2. Materials and Research Methods
The synthesis of lithium-containing ceramics was carried out by using two types of compositions of the initial mixtures: (1) ZrO2 and LiClO4·3H2O and (2) ZrO2 and Li2CO3 with varying the molar ratio of the components from 0.25 to 0.75 M. In the future, these compositions will be designated as 1 and 2. The selection of these two compositions, featuring different components, stems from the potential to create lithium-containing ceramics with diverse phase compositions, strength, and thermophysical properties. Additionally, the choice of using LiClO4·3H2O and Li2CO3 as the lithium component was made to evaluate the feasibility of the mechanochemical synthesis method in producing stable ceramics with high lithium concentrations, which constitutes a crucial criterion when selecting ceramics for tritium production.
The primary approach employed to manufacture lithium-containing ceramics was the mechanochemical solid-phase synthesis method. This method entails mechanically blending the initial components within a PULVERISETTE 6 planetary mill (Fritsch, Berlin, Germany) for 5 h at a grinding speed of 300 rpm, with the aim of achieving a uniform particle size distribution. Subsequently, the resulting powders were compacted into 10 mm diameter and 1 mm thickness tablets, considering the requirements of the measuring instruments used to determine properties such as hardness, fracture resistance, and thermal conductivity. The tablets were pressed using a mold specially made for this purpose under a pressure of 250 MPa. After pressing, the resulting tablets were subjected to thermal annealing at a temperature of 900 °C in a muffle furnace SNOL 39/1100 (AB UMEGA-GROUP, Ukmergė, Lithuania). Annealing was carried out for 8 h in an air atmosphere, followed by cooling for 24 h with the furnace. The selection of these annealing conditions, encompassing temperature, duration, and cooling process, was informed by prior experimental investigations [
26,
27], alongside a priori knowledge concerning phase and structural transformations in lithium-containing ceramics derived through mechanochemical or solid-phase synthesis.
The analysis of structural parameters and phase composition in the examined ceramics, contingent on the type of initial component composition and its variations, was conducted through an assessment of the X-ray diffraction patterns. These patterns were captured in the Bragg-Brentano configuration (2θ = 25–100°) with an increment of 0.03°. A Cu—kα X-ray tube with a wavelength of 1.54 Å was used as an X-ray source. The diffraction data was collected using a D8 Advance ECO X-ray diffractometer (Bruker, Berlin, Germany). The interpretation of the acquired diffraction patterns was facilitated by the Diffrac EVA v.4.2 software (Bruker, Berlin, Germany), enabling the determination of structural parameters such as crystal lattice dimensions, volume, and the structural order degree, along with the calculation of phase contributions within the samples. The weight contributions of each identified phase within the samples were ascertained by calculating the areas of all diffraction reflections characteristic of a given phase and then determining their ratio relative to other established phases. The degree of structural ordering (degree of crystallinity) was determined as the ratio of the contributions of the intensities of all diffraction reflections to the area of background radiation recorded during the angular dependence of the change in the intensity of X-ray diffraction patterns.
The mechanical properties, including alterations in hardness and resistance to cracking during a single compression, were evaluated for the investigated ceramics with regard to variations in their phase composition using established methods. Hardness assessments were conducted by applying a load of 100 N on a Vickers diamond pyramid indenter for 15 s, followed by the evaluation of the resultant indenter imprint on the sample. These experiments were carried out using a Duroline M1 microhardness tester (Metkon, Bursa, Turkey). The assessments of the hardening factors were based on the data concerning changes in hardness, attributable to adjustments in component ratios, as well as the introduction of impurity phases within the structure.
A single compression, based on the data of which the dynamics of changes in cracking with increasing pressure on the sample was established, was carried out using a mechanical testing machine LFM-L 10 kN (Walter + Bai AG, Leuningen, Switzerland). At the same time, to establish the effect of crack resistance when varying the compression rate, a series of experiments were carried out on samples in which the samples were subjected to compression at different compression rates. The influence of thermal expansion on crack resistance was determined by conducting experiments on single compression of samples heated in a chamber at a temperature of 500 °C.
Thermal conductivity coefficient measurements were conducted in the temperature range of 25 to 700 °C using the longitudinal heat flow method. The KIT-800 instrument from Teplofon, Moscow, Russia, was employed for these measurements.
The coefficient of thermal expansion for the ceramic samples, influenced by prolonged thermal exposure during 500 h of heating at 700 °C, was determined by assessing changes in the crystal lattice volume before and after thermal exposure. Using Formula (1), alterations in material properties due to high-temperature degradation were calculated based on the acquired data.
where ∆
V is the change in the volume of the crystal lattice before and after high-temperature heating, ∆
T is the difference in measurement temperatures, and
Vinitial is the volume of the crystal lattice of the initial sample. The crystal lattice volume was determined by calculating the crystal lattice parameters for each test sample by analyzing the obtained X-ray diffraction patterns of the ceramic samples under study. Moreover, in the case of thermal tests, before they were carried out, the parameters and volume of the crystal lattice of each test sample were measured, after which, after thermal exposure, X-ray diffraction patterns were obtained, on the basis of which the parameters of the crystal lattice of the samples after thermal exposure were determined. Through comparative analysis, the values of changes in the volume of the crystal lattice were calculated, indicating changes in the properties of ceramics as a result of external influences.
3. Results and Discussion
In previous studies [
28,
29], it was demonstrated that modifications in the synthesis conditions, such as adjusting the proportions of the initial solution components, result in alterations in the ceramics’ phase composition. This, in turn, directly influences the material’s hardening effects and thermophysical properties. It is important to note that these studies explored the use of LiClO
4·3H
2O as a lithium-containing component. Changes in its concentration or variations in the thermal sintering conditions lead to the formation of impurity inclusions in the form of oxide phases. As indicated in [
28], these inclusions enhance resistance to radiation-induced swelling during the accumulation of helium in the surface layer.
