Tuning the Coefficient of Thermal Expansion of Transparent Lithium Aluminosilicate Glass-Ceramics by a Two-Stage Heat Treatment
Abstract
:1. Introduction
2. Materials and Methods
2.1. Glass Synthesis and Crystallization
2.2. Glass and Glass-Ceramics Characterization
3. Results and Discussion
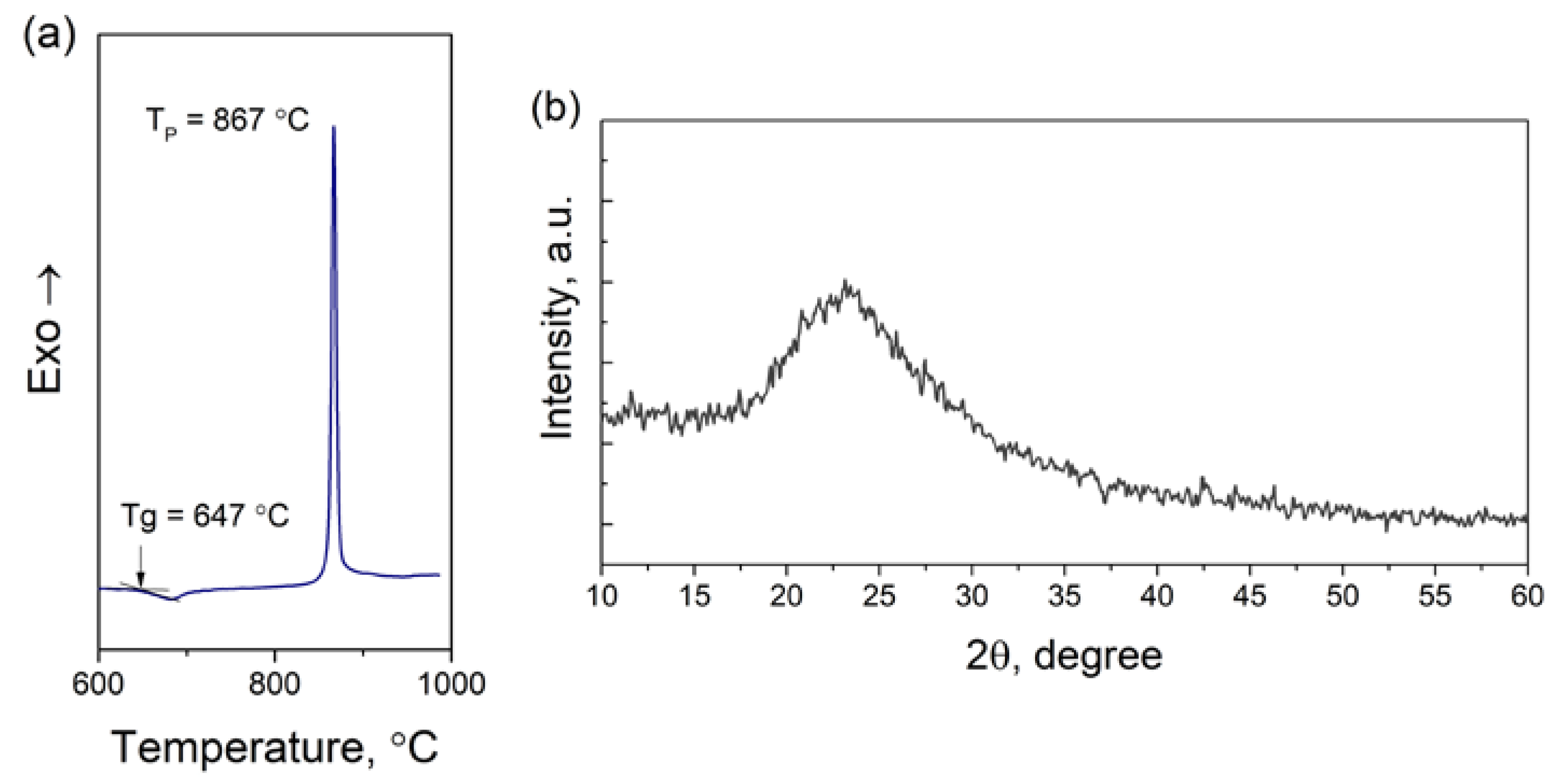
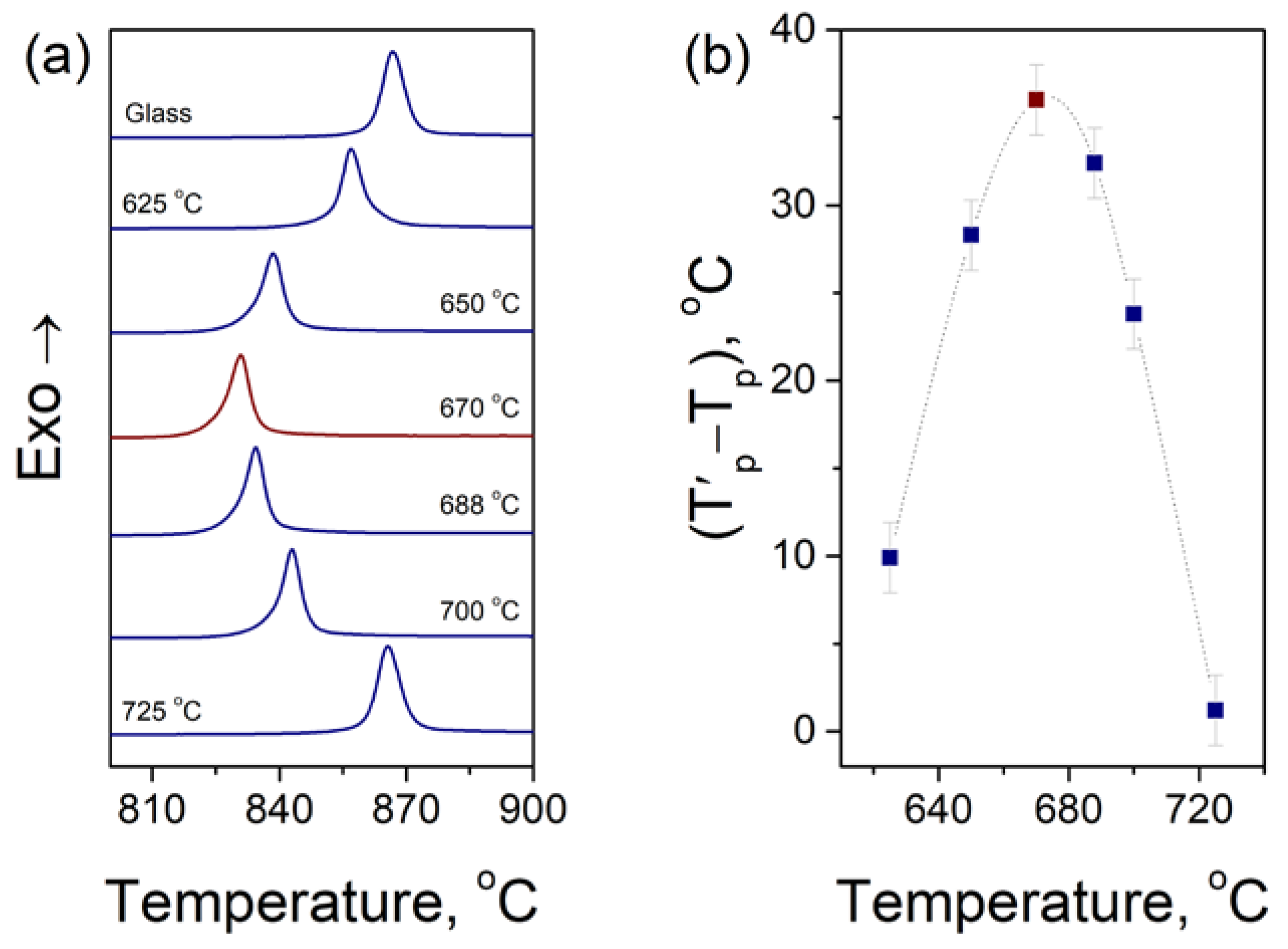
3.1. Determination of the Nucleation Temperature
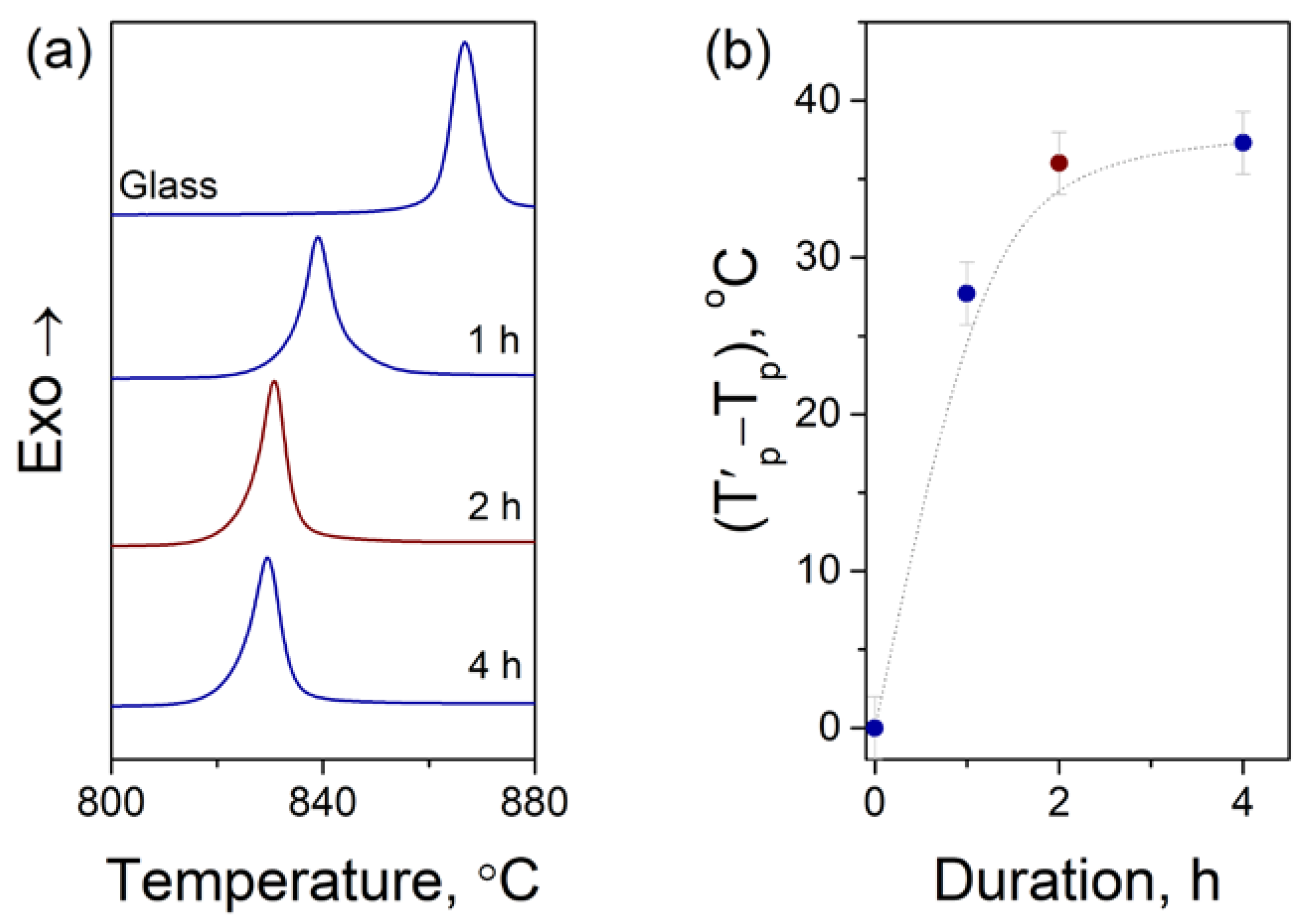
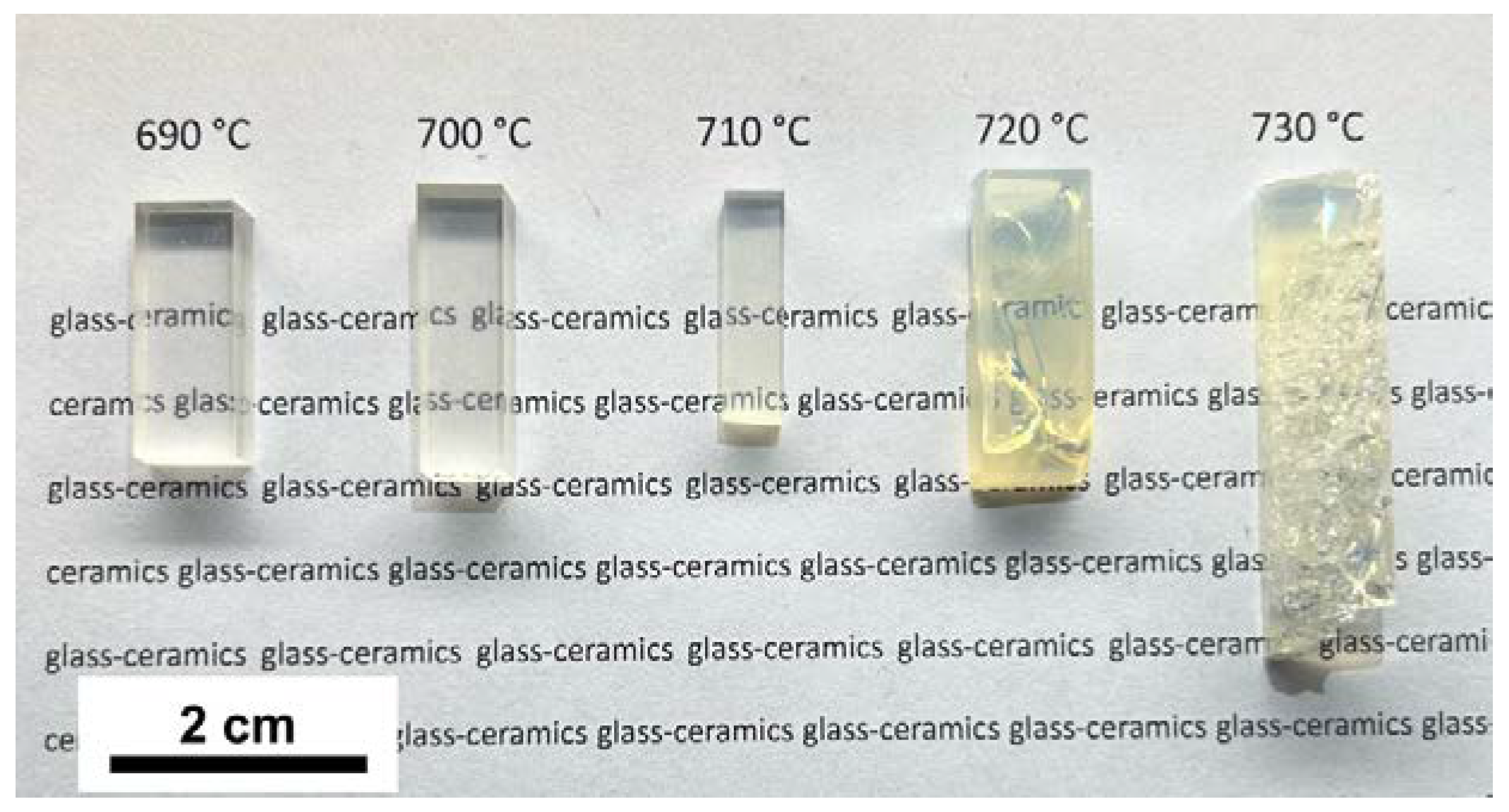
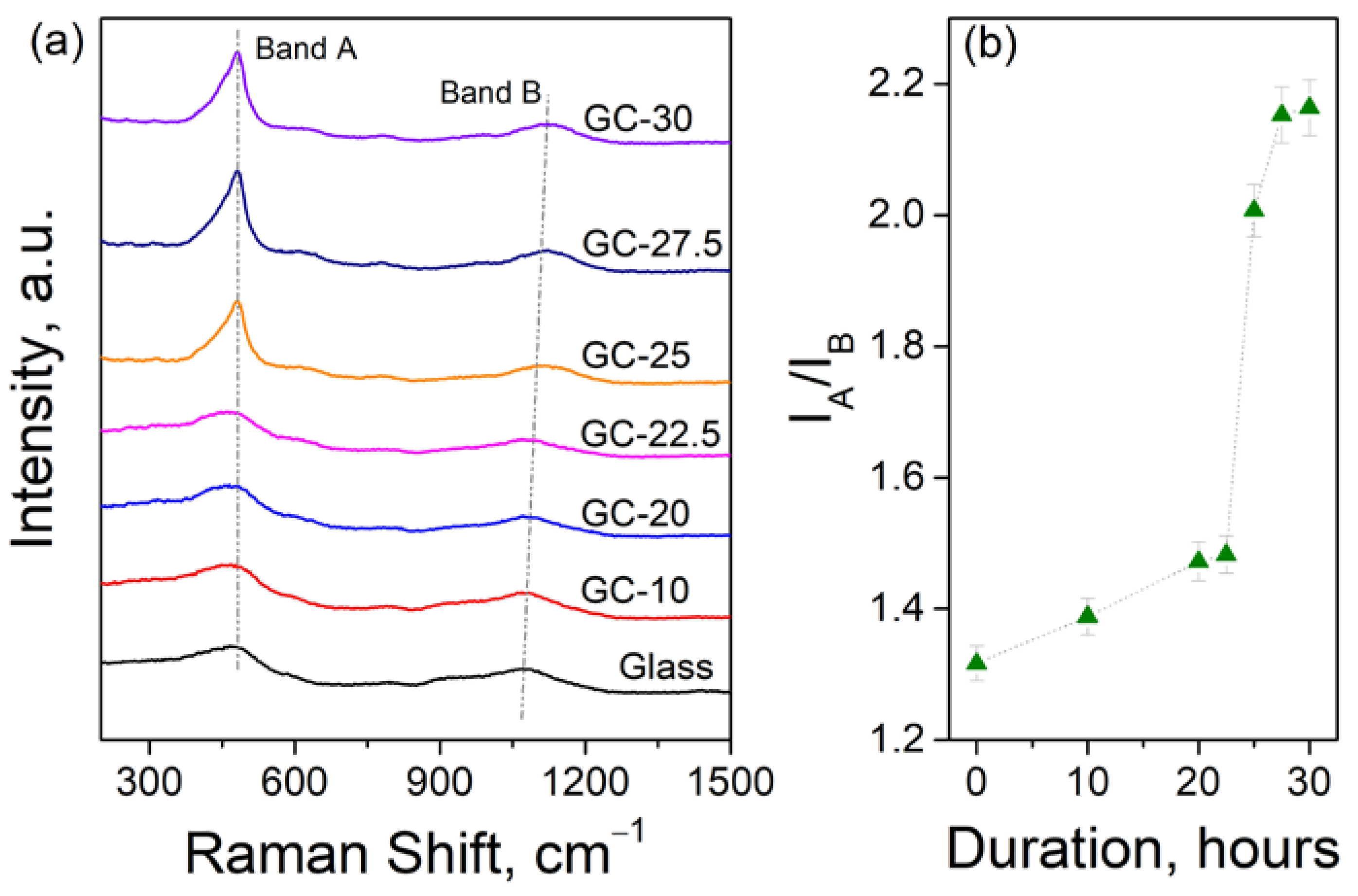
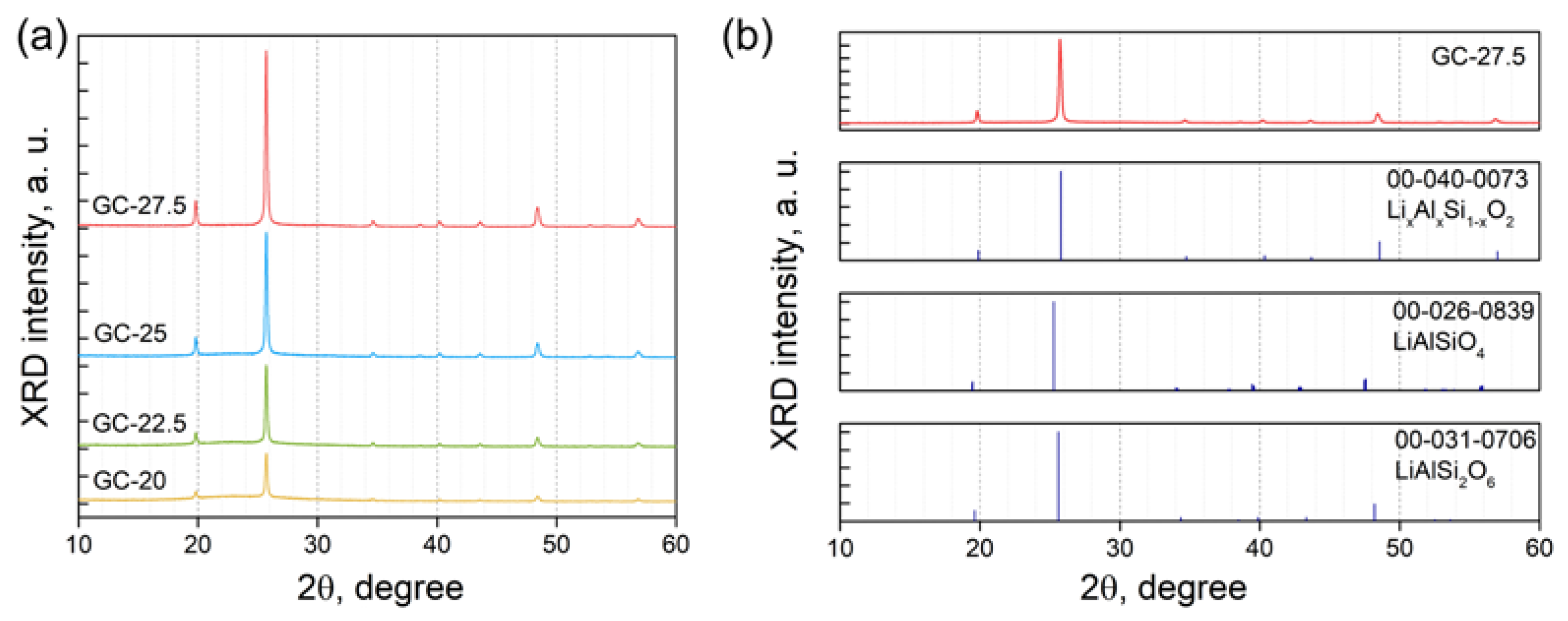
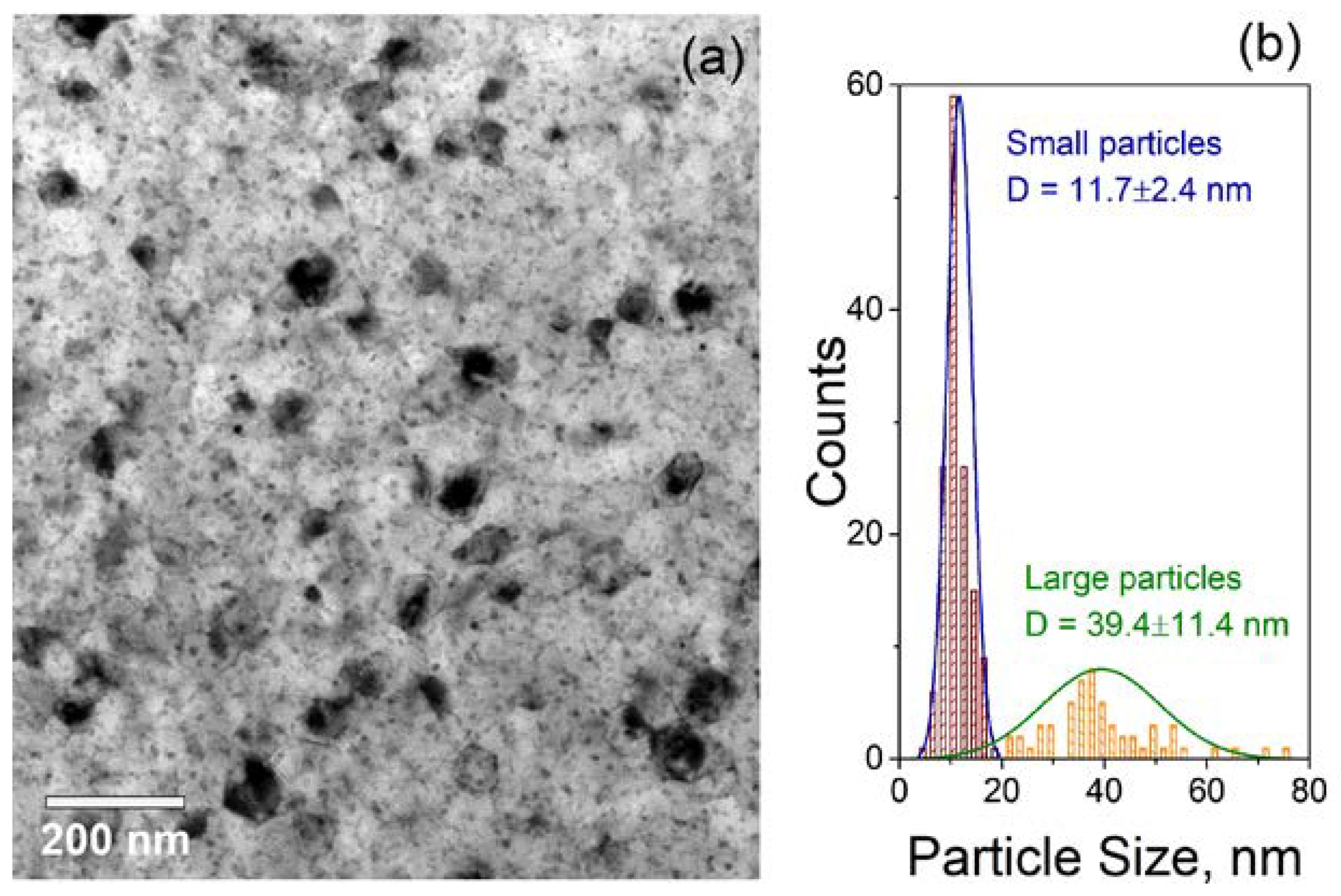
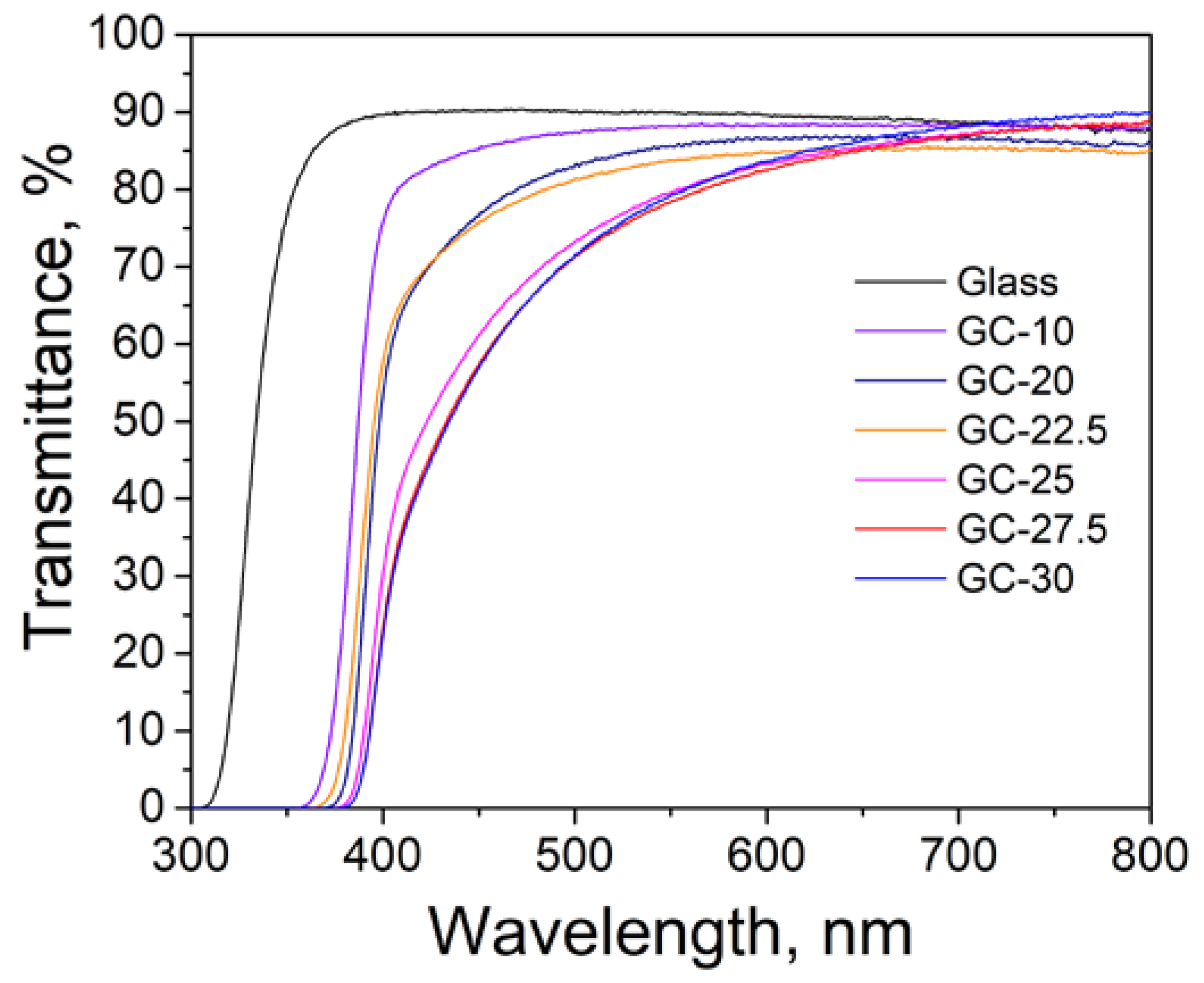
3.2. Effect of Crystallization Conditions on the Microstructure and Properties of Glass-Ceramics
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Venkateswaran, C.; Sreemoolanadhan, H.; Vaish, R. Lithium aluminosilicate (LAS) glass-ceramics: A review of recent progress. Int. Mater. Rev. 2022, 67, 620–657. [Google Scholar] [CrossRef]
- Dymshits, O.; Shepilov, M.; Zhilin, A. Transparent glass-ceramics for optical applications. MRS Bull. 2017, 42, 200–205. [Google Scholar] [CrossRef]
- Döhring, T.; Jedamzik, R.; Thomas, A.; Hartmann, P. Forty years of ZERODUR mirror substrates for astronomy: Review and outlook. In Proceedings of the SPIE 7018, Advanced Optical and Mechanical Technologies in Telescopes and Instrumentation, Marseille, France, 23–28 June 2008; Volume 7018, pp. 1154–1165. [Google Scholar] [CrossRef]
- Semenov, A.P.; Abdulkadyrov, M.A.; Ignatov, A.N.; Patrikeev, V.E.; Pridnya, V.V.; Polyanchikov, A.V.; Sharov, Y.A. Fabrication of blanks, figuring, polishing and testing of segmented astronomic mirrors for SALT and LAMOST projects. In Proceedings of the SPIE 5494, Optical Fabrication, Metrology, and Material Advancements for Telescopes, Glasgow, UK, 21–25 June 2004; Volume 10315, pp. 34–37. [Google Scholar] [CrossRef]
