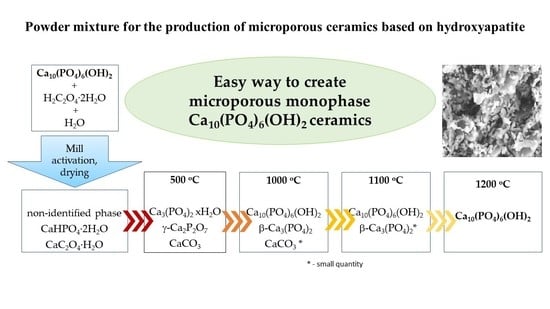
Powder Mixture for the Production of Microporous Ceramics Based on Hydroxyapatite
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Huang, J.; Best, S.M. Ceramic biomaterials for tissue engineering. In Tissue Engineering Using Ceramics and Polymers, 3rd ed.; Boccaccini, A.R., Ma, P.X., Liverani, L., Eds.; Woodhead Publishing: Cambridge, UK, 2022; Chapter 1; pp. 3–40. ISBN 9780128205082. [Google Scholar] [CrossRef]
- Matveichuk, I.V.; Rozanov, V.V.; Litvinov, Y.Y. Biophysical properties of bone tissue for biomedical applications. Al’manakh Klinich. Med. 2016, 44, 193–202. [Google Scholar] [CrossRef]
- Quelch, K.J.; Melick, R.A.; Bingham, P.J.; Mercuri, S.M. Chemical composition of human bone. Arch. Oral Biol. 1983, 28, 665–674. [Google Scholar] [CrossRef]
- Simske, S.J.; Ayers, R.A.; Bateman, T.A. Porous materials for bone engineering. Mater. Sci. Forum. 1997, 250, 151–182. [Google Scholar] [CrossRef]
- Yoshikawa, H.; Myoui, A. Bone tissue engineering with porous hydroxyapatite ceramics. J. Artif. Organs 2005, 8, 131–136. [Google Scholar] [CrossRef] [PubMed]
- Jones, J.R.; Lee, P.D.; Hench, L.L. Hierarchical porous materials for tissue engineering. Philos. Trans. R. Soc. A Math. Phys. Eng. Sci. 2006, 364, 263–281. [Google Scholar] [CrossRef]
- Putlyaev, V.I.; Safronova, T.V. Chemical Transformations of Calcium Phosphates during Production of Ceramic Materials on Their Basis. Inorg. Mater. 2019, 55, 1328–1341. [Google Scholar] [CrossRef]
- Nikolenko, M.V.; Vasylenko, K.V.; Myrhorodska, V.D.; Kostyniuk, A.; Likozar, B. Synthesis of calcium orthophosphates by chemical precipitation in aqueous solutions: The effect of the acidity, Ca/P molar ratio, and temperature on the phase composition and solubility of precipitates. Processes 2020, 8, 1009. [Google Scholar] [CrossRef]
- Yoon, S.Y.; Park, Y.M.; Park, S.S.; Stevens, R.; Park, H.C. Synthesis of hydroxyapatite whiskers by hydrolysis of α-tricalcium phosphate using microwave heating. Mater. Chem. Phys. 2005, 91, 48–53. [Google Scholar] [CrossRef]
- Earl, J.S.; Wood, D.J.; Milne, S.J. Hydrothermal synthesis of hydroxyapatite. In Journal of Physics: Conference Series; IOP Publishing: Bristol, UK, 2006; Volume 26, pp. 268–271. [Google Scholar] [CrossRef]
- Rabiee, S.M.; Moztarzadeh, F.; Solati-Hashjin, M. Synthesis and characterization of hydroxyapatite cement. J. Mol. Struct. 2010, 969, 172–175. [Google Scholar] [CrossRef]
- Javadinejad, H.R.; Ebrahimi-Kahrizsangi, R. Thermal and kinetic study of hydroxyapatite formation by solid-state reaction. Int. J. Chem. Kinet. 2021, 53, 583–595. [Google Scholar] [CrossRef]
- Pu’ad, N.M.; Haq, R.A.; Noh, H.M.; Abdullah, H.Z.; Idris, M.I.; Lee, T.C. Synthesis method of hydroxyapatite: A review. Mater. Today Proc. 2019, 29, 233–239. [Google Scholar] [CrossRef]
- Safronova, T.V.; Putlyaev, V.I.; Ivanov, V.K.; Knot’ko, A.V.; Shatalova, T.B. Powders Mixtures Based on Ammonium Pyrophosphate and Calcium Carbonate for Preparation of Biocompatible Porous Ceramic in the CaO–P2O5 System. Refract. Ind. Ceram. 2016, 56, 502–509. [Google Scholar] [CrossRef]
- Taş, A.C. Molten salt synthesis of calcium hydroxyapatite whiskers. J. Am. Ceram. Soc. 2001, 84, 295–300. [Google Scholar] [CrossRef] [Green Version]