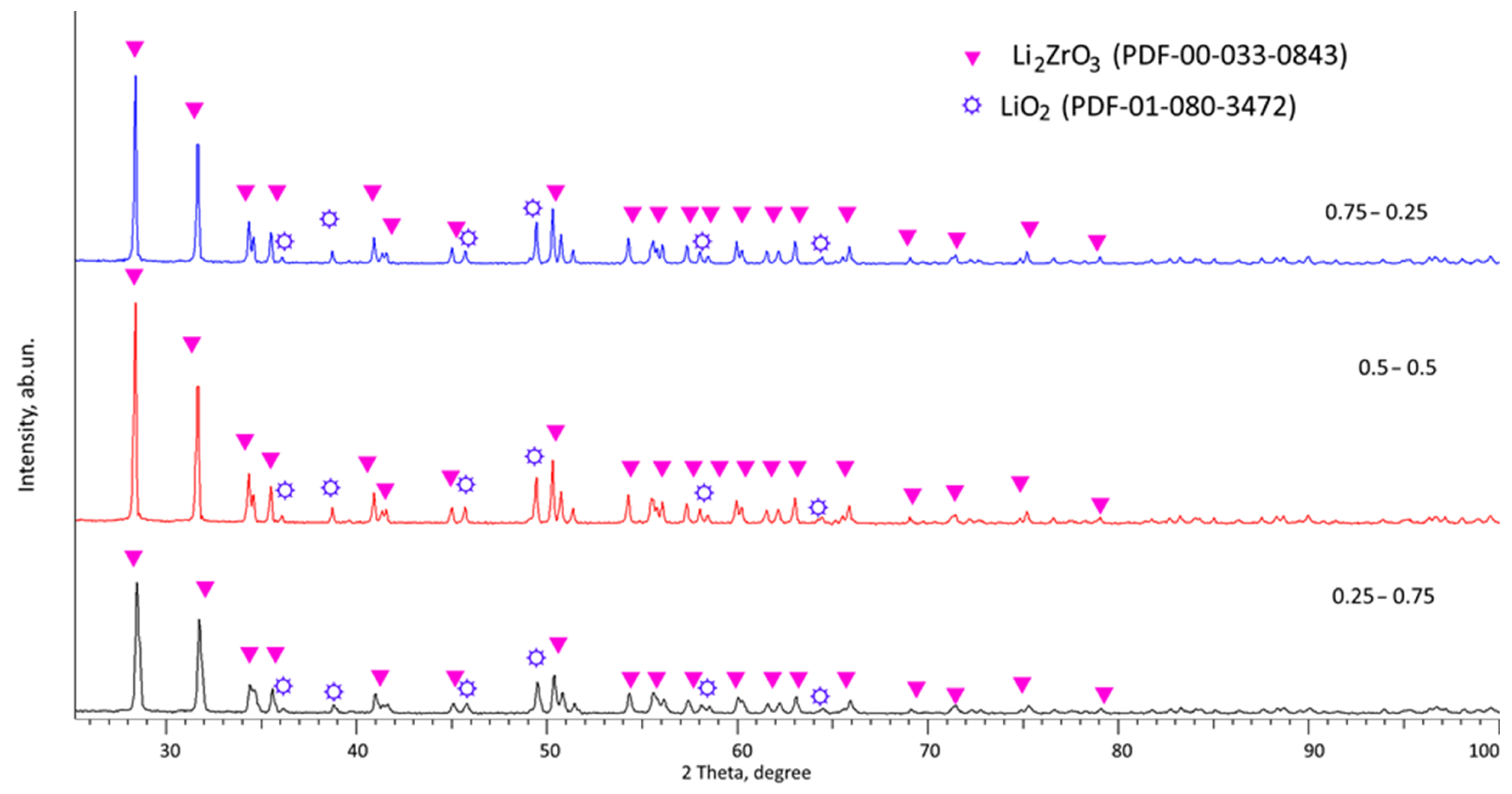
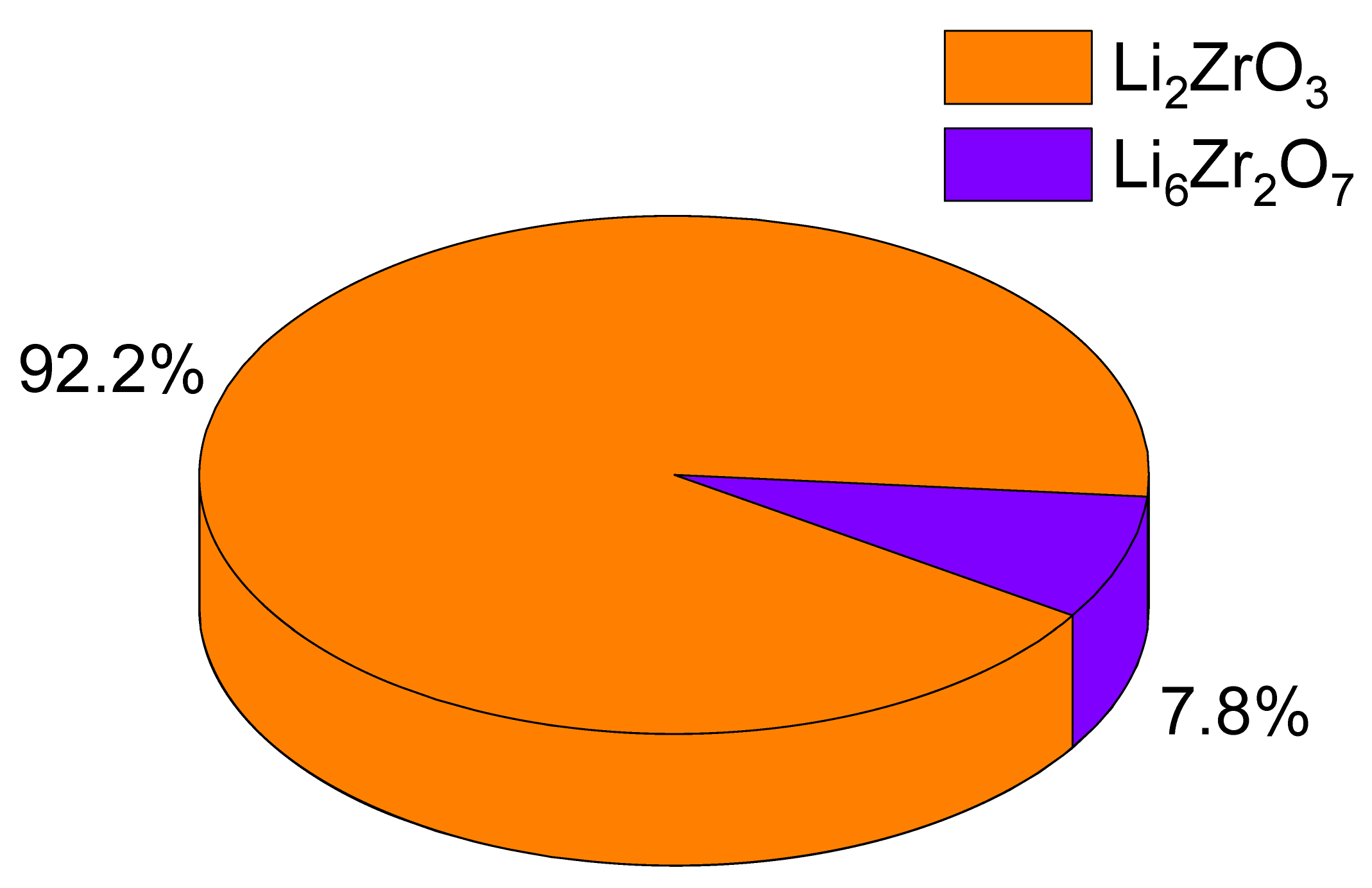
Figure 1 presents the X-ray diffraction results for the ceramic samples obtained from composition 1, with varying proportions of the components. These diffraction patterns reveal the impact of altering the initial component concentrations on the phase composition, specifically the changes in the relative amounts of the identified components within the samples. The X-ray diffraction data indicate that the primary phase in all three samples is the Li
2ZrO
3 (PDF-00-033-0843) monoclinic phase, and characteristic reflections of the LiO
2 (PDF-01-080-3472) orthorhombic phase are also detected. An analysis of the weight percentages of these phases, based on the determination of the weight ratio of the areas of diffraction reflections for each established phase, is detailed in
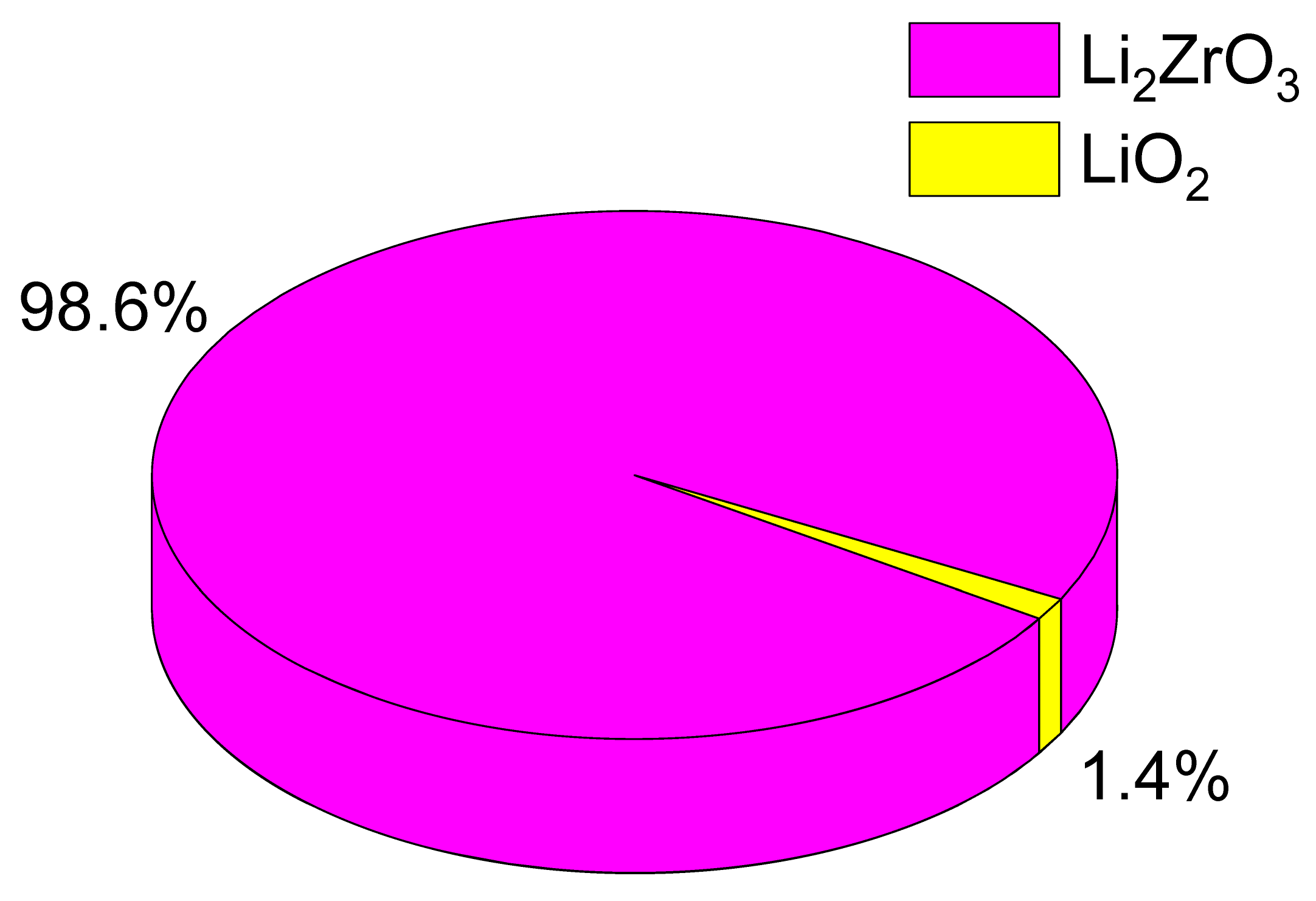
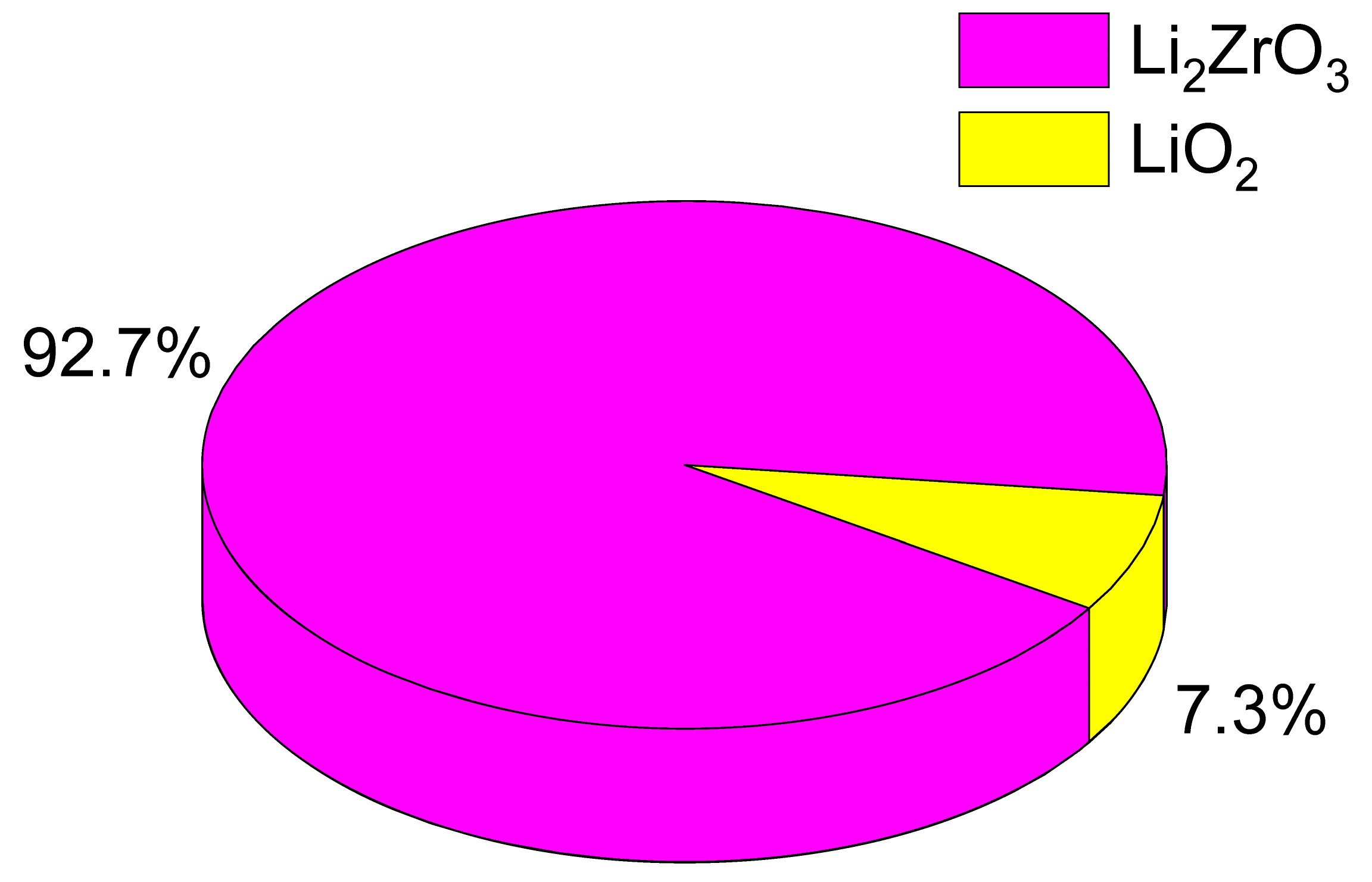
Table 1. As per the provided data, an increase in the LiClO
4·3H
2O mixture composition results in a rise in the contribution of the LiO
2 impurity phase, increasing from 1.4 wt% to 5.7 and 7.3 wt%. Concurrently, the assessment of structural parameters, also depicted in
Figure 2, signifies an improvement in the crystal lattice of the primary Li
2ZrO
3 phase, as evidenced by a reduction in the parameters and volume of the crystal lattice. Comparable alterations in structural parameters with variations in the component ratios are also observed for the LiO
2 impurity orthorhombic phase.
Analyzing the data obtained, we can conclude that when lithium perchlorate (LiClO
4·3H
2O) is used as a lithium-containing component, the main phase in the ceramics is the monoclinic phase Li
2ZrO
3. At the same time, at high concentrations of LiClO
4·3H
2O in the composition, the formation of the orthorhombic phase of LiO
2 in the form of inclusions is observed, the maximum content of which is no more than 7.3 wt%. Moreover, according to the data of works [
28,
29], the content of impurity inclusions, even in the case of varying thermal sintering conditions, does not exceed 10 wt%.
Therefore, upon examining the observed alterations in structural parameters, it can be deduced that as the concentration of LiClO4·3H2O increases, the ceramics become denser owing to the structural organization of the recognized phases. Furthermore, upon assessing the phase composition, an augmentation in the LiO2 impurity phase within the ceramic composition was ascertained. This, in turn, can potentially impact modifications in the thermophysical and strength properties of the ceramics.
Figure 1.