- Flügge, J.; Köning, R.; Schötka, E.; Weichert, C.; Köchert, P.; Bosse, H.; Kunzmann, H. Improved measurement performance of the Physikalisch-Technische Bundesanstalt nanometer comparator by integration of a new Zerodur sample carriage. Opt. Eng. 2014, 53, 122404. [Google Scholar] [CrossRef]
- Beverini, N.; Di Virgilio, A.; Belfi, J.; Ortolan, A.; Schreiber, K.U.; Gebauer, A.; Klügel, T. High-accuracy ring laser gyroscopes: Earth rotation rate and relativistic effects. J. Phys. Conf. Ser. 2016, 723, 012061. [Google Scholar] [CrossRef]
- Kuznetsov, A.G.; Molchanov, A.V.; Chirkin, M.V.; Izmailov, E.A. Precise laser gyroscope for autonomous inertial navigation. Quantum Electron. 2015, 45, 78–88. [Google Scholar] [CrossRef]
- Manske, E.; Fröhlich, T.; Füßl, R.; Ortlepp, I.; Mastylo, R.; Blumröder, U.; Dontsov, D.; Kuehnel, M.; Köchert, P. Progress of nanopositioning and nanomeasuring machines for cross-scale measurement with sub-nanometre precision. Meas. Sci. Technol. 2020, 31, 085005. [Google Scholar] [CrossRef]
- Mitra, I. ZERODUR: A glass-ceramic material enabling optical technologies. Opt. Mater. Express 2022, 12, 3563–3576. [Google Scholar] [CrossRef]
- Schulz, H. Thermal expansion of beta eucryptite. J. Am. Ceram. Soc. 1974, 57, 313–318. [Google Scholar] [CrossRef]
- Lichtenstein, A.I.; Jones, R.O.; Xu, H.; Heaney, P.J. Anisotropic thermal expansion in the silicate β-eucryptite: A neutron diffraction and density functional study. Phys. Rev. B 1998, 58, 6219–6223. [Google Scholar] [CrossRef]
- Roy, R.; Agrawal, D.K.; McKinstry, H.A. Very low thermal expansion coefficient materials. Annu. Rev. Mater. Sci. 1989, 19, 59–81. [Google Scholar] [CrossRef]
- Gillery, F.H.; Bush, E.A. Thermal contraction of β-eucryptite (Li2O⋅Al2O3⋅2SiO2) by X-ray and dilatometer methods. J. Am. Ceram. Soc. 1959, 42, 175–177. [Google Scholar] [CrossRef]
