Hydrogen Permeation Properties of Ternary Ni–BaCe0.9Y0.1O3–Ce0.9Gd0.1O2 Cermet Membranes
Abstract
1. Introduction
2. Experimental Procedure
2.1. Sample Powder Synthesis
2.2. Preparation of Ni–BCY–GDC Cermets
2.3. Conductivity Measurements
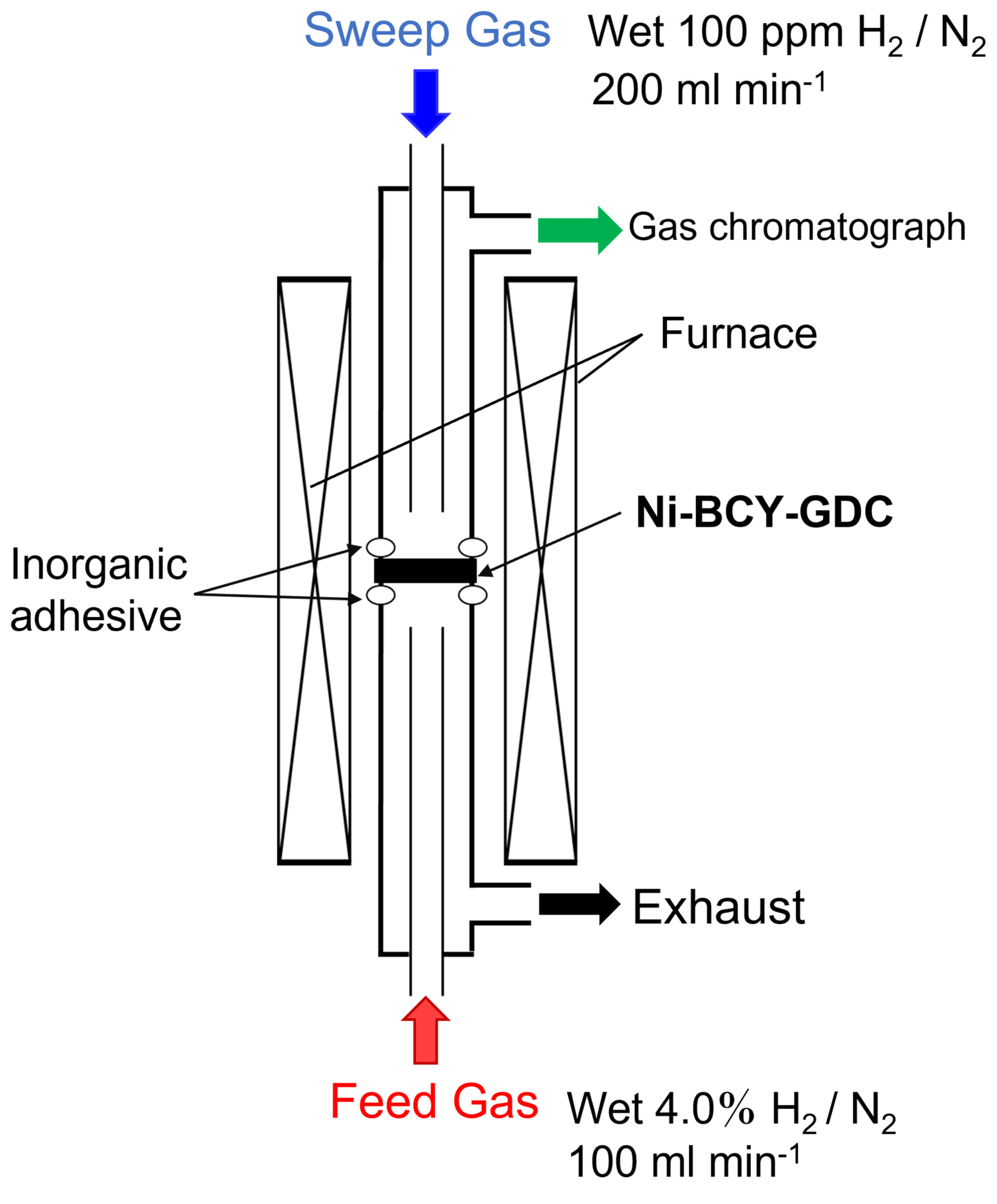
2.4. Fabrication and Evaluation of Hydrogen Permeation Cells
3. Results and Discussion
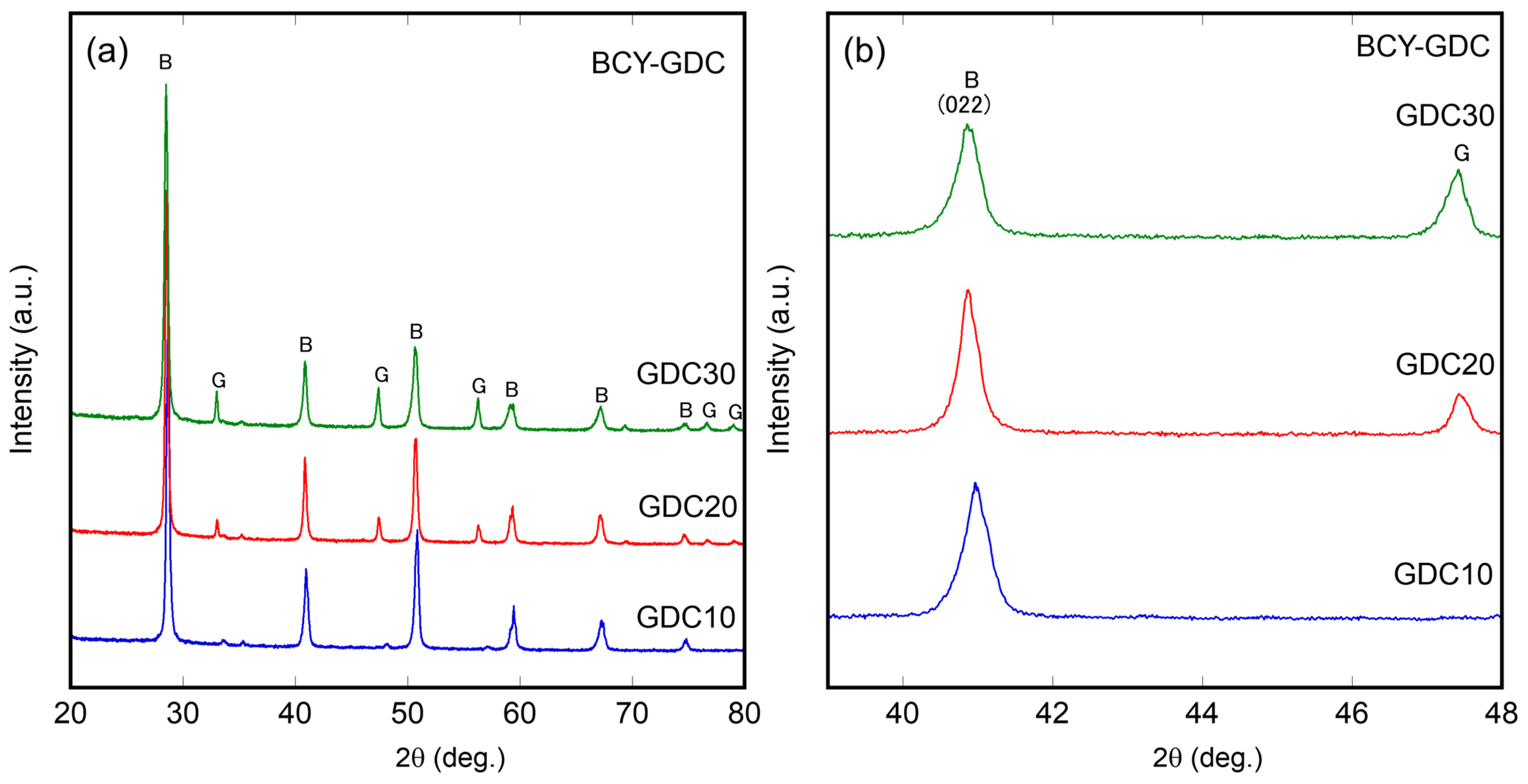
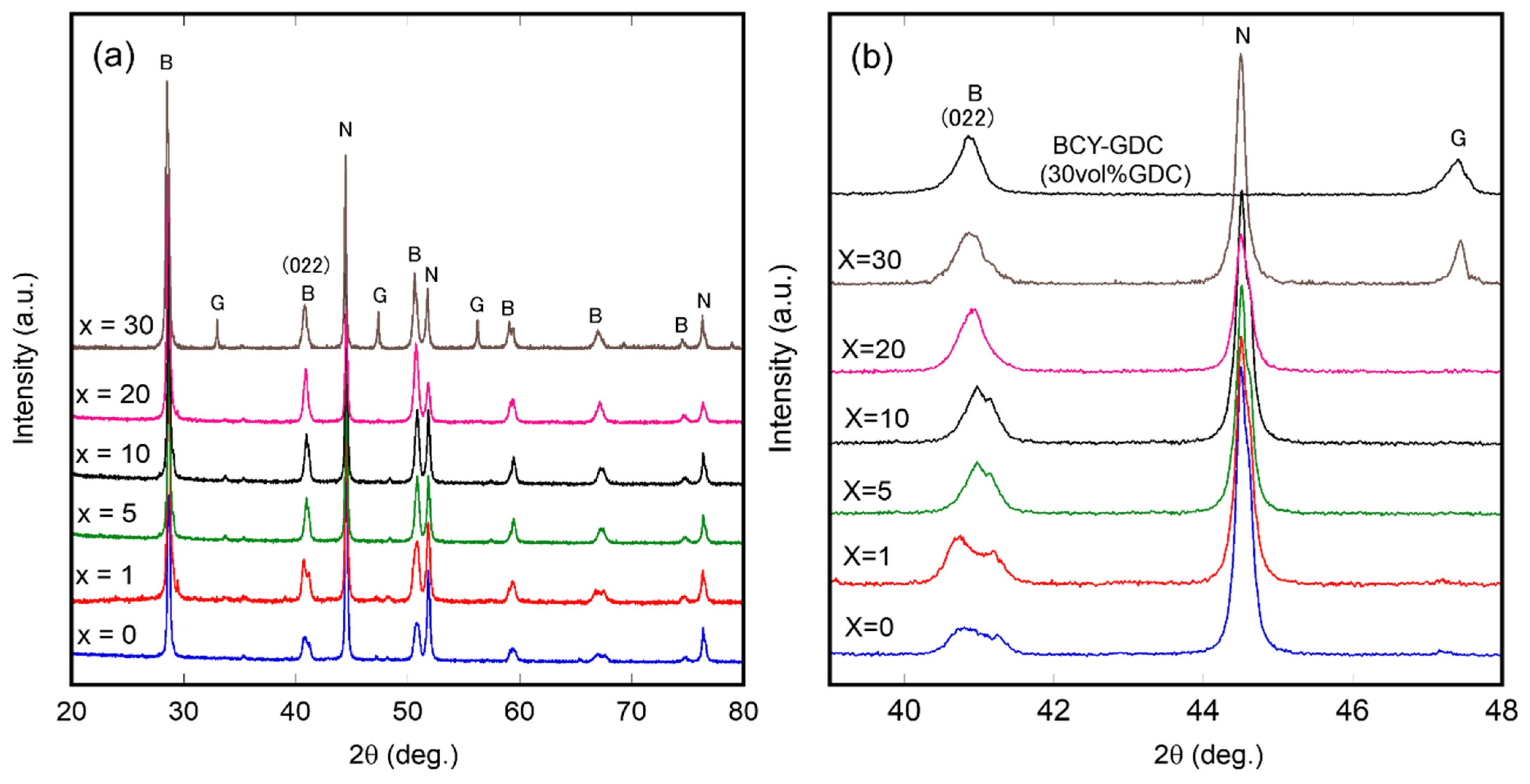
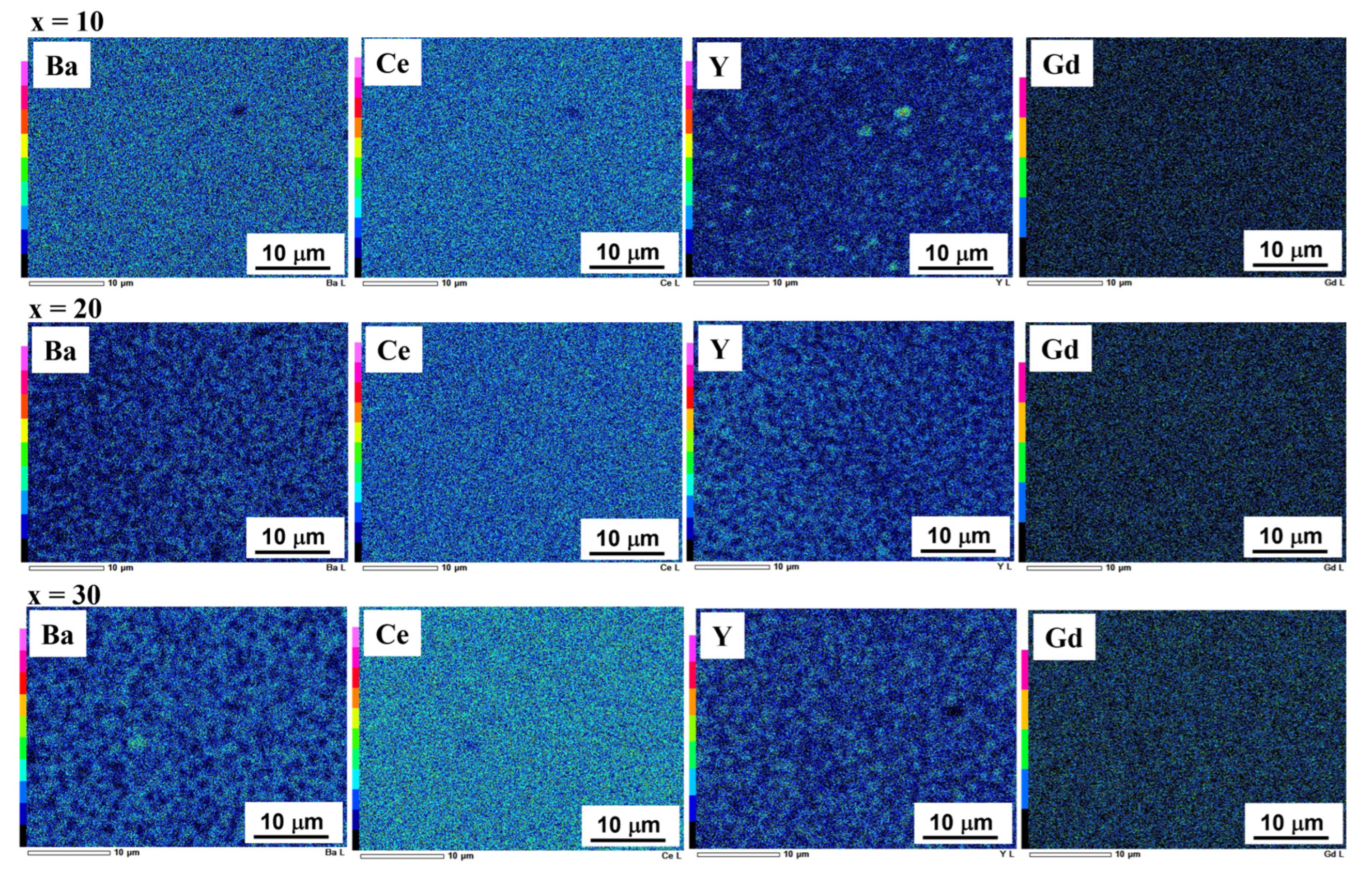
3.1. Characterization of Ni–BCY–GDC Composites
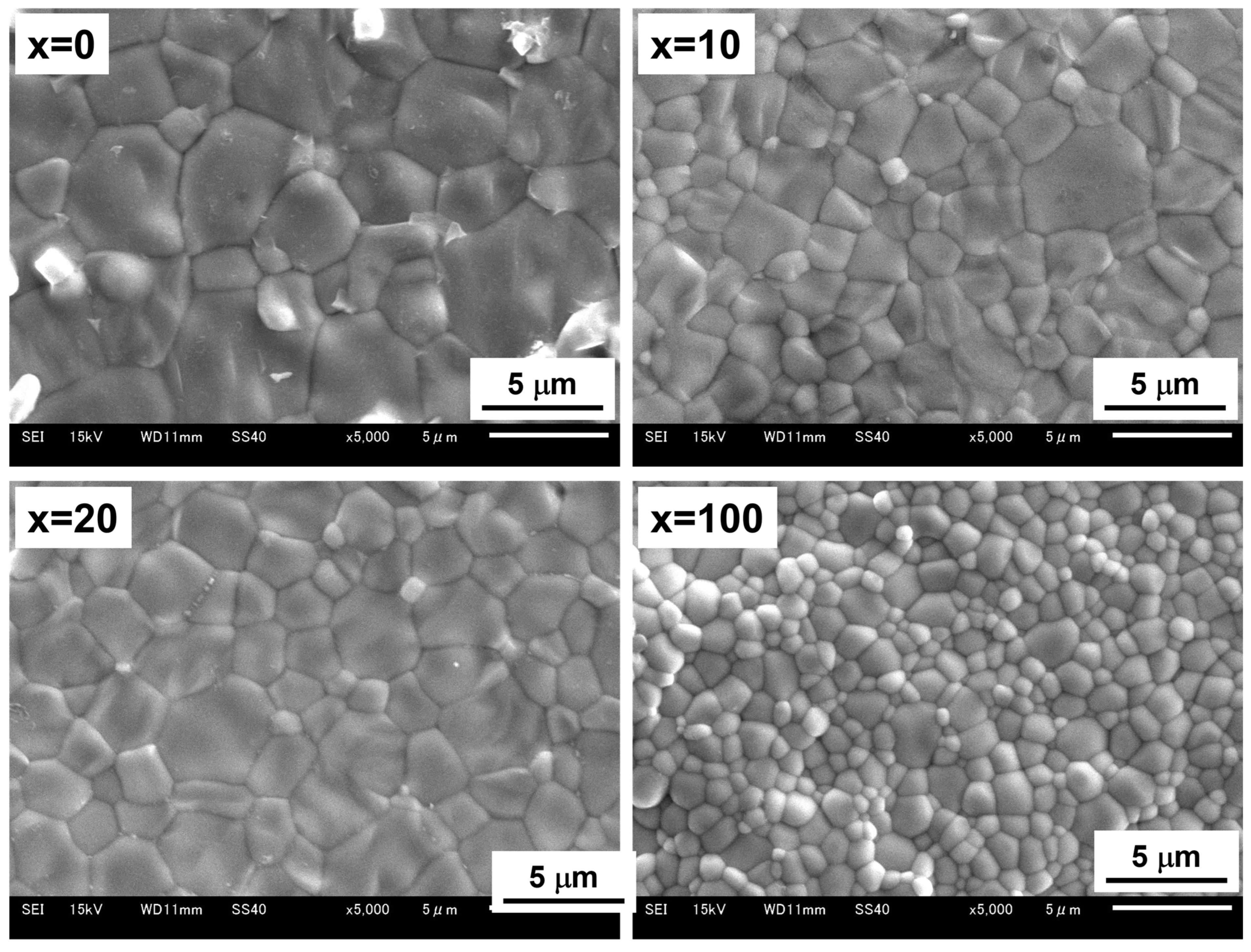
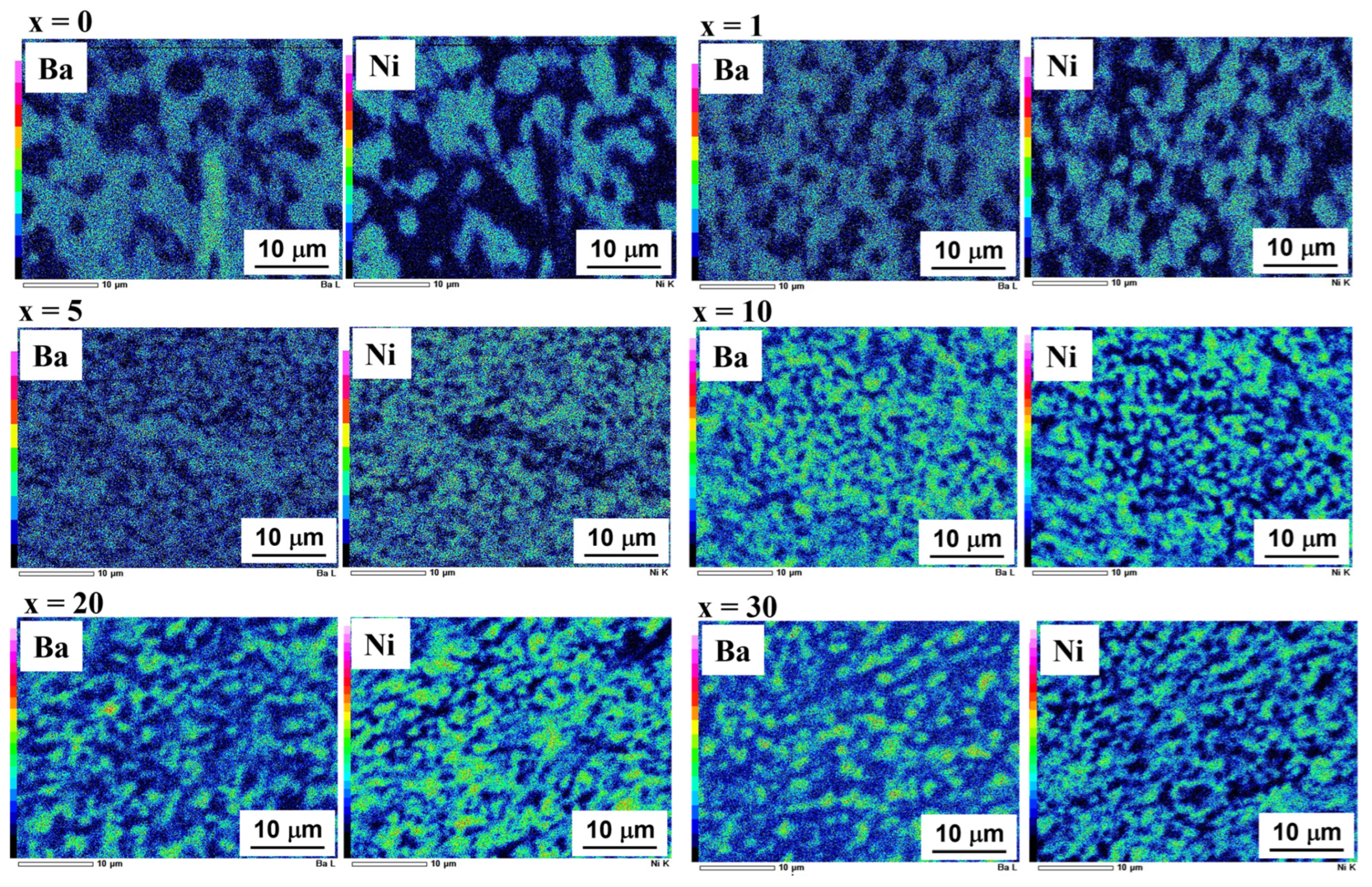
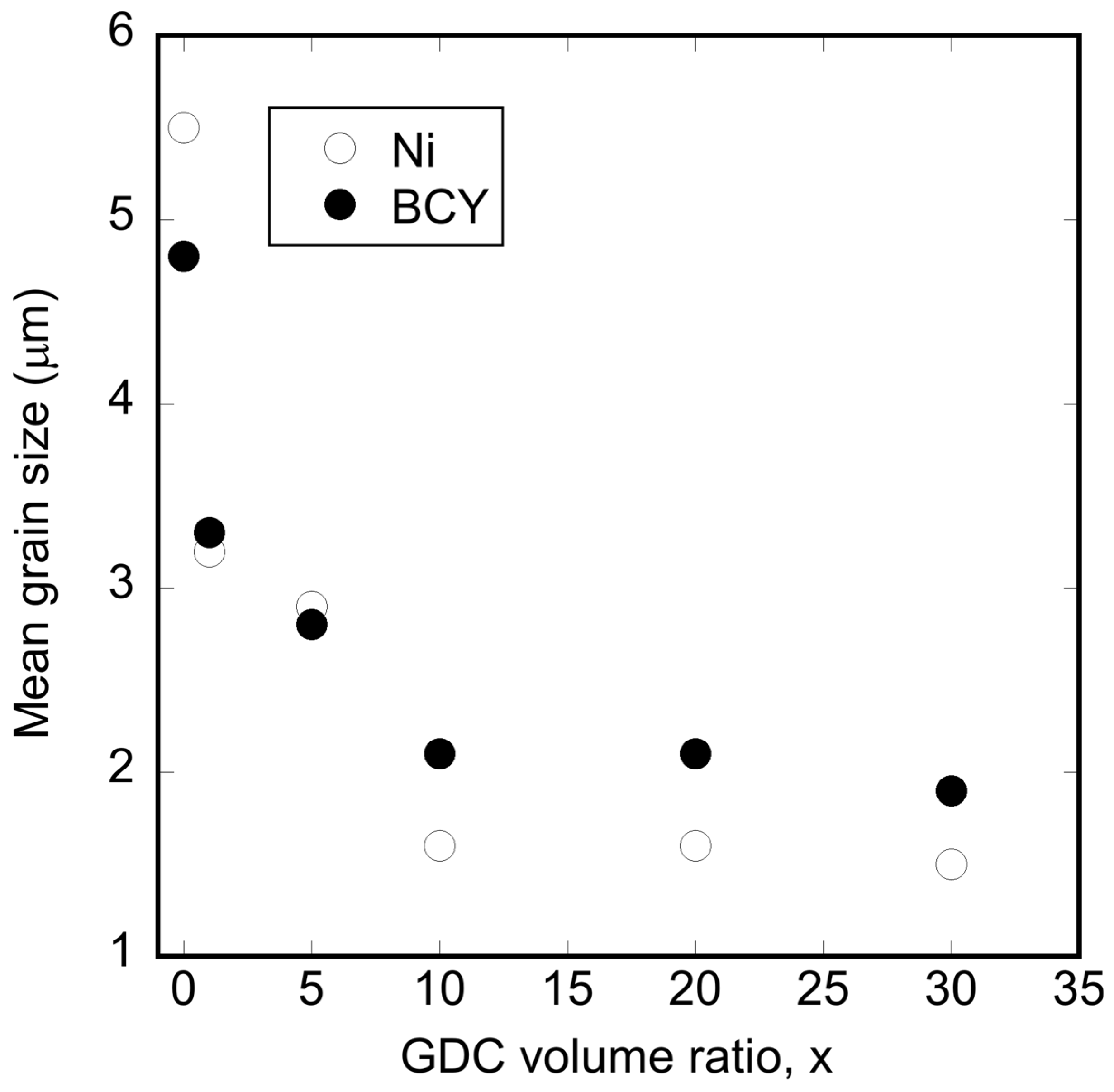
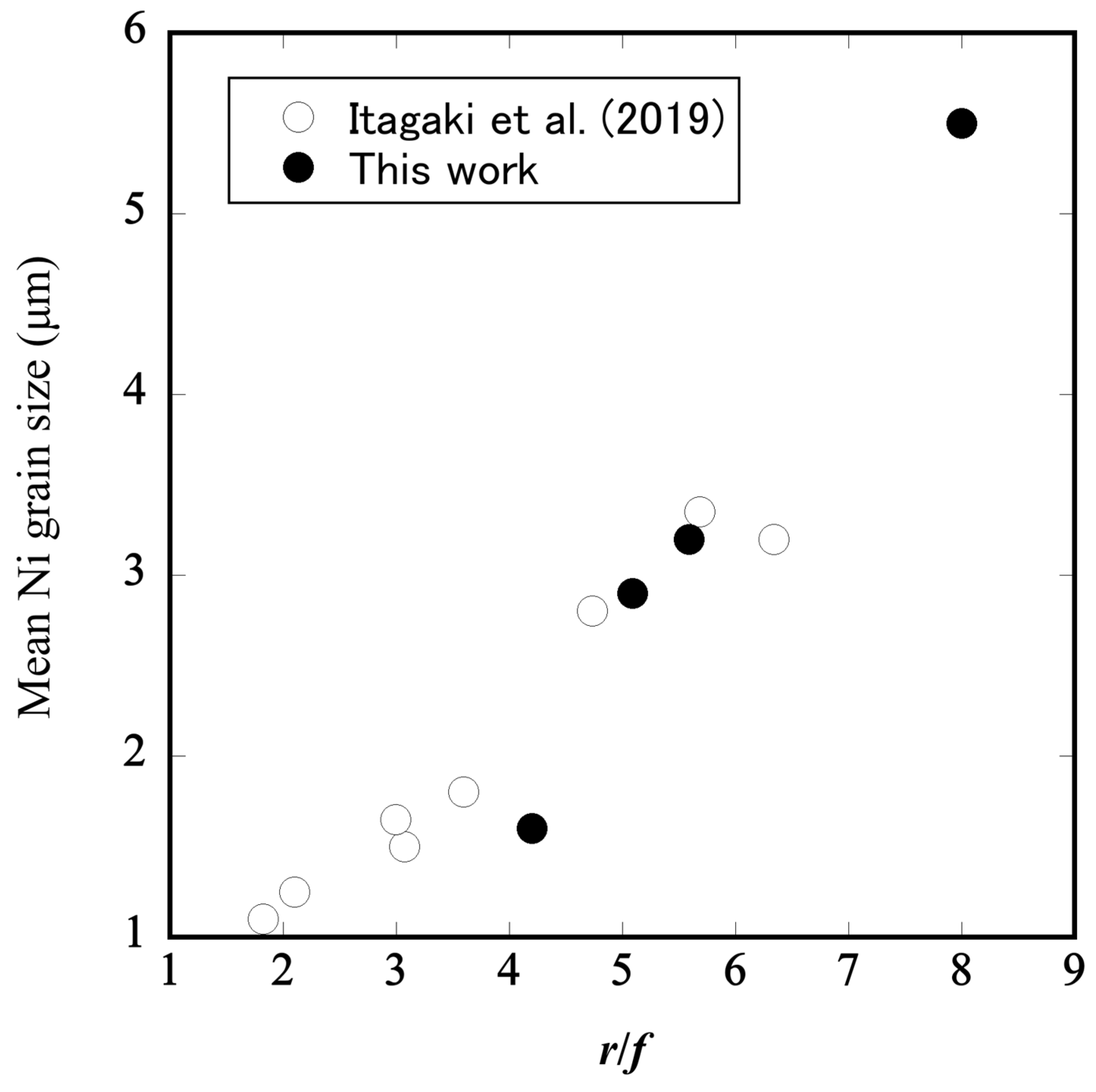
3.2. Microstructural Changes in Ni–BCY–GDC Cermets
3.3. Conductivity Measurements
3.4. Hydrogen Permeation Tests
3.5. Comparison with Other Ceramics Material for H2 Permeation
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Holladay, J.D.; Hu, J.; King, D.L.; Wang, Y. An overview of hydrogen production technologies. Catal. Today 2009, 139, 244–260. [Google Scholar] [CrossRef]
- Shu, J.; Grandjean, B.P.A.; Van Neste, A.; Kaliaguine, S. Catalytic palladium-based membrane reactors: A review. Can. J. Chem. Eng. 1991, 69, 1036–1060. [Google Scholar] [CrossRef]
- Cheng, X.; Shi, Z.; Glass, N.; Zhang, L.; Zhang, J.; Song, D.; Liu, Z.-S.; Wang, H.; Shen, J. A review of PEM hydrogen fuel cell contamination: Impacts, mechanisms, and mitigation. J. Power Sources 2007, 165, 739–756. [Google Scholar] [CrossRef]
- Hatlevik, Ø.; Gade, S.K.; Keeling, M.K.; Thoen, P.M.; Davidson, A.P.; Way, J.D. Palladium and palladium alloy membranes for hydrogen separation and production: History, fabrication strategies, and current performance. Sep. Purif. Technol. 2010, 73, 59–64. [Google Scholar] [CrossRef]
