Structural and Dielectric Properties of Titania Co-Doped with Yttrium and Niobium: Experimental Evidence and DFT Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of Materials
2.2. Characterization
2.3. Computational Details
3. Results
3.1. Sintering Temperature
3.2. Concentration of Co-Dopants
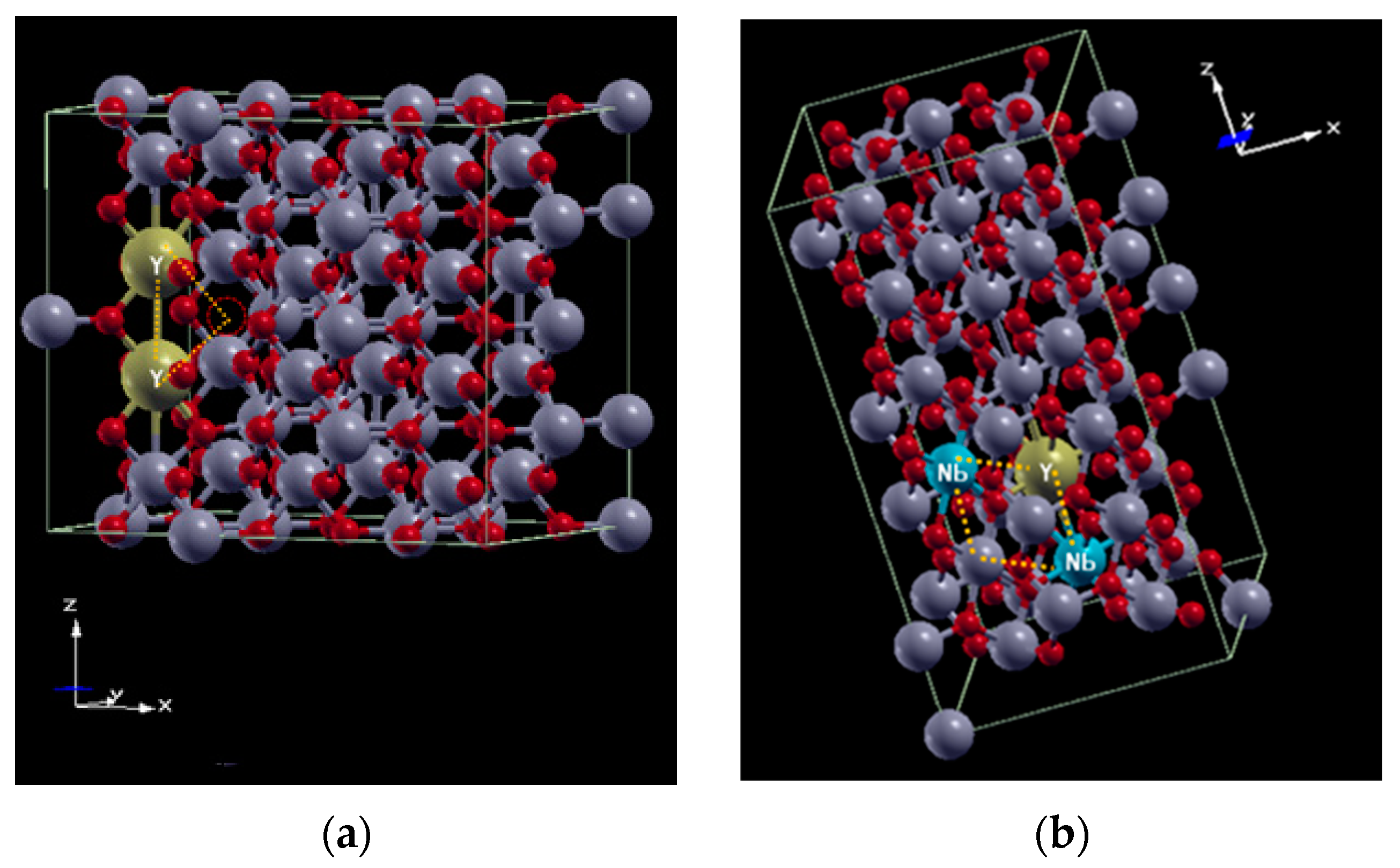
3.3. Computational Structural Modeling
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Board Review Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Buchanan, R.C. Ceramic Materials for Electronics; CRC Press: Boca Raton, FL, USA, 2018; ISBN 978-0-8247-4028-3. [Google Scholar]
- Li, J.; Cho, K.; Wu, N.; Ignatiev, A. Correlation between dielectric properties and sintering temperatures of polycrystalline CaCu3Ti4O12. IEEE Trans. Dielectr. Electr. Insul. 2004, 11, 534–541. [Google Scholar] [CrossRef]
- Krohns, S.; Lunkenheimer, P.; Meissner, S.; Reller, A.; Gleich, B.; Rathgeber, A.; Glauger, T.; Buhl, H.U.; Sinclair, D.C.; Loidl, A. The route to resource-efficient novel materials. Nat. Mater. 2011, 10, 899–901. [Google Scholar] [CrossRef] [PubMed]