- Tan, J.; Liu, Y.; Gong, J.; Jin, X.; Cheng, C.; Zhang, R.; Chen, M. Non-aqueous liquid crystals of hydroxyapatite nanorods. Acta Biomater. 2020, 116, 383–390. [Google Scholar] [CrossRef] [PubMed]
- Hassan, M.N.; Mahmoud, M.M.; El-Fattah, A.A.; Kandil, S. Microwave-assisted preparation of Nano-hydroxyapatite for bone substitutes. Ceram. Int. 2016, 42, 3725–3744. [Google Scholar] [CrossRef]
- Rhee, S.H. Synthesis of hydroxyapatite via mechanochemical treatment. Biomaterials 2002, 23, 1147–1152. [Google Scholar] [CrossRef]
- Kojima, Y.; Kitazawa, K.; Nishimiya, N. Synthesis of nano-sized hydroxyapatite by ultrasound irradiation. In Journal of Physics: Conference Series; IOP Publishing: Bristol, UK, 2012; Volume 339, p. 012001. [Google Scholar] [CrossRef]
- Batista, H.A.; Silva, F.N.; Lisboa, H.M.; Costa, A.C.F.M. Modeling and optimization of combustion synthesis for hydroxyapatite production. Ceram. Int. 2020, 46, 11638–11646. [Google Scholar] [CrossRef]
- DileepKumar, V.G.; Sridhar, M.S.; Aramwit, P.; Krut’ko, V.K.; Musskaya, O.N.; Glazov, I.E.; Reddy, N. A review on the synthesis and properties of hydroxyapatite for biomedical applications. J. Biomater. Sci. Polym. Ed. 2022, 33, 229–261. [Google Scholar] [CrossRef]
- Rial, R.; González-Durruthy, M.; Liu, Z.; Ruso, J.M. Advanced materials based on nanosized hydroxyapatite. Molecules 2021, 26, 3190. [Google Scholar] [CrossRef]
- Safronova, T.V.; Putlyaev, V.I. Powder systems for calcium phosphate ceramics. Inorg. Mater. 2017, 53, 17–26. [Google Scholar] [CrossRef]
- Safronova, T.V.; Putlyaev, V.I.; Shekhirev, M.A.; Kuznetsov, A.V. Disperse systems in calcium hydroxyapatite ceramics technology. Glass Ceram. 2007, 64, 22–26. [Google Scholar] [CrossRef]
- Layrolle, P.; Ito, A.; Tateishi, T. Sol-gel synthesis of amorphous calcium phosphate and sintering into microporous hydroxyapatite bioceramics. J. Am. Ceram. Soc. 1998, 81, 1421–1428. [Google Scholar] [CrossRef]
- Studart, A.R.; Gonzenbach, U.T.; Tervoort, E.; Gauckler, L.J. Processing routes to macroporous ceramics: A review. J. Am. Ceram. Soc. 2006, 89, 1771–1789. [Google Scholar] [CrossRef]
- Iwamoto, T.; Hieda, Y.; Kogai, Y. Effects of molecular weight on macropore sizes and characterization of porous hydroxyapatite ceramics fabricated using polyethylene glycol: Mechanisms to generate macropores and tune their sizes. Mater. Today Chem. 2021, 20, 100421. [Google Scholar] [CrossRef]
- Seesala, V.S.; Rajasekaran, R.; Dutta, A.; Vaidya, P.V.; Dhara, S. Dense-porous multilayer ceramics by green shaping and salt leaching. Open Ceram. 2021, 5, 100084. [Google Scholar] [CrossRef]
- Neeraj, V.S.; Wilson, P.; Vijayan, S.; Prabhakaran, K. Porous ceramics with a duplex pore structure by compression molding of alumina-NaCl paste in molten sucrose. Ceram. Int. 2017, 43, 14107–14113. [Google Scholar] [CrossRef]
- Barinov, S.M. Calcium phosphate-based ceramic and composite materials for medicine. Russ. Chem. Rev. 2010, 79, 13–29. [Google Scholar] [CrossRef]
- Beletskii, B.I.; Shumskii, V.I.; Nikitin, A.A.; Vlasova, E.B. Biocomposite calcium-phosphate materials used in osteoplastic surgery. Glass Ceram. 2000, 57, 322–325. [Google Scholar] [CrossRef]
- ICDD. PDF-4+ 2010 (Database), Ed. by Dr. Soorya Kabekkodu (International Centre for Diffraction Data, Newtown Square, PA, USA, 2010). Available online: https://www.icdd.com/pdf-2/ (accessed on 30 January 2022).