- Guan, J. Femtosecond-laser-written integrated photonics in bulk glass-ceramics Zerodur. Ceram. Int. 2021, 47, 10189–10192. [Google Scholar] [CrossRef]
- Ferreira, P.H.D.; Fabris, D.C.N.; Boas, M.V.; Bezerra, I.G.; Mendonça, C.R.; Zanotto, E.D. Transparent glass-ceramic waveguides made by femtosecond laser writing. Opt. Laser Technol. 2021, 136, 106742. [Google Scholar] [CrossRef]
- Lipatiev, A.; Fedotov, S.; Lotarev, S.; Naumov, A.; Lipateva, T.; Savinkov, V.; Shakhgildyan, G.; Sigaev, V. Direct laser writing of depressed-cladding waveguides in extremely low expansion lithium aluminosilicate glass-ceramics. Opt. Laser Technol. 2021, 138, 106846. [Google Scholar] [CrossRef]
- Khodakovskaya, R.Y. Chemistry of Titanium-Containing Glasses and Glass Ceramics; Khimiya: Moscow, Russia, 1978. [Google Scholar]
- Khodakovskaya, R.Y.; Sigaev, V.N.; Plutalov, N.F. Phase separation of Li2O–Al2O3–SiO2–TiO2 glass at the initial stages of crystallization. Fiz. Khim. Stekla. 1979, 5, 134–140. [Google Scholar]
- Sakamoto, A.; Yamamoto, S. Glass-ceramics: Engineering principles and applications. Int. J. Appl. Glas. Sci. 2010, 1, 237–247. [Google Scholar] [CrossRef]
- Bach, H.; Krause, D. (Eds.) Low Thermal Expansion Glass Ceramics; Springer: Berlin/Heidelberg, Germany, 2005; pp. 121–235. [Google Scholar]
- Liu, X.; Zhou, J.; Zhou, S.; Yue, Y.; Qiu, J. Transparent glass-ceramics functionalized by dispersed crystals. Prog. Mater. Sci. 2018, 97, 38–96. [Google Scholar] [CrossRef]
- Kleebusch, E.; Patzig, C.; Höche, T.; Rüssel, C. A modified B2O3 containing Li2O-Al2O3-SiO2 glass with ZrO2 as nucleating agent-crystallization and microstructure studied by XRD and (S)TEM-EDX. Ceram. Int. 2018, 44, 19818–19824. [Google Scholar] [CrossRef]
