Study of the Phase Formation Processes and Their Influence on the Change in the Optical and Shielding Characteristics of 0.25ZnO–0.25Al2O3–0.25WO3–0.25Bi2O3 Ceramics
Abstract
1. Introduction
2. Experimental Part
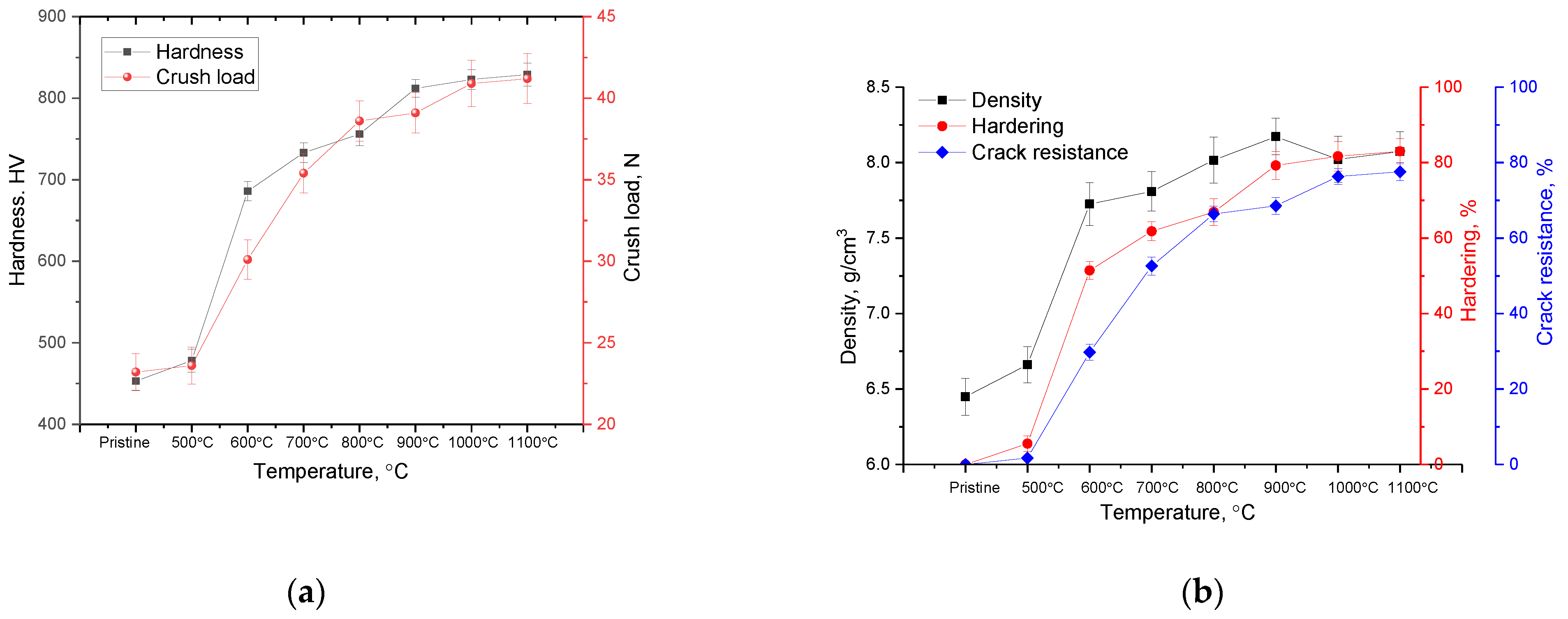
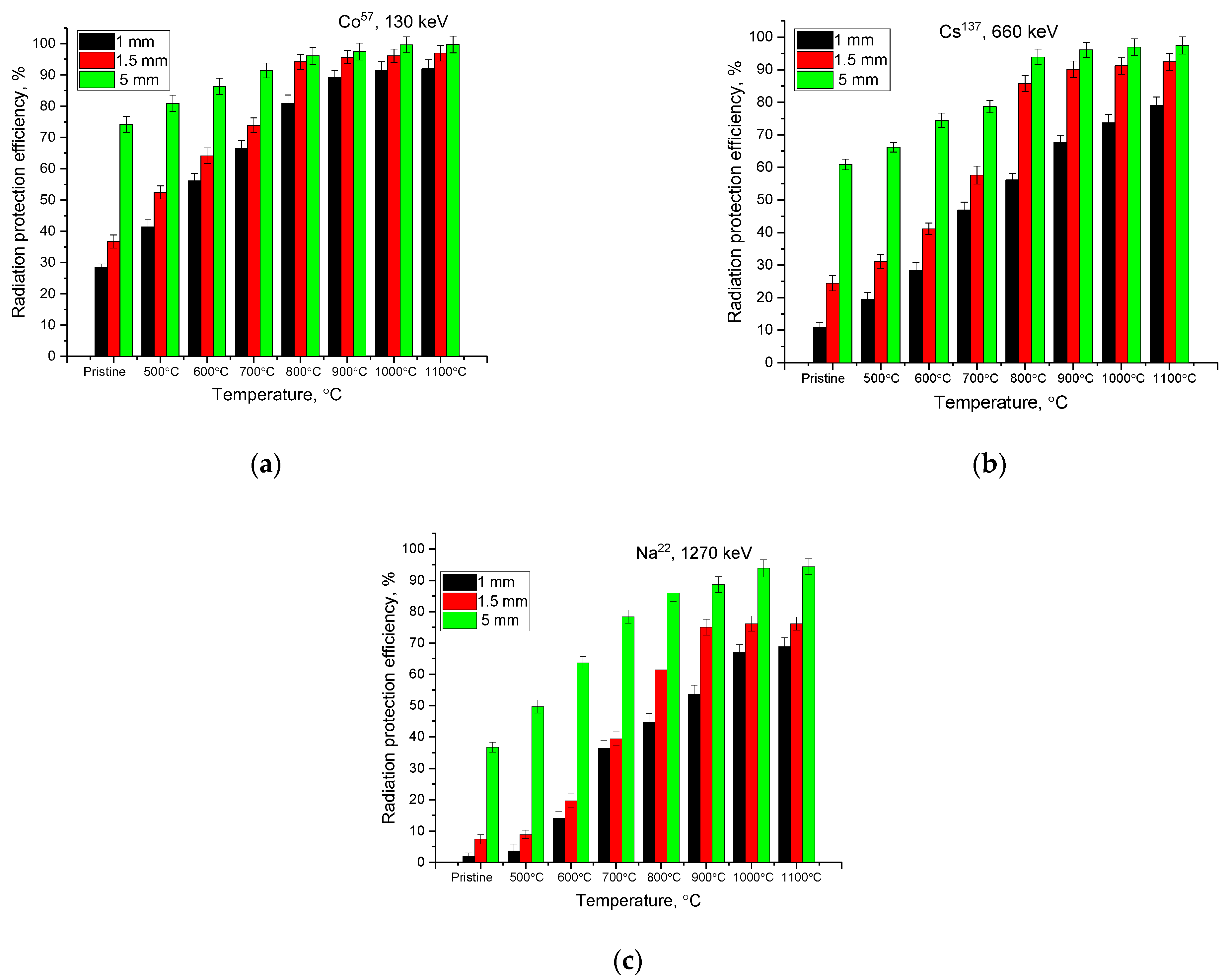
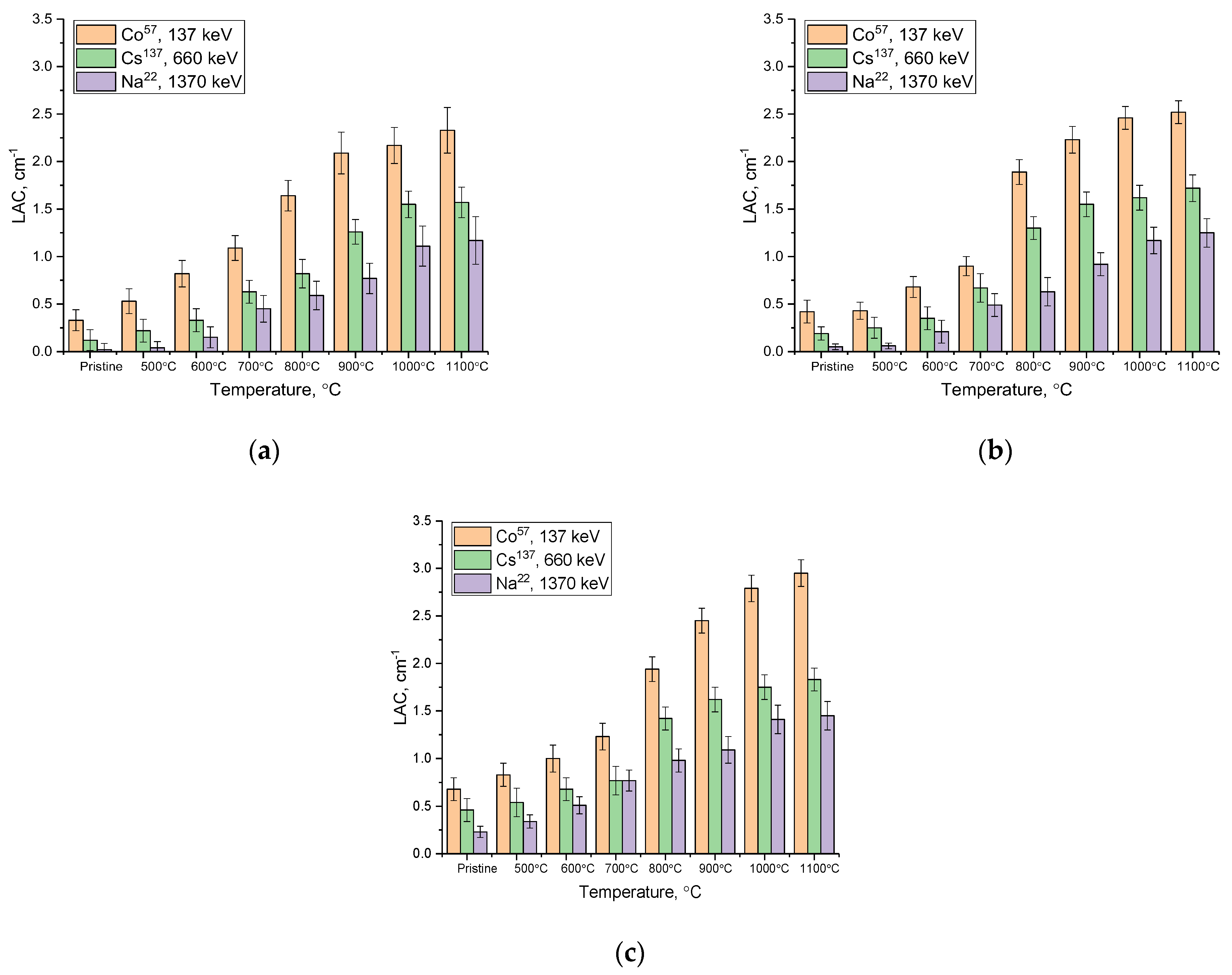
3. Results and Discussion

4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Prasad, K.N.; Cole, W.C.; Haase, G.M. Radiation protection in humans: Extending the concept of as low as reasonably achievable (ALARA) from dose to biological damage. Br. J. Radiol. 2004, 77, 97–99. [Google Scholar] [CrossRef] [PubMed]
- Newman, B.; Callahan, M.J. ALARA (as low as reasonably achievable) CT 2011—Executive summary. Pediatr. Radiol. 2011, 41, 453–455. [Google Scholar] [CrossRef]
- Strauss, K.J.; Kaste, S.C. The ALARA (as low as reasonably achievable) concept in pediatric interventional and fluoroscopic imaging: Striving to keep radiation doses as low as possible during fluoroscopy of pediatric patients—A white paper executive summary. Radiology 2006, 240, 621–622. [Google Scholar] [CrossRef] [PubMed]
- McGiff, T.J.; Danforth, R.A.; Herschaft, E.E. Maintaining radiation exposures as low as reasonably achievable (ALARA) for dental personnel operating portable hand-held x-ray equipment. Health Phys. 2012, 103, S179–S185. [Google Scholar] [CrossRef] [PubMed]
- Baker, S.I.; Kamboj, S. Applying ALARA Principles in the Design of New Radiological Facilities. Health Phys. 2022, 122, 452–462. [Google Scholar] [CrossRef]
- Abdalla, A.M.; Al-Naggar, T.I.; Bashiri, A.; Alsareii, S.A. Radiation shielding performance for local granites. Prog. Nucl. Energy 2022, 150, 104294. [Google Scholar] [CrossRef]
- Shultis, J.K.; Faw, R.E. Radiation shielding technology. Health Phys. 2005, 88, 297–322. [Google Scholar] [CrossRef]
- Oto, B.; Yıldız, N.; Akdemir, F.; Kavaz, E. Investigation of gamma radiation shielding properties of various ores. Prog. Nucl. Energy 2015, 85, 391–403. [Google Scholar] [CrossRef]