- Beevers, C.A. The crystal structure of dicalcium phosphate dihydrate, CaHPO4·2H2O. Acta Crystallogr. 1958, 11, 273–277. [Google Scholar] [CrossRef]
- Sterling, C. Crystal structure analysis of weddellite, CaC2O4·(2+x)H2O. Acta Crystallogr. 1965, 18, 917–921. [Google Scholar] [CrossRef]
- Safronova, T.V.; Shatalova, T.B.; Tikhonova, S.A.; Filippov, Y.Y.; Krut’ko, V.K.; Musskaya, O.N.; Kononenko, N.E. Synthesis of Calcium Pyrophosphate Powders from Phosphoric Acid and Calcium Carbonate. Inorg. Mater. Appl. Res. 2021, 12, 986–992. [Google Scholar] [CrossRef]
- Chang, H.; Huang, P.J. Thermal Decomposition of CaC2O4·H2O Studied by Thermo-Raman Spectroscopy with TGA/DTA. Anal. Chem. 1997, 69, 1485–1491. [Google Scholar] [CrossRef]
- Dosen, A.; Giese, R.F. Thermal decomposition of brushite, CaHPO4·2H2O to monetite CaHPO4 and the formation of an amorphous phase. Am. Miner. 2011, 96, 368–373. [Google Scholar] [CrossRef]
- Safronova, T.V.; Putlyaev, V.I.; Avramenko, O.A.; Shekhirev, M.A.; Veresov, A.G. Ca-deficient hydroxyapatite powder for producing tricalcium phosphate based ceramics. Glass Ceram. 2011, 68, 28–32. [Google Scholar] [CrossRef]