Results of X-ray phase analysis of the studied samples of lithium-containing ceramics depending on the variation in the concentration of the initial components.
Figure 1.
Results of X-ray phase analysis of the studied samples of lithium-containing ceramics depending on the variation in the concentration of the initial components.
Table 1.
Data from phase analysis and structural parameters of the studied ceramics obtained from composition 1.
Table 1.
Data from phase analysis and structural parameters of the studied ceramics obtained from composition 1.
| 0.25–0.75 | 0.5–0.5 | 0.75–0.25 |
![Ceramics 06 00147 i001]() | ![Ceramics 06 00147 i002]() | ![Ceramics 06 00147 i003]() |
| Li2ZrO3 Monoclinic, C2/c(15) (PDF-00-033-0843) |
| a = 5.3936 Å, b = 8.9584 Å, c = 5.3201 Å, β = 112.477°, V = 237.53 Å3 | a = 5.3883 Å, b = 8.9548 Å,
c = 5.3191 Å, β = 112.387°,
V = 237.30 Å3 | a = 5.3839 Å, b = 8.9477 Å,
c = 5.3148 Å, β = 112.297°,
V = 236.89 Å3 |
| LiO2 Orthorhombic, Pnnm(58) (PDF-01-080-3472) |
| a = 3.9875 Å, b = 4.8722 Å, c = 2.9581 Å, V = 57.37 Å3 | a = 3.9803 Å, b = 4.8742 Å,
c = 2.9558 Å, V = 57.34 Å3 | a = 3.9771 Å, b = 4.8683 Å, c = 2.9534 Å, V = 57.18 Å3 |
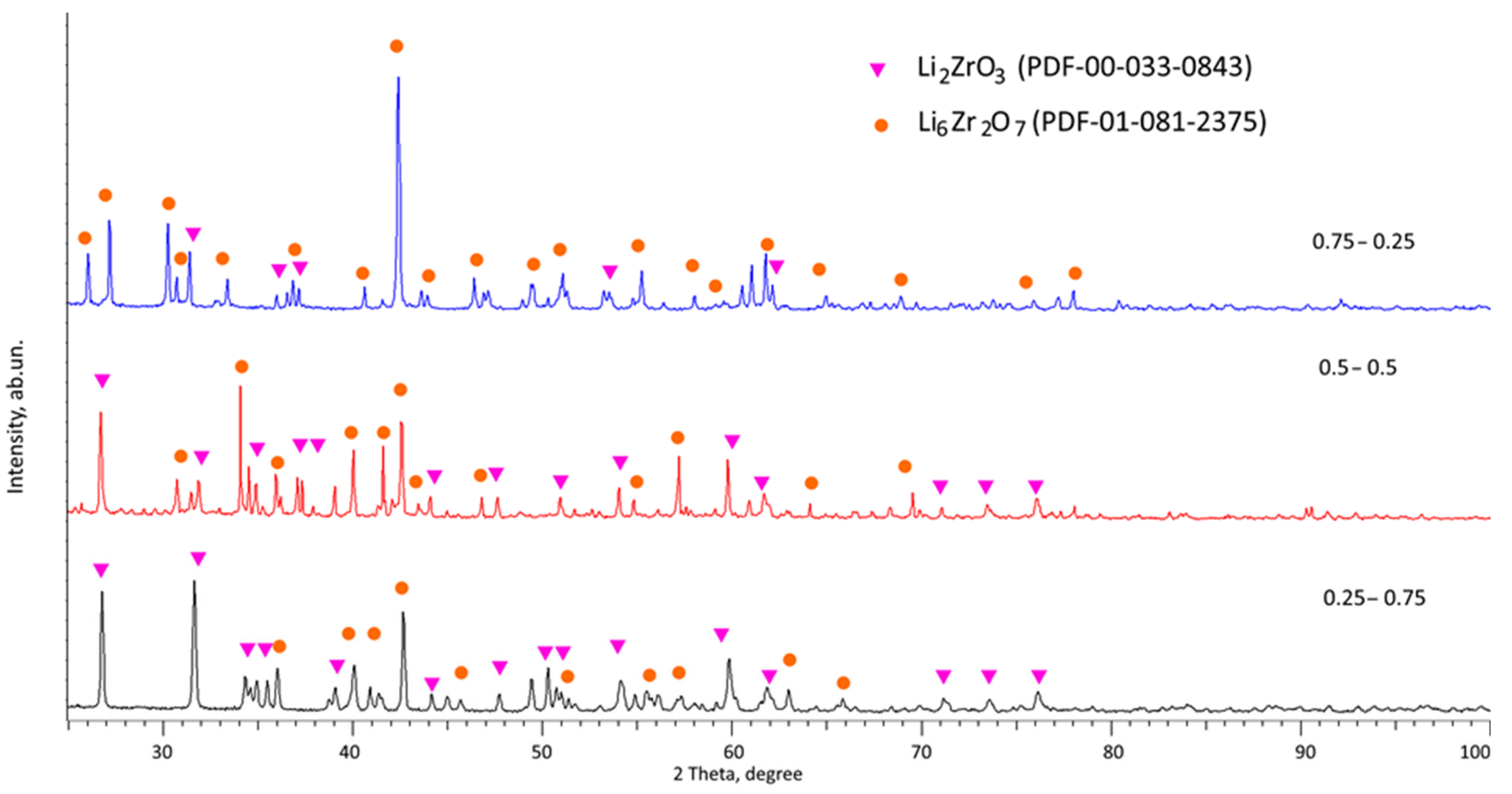
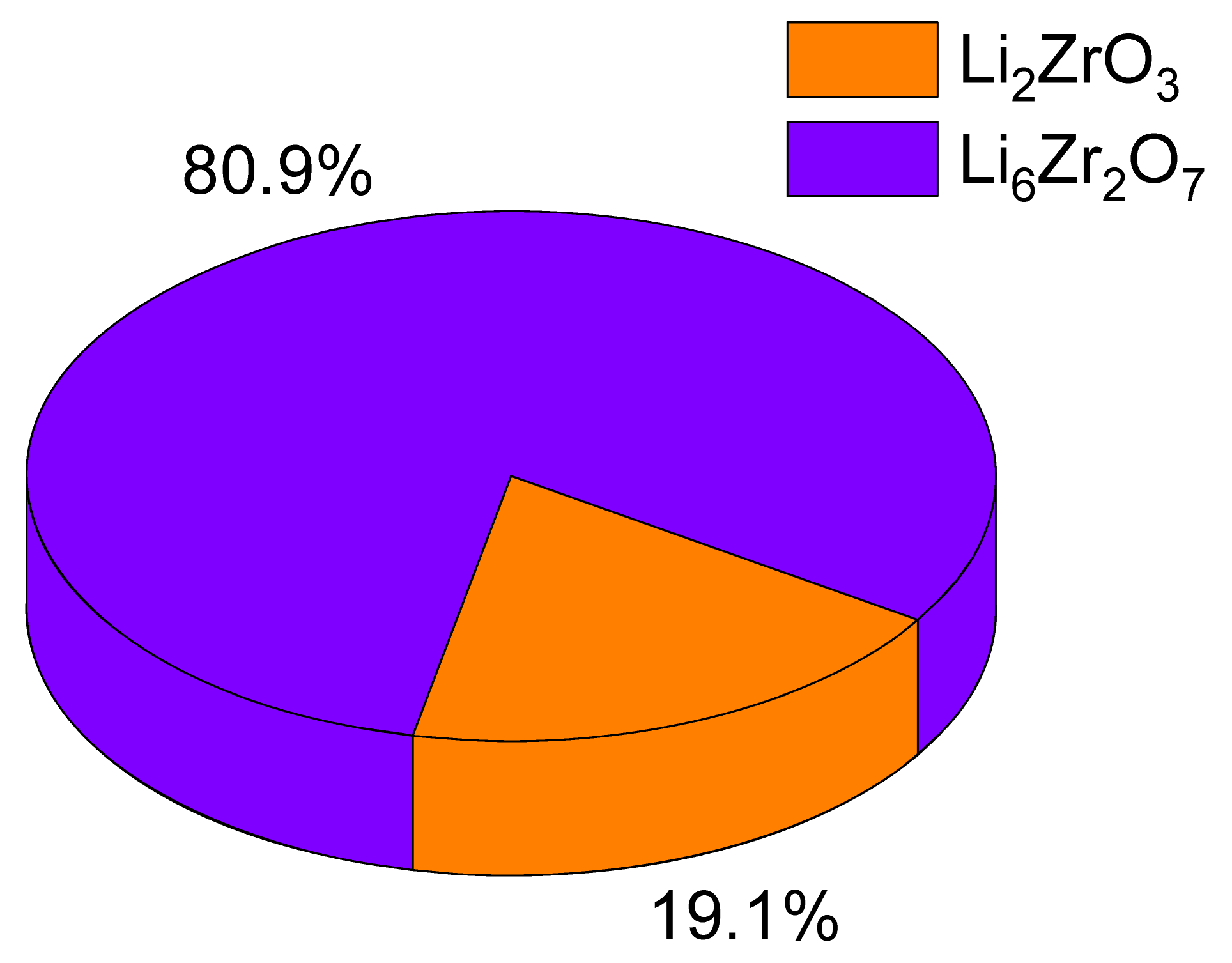
Figure 2 shows the data on changes in X-ray phase analysis of the studied ceramic samples obtained from composition 2 (in which Li
2CO
3 was used as a lithium-containing component). According to the assessment of structural parameters, as well as the shape and position of diffraction reflections, a change in the concentration of the Li
2CO
3 component (due to its increase in content) leads to more pronounced changes in the phase composition during mechanochemical stirring and subsequent thermal annealing. At a low concentration of Li
2CO
3 in the ceramic composition, the presence of two monoclinic phases, Li
2ZrO
3 (PDF-00-033-0843) and Li
6Zr
2O
7 (PDF-01-081-2375), is observed. Simultaneously, the dominant phase, Li
2ZrO
3, comprises over 90% of the composition. It is noteworthy that the close-to-symmetrical configuration of the diffraction reflections suggests a minimal presence of structural distortions within the structure. As the Li
2CO
3 concentration within the ceramic composition changes, there is a more than twofold surge in the Li
6Zr
2O
7 phase contribution in the composition when the component ratios in composition 2 are equal. It exhibits dominance, exceeding 80%, when the component ratio is 0.75 to 0.25. This shift in the phase composition signifies that the inclusion of Li
2CO
3 in the initial mixtures, followed by mechanochemical milling and subsequent thermal annealing, triggers phase transformation processes. These processes are linked to the transformation of the Li
2ZrO
3 phase into Li
6Zr
2O
7, all while preserving the structural motif and crystal lattice type. Simultaneously, an examination of the crystal lattice parameters and volume, as detailed in
Table 2, highlights that the prevalence of Li
6Zr
2O
7 results in more pronounced structural ordering. The changes in lattice parameters and volume are more significant at concentrations of 0.75 to 0.25 than at other concentrations.