- Singh, N.; Singh, K.J.; Singh, K.; Singh, H. Comparative study of lead borate and bismuth lead borate glass systems as gamma-radiation shielding materials. Nucl. Instrum. Methods Phys. Res. Sect. B Beam Interact. Mater. At. 2004, 225, 305–309. [Google Scholar] [CrossRef]
- Al-Hadeethi, Y.; Sayyed, M.I. Analysis of borosilicate glasses doped with heavy metal oxides for gamma radiation shielding application using Geant4 simulation code. Ceram. Int. 2019, 45, 24858–24864. [Google Scholar] [CrossRef]
- Kamislioglu, M. An investigation into gamma radiation shielding parameters of the (Al: Si) and (Al+ Na): Si-doped international simple glasses (ISG) used in nuclear waste management, deploying Phy-X/PSD and SRIM software. J. Mater. Sci. Mater. Electron. 2021, 32, 12690–12704. [Google Scholar] [CrossRef]
- Yasaka, P.; Pattanaboonmee, N.; Kim, H.J.; Limkitjaroenporn, P.; Kaewkhao, J. Gamma radiation shielding and optical properties measurements of zinc bismuth borate glasses. Ann. Nucl. Energy 2014, 68, 4–9. [Google Scholar] [CrossRef]
- Çağlar, İ.; Cengiz, G.B.; Bilir, G. Gamma radiation shielding properties of some binary tellurite glasses. J. Non-Cryst. Solids 2021, 574, 121139. [Google Scholar] [CrossRef]
- Abouhaswa, A.S.; Rammah, Y.S.; Sayyed, M.I.; Tekin, H.O. Synthesis, structure, optical and gamma radiation shielding properties of B2O3-PbO2-Bi2O3 glasses. Compos. Part B Eng. 2019, 172, 218–225. [Google Scholar] [CrossRef]
- Issa, S.A.; Sayyed, M.I.; Mostafa, A.M.A.; Lakshminarayana, G.; Kityk, I.V. Investigation of mechanical and radiation shielding features of heavy metal oxide based phosphate glasses for gamma radiation attenuation applications. J. Mater. Sci. Mater. Electron. 2019, 30, 12140–12151. [Google Scholar] [CrossRef]
- Sayyed, M.I.; Al-Hadeethi, Y.; AlShammari, M.M.; Ahmed, M.; Al-Heniti, S.H.; Rammah, Y.S. Physical, optical and gamma radiation shielding competence of newly boro-tellurite based glasses: TeO2–B2O3–ZnO–Li2O3–Bi2O3. Ceram. Int. 2021, 47, 611–618. [Google Scholar] [CrossRef]