- Wu, J.; Lin, C.; Liu, J.; Han, L.; Gui, H.; Li, C.; Liu, T.; Lu, A. The effect of complex nucleating agent on the crystallization, phase formation and performances in lithium aluminum silicate (LAS) glasses. J. Non-Cryst. Solids 2019, 521, 119486. [Google Scholar] [CrossRef]
- Dymshits, O.; Bachina, A.; Alekseeva, I.; Golubkov, V.; Tsenter, M.; Zapalova, S.; Bogdanov, K.; Danilovich, D.; Zhilin, A. Phase transformations upon formation of transparent lithium alumosilicate glass-ceramics nucleated by yttrium niobates. Ceramics 2023, 6, 1490–1507. [Google Scholar] [CrossRef]
- Maier, V.; Muller, G. Mechanism of oxide nucleation in lithium aluminosilicate glass-ceramics. J. Am. Ceram. Soc. 1987, 70, 176–178. [Google Scholar] [CrossRef]
- Venkateswaran, C.; Sharma, S.C.; Pant, B.; Chauhan, V.S.; Vaish, R. Crystallisation studies on site saturated lithium aluminosilicate (LAS) glass. Thermochim. Acta 2019, 679, 178311. [Google Scholar] [CrossRef]
- Kumar, A.; Chakrabarti, A.; Shekhawat, M.S.; Molla, A.R. Transparent ultra-low expansion lithium aluminosilicate glass-ceramics: Crystallization kinetics, structural and optical properties. Thermochim. Acta 2019, 676, 155–163. [Google Scholar] [CrossRef]
- Zhang, R.; Yi, L.; Kong, F.; Liang, X.; Yin, Z.; Rao, Y.; Wang, D.; Chen, Z.; Yu, X.; Jiang, H.; et al. Rapid preparation of low thermal expansion transparent LAS nanocrystalline glass by one-step thermoelectric treatment. Ceram. Int. 2021, 47, 34380–34387. [Google Scholar] [CrossRef]
- Komatsu, T.; Honma, T. Laser patterning and growth mechanism of orientation designed crystals in oxide glasses: A review. J. Solid State Chem. 2019, 275, 210–222. [Google Scholar] [CrossRef]
- Wang, X.; Yu, H.; Li, P.; Zhang, Y.; Wen, Y.; Qiu, Y.; Liu, Z.; Li, Y.; Liu, L. Femtosecond laser-based processing methods and their applications in optical device manufacturing: A review. Opt. Laser Technol. 2021, 135, 106687. [Google Scholar] [CrossRef]