- Rahimpour, M.R.; Samimi, F.; Babapoor, A.; Tohidian, T.; Mohebi, S. Palladium membranes applications in reaction systems for hydrogen separation and purification: A review. Chem. Eng. Process 2017, 121, 24–49. [Google Scholar] [CrossRef]
- Howard, B.H.; Killmeyer, R.P.; Rothenberger, K.S.; Cugini, A.V.; Morreale, A.V.; Enick, R.M.; Bustamante, F. Hydrogen permeance of palladium–copper alloy membranes over a wide range of temperatures and pressures. J. Membr. Sci. 2004, 241, 207–218. [Google Scholar] [CrossRef]
- Kulprathipanja, A.; Alptekin, G.O.; Falconer, J.L.; Way, J.D. Pd and Pd–Cu membranes: Inhibition of H2 permeation by H2S. J. Membr. Sci. 2005, 254, 49–62. [Google Scholar] [CrossRef]
- Roa, F.; Block, M.J.; Way, J.D. The influence of alloy composition on the H2 flux of composite Pd-Cu membranes. Desalination 2002, 147, 411–416. [Google Scholar] [CrossRef]
- Li, Z.Y.; Maeda, H.; Kusakabe, K.; Morooka, S.; Anzai, H.; Akiyama, S. Preparation of palladium-silver alloy membranes for hydrogen separation by the spray pyrolysis method. J. Membr. Sci. 1993, 78, 247–254. [Google Scholar] [CrossRef]
- Cheng, Y.S.; Yeung, K.L. Palladium–silver composite membranes by electroless plating technique. J Membr. Sci. 1999, 158, 127–141. [Google Scholar] [CrossRef]
- Coulter, K. Cost-effective method for producing self-supporting Pd alloy membrane for use in the efficient production of coal-derived hydrogen. In FY 2008 Annual Progress Report; Hydrogen Program, US Department of Energy (DOE): Washington, DC, USA, 2008; pp. 212–214. [Google Scholar]