- Guillemet-Fritsch, S.; Valdez-Nava, Z.; Tenailleau, C.; Lebey, T.; Durand, B.; Chane-Ching, J.Y. Colossal permittivity in ultrafine grain size BaTiO3−x and Ba0.95La0.05TiO3−x materials. Adv. Mater. 2008, 20, 551–555. [Google Scholar] [CrossRef]
- Wu, J.; Nan, C.W.; Lin, Y.; Deng, Y. Giant dielectric permittivity observed in Li and Ti doped NiO. Phys. Rev. Lett. 2002, 89, 217601. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Tang, Z.J. Polaron relaxation and variable-range-hopping conductivity in the giant-dielectric-constant material CaCu3Ti4O12. Phys. Rev. B 2004, 70, 174306. [Google Scholar] [CrossRef]
- Bak, T.; Nowotny, J.; Rekas, M.; Sorrell, C. Defect chemistry and semiconducting properties of titanium dioxide: II Defect diagrams. J. Phys. Chem. Solids 2003, 64, 1057–1067. [Google Scholar] [CrossRef]
- Bonkerud, J.; Zimmermann, C.; Weiser, P.M.; Vines, L.; Monakhov, E.V. On the permittivity of titanium dioxide. Sci. Rep. 2021, 11, 12443. [Google Scholar] [CrossRef]
- Hu, W.; Liu, Y.; Withers, R.L.; Frankcombe, T.J.; Norén, L.; Snashall, A.; Kitchin, M.; Smith, P.; Gong, B.; Chen, H.; et al. Electron-pinned defect-dipoles for high-performance colossal permittivity materials. Nat. Mater. 2013, 12, 821–826. [Google Scholar] [CrossRef]
- Dong, W.; Hu, W.; Berlie, A.; Lau, K.; Chen, H.; Withers, R.L.; Liu, Y. Colossal dielectric behavior of Ga+Nb co-doped rutile TiO2. Appl. Mater. Interfaces 2015, 7, 25321–25325. [Google Scholar] [CrossRef]
- Peng, H.; Liang, P.; Zhou, X.; Peng, Z.; Xiang, Y. Good thermal stability giant permittivity, and low dielectric loss for X9R-type (Ag1/4Nb3/4)0.005Ti0.995O2 ceramics. J. Am. Ceram. Soc. 2019, 102, 970–975. [Google Scholar] [CrossRef]