- Issa, S.A.M.; Sayyed, M.I.; Kurudirek, M. Study of gamma radiation shielding properties of ZnO-\mathbf TeO2 ZnO-TeO2 glasses. Bull. Mater. Sci. 2017, 40, 841–857. [Google Scholar] [CrossRef]
- Dong, M.G.; Sayyed, M.I.; Lakshminarayana, G.; Ersundu, M.Ç.; Ersundu, A.E.; Nayar, P.; Mahdi, M.A. Investigation of gamma radiation shielding properties of lithium zinc bismuth borate glasses using XCOM program and MCNP5 code. J. Non-Cryst. Solids 2017, 468, 12–16. [Google Scholar] [CrossRef]
- Kaky, K.M.; Sayyed, M.I.; Ati, A.A.; Mhareb, M.H.A.; Mahmoud, K.A.; Baki, S.O.; Mahdi, M.A. Germanate oxide impacts on the optical and gamma radiation shielding properties of TeO2-ZnO-Li2O glass system. J. Non-Cryst. Solids 2020, 546, 120272. [Google Scholar] [CrossRef]
- D’souza, A.N.; Sayyed, M.I.; Karunakara, N.; Al-Ghamdi, H.; Almuqrin, A.H.; Elsafi, M.; Kamath, S.D. TeO2–SiO2–B2O3 glasses doped with CeO2 for gamma radiation shielding and dosimetry application. Radiat. Phys. Chem. 2022, 200, 110233. [Google Scholar] [CrossRef]
- Rammah, Y.S.; Kumar, A.; Mahmoud, K.A.A.; El-Mallawany, R.; El-Agawany, F.I.; Susoy, G.; Tekin, H.O. SnO-reinforced silicate glasses and utilization in gamma-radiation-shielding applications. Emerg. Mater. Res. 2020, 9, 1000–1008. [Google Scholar] [CrossRef]
- Kavaz, E.; Ekinci, N.; Tekin, H.O.; Sayyed, M.I.; Aygün, B.; Perişanoğlu, U. Estimation of gamma radiation shielding qualification of newly developed glasses by using WinXCOM and MCNPX code. Prog. Nucl. Energy 2019, 115, 12–20. [Google Scholar] [CrossRef]
- Al-Harbi, N.; Sayyed, M.I.; Kurtulus, R.; Kamışlıoğlu, M.; Kumar, A.; Alhuthali, A.M.S.; Kavas, T.; Al-Hadeethi, Y. Understanding the role of Bi2O3 in the P2O5–CaO–Na2O–K2O glass system in terms of physical, structural and radiation shielding properties. J. Mater. Sci. Mater. Electron. 2021, 32, 11649–11665. [Google Scholar] [CrossRef]
- El-Mallawany, R.; El-Agawany, F.I.; Al-Buriahi, M.S.; Muthuwong, C.; Novatski, A.; Rammah, Y.S. Optical properties and nuclear radiation shielding capacity of TeO2-Li2O-ZnO glasses. Opt. Mater. 2020, 106, 109988. [Google Scholar] [CrossRef]