Figure 2.
Results of X-ray phase analysis of the studied samples of lithium-containing ceramics depending on the variation in the concentration of the initial components.
Figure 2.
Results of X-ray phase analysis of the studied samples of lithium-containing ceramics depending on the variation in the concentration of the initial components.
Table 2.
Data from phase analysis and structural parameters of the studied ceramics obtained from composition 2.
Table 2.
Data from phase analysis and structural parameters of the studied ceramics obtained from composition 2.
| 0.25–0.75 | 0.5–0.5 | 0.75–0.25 |
![Ceramics 06 00147 i004]() | ![Ceramics 06 00147 i005]() | ![Ceramics 06 00147 i006]() |
| Li2ZrO3 Monoclinic, C2/c(15) (PDF-00-033-0843) |
| a = 5.3953 Å, b = 9.0068 Å, c = 5.3889 Å, β = 112.414°, V = 242.09 Å3 | a = 5.4006 Å, b = 8.9803 Å,
c = 5.3773 Å, β = 112.171°,
V = 241.51 Å3 | a = 5.3826 Å, b = 8.9645 Å,
c = 5.3657 Å, β = 111.973°,
V = 240.10 Å3 |
| Li6Zr2O7 Monoclinic, C2/c(15) (PDF-01-081-2375) |
| a = 10.4216 Å, b = 5.9710 Å, c = 10.1699 Å, β = 99.994°, V = 623.25 Å3 | a = 10.3909 Å, b = 5.9559 Å, c = 10.2358 Å, β = 99.975°, V = 623.88 Å3 | a = 10.3644 Å, b = 5.9336 Å, c = 10.1937 Å, β = 100.034°, V = 617.31 Å3 |
The main difference in the use of lithium-containing components in the form of Li2CO3 and LiClO4·3H2O is that when lithium carbonate is used, the phase composition of the ceramics is presented in the form of two monoclinic phases Li2ZrO3 and Li6Zr2O7, the contributions of which vary with changes in the concentration of lithium carbonate. Moreover, in the case of using lithium perchlorate, the dominant phase in the composition of the resulting ceramics is the monoclinic phase Li2ZrO3, and also in the composition of the ceramics, the presence of impurity inclusions in the form of the orthorhombic phase LiO2 is observed, the content of which varies from 1.4 to 7.3 wt%.
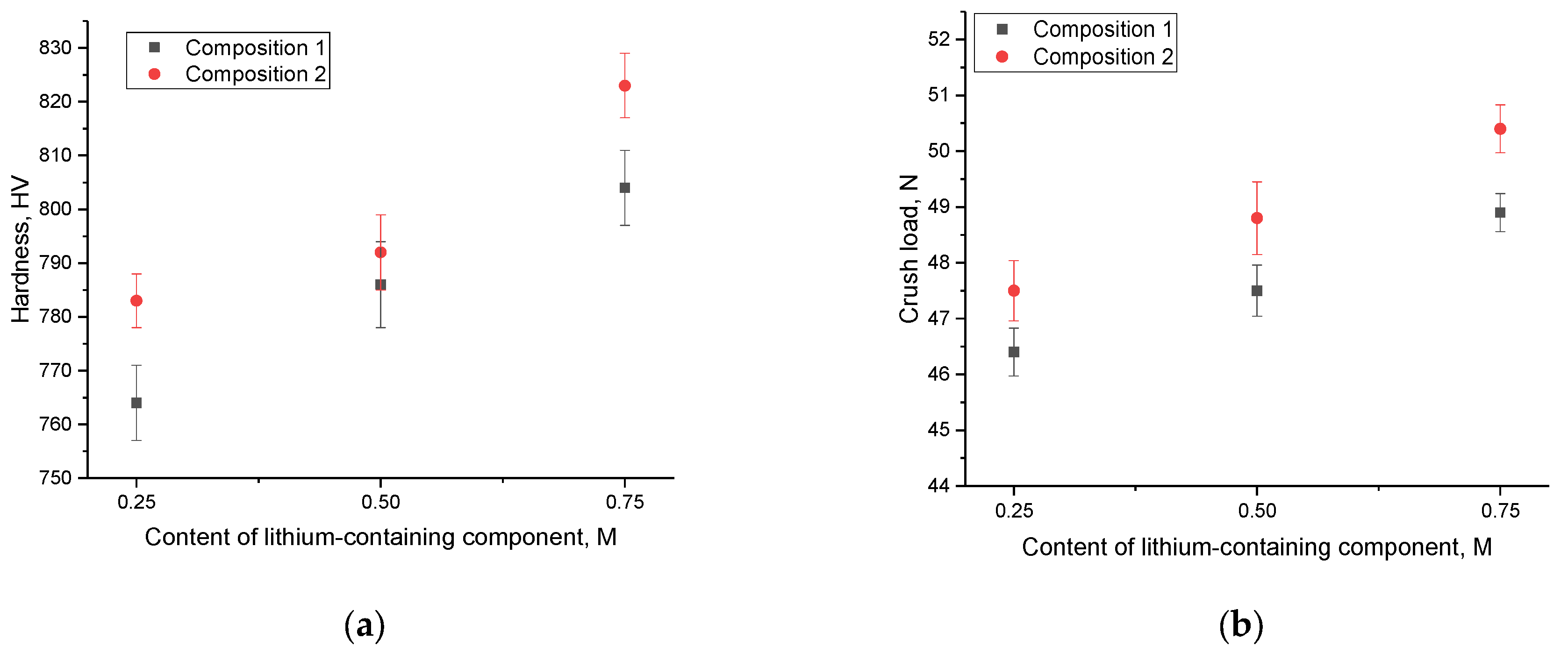
Figure 3 depicts the results concerning the variations in the strength characteristics of the lithium-containing ceramics studied. These ceramics were obtained from different compositions where the components were altered to achieve two-phase ceramics. The general trend observed in the strength parameter data indicates a favorable impact from the introduction of impurity phases, such as LiO
2 (for composition 1) and Li
6Zr
2O
7 (for composition 2). Furthermore, in the case of composition 2, where Li
6Zr
2O
7 predominates in the composition of the ceramic, a more noticeable shift in strength parameters is observed. This can be attributed to the effects of structural ordering, which ranges from 89% to 93% in ceramic samples. Additionally, the formation of interphase boundaries hinders the propagation of microcracks and fractures within the ceramics. Moreover, in the case of using composition 1, the formation of the LiO
2 impurity phase at high concentrations of the lithium-containing component LiClO
4·3H
2O leads to maximum strengthening of no more than 7–8%, while when using composition 2, strengthening and resistance to cracking under external load exceeds 9–11%.