- Zhang, B.; Wang, L.; Chen, F. Recent advances in femtosecond laser processing of LiNbO3 crystals for photonic applications. Laser Photonics Rev. 2020, 14, 1900407. [Google Scholar] [CrossRef]
- Lei, Y.; Wang, H.; Skuja, L.; Kühn, B.; Franz, B.; Svirko, Y.; Kazansky, P.G. Ultrafast laser writing in different types of silica glass. Laser Photonics Rev. 2023, 17, 2200978. [Google Scholar] [CrossRef]
- Stoian, R.; Colombier, J.-P. Advances in ultrafast laser structuring of materials at the nanoscale. Nanophotonics 2020, 9, 4665–4688. [Google Scholar] [CrossRef]
- Khalil, A.A.; Bérubé, J.-P.; Danto, S.; Desmoulin, J.-C.; Cardinal, T.; Petit, Y.; Vallée, R.; Canioni, L. Direct laser writing of a new type of waveguides in silver containing glasses. Sci. Rep. 2017, 7, 11124. [Google Scholar] [CrossRef]
- Shakhgildyan, G.Y.; Alekseev, R.O.; Golubev, N.V.; Savinkov, V.I.; Naumov, A.S.; Presnyakova, N.N.; Sigaev, V.N. One-step crystallization of gahnite glass-ceramics in a wide thermal gradient. Chemengineering 2023, 7, 37. [Google Scholar] [CrossRef]
- Langford, J.I.; Wilson, A.J.C. Scherrer after sixty years: A survey and some new results in the determination of crystallite size. J. Appl. Crystallogr. 1978, 11, 102–113. [Google Scholar] [CrossRef]
- Kleebusch, E.; Patzig, C.; Höche, T.; Rüssel, C. The evidence of phase separation droplets in the crystallization process of a Li2O-Al2O3-SiO2 glass with TiO2 as nucleating agent—An X-ray diffraction and (S)TEM-study supported by EDX-analysis. Ceram. Int. 2018, 44, 2919–2926. [Google Scholar] [CrossRef]
- Hartmann, P.; Jedamzik, R.; Carré, A.; Krieg, J.; Westerhoff, T. Glass ceramic ZERODUR®: Even closer to zero thermal expansion: A Review, Part 1. J. Astron. Telesc. Instrum. Syst. 2021, 7, 020901. [Google Scholar] [CrossRef]
