google Scholar] [CrossRef]
- Gonzalez-Pedro, V.; Juarez-Perez, E.J.; Arsyad, W.-S.; Barea, E.M.; Fabregat-Santiago, F.; Mora-Sero, I.; Bisquert, J. General working principles of CH3NH3PbX3 perovskite solar cells. Nano Lett. 2014, 14, 888–893. [Google Scholar] [CrossRef]
- Imran, M.; Haider, S.; Ahmad, K.; Mahmood, A.; Al-Masry, W.A. Fabrication and characterization of zinc oxide nanofibers for renewable energy applications. Arab. J. Chem. 2017, 10, S1067–S1072. [Google Scholar] [CrossRef]
- Valadi, K.; Gharibi, S.; Taheri-Ledari, R.; Akin, S.; Maleki, A.; Shalan, A.E. Metal oxide electron transport materials for perovskite solar cells: A review. Environ. Chem. Lett. 2021, 19, 2185–2207. [Google Scholar] [CrossRef]
- Son, D.-Y.; Im, J.-H.; Kim, H.-S.; Park, N.-G. 11% efficient perovskite solar cell based on ZnO nanorods: An effective charge collection system. J. Phys. Chem. C 2014, 118, 16567–16573. [Google Scholar] [CrossRef]
- Amna, T.; Hassan, M.S.; Barakat, N.A.; Pandeya, D.R.; Hong, S.T.; Khil, M.-S.; Kim, H.Y. Antibacterial activity and interaction mechanism of electrospun zinc-doped titania nanofibers. Appl. Microbiol. Biotechnol. 2012, 93, 743–751. [Google Scholar] [CrossRef]
- Zhen, C.; Wu, T.; Chen, R.; Wang, L.; Liu, G.; Cheng, H.-M. Strategies for modifying TiO2 based electron transport layers to boost perovskite solar cells. ACS Sustain. Chem. Eng. 2019, 7, 4586–4618. [Google Scholar] [CrossRef]
- Qiu, L.; Zhuang, Z.; Yang, S.; Chen, W.; Song, L.; Ding, M.; Xia, G.; Du, P.; Xiong, J. Fabrication of high efficiency perovskite solar cells based on mesoporous TiO2 nanofibrous film under high humidity conditions. Mater. Res. Bull. 2018, 106, 439–445. [Google Scholar] [CrossRef]
- Park, J.Y.; Kim, S.S. Growth of nanograins in electrospun ZnO nanofibers. J. Am. Ceram. Soc. 2009, 92, 1691–1694. [Google Scholar] [CrossRef]
- Mali, S.S.; Su Shim, C.; Kook Hong, C. Highly porous Zinc Stannate (Zn2SnO4) nanofibers scaffold photoelectrodes for efficient methyl ammonium halide perovskite solar cells. Sci. Rep. 2015, 5, 11424. [Google Scholar] [CrossRef]
- Erdogar, K.; Yucel, O.; Oruc, M.E. Investigation of Structural, Morphological, and Optical Properties of Novel Electrospun Mg-Doped TiO2 Nanofibers as an Electron Transport Material for Perovskite Solar Cells. Nanomaterials 2023, 13, 2255. [Google Scholar] [CrossRef]
- Aryal, S.; Kim, C.K.; Kim, K.-W.; Khil, M.S.; Kim, H.Y. Multi-walled carbon nanotubes/TiO2 composite nanofiber by electrospinning. Mater. Sci. Eng. C 2008, 28, 75–79. [Google Scholar] [CrossRef]
- Siddheswaran, R.; Sankar, R.; Ramesh Babu, M.; Rathnakumari, M.; Jayavel, R.; Murugakoothan, P.; Sureshkumar, P. Preparation and characterization of ZnO nanofibers by electrospinning. Cryst. Res. Technol. 2006, 41, 446–449. [Google Scholar] [CrossRef]
- Xu, A.-W.; Gao, Y.; Liu, H.-Q. The preparation, characterization, and their photocatalytic activities of rare-earth-doped TiO2 nanoparticles. J. Catal. 2002, 207, 151–157. [Google Scholar] [CrossRef]
- Liao, D.; Liao, B. Shape, size and photocatalytic activity control of TiO2 nanoparticles with surfactants. J. Photochem. Photobiol. A Chem. 2007, 187, 363–369. [Google Scholar] [CrossRef]
- Liu, L.; Li, S.; Zhuang, J.; Wang, L.; Zhang, J.; Li, H.; Liu, Z.; Han, Y.; Jiang, X.; Zhang, P. Improved selective acetone sensing properties of Co-doped ZnO nanofibers by electrospinning. Sens. Actuators B Chem. 2011, 155, 782–788. [Google Scholar] [CrossRef]
- Tai, M.; Zhao, X.; Shen, H.; Guo, Y.; Zhang, M.; Zhou, Y.; Li, X.; Yao, Z.; Yin, X.; Han, J. Ultrathin Zn2SnO4 (ZTO) passivated ZnO nanocone arrays for efficient and stable perovskite solar cells. Chem. Eng. J. 2019, 361, 60–66. [Google Scholar] [CrossRef]