- Al-Hadeethi, Y.; Sayyed, M.I.; Rammah, Y.S. Fabrication, optical, structural and gamma radiation shielding characterizations of GeO2-PbO-Al2O3–CaO glasses. Ceram. Int. 2020, 46, 2055–2062. [Google Scholar] [CrossRef]
- Sayyed, M.I.; Qashou, S.I.; Khattari, Z.Y. Radiation shielding competence of newly developed TeO2-WO3 glasses. J. Alloys Compd. 2017, 696, 632–638. [Google Scholar] [CrossRef]
- Temir, A.; Zhumadilov, K.; Zdorovets, M.; Kozlovskiy, A.; Trukhanov, A. Study of gamma radiation shielding efficiency with radiation-resistant Bi2O3-TeO2-WO3 ceramics. Solid State Sciences. 2021, 115, 106604. [Google Scholar] [CrossRef]
- Wu, P. Optimization and Calculation of Thermodynamic Properties and Phase Diagrams of Multi-Component Oxide Systems; Ecole polytechnique de Montreal: Montréal, QC, Canada, 1992. [Google Scholar]
- Harwig, H.A. On the Structure of Bismuthsesquioxide: The α, β, γ, and δ-Phase. Z. Anorg. Allg. Chem. 1978, 444, 151–166. [Google Scholar] [CrossRef]
- Kaurova, I.A.; Kuz’micheva, G.M.; Rybakov, V.B. Growth and structural, optical, and electrical properties of zincite crystals. Crystallogr. Rep. 2013, 58, 226–233. [Google Scholar] [CrossRef]
- Guse, W.; Saalfeld, H. X-ray characterization and structure refinement of a new cubic alumina phase (σ-Al2O3) with spinel-type structure. Neues Jahrb. Mineral. Mon. 1990, 5, 217–226. [Google Scholar]
- Salje, E.; Viswanathan, K. Physical properties and phase transitions in WO3. Acta Crystallogr. Sect. A Cryst. Phys. Diffr. Theor. Gen. Crystallogr. 1975, 31, 356–359. [Google Scholar] [CrossRef]
- Craig, D.C.; Stephenson, N.C. Structural studies of some body-centered cubic phases of mixed oxides involving Bi2O3: The structures of Bi25FeO40 and Bi38ZnO60. J. Solid State Chem. 1975, 15, 1–8. [Google Scholar] [CrossRef]
- Curti, M.; Gesing, T.M.; Murshed, M.M.; Bredow, T.; Mendive, C.B. Liebau density vector: A new approach to characterize lone electron pairs in mullite-type materials. Z. Krist. -Cryst. Mater. 2013, 228, 629–634. [Google Scholar] [CrossRef]