- Iwahara, H.; Esaka, T.; Uchida, H.; Maeda, N. Proton conduction in sintered oxides and its application to steam electrolysis for hydrogen production. Solid State Ionics 1981, 3–4, 359–363. [Google Scholar] [CrossRef]
- Iwahara, H. Proton conducting ceramics and their applications. Solid State Ionics 1996, 86–88, 9–15. [Google Scholar] [CrossRef]
- Iwahara, H. Technological challenges in the application of proton conducting ceramics. Solid State Ionics 1995, 77, 289–298. [Google Scholar] [CrossRef]
- Duan, C.; Kee, R.; Zhu, H.; Sullivan, N.; Zhu, L.; Bian, L.; Jennings, D.; O’Hayre, R. Highly efficient reversible protonic ceramic electrochemical cells for power generation and fuel production. Nat. Energy 2019, 4, 230–240. [Google Scholar] [CrossRef]
- Bi, L.; Boulfrad, S.; Traversa, E. Steam electrolysis by solid oxide electrolysis cells (SOECs) with proton-conducting oxides. Chem. Soc. Rev. 2014, 43, 8255–8270. [Google Scholar] [CrossRef]
- Duan, C.; Tong, J.; Shang, M.; Nikodemski, S.; Sanders, M.; Ricote, S.; Almansoori, A.; O’Hayre, R. Readily processed protonic ceramic fuel cells with high performance at low temperatures. Science 2015, 349, 1321–1326. [Google Scholar] [CrossRef]
- Fabbri, E.; D’Epifanio, A.; Di Bartolomeo, E.; Licoccia, S.; Traversa, E. Tailoring the chemical stability of Ba(Ce0.8−xZrx)Y0.2O3−δ protonic conductors for intermediate temperature solid oxide fuel cells (IT-SOFCs). Solid State Ionics 2008, 179, 558–564. [Google Scholar] [CrossRef]
- Kreuer, K.D. Proton-conducting oxides. Annu. Rev. Mater. Res. 2003, 33, 333–359. [Google Scholar] [CrossRef]
- Norby, T. Solid-state protonic conductors: Principles, properties, progress and prospects. Solid State Ionics 1999, 125, 1–11. [Google Scholar] [CrossRef]