- Hartmann, P.; Jedamzik, R.; Carré, A.; Krieg, J.; Westerhoff, T. Glass ceramic ZERODUR®: Even closer to zero thermal expansion: A Review, Part 2. J. Astron. Telesc. Instrum. Syst. 2021, 7, 020902. [Google Scholar] [CrossRef]
- Wurth, R.; Muñoz, F.; Müller, M.; Rüssel, C. Crystal growth in a multicomponent lithia aluminosilicate glass. Mater. Chem. Phys. 2009, 116, 433–437. [Google Scholar] [CrossRef]
- Li, M.; Xiong, C.; Ma, Y.; Jiang, H. Study on crystallization process of Li2O–Al2O3–SiO2 glass-ceramics based on in situ analysis. Materials 2022, 15, 8006. [Google Scholar] [CrossRef]
- Marotta, A.; Buri, A.; Branda, F. Nucleation in glass and differential thermal analysis. J. Mater. Sci. 1981, 16, 341–344. [Google Scholar] [CrossRef]
- Loshmanov, A.A.; Sigaev, V.N.; Khodakovskaya, R.Y.; Pavlushkin, N.M.; Yamzin, I.I. Small-angle neutron scattering on silica glasses containing titania. J. Appl. Crystallogr. 1974, 7, 207–210. [Google Scholar] [CrossRef]
- Kleebusch, E.; Patzig, C.; Höche, T.; Rüssel, C. Effect of the concentrations of nucleating agents ZrO2 and TiO2 on the crystallization of Li2O–Al2O3–SiO2 glass: An X-ray diffraction and TEM investigation. J. Mater. Sci. 2016, 51, 10127–10138. [Google Scholar] [CrossRef]
- Kleebusch, E.; Thieme, C.; Patzig, C.; Höche, T.; Rüssel, C. Crystallization of lithium aluminosilicate and microstructure of a lithium alumino borosilicate glass designed for zero thermal expansion. Ceram. Int. 2023, 49, 21246–21254. [Google Scholar] [CrossRef]
- Bhattacharyya, S.; Höche, T.; Jinschek, J.R.; Avramov, I.; Wurth, R.; Müller, M.; Rüssel, C. Direct evidence of Al-rich layers around nanosized ZrTiO4 in glass: Putting the role of nucleation agents in perspective. Cryst. Growth Des. 2010, 10, 379–385. [Google Scholar] [CrossRef]