Figure 4 demonstrates the results of a comparative analysis of alterations in the strengthening efficiency of ceramics obtained from different compositions, depending on the concentration of the impurity phase (in the case of composition 1—LiO
2 phase, in the case of composition 2—Li
6Zr
2O
7 phase). As a comparison, the values of hardness and resistance of ceramics to cracking under single compression were taken from works [
28,
29]. The hardening values were calculated taking into account changes in hardness values and the maximum pressure that the ceramics could withstand during a single compression at a speed of 0.1 mm/min. The overall trend observed in the data suggests that the most significant alterations in the strength characteristics of ceramics due to variations in impurity inclusions occur at low concentrations. This is true for ceramics obtained from both composition 1 and composition 2. Furthermore, in the case of composition 2, when the Li
6Zr
2O
7 phase dominates at high concentrations, the strengthening effect is minimal, not exceeding 10%. This can be attributed to the dominance of the Li
6Zr
2O
7 phase in the ceramic composition, which results in a reduction of its strengthening properties. The strengthening effect in ceramics resulting from an increase in impurity inclusions is attributed to the presence of interphase boundaries. A higher concentration of these boundaries hinders the propagation of microcracks and cleavages under external loads and compression of the samples. However, when the Li
6Zr
2O
7 phase prevails in the ceramic composition, a slight decrease in the rate of strength characteristics’ growth is observed. This can be explained by the dominance of this phase, which has lower strength and a decrease in interphase boundaries. Thus, by analyzing the general alterations in strength characteristics depending on the production conditions (i.e., with variations in the ratio of the initial components), the following conclusion can be drawn. The formation of impurity inclusions in the form of LiO
2 or Li
6Zr
2O
7 results in a growth in strength characteristics (hardening of ceramics), which can be explained by the effects of interphase boundaries, the formation of which leads to a rise in resistance to cracking under external mechanical influences. In this case, the most pronounced changes are observed for two-phase ceramics, in which the Li
6Zr
2O
7 phase dominates.
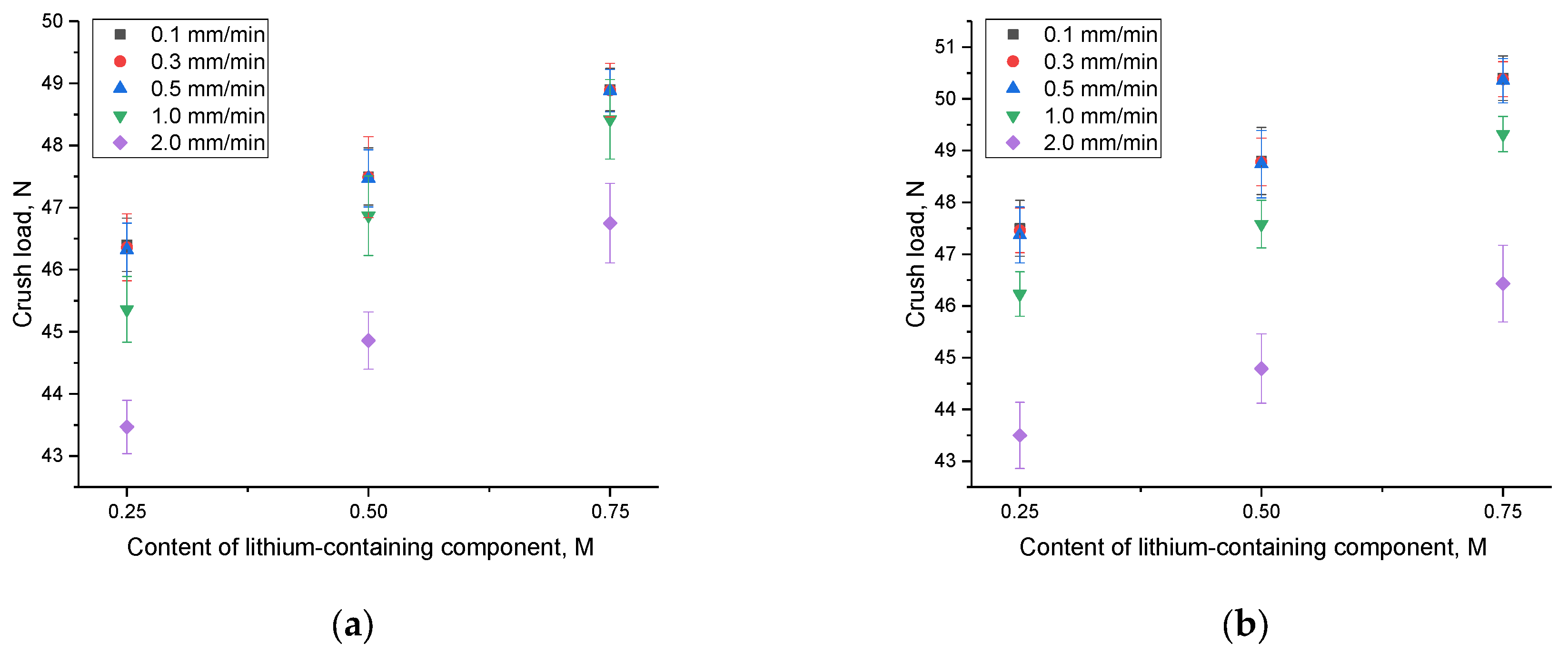
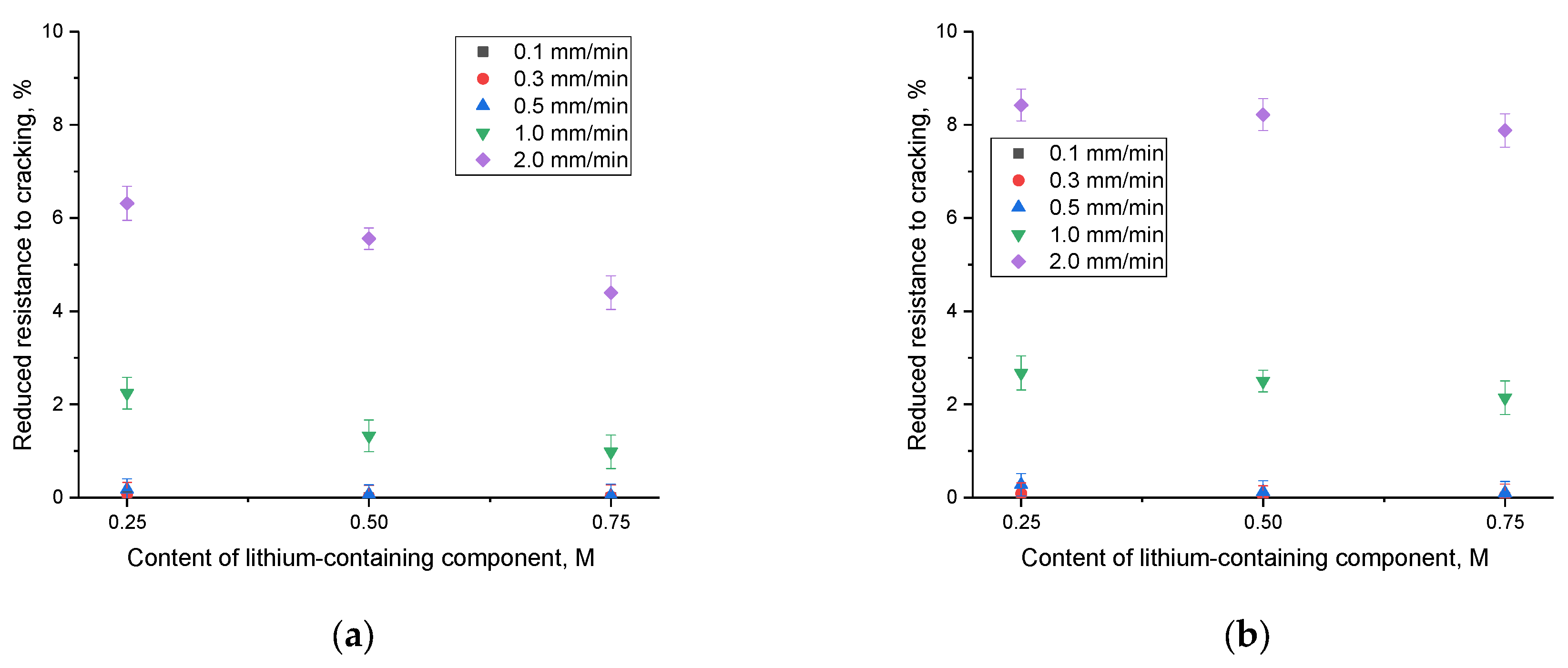