- Taillades, G.; Jacquin, M.; Khani, Z.; Jones, D.; Marrony, M.; Roziere, J. Development of proton conducting thin films from nanoparticulate precursors. ECS Trans. 2007, 7, 2291–2298. [Google Scholar] [CrossRef]
- Kim, H.; Kim, B.; Lee, J.; Ahn, K.; Kim, H.-R.; Yoon, K.J.; Kim, B.-K.; Cho, Y.W.; Lee, H.-W.; Lee, J.-H. Microstructural adjustment of Ni–BaCe0.9Y0.1O3−δ cermet membrane for improved hydrogen permeation. Ceram. Int. 2014, 40, 4117–4126. [Google Scholar] [CrossRef]
- Song, S.-J.; Moon, J.-H.; Lee, T.H.; Dorris, S.E.; Balachandran, U. Thickness dependence of hydrogen permeability for Ni–BaCe0.8Y0.2O3−δ. Solid State Ionics 2008, 179, 1854–1857. [Google Scholar] [CrossRef]
- Zhang, G.; Dorris, S.E.; Balachandran, U.; Liu, M. Interfacial resistances of Ni–BCY mixed-conducting membranes for hydrogen separation. Solid State Ionics 2003, 159, 121–134. [Google Scholar] [CrossRef]
- Scholten, M.J.; Schoonman, J.; van Miltenburg, J.C.; Oonk, H.A.J. Synthesis of strontium and barium cerate and their reaction with carbon dioxide. Solid State Ionics 1993, 61, 83–91. [Google Scholar] [CrossRef]
- Guo, Y.; Lin, Y.; Ran, R.; Shao, Z. Zirconium doping effect on the performance of proton-conducting BaZryCe0.8−yY0.2O3−δ (0.0 ≤ y ≤ 0.8) for fuel cell applications. J. Power Sources 2009, 193, 400–407. [Google Scholar] [CrossRef]
- Zuo, C.; Lee, T.H.; Dorris, S.E.; Balachandran, U.; Liu, M. Composite Ni–Ba(Zr0.1Ce0.7Y0.2)O3 membrane for hydrogen separation. J. Power Sources 2006, 159, 1291–1295. [Google Scholar] [CrossRef]
- Itagaki, Y.; Hiraoka, A.; Aono, H.; Yahiro, H. Hydrogen permeation of BaCe0.80Y0.20O3−δ–Gd0.1Ce0.9Ox dual-phase membranes. J. Ceram. Soc. Jpn. 2017, 125, 338–342. [Google Scholar] [CrossRef]