- Höche, T.; Patzig, C.; Gemming, T.; Wurth, R.; Rüssel, C.; Avramov, I. Temporal evolution of diffusion barriers surrounding ZrTiO4 nuclei in lithia aluminosilicate glass-ceramics. Cryst. Growth Des. 2012, 12, 1556–1563. [Google Scholar] [CrossRef]
- Höche, T.; Mäder, M.; Bhattacharyya, S.; Henderson, G.S.; Gemming, T.; Wurth, R.; Rüssel, C.; Avramov, I. ZrTiO4 crystallisation in nanosized liquid–liquid phase-separation droplets in glass—A quantitative XANES study. CrystEngComm 2011, 13, 2550–2556. [Google Scholar] [CrossRef]
- Serbena, F.C.; Zanotto, E.D. Internal residual stresses in glass-ceramics: A review. J. Non-Cryst. Solids 2012, 358, 975–984. [Google Scholar] [CrossRef]
- Serbena, F.C.; Soares, V.O.; Peitl, O.; Pinto, H.; Muccillo, R.; Zanotto, E.D. Internal residual Stresses in sintered and commercial low expansion Li2O-Al2O3-SiO2 glass-ceramics. J. Am. Ceram. Soc. 2011, 94, 1206–1214. [Google Scholar] [CrossRef]
- Ross, S.; Welsch, A.-M.; Behrens, H. Lithium conductivity in glasses of the Li2O–Al2O3–SiO2 system. Phys. Chem. Chem. Phys. 2015, 17, 465–474. [Google Scholar] [CrossRef]
- Wei, A.; Liu, Z.; Zhang, F.; Ma, M.; Chen, G.; Li, Y. Thermal expansion coefficient tailoring of LAS glass-ceramic for anodic bondable low temperature co-fired ceramic application. Ceram. Int. 2020, 46, 4771–4777. [Google Scholar] [CrossRef]

| Glass Composition | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Oxides | SiO2 | Al2O3 | Li2O | P2O5 | TiO2 | ZrO2 | ZnO | MgO | BaO | CaO | As2O3 | Sb2O3 |
| Batch composition, mol. % | 61.1 | 15.9 | 11.1 | 4.9 | 2.1 | 0.9 | 0.4 | 2.0 | 0.8 | 0.4 | 0.2 | 0.2 |
| Analytical composition, mol. % | 60.0 | 19.2 | 10.1 | 4.6 | 1.9 | 0.8 | 0.4 | 1.9 | 0.7 | 0.3 | 0.1 | 0.1 |
| Glass properties | ||||||||||||
| Glass transition temperature Tg, °C | Crystallization peak TP, °C | TP − Tg, °C | CTE20…500 °C, ppm/K | Density, g/cm3 | Refractive index (nD) | |||||||
| 647 | 867 | 220 | 5.17 | 2.46 | 1.52 | |||||||