- Kraus, H.; Mikhailik, V.B.; Vasylechko, L.; Day, D.; Hutton, K.B.; Telfer, J.; Prots, Y. Effect of Ca doping on the structure and scintillation properties of ZnWO4. Phys. Status Solidi (a) 2007, 204, 730–736. [Google Scholar] [CrossRef]
- Levy, D.; Pavese, A.; Sani, A.; Pischedda, V. Structure and compressibility of synthetic ZnAl2O4 (gahnite) under high-pressure conditions, from synchrotron X-ray powder diffraction. Phys. Chem. Miner. 2001, 28, 612–618. [Google Scholar] [CrossRef]
- Watanabe, A.; Goto, M. Characterization of Bi2W2O9 having a unique layered structure. J. Less Common Met. 1978, 61, 265–272. [Google Scholar] [CrossRef]
- Knight, K.S. The crystal structure of ferroelectric Bi2WO6 at 961 K. Ferroelectrics 1993, 150, 319–330. [Google Scholar] [CrossRef]
- Atuchin, V.V.; Isaenko, L.I.; Kesler, V.G.; Lin, Z.S.; Molokeev, M.S.; Yelisseyev, A.P.; Zhurkov, S.A. Exploration on anion ordering, optical properties and electronic structure in K3WO3F3 elpasolite. J. Solid State Chem. 2012, 187, 159–164. [Google Scholar] [CrossRef]
- Ji, H.; Huang, Z.; Xia, Z.; Molokeev, M.S.; Jiang, X.; Lin, Z.; Atuchin, V.V. Comparative investigations of the crystal structure and photoluminescence property of eulytite-type Ba3Eu(PO4)3 and Sr3Eu(PO4)3. Dalton Trans. 2015, 44, 7679–7686. [Google Scholar] [CrossRef]
- Grossman, V.; Atuchin, V.; Bazarov, B.G.; Aleksandrovsky, A.; Eremin, E.; Krylov, A.; Kuratieva, N.; Bazarova, J.G.; Maximov, N.; Molokeev, M.M.; et al. Structural, Spectroscopic, Electric and Magnetic Properties of New Trigonal K5FeHf(MoO4)6 Orthomolybdate. Molecules 2023, 28, 1629. [Google Scholar] [CrossRef]
- Sayyed, M.I.; Kaky, K.M.; Gaikwad, D.K.; Agar, O.; Gawai, U.P.; Baki, S.O. Physical, structural, optical and gamma radiation shielding properties of borate glasses containing heavy metals (Bi2O3/MoO3). J. Non-Cryst. Solids 2019, 507, 30–37. [Google Scholar] [CrossRef]