- Itagaki, Y.; Cui, J.; Tani, Y.; Aono, H.; Yahiro, H. Inhibition of Ni grain growth in Ni-BCY anode substrate for solid oxide fuel cell. ECS Trans. 2019, 91, 1693–1971. [Google Scholar] [CrossRef]
- Zhu, B.; Albinsson, I.; Mellander, B.-E. Electrical properties and proton conduction of gadolinium doped ceria. Ionics 1998, 4, 261–266. [Google Scholar] [CrossRef]
- Lin, D.; Wang, Q.; Peng, K.; Shaw, L.L. Phase formation and properties of composite electrolyte BaCe0.8Y0.2O3−δ–Ce0.8Gd0.2O1.9 for intermediate temperature solid oxide fuel cells. J Power Sources 2012, 205, 100–107. [Google Scholar] [CrossRef]
- Dubal, S.U.; Bhosale, C.H.; Jadhav, L.D. Performance of spray deposited Gd-doped barium cerate thin films for proton conducting SOFC. Ceram. Int. 2015, 41, 5607–5613. [Google Scholar] [CrossRef]
- Shannon, R.D. Revised effective ionic radii and systematic studies of interatomic distances in halides and chalcogenides. Acta Cryst. A 1976, 32, 751–767. [Google Scholar] [CrossRef]
- Caldes, M.T.; Kravchyk, K.V.; Benamira, M.; Besnard, N.; Gunes, V.; Bohnke, O.; Joubert, O. Metallic Nanoparticles and proton conductivity: Improving proton conductivity of BaCe0.9Y0.1O3−δ using a catalytic approach. Chem. Mater. 2012, 24, 4641–4646. [Google Scholar] [CrossRef]
- Wang, B.; Bi, L.; Zhao, X.S. Exploring the role of NiO as a sintering aid in BaZr0.1Ce0.7Y0.2O3−δ electrolyte for proton-conducting solid oxide fuel cells. J. Power Sources 2018, 399, 207–214. [Google Scholar] [CrossRef]