- Choi, H.C.; Jung, Y.M.; Kim, S.B. Size effects in the Raman spectra of TiO2 nanoparticles. Vib. Spectrosc. 2005, 37, 33–38. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, H.; An, X.; Gao, C.; Zhang, Z.; Zhou, J.; Zhou, M.; Xie, E. Effect of Al doping on the visible photoluminescence of ZnO nanofibers. J. Alloys Compd. 2010, 506, 772–776. [Google Scholar] [CrossRef]
- Zhang, X.; Thavasi, V.; Mhaisalkar, S.; Ramakrishna, S. Novel hollow mesoporous 1D TiO2 nanofibers as photovoltaic and photocatalytic materials. Nanoscale 2012, 4, 1707–1716. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Zhang, Z.; Cai, Y.; Chen, J.; Wang, J.; Huang, R.; Lu, X.; Gao, X.; Shui, L.; Wu, S. Enhanced performance of CH3NH3PbI3 − x Cl x perovskite solar cells by CH3NH3I modification of TiO2-perovskite layer interface. Nanoscale Res. Lett. 2016, 11, 316. [Google Scholar] [CrossRef]
- Mahmood, K.; Khalid, A.; Mehran, M.T. Nanostructured ZnO electron transporting materials for hysteresis-free perovskite solar cells. Sol. Energy 2018, 173, 496–503. [Google Scholar] [CrossRef]
- Onozuka, K.; Ding, B.; Tsuge, Y.; Naka, T.; Yamazaki, M.; Sugi, S.; Ohno, S.; Yoshikawa, M.; Shiratori, S. Electrospinning processed nanofibrous TiO2 membranes for photovoltaic applications. Nanotechnology 2006, 17, 1026. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, X.; Li, P.; Duan, Y.; Hu, X.; Li, F.; Song, Y. Dopamine-crosslinked TiO2/perovskite layer for efficient and photostable perovskite solar cells under full spectral continuous illumination. Nano Energy 2019, 56, 733–740. [Google Scholar] [CrossRef]
- Duan, L.; Liu, S.; Wang, X.; Zhang, Z.; Luo, J. Interfacial Crosslinking for Efficient and Stable Planar TiO2 Perovskite Solar Cells. Adv. Sci. 2024, 11, 2402796. [Google Scholar] [CrossRef]
- Shakoor, A.; Nowsherwan, G.A.; Alam, W.; Bhatti, S.Y.; Bilal, A.; Nadeem, M.; Zaib, A.; Hussain, S.S. Fabrication and characterization of TiO2: ZnO thin films as electron transport material in perovskite solar cell (PSC). Phys. B Condens. Matter 2023, 654, 414690. [Google Scholar] [CrossRef]
- Hu, W.; Zhou, W.; Lei, X.; Zhou, P.; Zhang, M.; Chen, T.; Zeng, H.; Zhu, J.; Dai, S.; Yang, S. Low-temperature in situ amino functionalization of TiO2 nanoparticles sharpens electron management achieving over 21% efficient planar perovskite solar cells. Adv. Mater. 2019, 31, 1806095. [Google Scholar] [CrossRef] [PubMed]
- Yakuphanoglu, F. Electrical and photovoltaic properties of cobalt doped zinc oxide nanofiber/n-silicon diode. J. Alloys Compd. 2010, 494, 451–455. [Google Scholar] [CrossRef]
- Son, D.-Y.; Bae, K.-H.; Kim, H.-S.; Park, N.-G. Effects of seed layer on growth of ZnO nanorod and performance of perovskite solar cell. J. Phys. Chem. C 2015, 119, 10321–10328. [Google Scholar] [CrossRef]
- Laila, I.K.; Mufti, N.; Maryam, S.; Fuad, A.; Taufiq, A. Synthesis and characterization of ZnO nanorods by hydrothermal methods and its application on perovskite solar cells. J. Phys. Conf. Ser. 2018, 1093, 012012. [Google Scholar] [CrossRef]
- Dharani, S.; Mulmudi, H.K.; Yantara, N.; Trang, P.T.T.; Park, N.G.; Graetzel, M.; Mhaisalkar, S.; Mathews, N.; Boix, P.P. High efficiency electrospun TiO2 nanofiber based hybrid organic–inorganic perovskite solar cell. Nanoscale 2014, 6, 1675–1679. [Google Scholar] [CrossRef]
- Cao, F.; Tian, W.; Gu, B.; Ma, Y.; Lu, H.; Li, L. High-performance UV–vis photodetectors based on electrospun ZnO nanofiber-solution processed perovskite hybrid structures. Nano Res. 2017, 10, 2244–2256. [Google Scholar] [CrossRef]