| Sample | Duration, h | Crystallized Fraction, % | Crystallite Size, nm | CTE 0…50 °C, ppm/K | CTE −50…400 °C, ppm/K | CTE 20…500 °C, ppm/K |
|---|---|---|---|---|---|---|
| GC-20 | 20 | 14 | 33 | 3.64 | 4.34 | 4.73 |
| GC-22.5 | 22.5 | 24 | 33 | 3.13 | 3.88 | 4.25 |
| GC-25 | 25 | 32 | 35 | 0.51 | 0.75 | 0.94 |
| GC-27.5 | 27.5 | 49 | 35 | −0.77 | −0.49 | −0.34 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Naumov, A.S.; Shakhgildyan, G.Y.; Golubev, N.V.; Lipatiev, A.S.; Fedotov, S.S.; Alekseev, R.O.; Ingat’eva, E.S.; Savinkov, V.I.; Sigaev, V.N. Tuning the Coefficient of Thermal Expansion of Transparent Lithium Aluminosilicate Glass-Ceramics by a Two-Stage Heat Treatment. Ceramics 2024, 7, 1-14. https://doi.org/10.3390/ceramics7010001
Naumov AS, Shakhgildyan GY, Golubev NV, Lipatiev AS, Fedotov SS, Alekseev RO, Ingat’eva ES, Savinkov VI, Sigaev VN. Tuning the Coefficient of Thermal Expansion of Transparent Lithium Aluminosilicate Glass-Ceramics by a Two-Stage Heat Treatment. Ceramics. 2024; 7(1):1-14. https://doi.org/10.3390/ceramics7010001
Chicago/Turabian StyleNaumov, Andrey S., Georgiy Yu. Shakhgildyan, Nikita V. Golubev, Alexey S. Lipatiev, Sergey S. Fedotov, Roman O. Alekseev, Elena S. Ingat’eva, Vitaliy I. Savinkov, and Vladimir N. Sigaev. 2024. "Tuning the Coefficient of Thermal Expansion of Transparent Lithium Aluminosilicate Glass-Ceramics by a Two-Stage Heat Treatment" Ceramics 7, no. 1: 1-14. https://doi.org/10.3390/ceramics7010001
APA StyleNaumov, A. S., Shakhgildyan, G. Y., Golubev, N. V., Lipatiev, A. S., Fedotov, S. S., Alekseev, R. O., Ingat’eva, E. S., Savinkov, V. I., & Sigaev, V. N. (2024). Tuning the Coefficient of Thermal Expansion of Transparent Lithium Aluminosilicate Glass-Ceramics by a Two-Stage Heat Treatment. Ceramics, 7(1), 1-14. https://doi.org/10.3390/ceramics7010001