- Lee, K.-R.; Tseng, C.-J.; Jang, S.-C.; Lin, J.-C.; Wang, K.-W.; Chang, J.-K.; Chen, T.-C.; Lee, S.-W. Fabrication of anode-supported thin BCZY electrolyte protonic fuel cells using NiO sintering aid. Int. J. Hydrogen Energy 2019, 44, 23784–23792. [Google Scholar] [CrossRef]
- Li, Y.; Guo, R.; Wang, C.; Liu, Y.; Shao, Z.; An, J.; Liu, C. Stable and easily sintered BaCe0.5Zr0.3Y0.2O3−δ electrolytes using ZnO and Na2CO3 additives for protonic oxide fuel cells. Electrochim. Acta 2013, 95, 95–101. [Google Scholar] [CrossRef]
- Baral, A.K.; Tsur, Y. Sintering aid (ZnO) effect on proton transport in BaCe0.35Zr0.5Y0.15O3−δ and electrode phenomena studied by distribution function of relaxation times. J. Am. Ceram. Soc. 2019, 102, 239–250. [Google Scholar] [CrossRef]
- Hara, S.; Shikata, K.; Shikzono, N.; Izumi, S.; Sakai, S. Monte Carlo study on the constraint effect of YSZ phase on Ni sintering in Ni-YSZ composite system. ECS Trans. 2013, 57, 2857–2863. [Google Scholar] [CrossRef]
- Wang, J.B.; Tai, Y.-L.; Dow, W.-P.; Huang, T.-J. Study of ceria-supported nickel catalyst and effect of yttria doping on carbon dioxide reforming of methane. Appl. Catal. A Gen. 2001, 218, 69–79. [Google Scholar] [CrossRef]
- Liu, Z.; Grinter, D.C.; Lustemberg, P.G.; Nguyen-Phan, T.-D.; Zhou, Y.; Luo, S.; Waluyo, I.; Crumlin, E.J.; Stacchiola, J.D.; Zhou, J.; et al. Dry Reforming of Methane on a Highly-Active Ni-CeO2 Catalyst: Effects of metal-support interactions on C–H Bond breaking. Angew. Chem. Int. Ed. 2016, 55, 7455–7459. [Google Scholar] [CrossRef]
- Maide, M.; Korjus, O.; Vestli, M.; Romann, T.; Aruväli, J.; Kirsimäe, K.; Nurk, G. Comparative study of BaY0.1Zr0.9O3−δ protective layers deposited to BaY0.1Ce0.9O3−δ membrane using ultrasonic spray pyrolysis and magnetron sputtering methods. J. Electrochem. Soc. 2016, 163, F443–F447. [Google Scholar] [CrossRef]