A change in the speed of mechanical influence on samples leads to an acceleration of the propagation of microcracks in the samples, which in turn leads to accelerated cracking at lower values of external load. In this case, the accelerated mechanical impact on the samples leads to the rapid spread of deformations and an increase in stress in the samples, which is accompanied by an acceleration of the destructive propagation of cracks. The results of experiments on changing the loading rate on samples under single compression are presented in
Figure 5. The compression rate varied within the range of 0.1 mm/min, 0.3 mm/min, 0.5 mm/min, 1 mm/min, and 2 mm/min.
An examination of the data displayed in
Figure 5 revealed that at low compression rates, there are minimal alterations in the crack resistance value (the maximum pressure ceramics can endure during a single compression). This suggests that ceramics exhibit remarkable resistance to minor fluctuations in the speed of external influences. However, as the compression rate escalates, a reduction in crack resistance becomes evident (refer to
Figure 6), signifying that at elevated rates of external influences, there is a rapid surge in the propagation of microcracks and structural deformations, resulting in cracking at lower external load values. It is worth mentioning that when the concentration of impurity inclusions increases, a reduction in the extent of changes in crack resistance at high speeds is evident. This signifies a favorable trend in the impact of interphase boundaries on resistance to structural deformations under external loads and underscores the enhanced stability of ceramics’ strength properties. Additionally, an analysis of these variations, as depicted in
Figure 5 and
Figure 6, highlights that the ceramics obtained from composition 1 exhibit the highest resistance to external influences. This is evident as the decline in resistance to external influences under heavy loads is less pronounced for them.
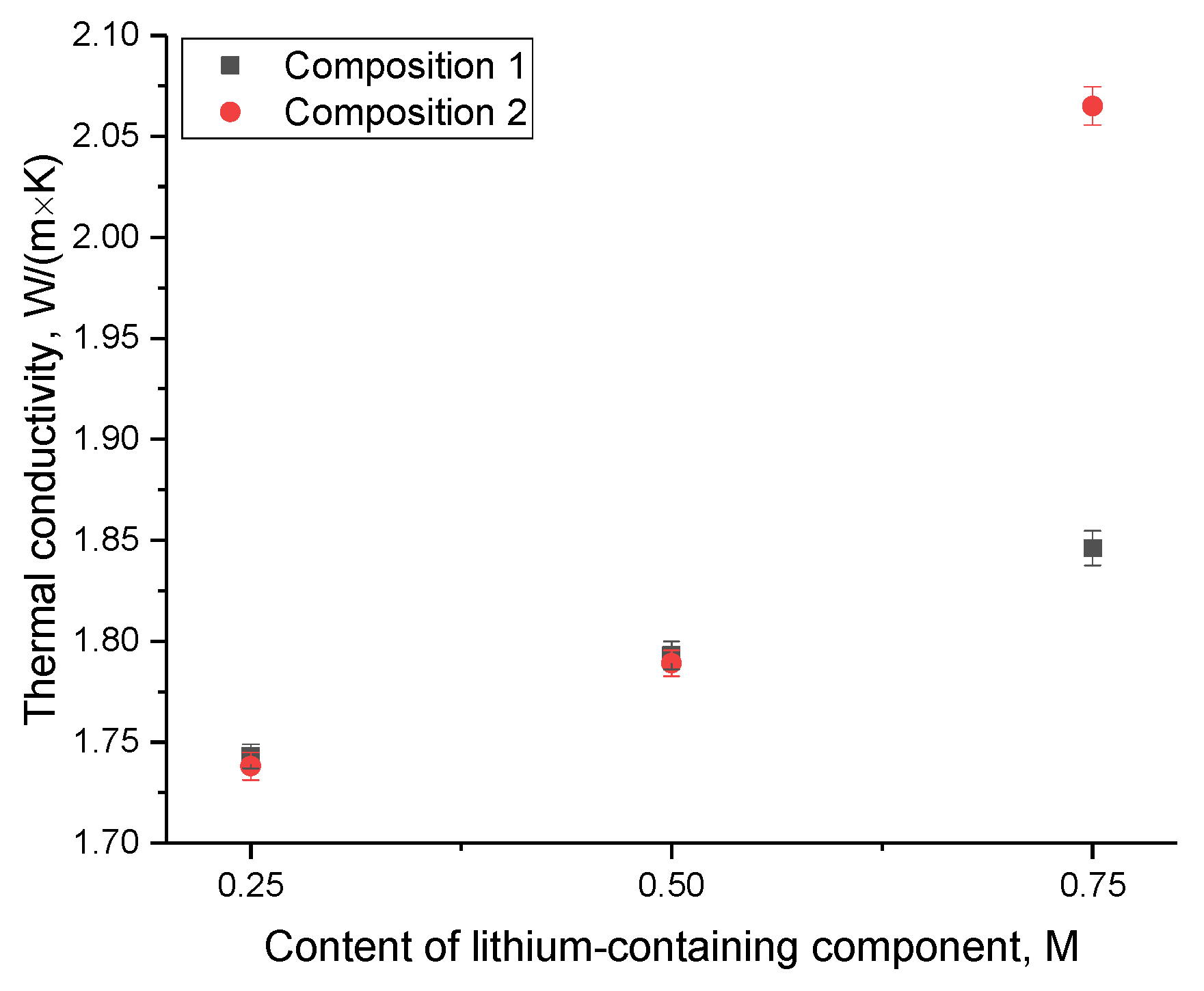
An evaluation of the thermal conductivity variation in ceramics concerning different compositions and component concentrations is depicted in
Figure 7. These measurements encompassed a temperature range from 25 to 700 °C and employed the longitudinal thermal conductivity flow measurement method. It is noteworthy that the increase in temperature from 25 to 700 °C did not yield substantial alterations in the thermal conductivity coefficient. This underscores the remarkable stability of thermophysical parameters across the entire temperature range under consideration.