- Safronova, T.; Putlayev, V.; Filippov, Y.; Shatalova, T.; Karpushkin, E.; Larionov, D.; Kazakova, G.; Shakhtarin, Y. Calcium phosphate powder synthesized from calcium acetate and ammonium hydrophosphate for bioceramics application. Ceramics 2018, 1, 375–392. [Google Scholar] [CrossRef] [Green Version]
- Champion, E. Sintering of calcium phosphate bioceramics. Acta Biomater. 2013, 9, 5855–5875. [Google Scholar] [CrossRef]
- Mitsionis, A.I.; Vaimakis, T.C.; Trapalis, C.C. The effect of citric acid on the sintering of calcium phosphate bioceramics. Ceram. Int. 2010, 36, 623–634. [Google Scholar] [CrossRef]
- Safronova, T.V.; Shekhirev, M.A.; Putlyaev, V.I.; Tret’yakov, Y.D. Hydroxyapatite-based ceramic materials prepared using solutions of different concentrations. Inorg. Mater. 2007, 43, 901–909. [Google Scholar] [CrossRef]
- Raynaud, S.; Champion, E.; Bernache-Assollant, D. Calcium phosphate apatites with variable Ca/P atomic ratio II. Calcination and sintering. Biomaterials 2002, 23, 1073–1080. [Google Scholar] [CrossRef]
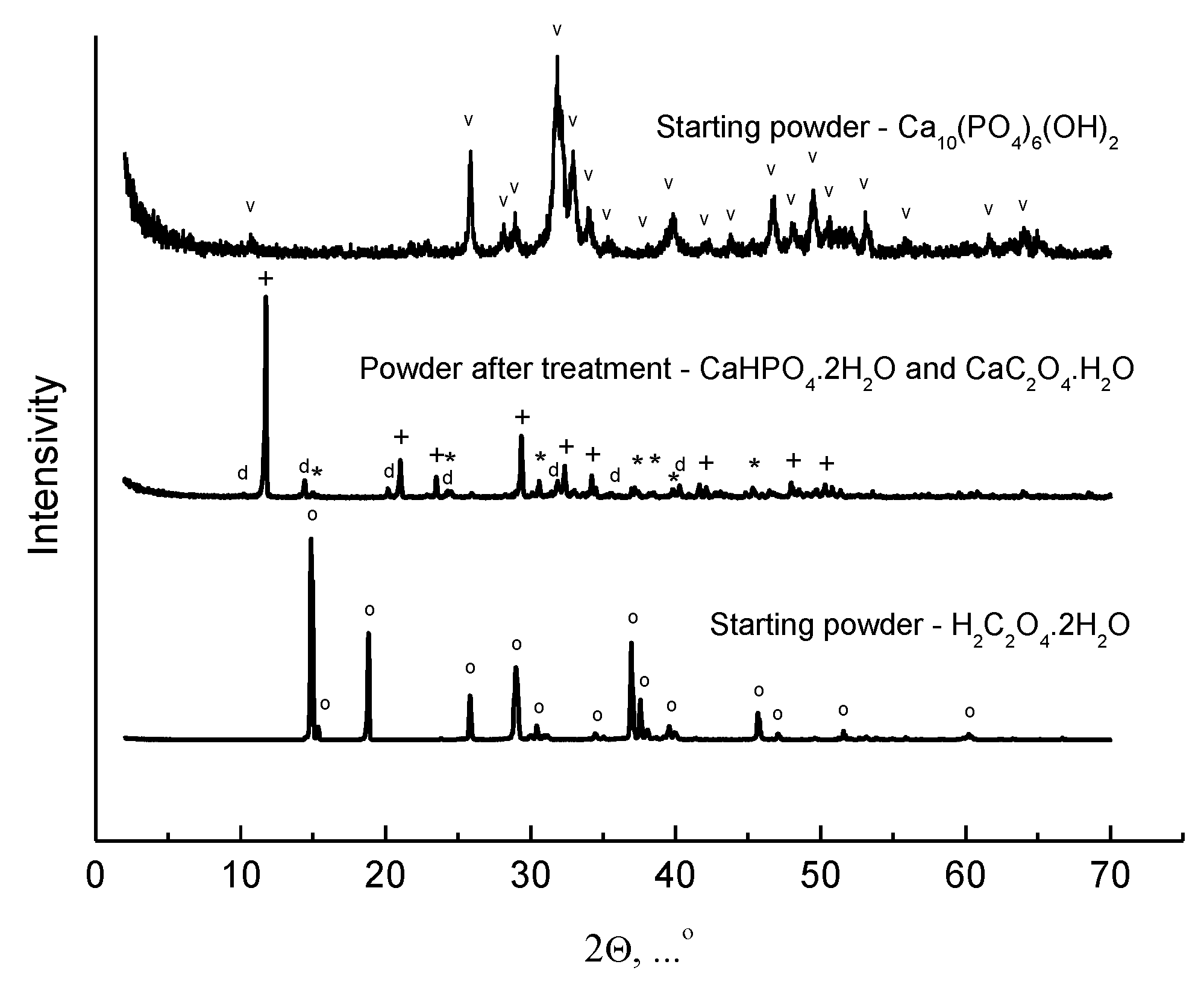
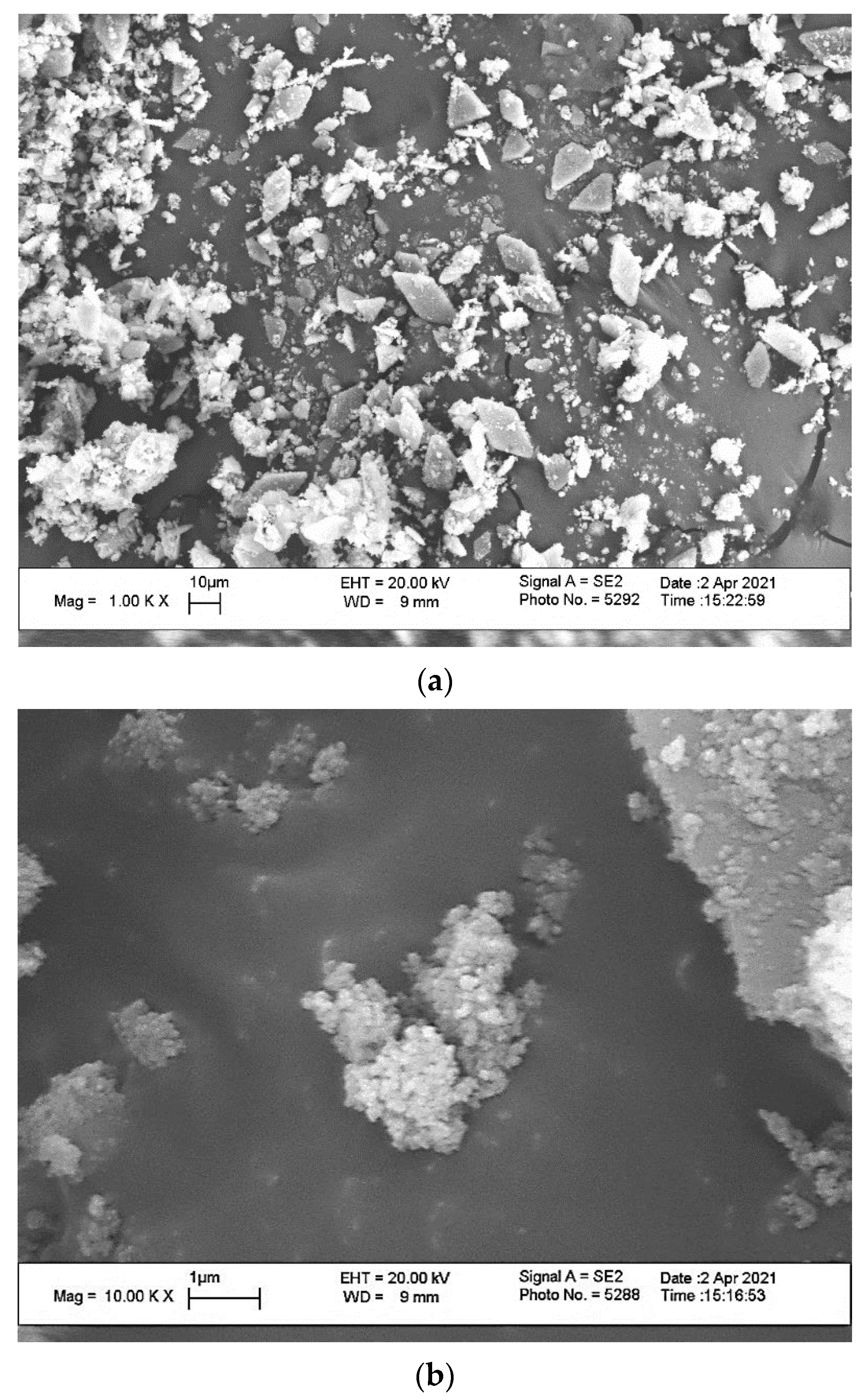
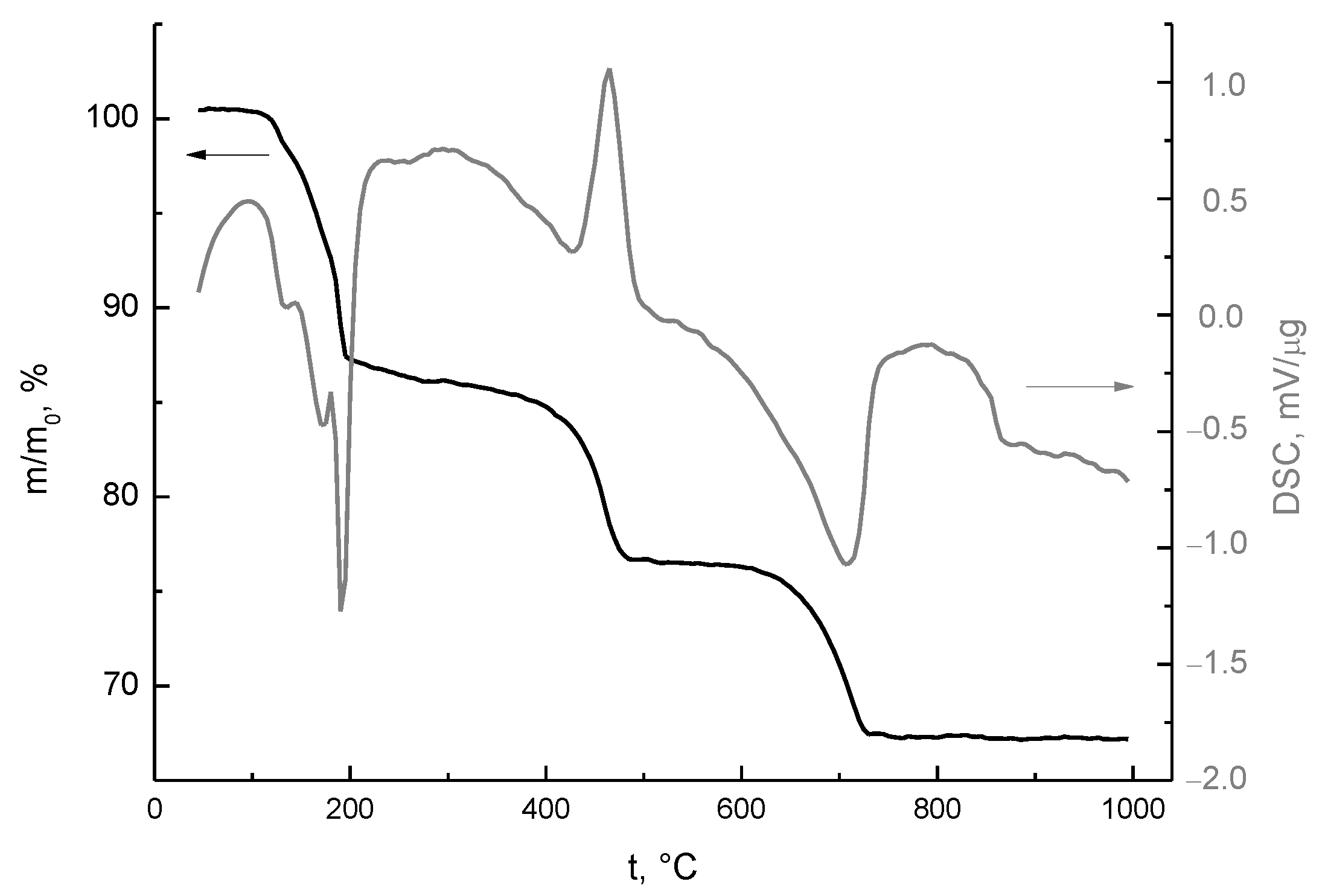
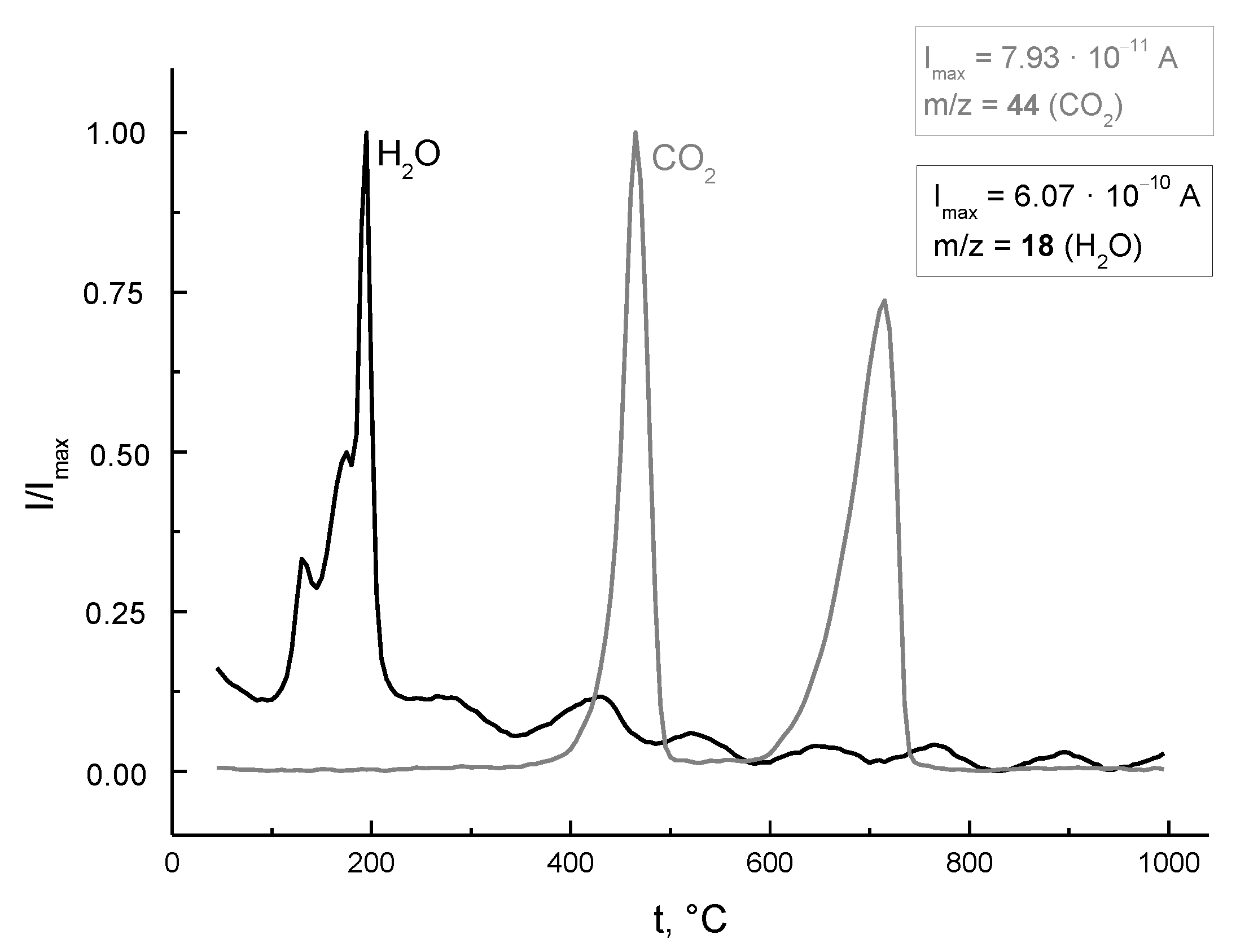

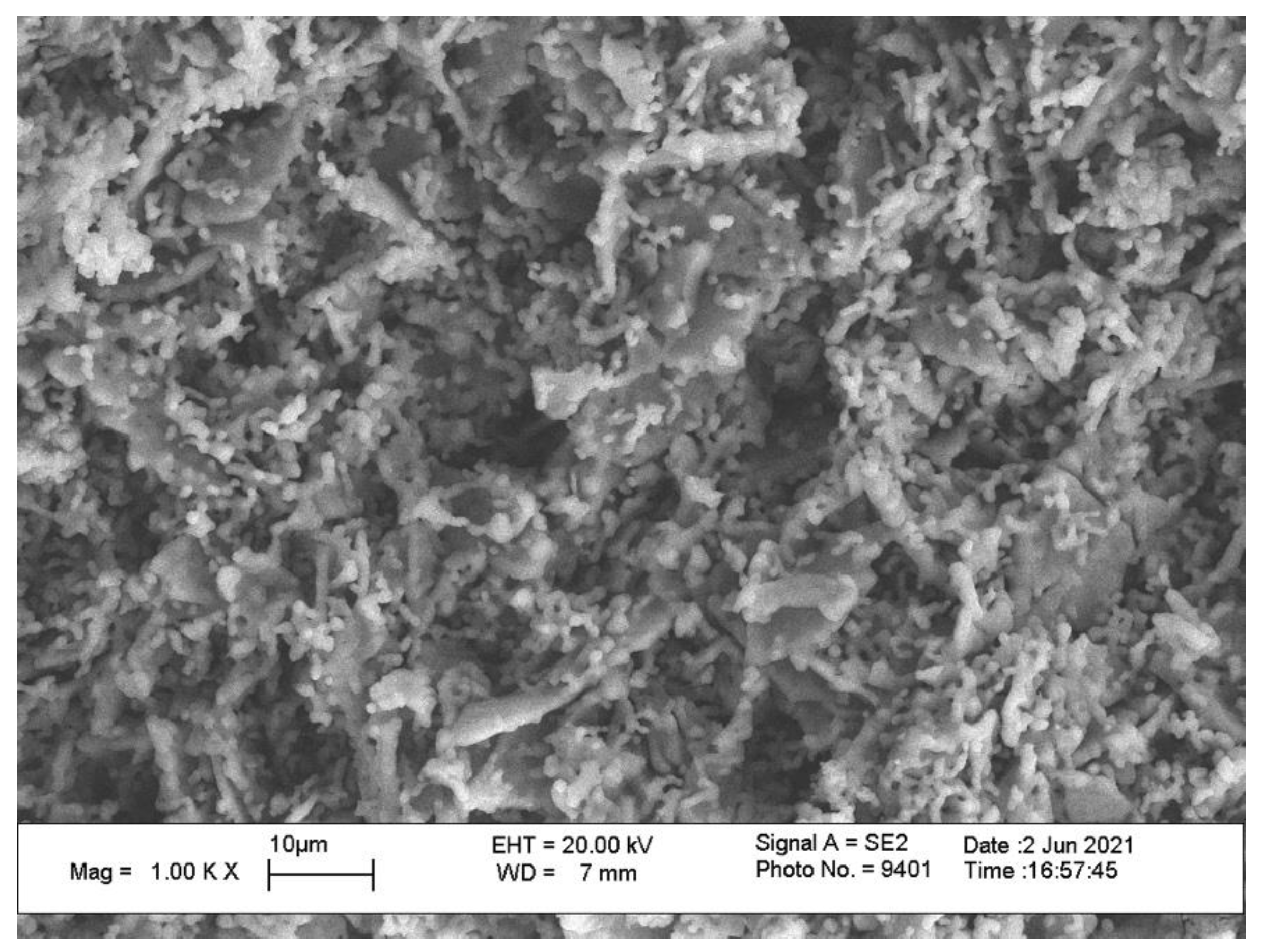