- Inaba, H.; Tagawa, H. Review Ceria-based solid electrolytes. Solid State Ionics 1996, 83, 1–16. [Google Scholar] [CrossRef]
- Zha, S.; Xia, C.; Meng, G. Effect of Gd (Sm) doping on properties of ceria lectrolyte for solid oxide fuel cells. J. Power Sources 2003, 115, 44–48. [Google Scholar] [CrossRef]
- Mortalò, C.; Boaro, M.; Rebollo, E.; Zin, V.; Aneggi, E.; Fabrizio, M.; Trovarelli, A. Insights on the interfacial processes involved in the mechanical and redox stability of the BaCe0.65Zr0.20Y0.15O3−δ–Ce0.85Gd0.15O2−δ composite. Appl. Energy Mater. 2020, 3, 9877–9888. [Google Scholar] [CrossRef]
- Mercandri, E.; Gondolini, A.; Arbit, M.; Cruciani, G.; Melandri, C.; Escolástico, S.; Serra, J.M.; Sanson, A. Chemical and mechanical stability of BCZY-GDC membranes for hydrogen separation. Sep. Purif. Technol. 2022, 289, 120795. [Google Scholar]
- Rosensteel, A.; Ricote, A.; Sullivan, N.P. Hydrogen permeation through dense BaCe0.8Y0.2O3−δ–Ce0.8Y0.2O2−δ composite-ceramic hydrogen separation membranes. Int. J. Hydrogen Energy 2016, 41, 2598–2606. [Google Scholar] [CrossRef]
- Rebollo, E.; Mortalò, C.; Escolástico, S.; Boldrini, S.; Barison, S.; Serrab, J.M.; Fabrizio, M. Exceptional hydrogen permeation of all-ceramic composite robust membranes based onBaCe0.65Zr0.20Y0.15O3−δ and Y- or Gd-doped ceria. Energy Environ. Sci. 2015, 8, 3675–3686. [Google Scholar] [CrossRef]




| Membranes | T [°C] | Thickness [μm] | Concentration (Feed Gas–Sweep Gas) | Hydrogen Flux * [mL min−1 cm−2] | Ref. |
|---|---|---|---|---|---|
| Ni-Cermets | |||||