As evident from the data presented, the variations in the thermal conductivity coefficient are indicative of the beneficial impact of minor impurity inclusions, which contribute to an increase in thermal conductivity. When using Li
2CO
3 as the lithium-containing component at elevated concentrations, leading to the dominance of the Li
6Zr
2O
7 phase, a marked escalation in thermal conductivity is observed in comparison to composition 1. This decrease can be elucidated by the predominance of the Li
6Zr
2O
7 phase, characterized by superior thermal conductivity properties when contrasted with the Li
2ZrO
3 phase [
30]. It is noteworthy that the thermal conductivity values for the acquired ceramic samples surpass the thermal conductivity values of analogous samples obtained through the Hot Wire Method [
31]. These disparities can be ascribed to not only the presence of interphase boundaries but also an upsurge in the degree of structural ordering. A modification in the degree of structural ordering results in fewer structural deformations in the crystal lattice, subsequently leading to a reduction in the defective inclusions that impede phonon heat transfer.
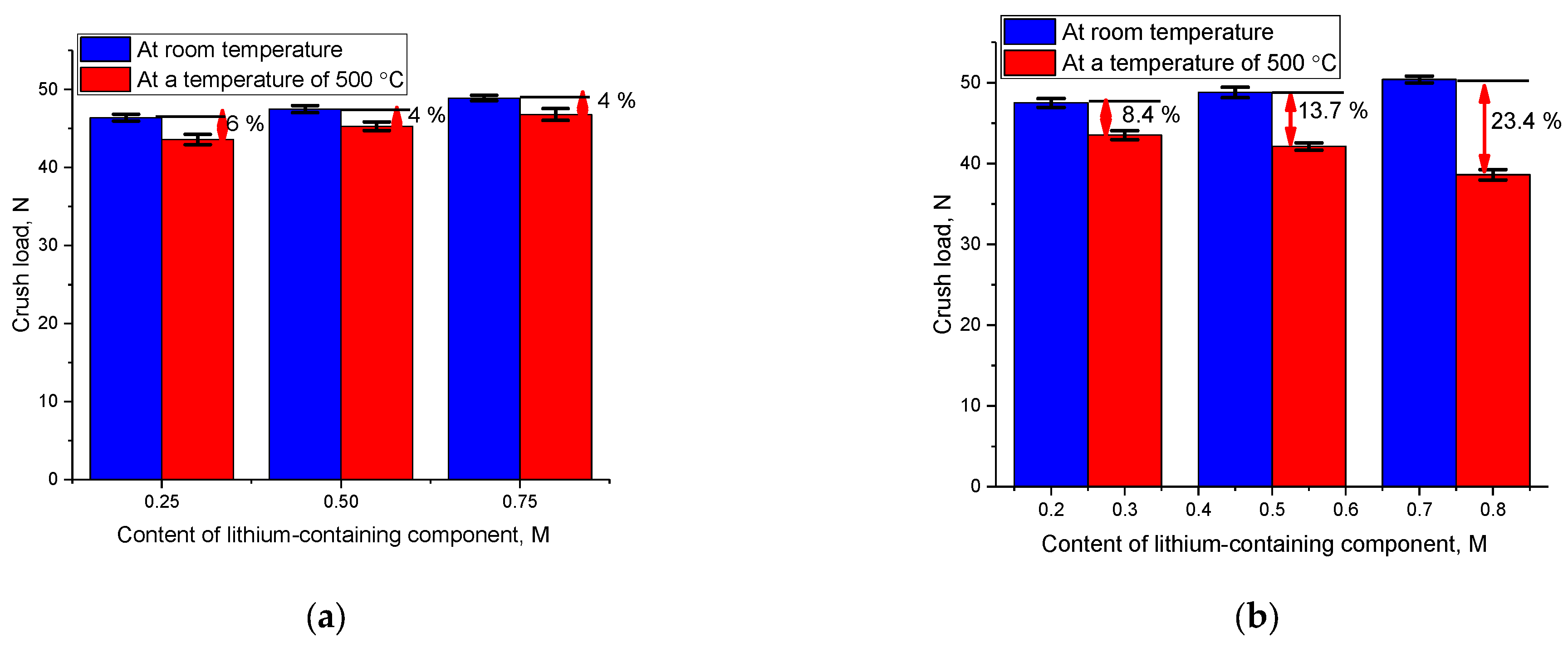
One of the important parameters for assessing the resistance of ceramics to external influences is the determination of their resistance to external influences under conditions of elevated temperatures corresponding to conditions as close as possible to operating conditions. Under such circumstances, the impact on the samples may be more severe, given that the ceramic specimens are subject to thermal expansion. This, in turn, can have adverse effects on the ceramics’ strength properties and stability dynamics. The experiments were conducted using a testing apparatus equipped with a heating chamber, which facilitates the heating of samples up to 500 °C, enabling temperature stabilization, followed by a single compression test.
Figure 8 illustrates a comparative assessment of variations in the resistance to single compression, both at room temperature and at 500 °C, offering insights into the data related to resistance to external forces.
The data presented indicate that when dealing with ceramics containing the LiO2 impurity phase, raising the testing temperature from room temperature to 500 °C results in a minor reduction in the resistance to single compression (within a range of 4–6%). Furthermore, an increase in the concentration of the LiO2 impurity phase diminishes the disparities in the data concerning variations in resistance to single compression, which can be attributed to heightened resistance to thermal expansion of ceramics when subjected to heat. For two-phase ceramics of the Li2ZrO3/Li6Zr2O7 composition, an elevated concentration of the Li6Zr2O7 phase with rising test temperatures during single compression results in a significant decrease in stability. This phenomenon can be attributed to variations in the thermal expansion of the crystal lattice, alongside a reduced resistance of ceramics to external forces stemming from thermal expansion.
Figure 9 shows the results of changes in the value of volumetric expansion of samples after thermal tests for resistance to long-term temperature heating of 500 h.
The data analysis reveals that an increase in the concentration of impurity inclusions in the form of the LiO
2 phase in the ceramic composition leads to enhanced stabilization of the crystal lattice’s thermal expansion. This is substantiated by the findings on the stability of strength characteristics, as shown in
Figure 9b. Conversely, the prevalence of the Li
6Zr
2O
7 phase in the ceramic composition (derived from composition 2) disrupts both thermal stability (significantly elevating the coefficient of volumetric thermal expansion by over 1.5 times) and the hardness of the ceramics. These findings indicate a decline in strength characteristics’ stability during high-temperature aging.
In the case of two-phase ceramics, it was found that an increase in the contribution of the Li6Zr2O7 phase leads to increased stability to high-temperature aging and degradation, due to increased stability to softening, as well as a decrease in the effect of thermal volumetric expansion of the crystal structure. In turn, the increase in resistance to cracking during long-term high-temperature degradation (high-temperature aging) for two-phase ceramics in the case of the dominance of the Li6Zr2O7 phase can be explained by interphase boundaries, the presence of which is an obstacle to the propagation of microcracks under external influences (mechanical pressure), and the presence of interphase boundaries leads to an increase in resistance to volumetric thermal expansion of the crystal structure as a result of prolonged thermal effects.