- Chen, D.; Zhu, Y. Electrospun perovskite nanofibers. Nanoscale Res. Lett. 2017, 12, 114. [Google Scholar] [CrossRef]
- Yang, H.; Kwon, H.-C.; Ma, S.; Kim, K.; Yun, S.-C.; Jang, G.; Park, J.; Lee, H.; Goh, S.; Moon, J. Energy level-graded Al-doped ZnO protection layers for copper nanowire-based window electrodes for efficient flexible perovskite solar cells. ACS Appl. Mater. Interfaces 2020, 12, 13824–13835. [Google Scholar] [CrossRef]
- Mahmood, K.; Khalid, A.; Ahmad, S.W.; Mehran, M.T. Indium-doped ZnO mesoporous nanofibers as efficient electron transporting materials for perovskite solar cells. Surf. Coat. Technol. 2018, 352, 231–237. [Google Scholar] [CrossRef]
- Zhuang, Z.; Qiu, L.; Dong, L.; Chen, Y.; Chu, Z.; Ma, X.; Du, P.; Xiong, J. Preparation of high-efficiency perovskite solar cells via doping Ag into CuO nanofibers as hole buffer layer. Polym. Compos. 2020, 41, 2145–2153. [Google Scholar] [CrossRef]
- Mohtaram, F.; Borhani, S.; Ahmadpour, M.; Fojan, P.; Behjat, A.; Rubahn, H.-G.; Madsen, M. Electrospun ZnO nanofiber interlayers for enhanced performance of organic photovoltaic devices. Sol. Energy 2020, 197, 311–316. [Google Scholar] [CrossRef]
- Abbas, A.; Shar, A.H.; Junejo, M.A. Why Brands Fail? Antecedents and Consequences of Brand Hate. A Study of Fashion Industry in Pakistan. J. Manag. Sci. 2023, 17, 1–26. [Google Scholar]


| Sr | Conductivity 1/Ohm-cm | Resistivity Ohm-cm | Sheet Resistance Ohm-sq | Sheet Carrier Mobility |
|---|---|---|---|---|
| TiO2 | 1.28 × 10−4 | 7.6 × 103 | 4.3 × 108 | 1.52 × 10−2 |
| ZnO | 1.12 × 10−4 | 9.8 × 103 | 5.3 × 108 | 1.4 × 10−2 |
| Sr | Rrec/Ohm | Rs/Ohm | Rco/Ohm | CPE-T/F |
|---|---|---|---|---|
| TiO2 | 12.8 | 27.1 | 25.9 | 5.7 × 10−6 |
| ZnO | 68.8 | 33.9 | 57.9 | 6.5 × 10−6 |
| PSC | Voc (V) | Jsc (mA/cm2) | Shunt Resistance (Ohm) | Series Resistance (Ohm) | FF | η (%) |
|---|---|---|---|---|---|---|
| ZnO | 0.82 | 17.77 | 52,278.68 | 205.423 | 0.59 | 8.5 |
| TiO2 | 0.83 | 20.68 | 53,469.8 | 197.553 | 0.61 | 10.4 |
| PSC | Jsc (mA cm−2) | Voc (mV) | FF | η (%) | Ref |
|---|---|---|---|---|---|
| In-doped ZnO nanofibers | 23.0 | 1000 | 70 | 16.10 | [39] |
| Al doped Cu-ZnO | 18.6 | 1080 | 70.77 | 14.18 | [38] |
| TiO2 fiber | 4.02 | 1060 | 73.0 | 3.11 | [35] |
| Pristine-TiO2 NFs | 23.32 | 1013 | 67.0 | 15.82 | [3] |
| Ag doped CuO NFs | 17.8 | 890 | 53.8 | 8.7 | [40] |
| ZnO NFs | 18.1 | 670 | 58.1 | 7.05 | [41,42] |
| TiO2 NFs | 20.68 | 830 | 0.61 | 10.4 | Our work |
| ZnO NFs | 17.77 | 820 | 0.59 | 8.5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zafar, A.; Iqbal, W.; Khan, S.; Alhodaib, A.; Fatima, M. Comparing the Efficacies of Electrospun ZnO and TiO2 Nanofibrous Interlayers for Electron Transport in Perovskite Solar Cells. Ceramics 2024, 7, 1742-1757. https://doi.org/10.3390/ceramics7040111
Zafar A, Iqbal W, Khan S, Alhodaib A, Fatima M. Comparing the Efficacies of Electrospun ZnO and TiO2 Nanofibrous Interlayers for Electron Transport in Perovskite Solar Cells. Ceramics. 2024; 7(4):1742-1757. https://doi.org/10.3390/ceramics7040111
Chicago/Turabian StyleZafar, Abdullah, Waqar Iqbal, Shahzaib Khan, Aiyeshah Alhodaib, and Mahvish Fatima. 2024. "Comparing the Efficacies of Electrospun ZnO and TiO2 Nanofibrous Interlayers for Electron Transport in Perovskite Solar Cells" Ceramics 7, no. 4: 1742-1757. https://doi.org/10.3390/ceramics7040111
APA StyleZafar, A., Iqbal, W., Khan, S., Alhodaib, A., & Fatima, M. (2024). Comparing the Efficacies of Electrospun ZnO and TiO2 Nanofibrous Interlayers for Electron Transport in Perovskite Solar Cells. Ceramics, 7(4), 1742-1757. https://doi.org/10.3390/ceramics7040111