| After Mill | 200 °C | 500 °C | 1000 °C | 1100 °C | 1200 °C |
|---|---|---|---|---|---|
| CaHPO4·2H2O non-identified phase CaC2O4·H2O | CaHPO4 Ca3(PO4)2·xH2O CaC2O4·H2O | γ-Ca2P2O7 Ca3(PO4)2 xH2O CaCO3 | Ca10(PO4)6(OH)2 β-Ca3(PO4)2 CaCO3 * | Ca10(PO4)6(OH)2 β-Ca3(PO4)2 * | Ca10(PO4)6(OH)2 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Safronova, T.; Chichulin, S.; Shatalova, T.; Filippov, Y. Powder Mixture for the Production of Microporous Ceramics Based on Hydroxyapatite. Ceramics 2022, 5, 108-119. https://doi.org/10.3390/ceramics5010010
Safronova T, Chichulin S, Shatalova T, Filippov Y. Powder Mixture for the Production of Microporous Ceramics Based on Hydroxyapatite. Ceramics. 2022; 5(1):108-119. https://doi.org/10.3390/ceramics5010010
Chicago/Turabian StyleSafronova, Tatiana, Stepan Chichulin, Tatiana Shatalova, and Yaroslav Filippov. 2022. "Powder Mixture for the Production of Microporous Ceramics Based on Hydroxyapatite" Ceramics 5, no. 1: 108-119. https://doi.org/10.3390/ceramics5010010
APA StyleSafronova, T., Chichulin, S., Shatalova, T., & Filippov, Y. (2022). Powder Mixture for the Production of Microporous Ceramics Based on Hydroxyapatite. Ceramics, 5(1), 108-119. https://doi.org/10.3390/ceramics5010010