- Sayyed, M.; Mhareb, M.; Alajerami, Y.; Mahmoud, K.; Imheidat, M.A.; Alshahri, F.; Alqahtani, M.; Al-Abdullah, T. Optical and radiation shielding features for a new series of borate glass samples. Optik 2021, 239, 166790. [Google Scholar] [CrossRef]
- Sayyed, M.I.; Lakshminarayana, G.; Dong, M.G.; Çelikbilek Ersundu, M.; Ersundu, A.E.; Kityk, I.V. Investigation on gamma and neutron radiation shielding parameters for BaO/SrO–Bi2O3–B2O3 glasses. Radiat. Phys. Chem. 2018, 145, 26–33. [Google Scholar] [CrossRef]
- Sayyed, M.; Elbashir, B.; Tekin, H.; Altunsoy, E.; Gaikwad, D. Radiation shielding properties of pentaternary borate glasses using MCNPX code. J. Phys. Chem. Solids 2018, 121, 17–21. [Google Scholar] [CrossRef]
- Sayyed, M.I.; Kumar, A.; Alhuthali, A.M.S.; Mahmoud, K.A.; Al-Hadeethi, Y. Tailoring Dy3+/Tb3+-doped lead telluride borate glasses for gamma-ray shielding applications. Eur. Phys. J. Plus 2021, 136, 1–16. [Google Scholar] [CrossRef]
- Alsaif, N.A.M.; Alotiby, M.; Hanfi, M.Y.; Mahmoud, K.A.; Al-Yousef, H.A.; Alotaibi, B.M.; Sayyed, M.I.; Al-Hadeethi, Y. Comprehensive study of radiation shielding and mechanical features of Bi2O3-TeO2-B2O3-GeO2 glasses. J. Aust. Ceram. Soc. 2021, 57, 1267–1274. [Google Scholar] [CrossRef]
- Alzahrani, J.S.; Muniz, R.F.; Alrowaili, Z.A.; Novatski, A.; Gunha, J.V.; Gonçalves, A.; Olarinoye, I.O.; Al-Buriahi, M.S. A synergistic effect of heavy metal oxides to enhance the physical, optical, and radiation-absorption properties of TeO2-Li2O-BaO glasses. Optik 2022, 261, 169189. [Google Scholar] [CrossRef]
- Rammah, Y.S.; Özpolat, Ö.F.; Alım, B.; Şakar, E.; El-Mallawany, R.; El-Agawany, F.I. Assessment of gamma-ray attenuation features for La+3 co-doped zinc borotellurite glasses. Radiat. Phys. Chem. 2020, 176, 109069. [Google Scholar] [CrossRef]
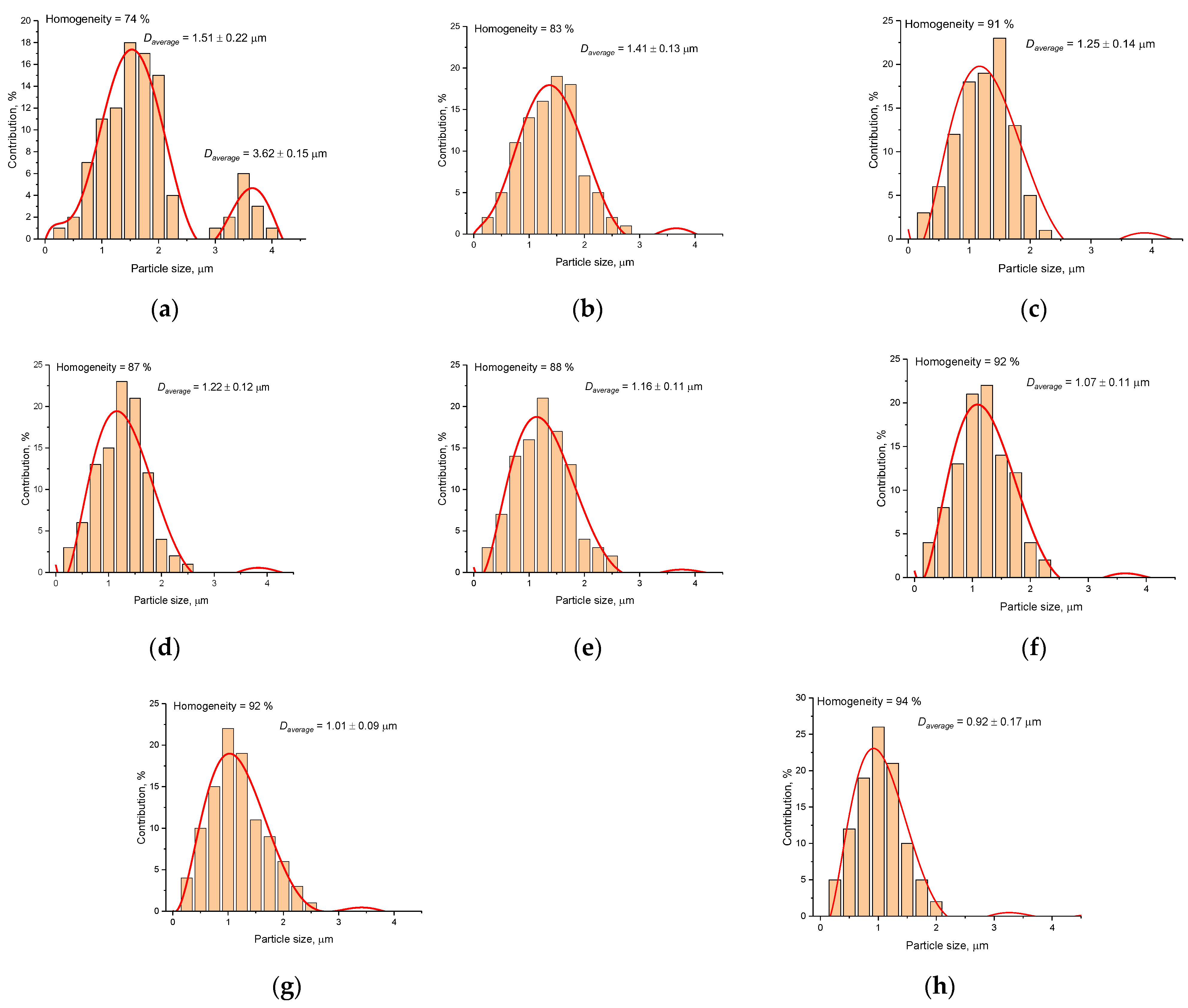



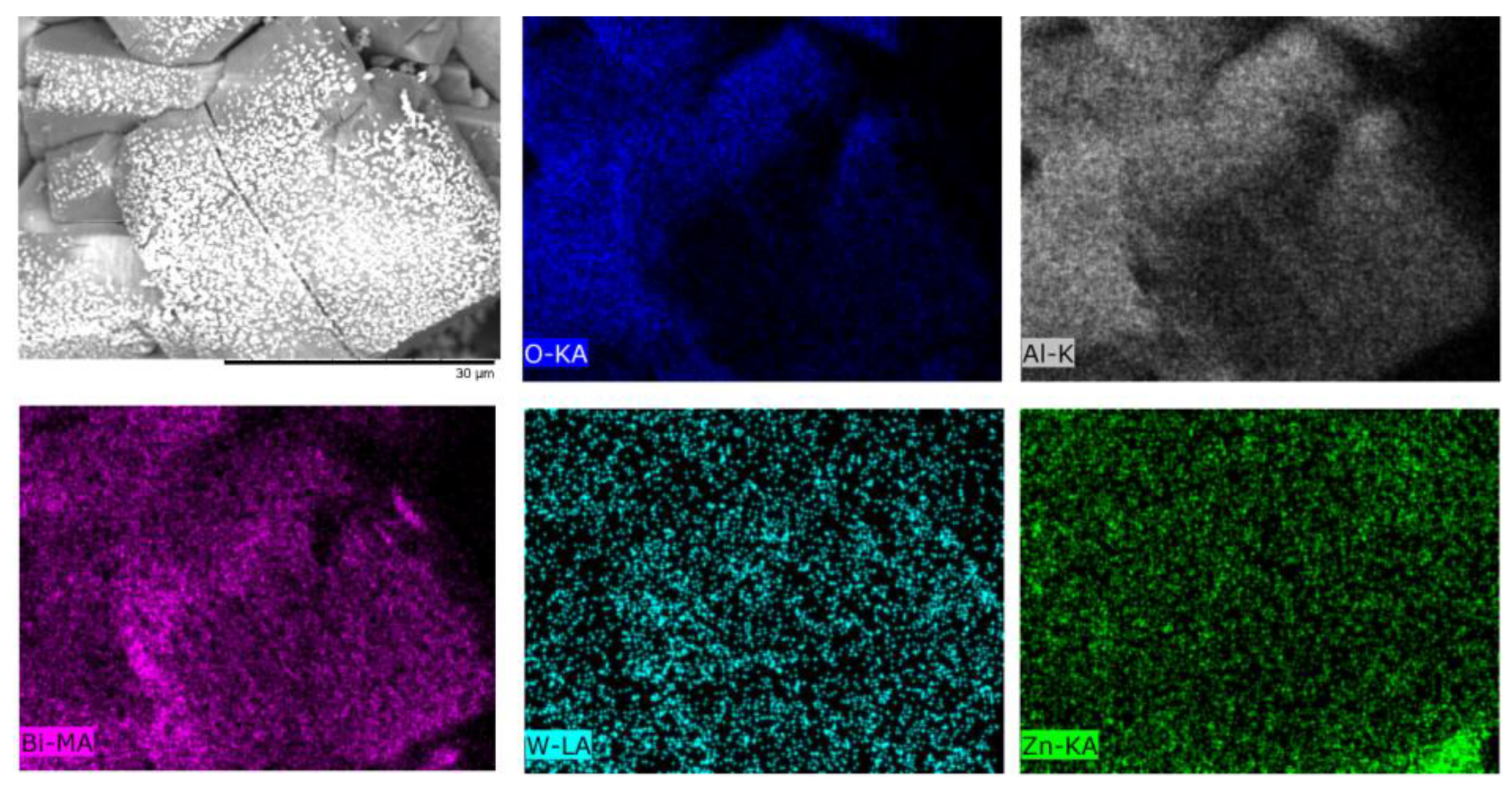
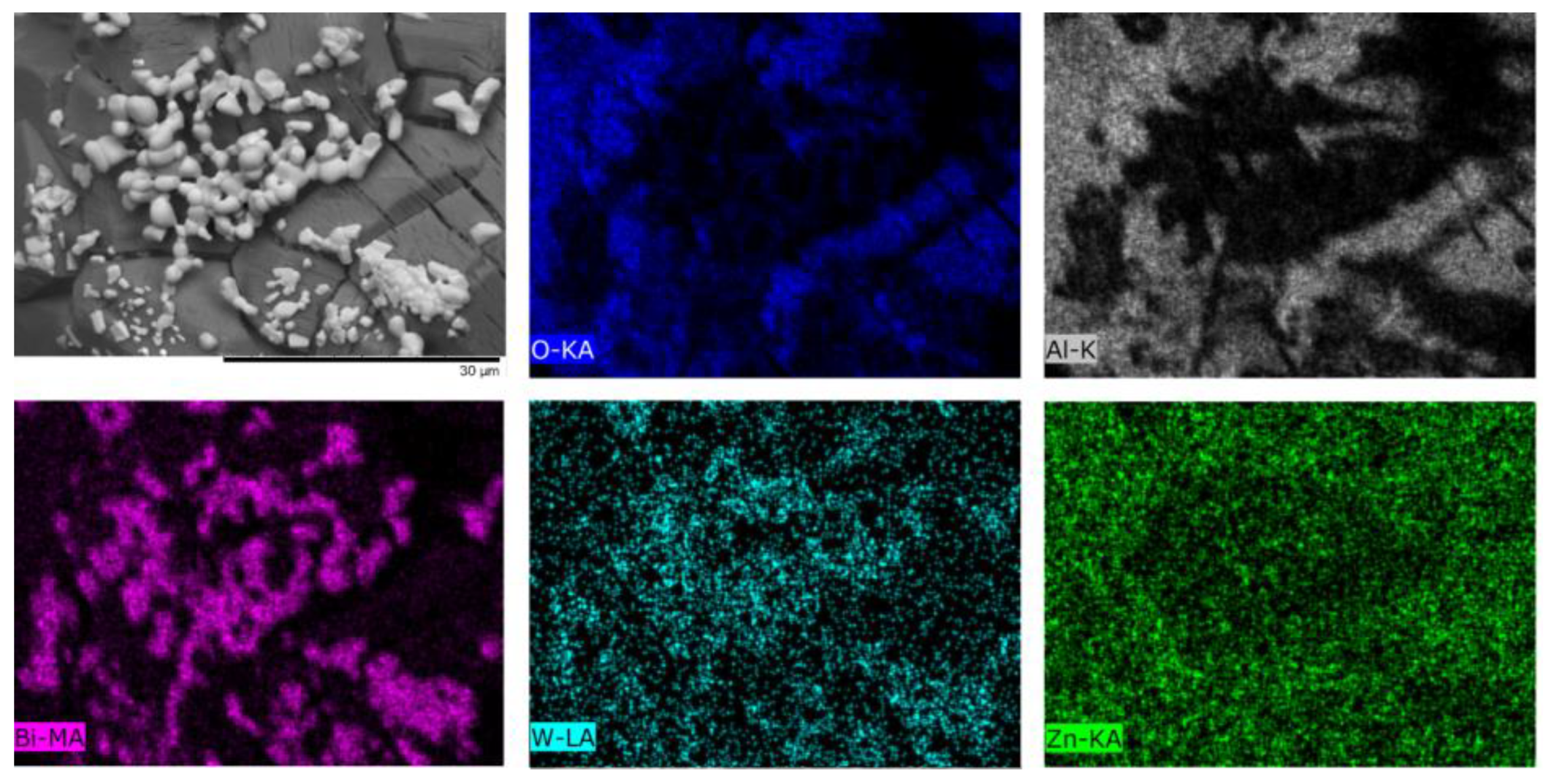


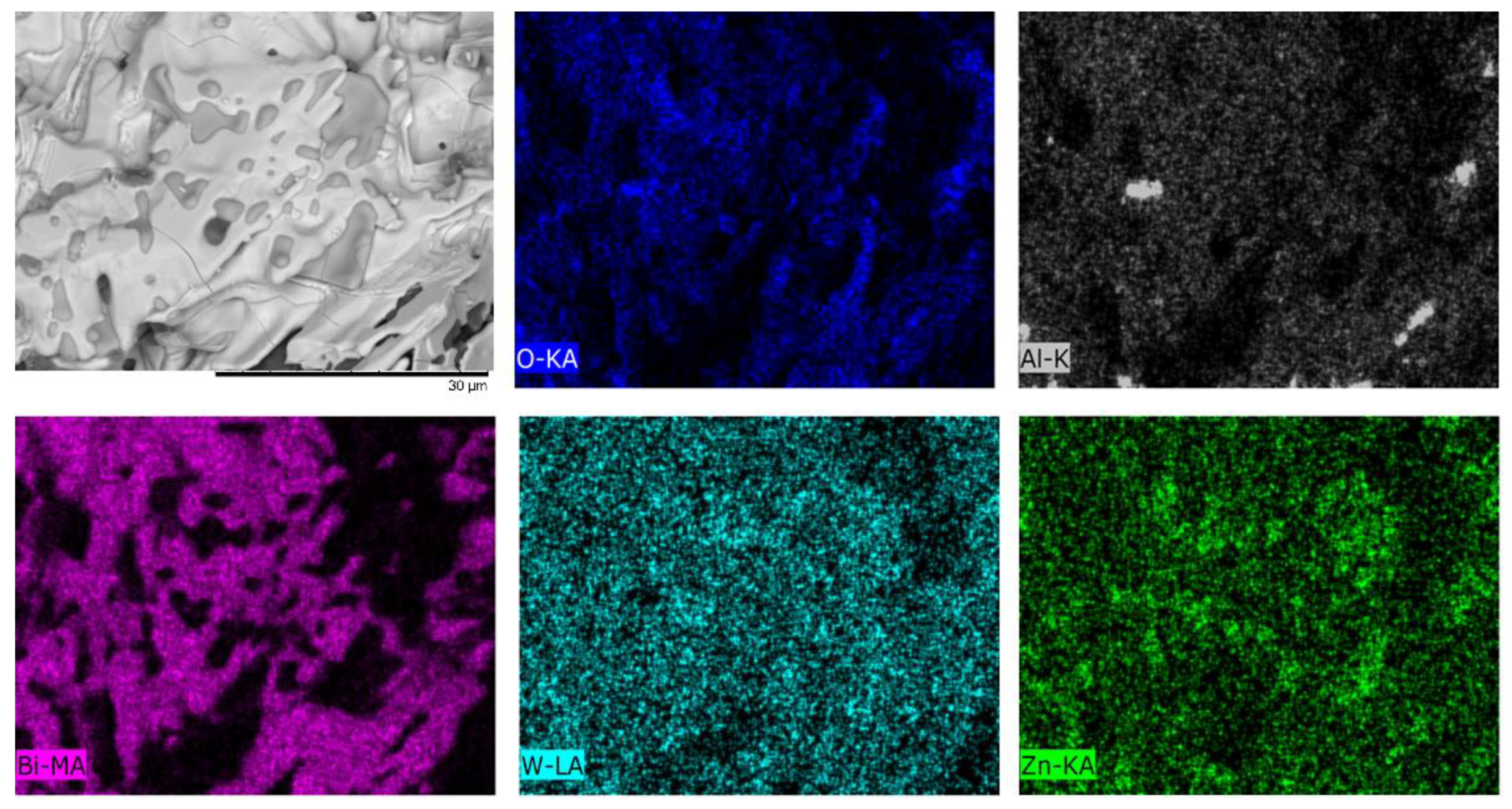


| Temperature, °C | Daverage, µm | SBET, m2/g |
|---|---|---|