| Ni–BaCe0.8Y0.2O3−δ-Gd0.1Ce0.9O2−δ (40:50:10vol.%) | 700 | 800 | Wet 4.0% H2/N2 –Wet 100 ppm/N2 | 0.142 (0.38) | This work |
| Ni–BaCe0.9Y0.1O3−δ (40:60vol.%) | 800 | 230 | Wet 100% H2 –Wet 100 ppmH2/N2 | 0.76 (0.16) | [22] |
| Ni–BaCe0.9Y0.1O3−δ (40:60vol.%) | 700 | 640 | Wet 3.8% H2/N2 –100 ppmH2/N2 | 0.05 (0.11) | [23] |
| Ni–Ba(Zr0.1Ce0.7Y0.2)O3−δ (40:60vol.%) | 900 | 266 | Wet 100% H2 –100 ppmH2/N2 | 0.805 (0.18) | [27] |
| Dual-phase ceramics | |||||
| BaCe0.8Y0.2O3−δ–Gd0.1Ce0.9O2−δ (54:46 vol%) | 800 | 1100 | Wet 100% H2 –Wet N2 | 0.30 (0.30) | [28] |
| BaCe0.8Y0.2O3−δ–Y0.2Ce0.8O2−δ (50:50 vol%) | 900 | 1440 | 0.5atm H2 partial pressure gradient | 0.0744 (0.10) | [47] |
| BaCe0.65Zr0.20Y0.15O3−δ–Gd0.15Ce0.85O2−δ (50:50 vol%) | 755 | 650 | Wet 50% H2/He –Wet Ar | 0.27 (0.20) | [48] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Itagaki, Y.; Mori, H.; Matsubayashi, T.; Aono, H. Hydrogen Permeation Properties of Ternary Ni–BaCe0.9Y0.1O3–Ce0.9Gd0.1O2 Cermet Membranes. Ceramics 2024, 7, 385-400. https://doi.org/10.3390/ceramics7010024
Itagaki Y, Mori H, Matsubayashi T, Aono H. Hydrogen Permeation Properties of Ternary Ni–BaCe0.9Y0.1O3–Ce0.9Gd0.1O2 Cermet Membranes. Ceramics. 2024; 7(1):385-400. https://doi.org/10.3390/ceramics7010024
Chicago/Turabian StyleItagaki, Yoshiteru, Hiroyuki Mori, Takumi Matsubayashi, and Hiromichi Aono. 2024. "Hydrogen Permeation Properties of Ternary Ni–BaCe0.9Y0.1O3–Ce0.9Gd0.1O2 Cermet Membranes" Ceramics 7, no. 1: 385-400. https://doi.org/10.3390/ceramics7010024
APA StyleItagaki, Y., Mori, H., Matsubayashi, T., & Aono, H. (2024). Hydrogen Permeation Properties of Ternary Ni–BaCe0.9Y0.1O3–Ce0.9Gd0.1O2 Cermet Membranes. Ceramics, 7(1), 385-400. https://doi.org/10.3390/ceramics7010024