- Nachaithong, T.; Moontragoon, P.; Chanlek, N.; Thongbai, P. Fe3+/Nb5+ co-doped rutile-TiO2 nanocrystalline powders prepared by a combustion process: Preparation and characterization and their giant dielectric response. RSC Adv. 2020, 10, 24784–24794. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; Peng, Z.; Zhu, J.; Shi, Q.; Liang, P.; Chao, X.; Yang, Z. High temperature stability and low dielectric loss in colossal permittivity TiO2 based ceramics co-doped with Ag+ and Mo6+. Mater. Chem. Phys. 2023, 295, 127072. [Google Scholar] [CrossRef]
- Wang, Z.; Li, Y.; Chen, H.; Fan, J.; Wang, X.; Ma, X. Correlation between the radius of acceptor ion and the dielectric properties of co-doped TiO2 ceramics. Ceram. Int. 2019, 45, 14625–14633. [Google Scholar] [CrossRef]
- Wang, W.; Li, L.; Lu, T.; Zhang, N.; Luo, W. Multifarious polarization in high-performance colossal permittivity titanium dioxide ceramics. J. Alloys Compd. 2019, 806, 89–98. [Google Scholar] [CrossRef]
- Hu, W.; Lau, K.; Liu, Y.; Withers, R.L.; Chen, H.; Fu, L.; Gong, B. Colossal dielectric permittivity in (Nb + Al) codoped rutile TiO2 ceramics: Compositional gradient and local structure. Chem. Mater. 2015, 27, 4934–4942. [Google Scholar] [CrossRef]
- Tuichai, W.; Danwittayakul, S.; Chanlek, N.; Thongbai, P. Effects of the sintering temperature on microstructure and giant dielectric properties of (V + Ta) co-doped TiO2 ceramics. J. Alloys Compd. 2017, 725, 310–317. [Google Scholar] [CrossRef]
- Nachaithong, T.; Tuichai, W.; Kidhhunthod, P.; Chanlek, N.; Thongbai, P.; Maensiri, S. Preparation, characterization, and giant dielectric permittivity of (Y3+ and Nb5+) co-doped TiO2 ceramics. J. Eur. Ceram. Soc. 2017, 37, 3521–3526. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, B.; Xu, L.; Wang, X.; Hu, Y.; Shen, G.; Sun, L. Dielectric properties of Y and Nb co-doped TiO2 ceramics. Sci. Rep. 2017, 7, 8517. [Google Scholar] [CrossRef]
- Wang, Q.; Lian, G.; Dickey, E.C. Grain boundary segregation in yttrium-doped polycrystalline TiO2. Acta Mater. 2004, 52, 809–820. [Google Scholar] [CrossRef]