| Pristine | 1.51 ± 022 | 0.61 ± 0.03 |
| 500 | 1.41 ± 0.13 | 0.63 ± 0.04 |
| 600 | 1.25 ± 0.14 | 0.62 ± 0.02 |
| 700 | 1.22 ± 0.12 | 0.63 ± 0.03 |
| 800 | 1.16 ± 0.11 | 0.65 ± 0.03 |
| 900 | 1.07 ± 0.11 | 0.68 ± 0.02 |
| 1000 | 1.01 ± 0.09 | 0.74 ± 0.04 |
| 1100 | 0.92 ± 0.17 | 0.81 ± 0.03 |
| Phase | Cell Parameter, Å | |||||||
|---|---|---|---|---|---|---|---|---|
| Temperature, °C | ||||||||
| Pristine | 500 °C | 600 °C | 700 °C | 800 °C | 900 °C | 1000 °C | 1100 °C | |
| ZnO–Hexagonal | a = 3.24489, c = 5.19884 | a = 3.23789, c = 5.19883 | a = 3.24189, c = 5.19776 | a = 3.24761, c = 5.21101 | a = 3.23484, c = 5.19364 | a = 3.22406, c = 5.17836 | – | – |
| Bi2O3–Monoclinic | a = 5.83269, b = 8.17140, c = 7.51377, β = 112.700° | a = 5.81553, b = 8.13135, c = 7.49756, β = 112.369° | – | – | – | – | – | – |
| WO3–Monoclinic | a = 7.27554, b = 7.55230, c = 7.67142, β = 90.857° | a = 7.25699, b = 7.51232, c = 7.64585, β = 90.589° | a = 7.27833, b = 7.54031, c = 7.67134, β = 90.820° | a = 7.25692, b = 7.52405, c = 7.66381, β = 90.660° | – | – | – | – |
| Al2O3–Cubic | a = 7.91675 | a = 7.89346 | – | – | – | – | – | – |
| ZnBi38O60–Cubic | – | – | a = 10.18689 | a = 10.22085 | – | – | – | – |
| Bi2Al4O9–Orthorhombic | – | – | a = 7.77947, b = 8.15873, c = 5.73945 | a = 7.77794, b = 8.17313, c = 5.74733 | – | – | – | – |
| Bi2WO6–Orthorhombic | – | – | a = 5.47499, b = 16.45805, c = 5.44780 | a = 5.46103, b = 16.43546, c = 5.44460 | a = 5.42355, b = 16.38712, c = 5.42645 | a = 5.39696, b = 16.28751, c = 5.41901 | a = 5.38744, b = 16.27154, c = 5.41156 | a = 5.39906, b = 16.28749, c = 5.42536 |
| ZnWO4–Monoclinic | – | – | – | – | a = 4.68416, b = 5.69505, c = 4.90631, β = 90.395° | a = 4.67773, b = 5.67830, c = 4.90150, β = 90.271° | a = 4.66947, b = 5.67719, c = 4.89478, β = 90.112° | – |
| ZnAl2O4–Cubic | – | – | – | – | a = 8.07055 | a = 8.05314 | a = 8.03777 | a = 8.05941 |
| Bi2W2O9–Orthorhombic | – | – | – | – | – | – | – | a = 5.42325, b = 5.44788, c = 23.75490 |
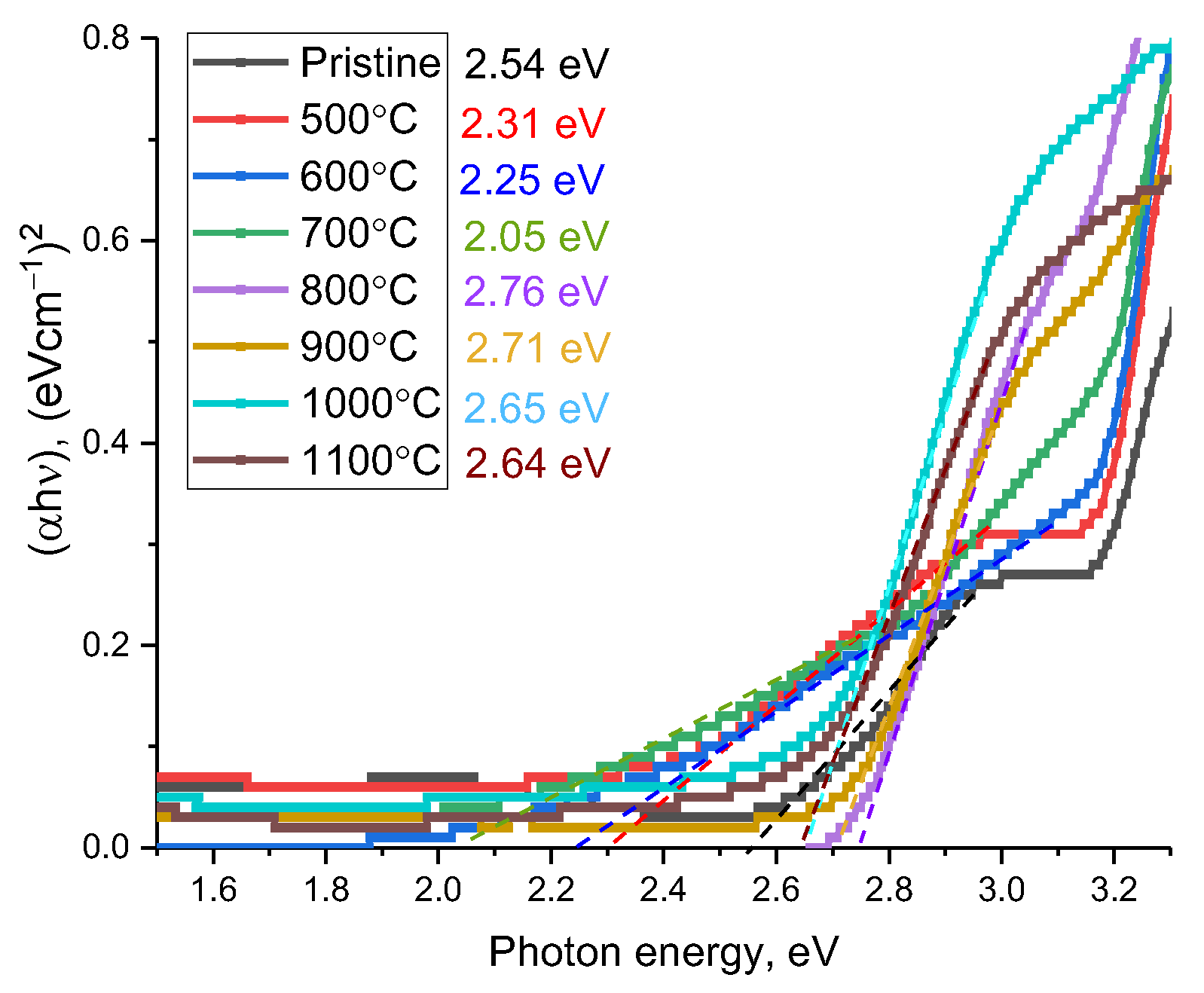