- Porfírio, T.C.; Muccillo, E.N.S. Effects of LiF addition on microstructure and dielectric properties of CaCu3Ti4O12 ceramics. Ceram. Int. 2016, 42, 12005–12009. [Google Scholar] [CrossRef]
- Gerhardt, R. Impedance and dielectric spectroscopy revisited: Distinguishing localized relaxation from long-range conductivity. J. Phys. Chem. Solids 1994, 55, 1491–1506. [Google Scholar] [CrossRef]
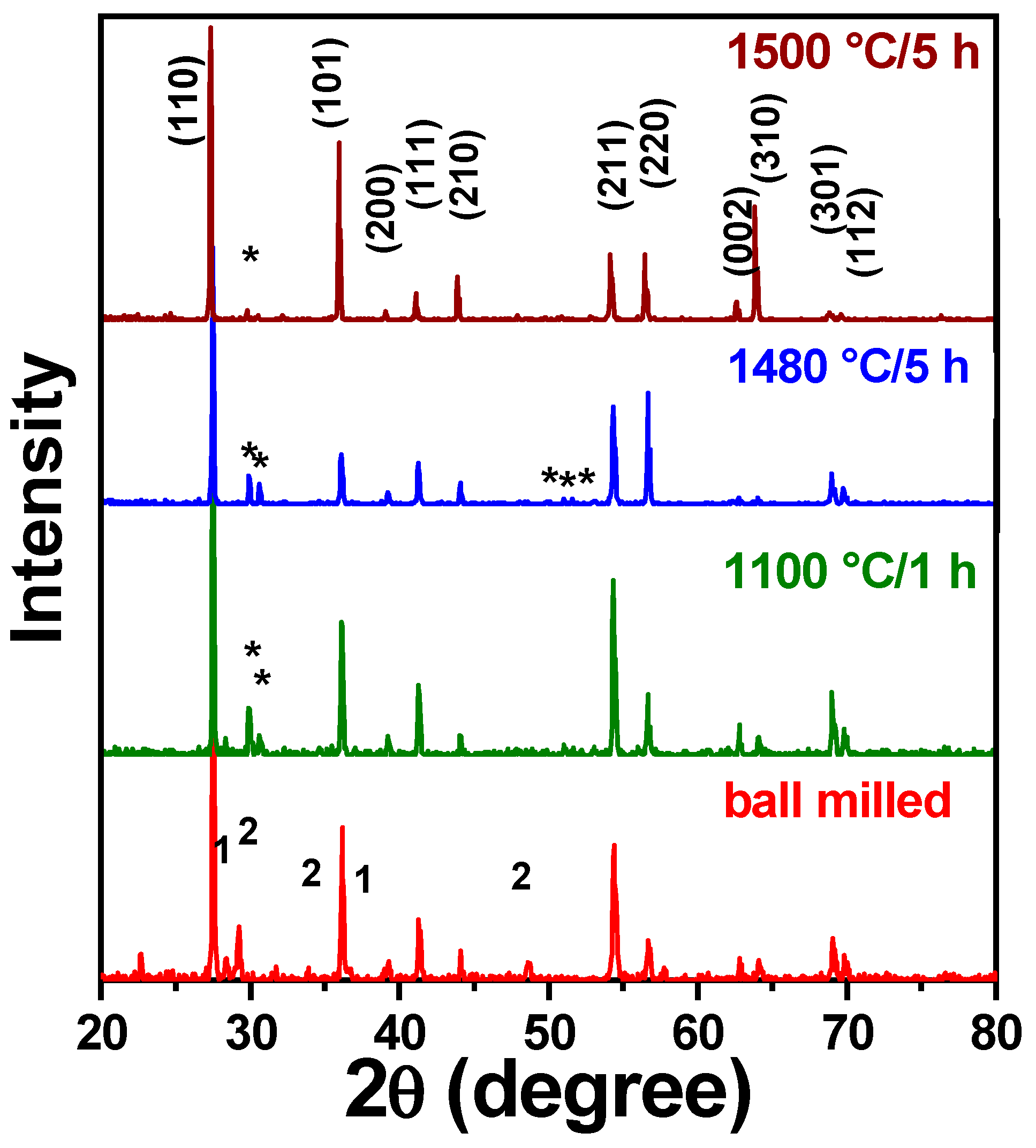
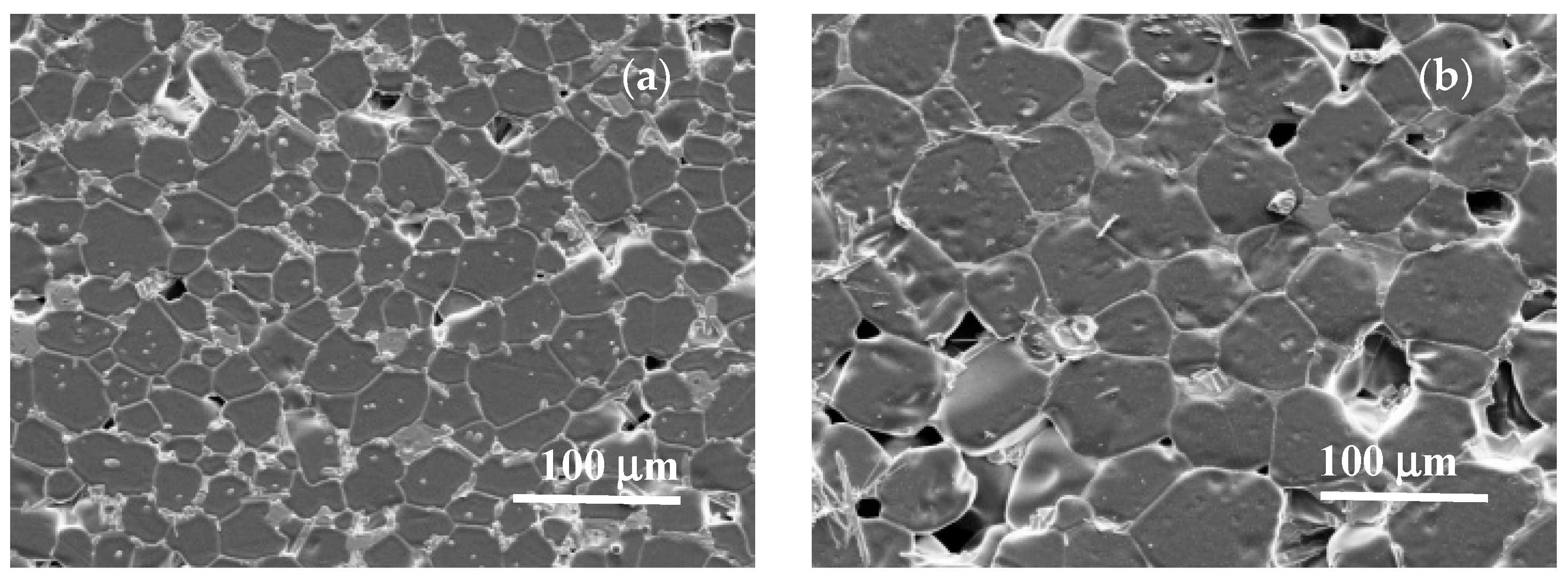
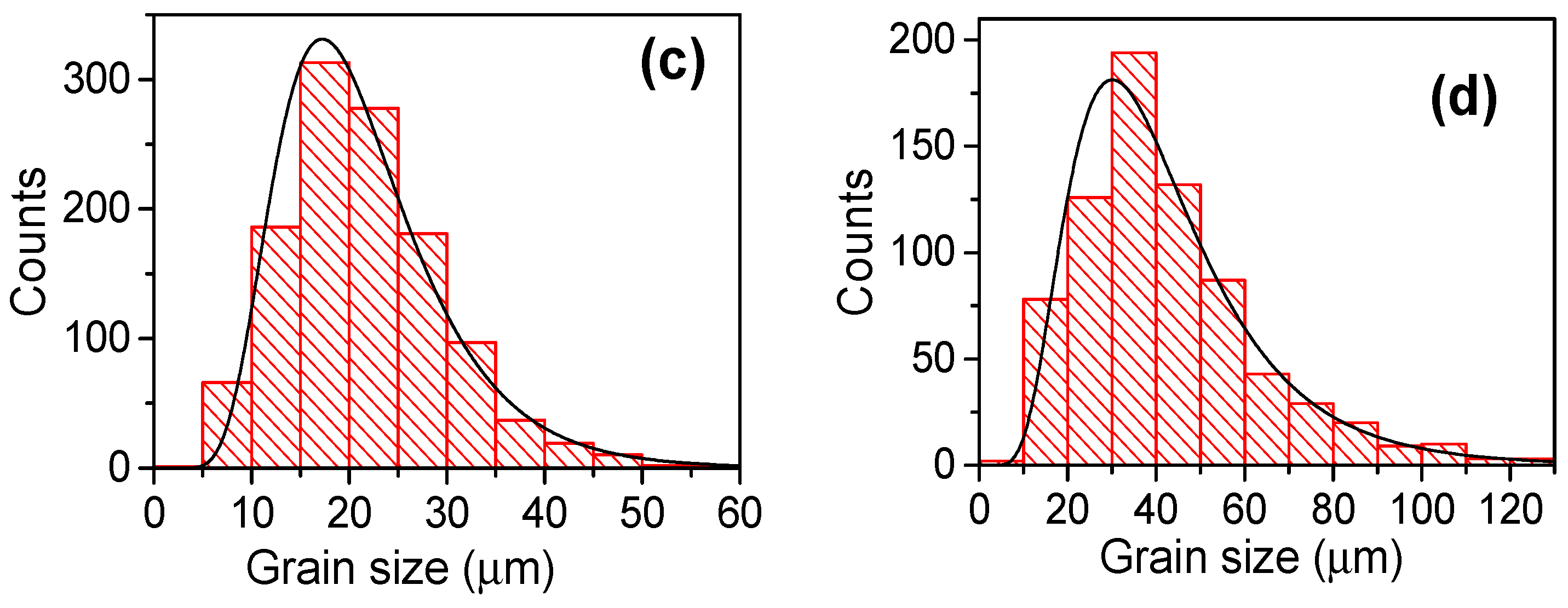

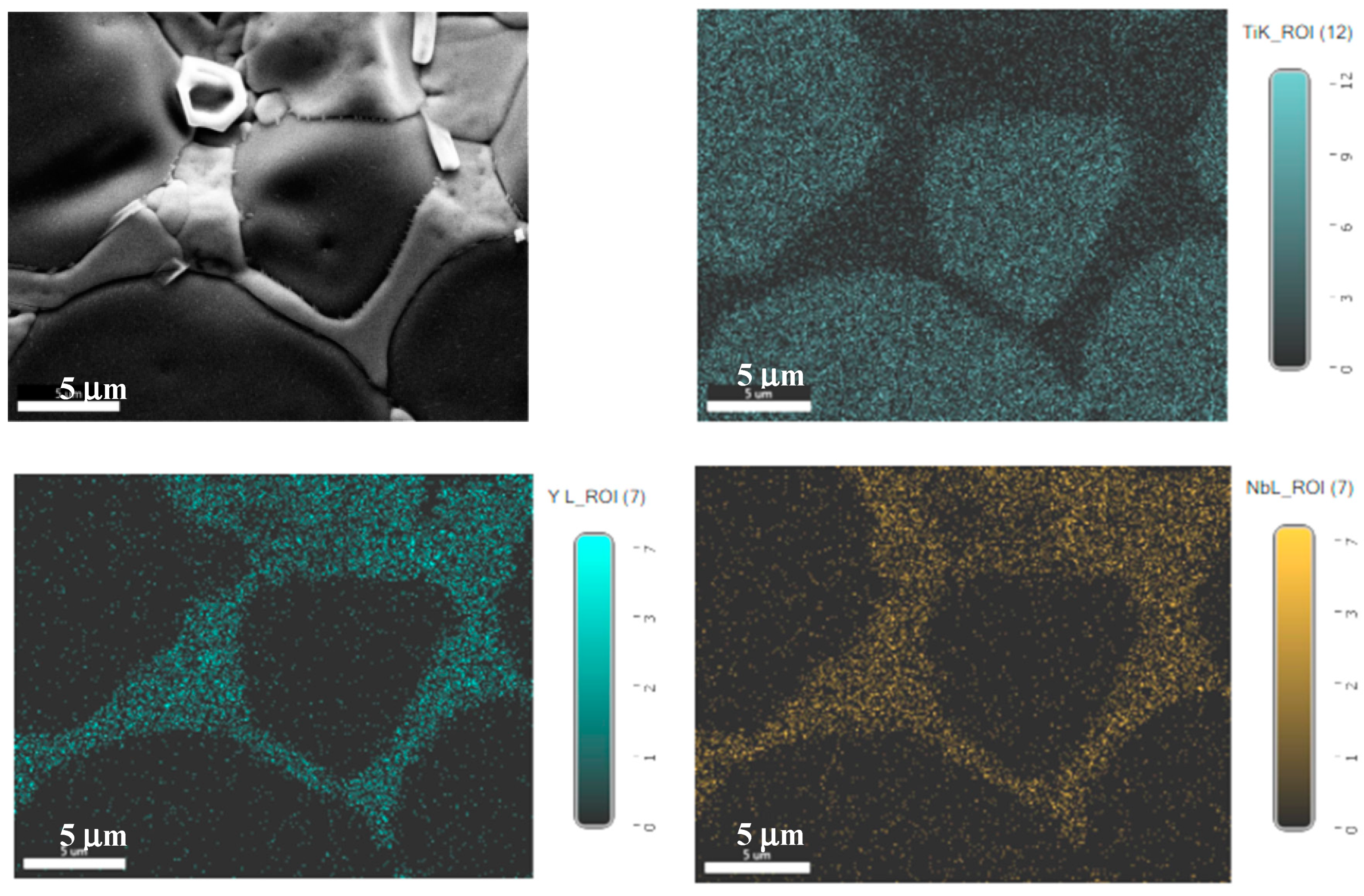
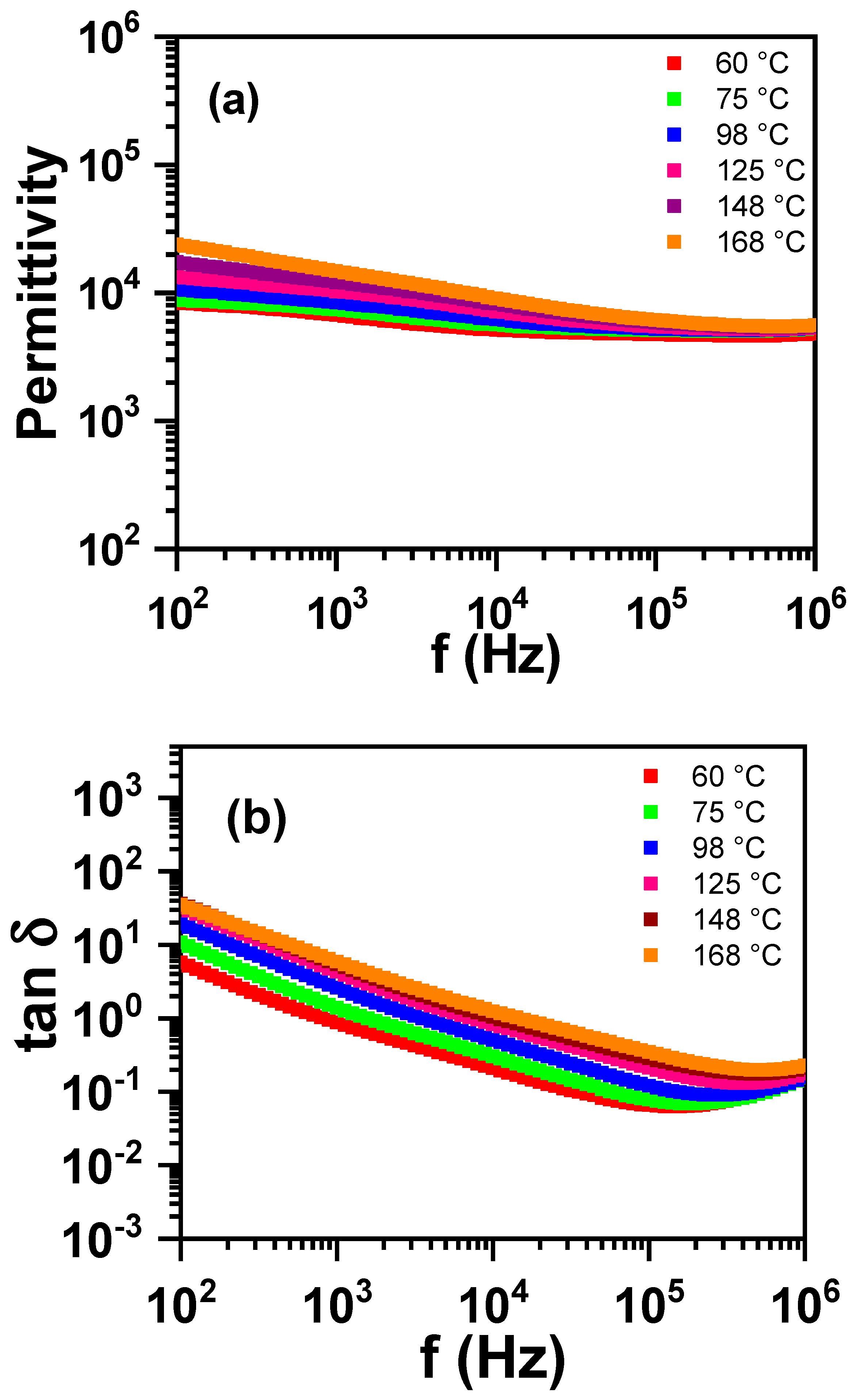
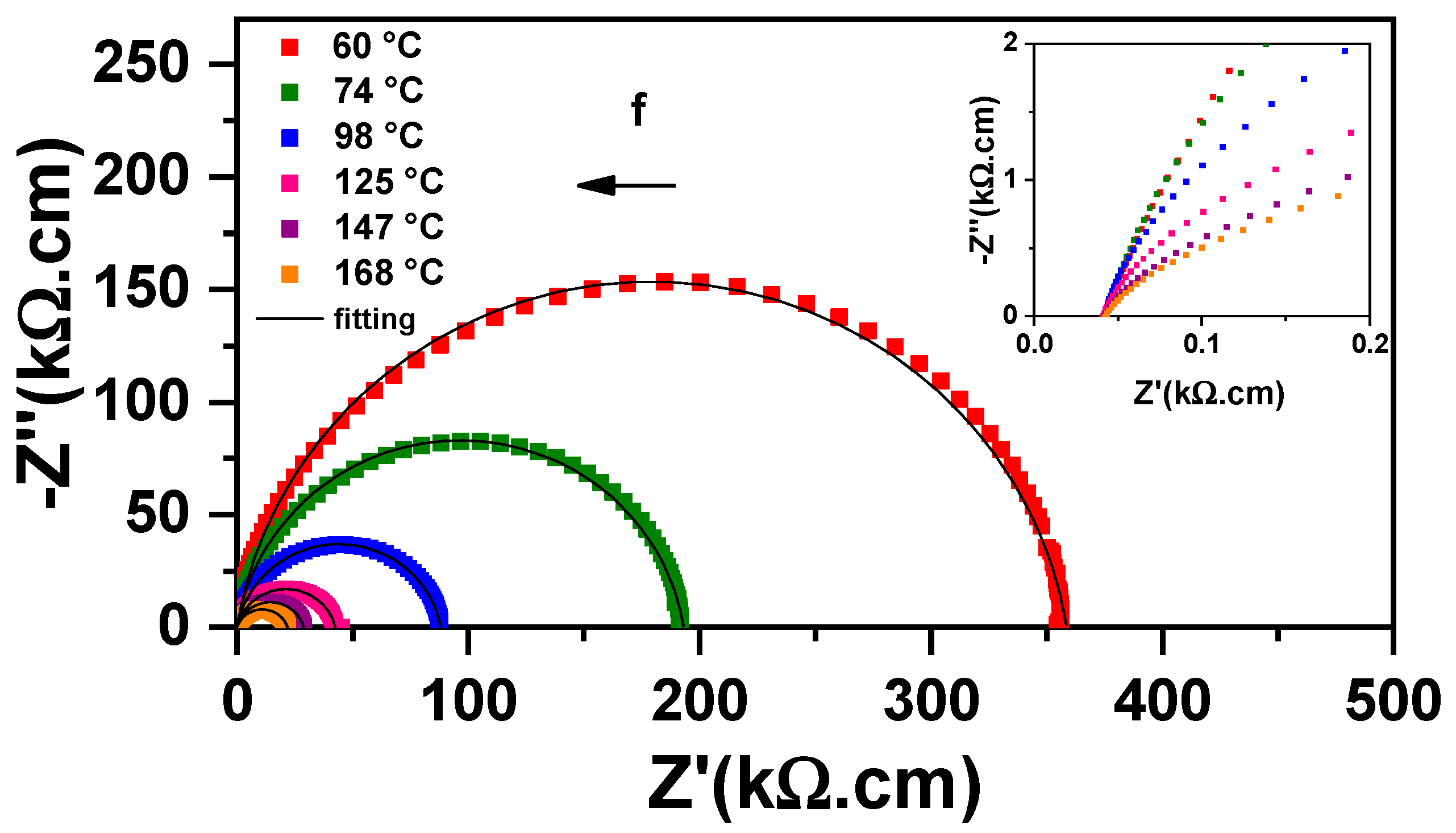

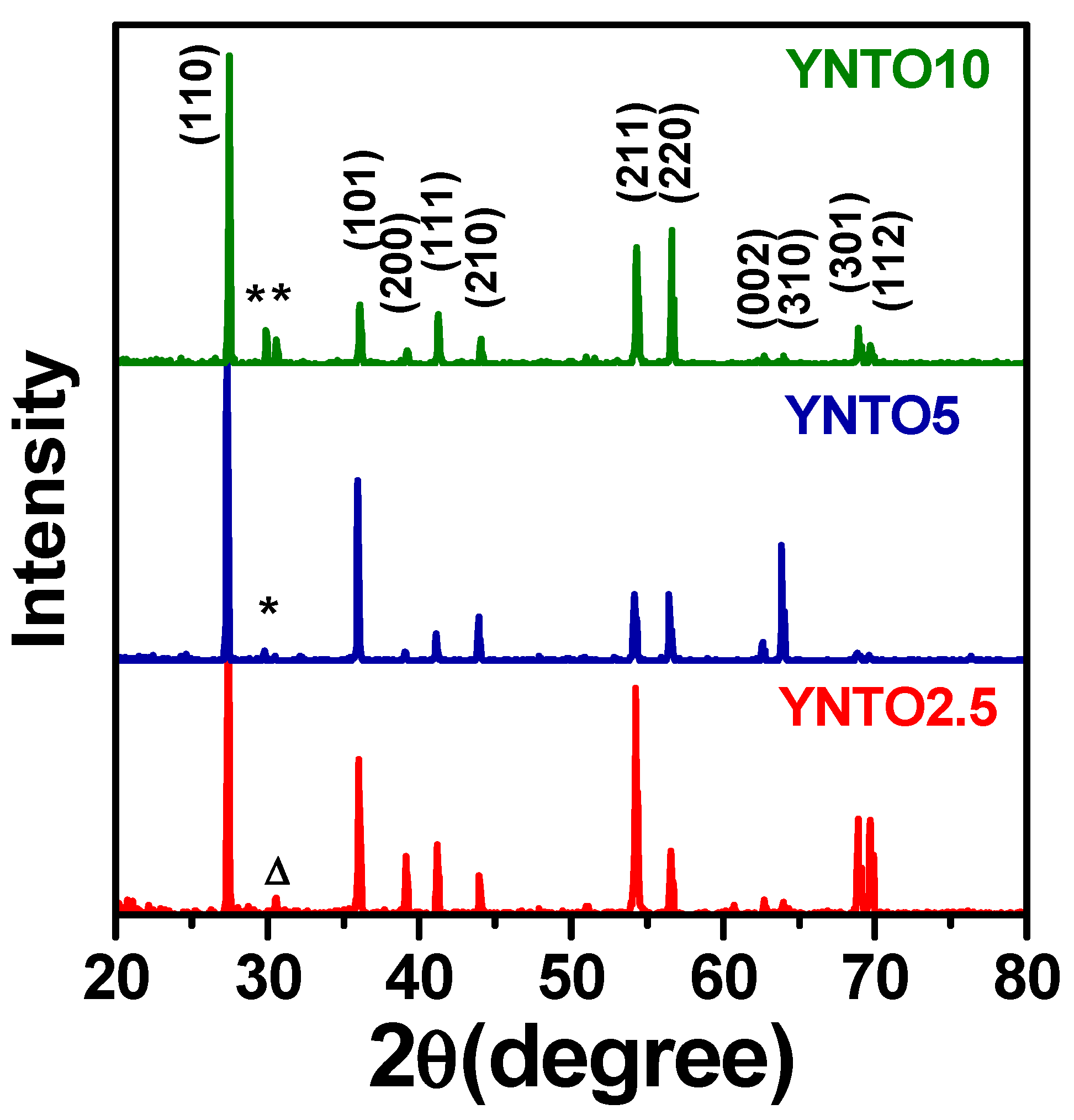
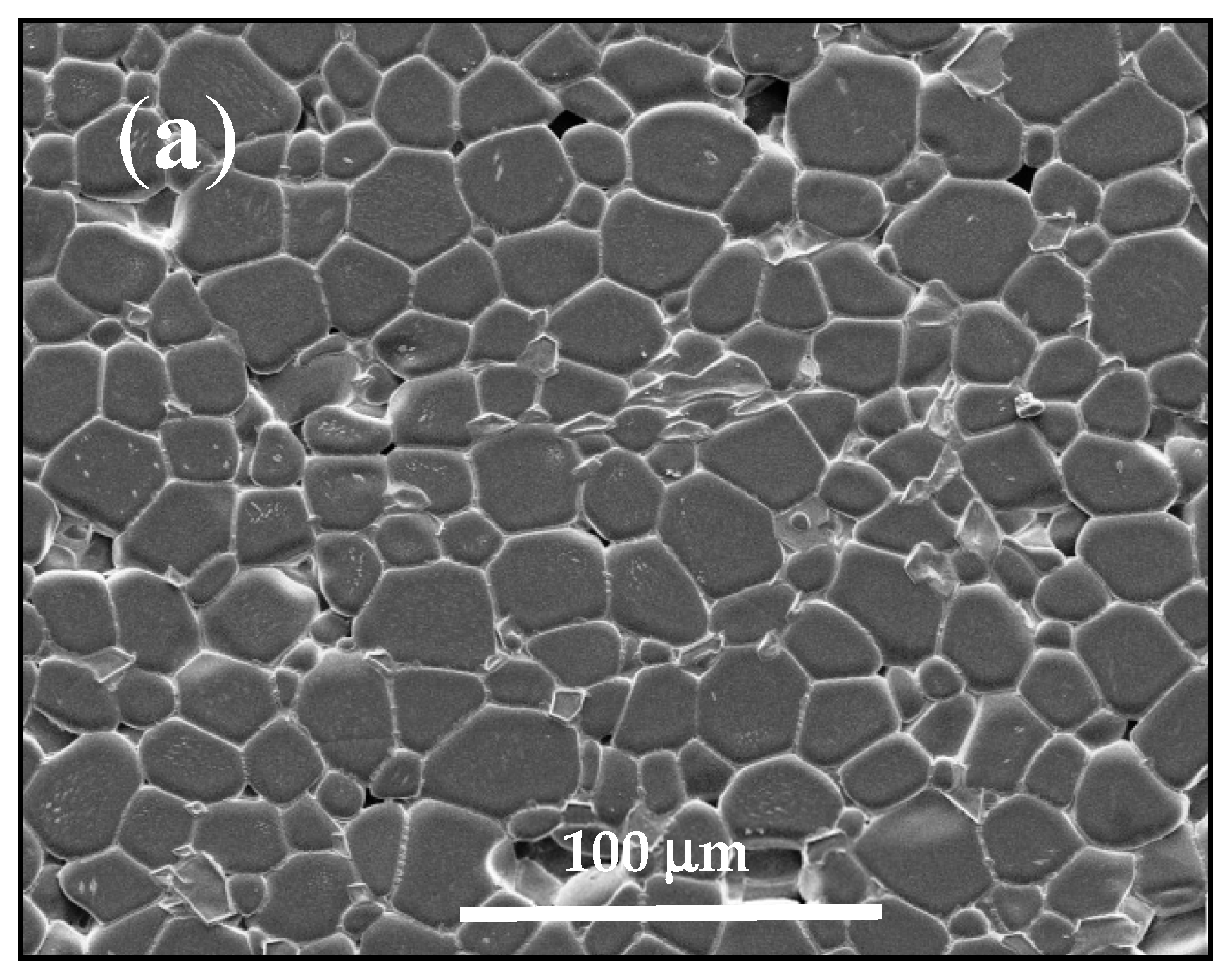
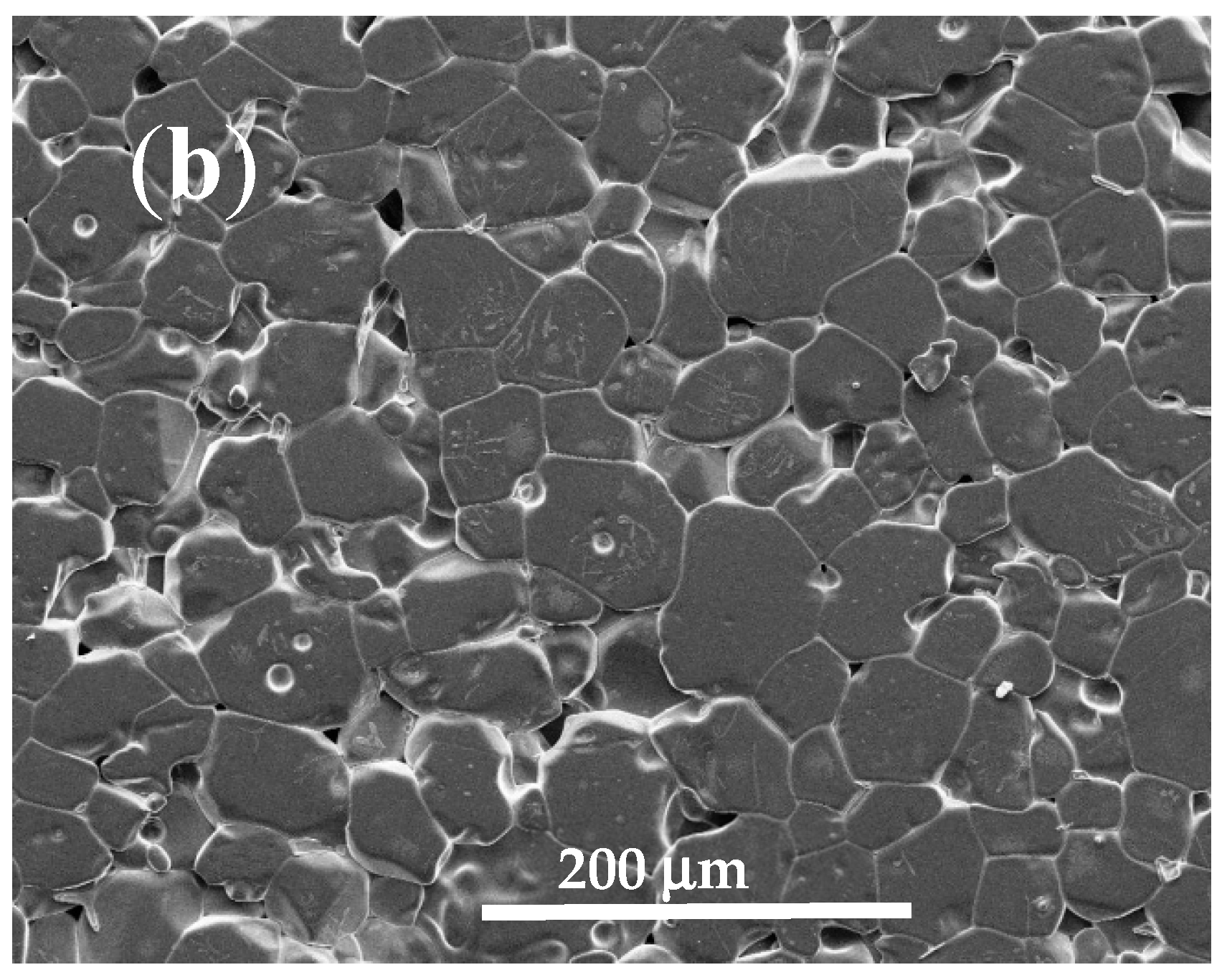
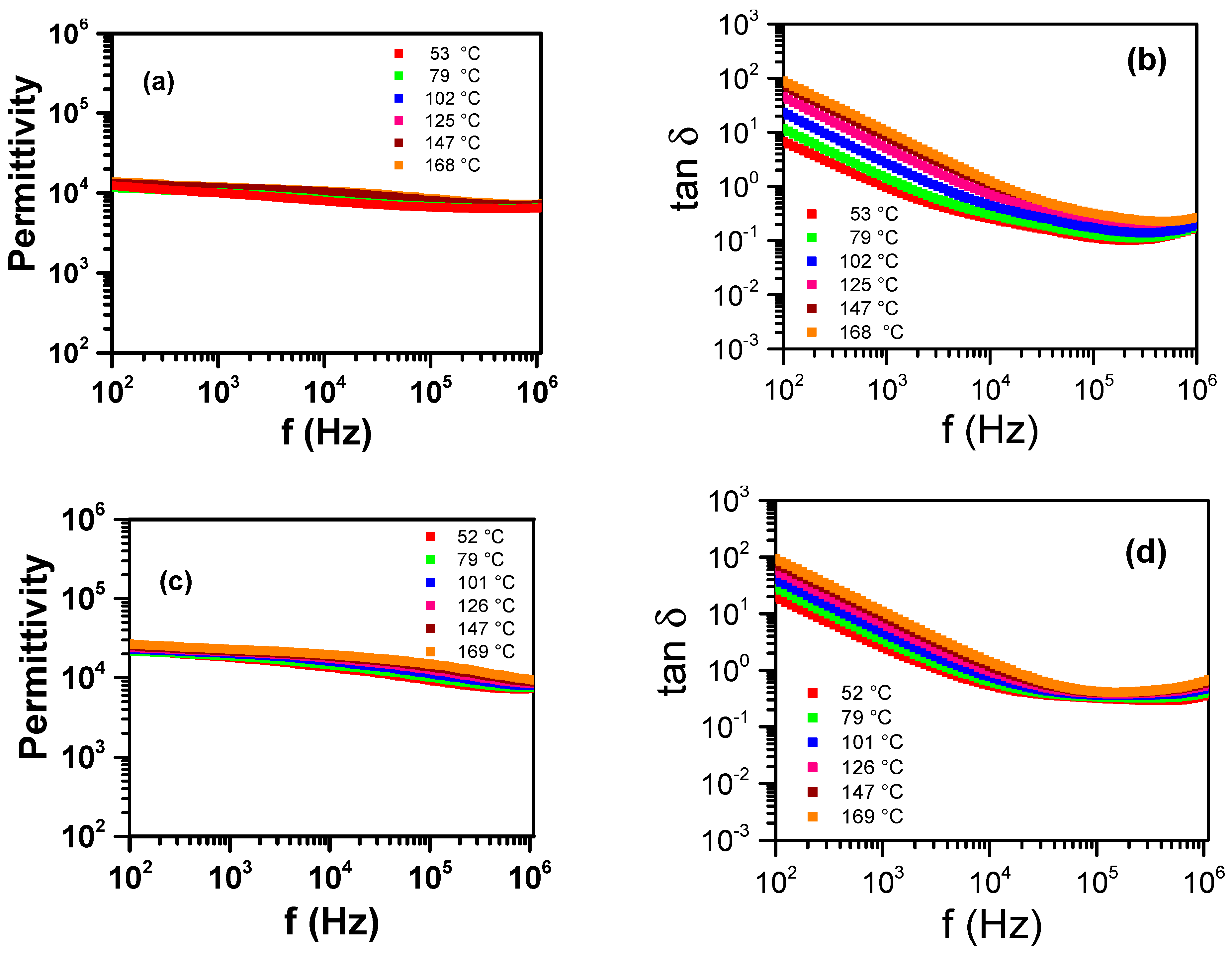
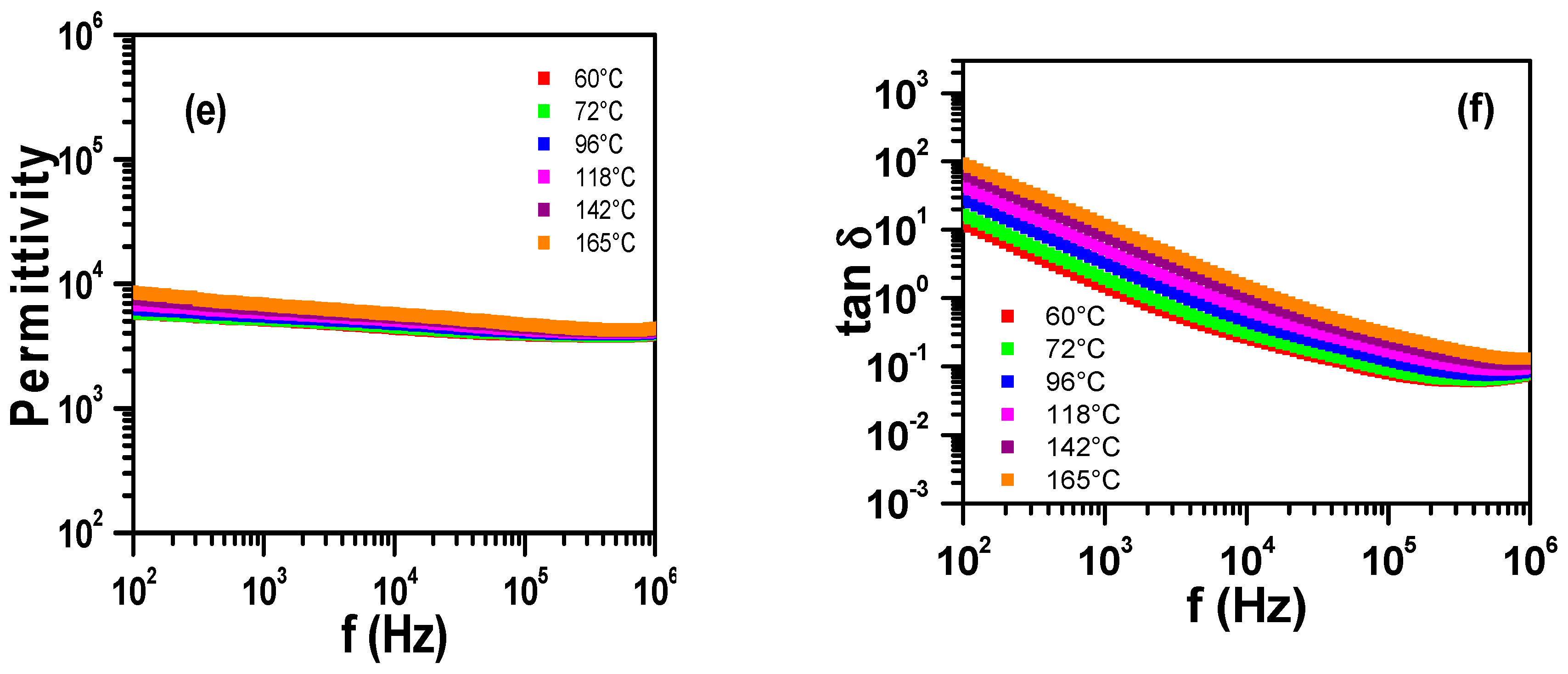
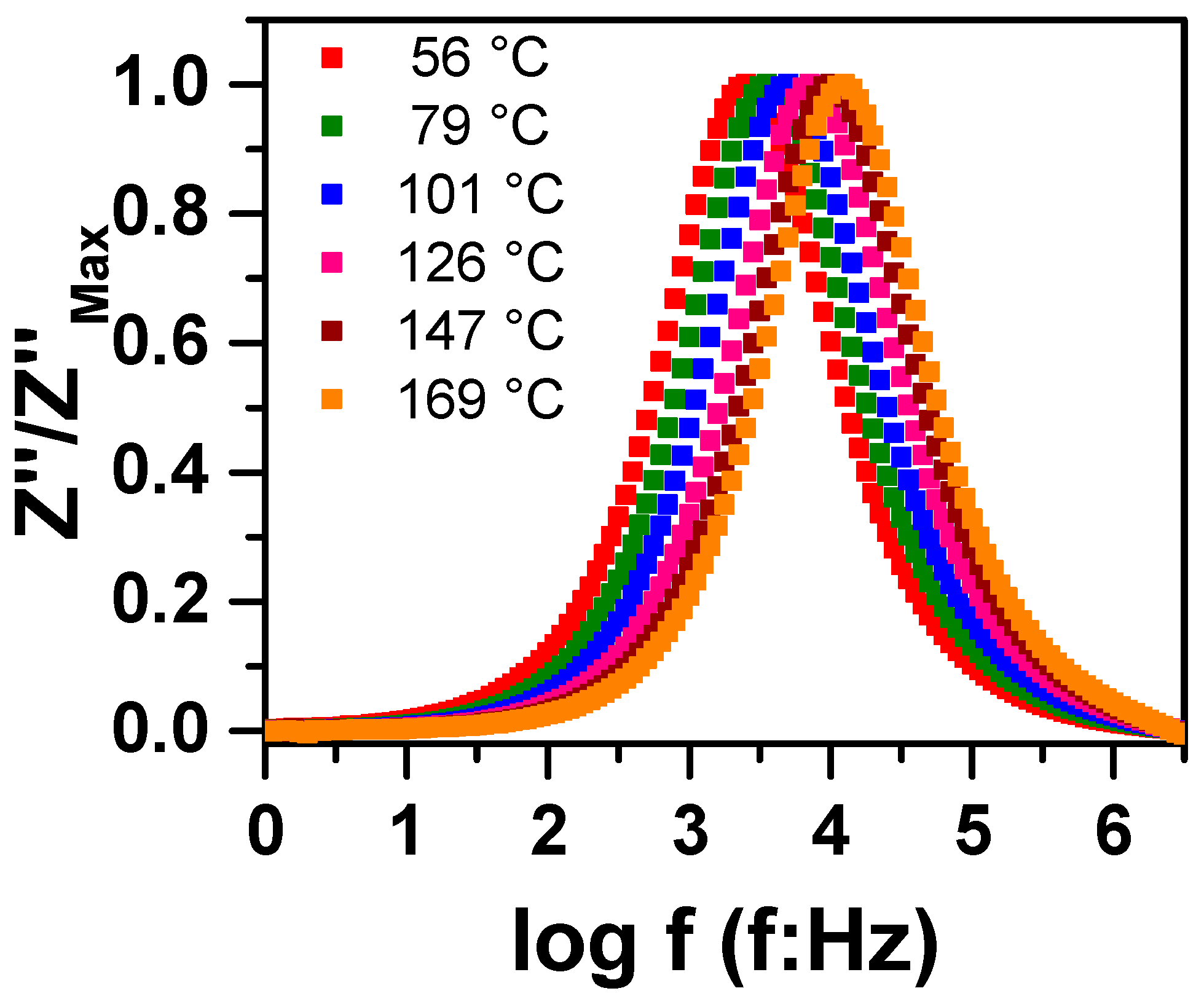
| Parameter | YNTO2.5 | YNTO5 | YNTO10 | YNTO10 |
|---|---|---|---|---|
| (1480 °C) | (1480 °C) | (1480 °C) | (1500 °C) | |
| G (μm) | 18.8 ± 0.1 | 37.1 ± 0.1 | 20 ± 0.1 | 37.9 ± 0.2 |
| ε′ (@ ~60 °C/10 kHz) | 7946 | 13,499 | 4410 | 5490 |
| tan δ (@ ~60 °C/10 kHz) | 0.26 | 0.54 | 0.27 | 0.21 |
| n | 0.92 ± 0.01 | 0.90 ± 0.02 | 0.94 ± 0.01 | 0.85 ± 0.05 |
| C (nF) | 3.1 ± 0.1 | 7.0 ± 0.5 | 2.1 ± 0.15 | 3.3 ± 0.5 |
| Ea (eV) | 0.31 ± 0.05 | 0.19 ± 0.05 | 0.27 ± 0.05 | 0.33 ± 0.05 |
| a,b (Å) | c (Å) | c/a | α (°) | β (°) | γ (°) | Ti-O (Å) | |
|---|---|---|---|---|---|---|---|
| 3 × 2 × 4 | 4.612 | 2.960 | 0.642 | 90 | 90 | 90 | 1.965 |
| triangular | 4.638 | 2.981 | 0.643 | 90 | 90 | 90.24 | 1.953 |
| 2 × 2 × 6 | 4.621 | 2.954 | 0.639 | 90 | 90 | 90 | 1.965 |
| diamond | 4.643 | 2.991 | 0.644 | 89.98 | 89.98 | 89.96 | 1.963 |
| YNTO2.5 | 4.604 | 2.961 | 0.643 | - | - | - | - |
| YNTO5 | 4.604 | 2.963 | 0.643 | - | - | - | - |