| Temperature, °C | Band Gap, eV | Linear Refractive index | Refraction Loss | Optical Transmission | Static Dielectric Constants | Molar Refraction | Metallization Criterion |
|---|---|---|---|---|---|---|---|
| Pristine | 2.54 | 2.53 | 0.188 | 0.684 | 6.401 | 20.88 | 0.35 |
| 500 °C | 2.31 | 2.61 | 0.199 | 0.668 | 6.812 | 20.33 | 0.40 |
| 600 °C | 2.25 | 2.63 | 0.202 | 0.664 | 6.917 | 28.27 | 0.42 |
| 700 °C | 2.05 | 2.71 | 0.212 | 0.650 | 7.344 | 31.28 | 0.48 |
| 800 °C | 2.76 | 2.46 | 0.178 | 0.698 | 6.052 | 36.61 | 0.31 |
| 900 °C | 2.71 | 2.48 | 0.181 | 0.694 | 6.150 | 39.86 | 0.32 |
| 1000 °C | 2.65 | 2.49 | 0.182 | 0.692 | 6.200 | 41.86 | 0.33 |
| 1100 °C | 2.64 | 2.50 | 0.184 | 0.690 | 6.250 | 66.76 | 0.33 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Seitbayev, A.S.; Kozlovskiy, A.L.; Borgekov, D.B.; Zdorovets, M.V. Study of the Phase Formation Processes and Their Influence on the Change in the Optical and Shielding Characteristics of 0.25ZnO–0.25Al2O3–0.25WO3–0.25Bi2O3 Ceramics. Ceramics 2023, 6, 798-817. https://doi.org/10.3390/ceramics6020046
Seitbayev AS, Kozlovskiy AL, Borgekov DB, Zdorovets MV. Study of the Phase Formation Processes and Their Influence on the Change in the Optical and Shielding Characteristics of 0.25ZnO–0.25Al2O3–0.25WO3–0.25Bi2O3 Ceramics. Ceramics. 2023; 6(2):798-817. https://doi.org/10.3390/ceramics6020046
Chicago/Turabian StyleSeitbayev, Aibek S., Artem L. Kozlovskiy, Daryn B. Borgekov, and Maxim V. Zdorovets. 2023. "Study of the Phase Formation Processes and Their Influence on the Change in the Optical and Shielding Characteristics of 0.25ZnO–0.25Al2O3–0.25WO3–0.25Bi2O3 Ceramics" Ceramics 6, no. 2: 798-817. https://doi.org/10.3390/ceramics6020046
APA StyleSeitbayev, A. S., Kozlovskiy, A. L., Borgekov, D. B., & Zdorovets, M. V. (2023). Study of the Phase Formation Processes and Their Influence on the Change in the Optical and Shielding Characteristics of 0.25ZnO–0.25Al2O3–0.25WO3–0.25Bi2O3 Ceramics. Ceramics, 6(2), 798-817. https://doi.org/10.3390/ceramics6020046