| YNTO10 | 4.604 | 2.965 | 0.644 | - | - | - | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Silva, D.Y.B.; Muccillo, R.; Muccillo, E.N.S. Structural and Dielectric Properties of Titania Co-Doped with Yttrium and Niobium: Experimental Evidence and DFT Study. Ceramics 2024, 7, 411-425. https://doi.org/10.3390/ceramics7010026
Silva DYB, Muccillo R, Muccillo ENS. Structural and Dielectric Properties of Titania Co-Doped with Yttrium and Niobium: Experimental Evidence and DFT Study. Ceramics. 2024; 7(1):411-425. https://doi.org/10.3390/ceramics7010026
Chicago/Turabian StyleSilva, Deborah Y. B., Reginaldo Muccillo, and Eliana N. S. Muccillo. 2024. "Structural and Dielectric Properties of Titania Co-Doped with Yttrium and Niobium: Experimental Evidence and DFT Study" Ceramics 7, no. 1: 411-425. https://doi.org/10.3390/ceramics7010026
APA StyleSilva, D. Y. B., Muccillo, R., & Muccillo, E. N. S. (2024). Structural and Dielectric Properties of Titania Co-Doped with Yttrium and Niobium: Experimental Evidence and DFT Study. Ceramics, 7(1), 411-425. https://doi.org/10.3390/ceramics7010026







