Influence of Aluminium and Boron Orthophosphate on the Setting and the Resulting Structure of Alkali Silicate Binders for Refractory Application
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. Dynamic Mechanical Analysis
2.2.2. Nuclear Magnetic Resonance Spectroscopy
3. Results
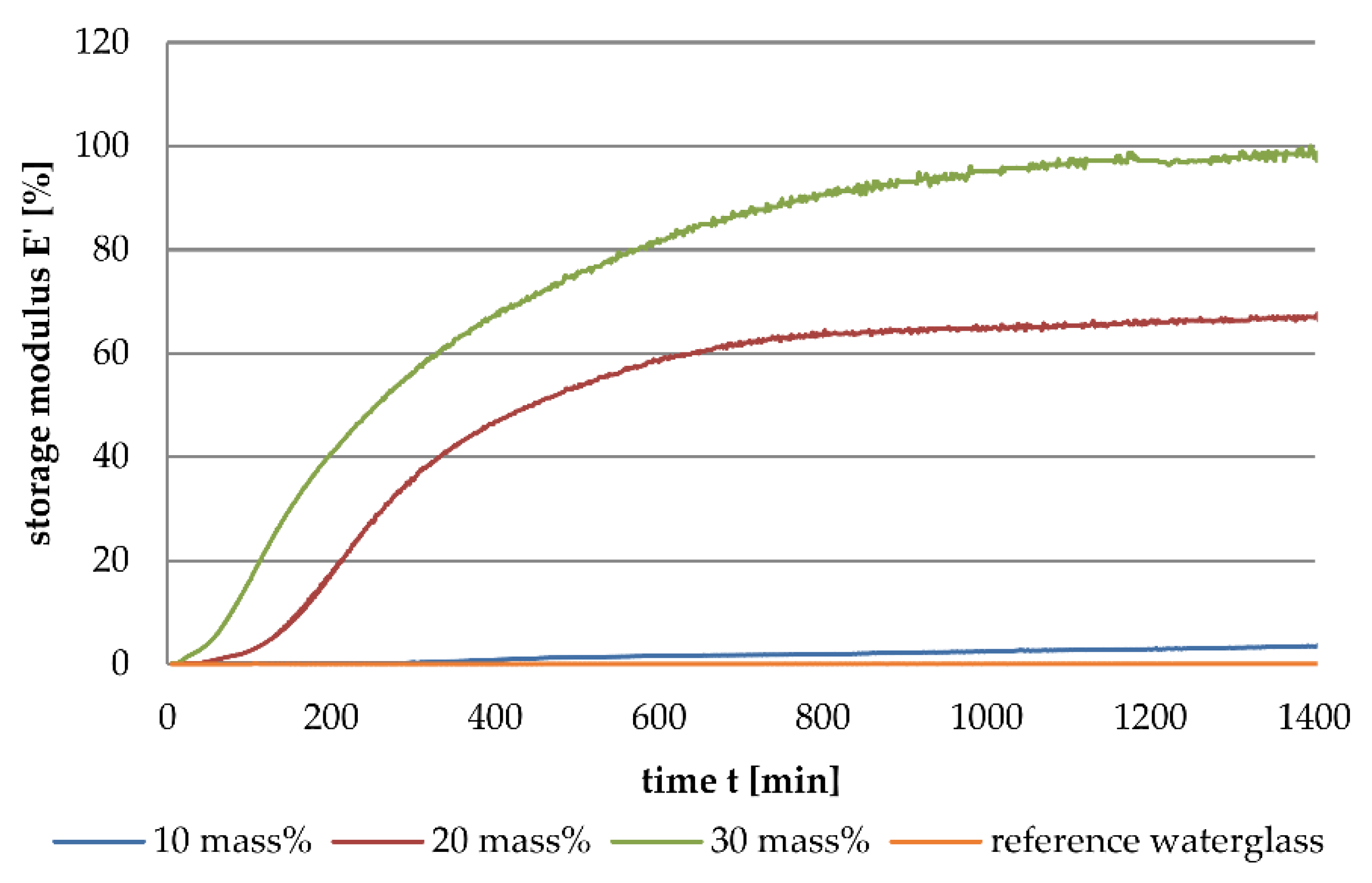
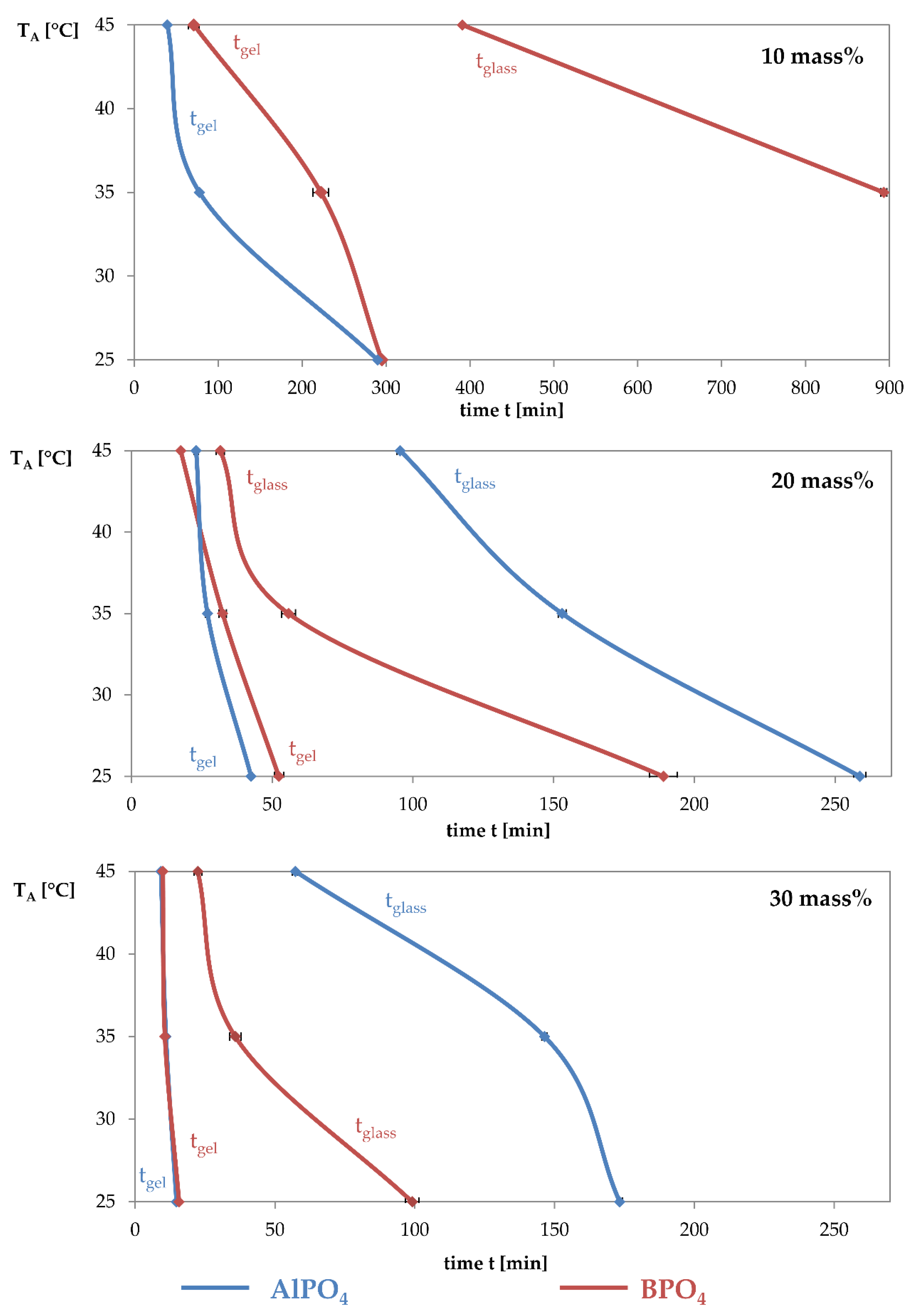
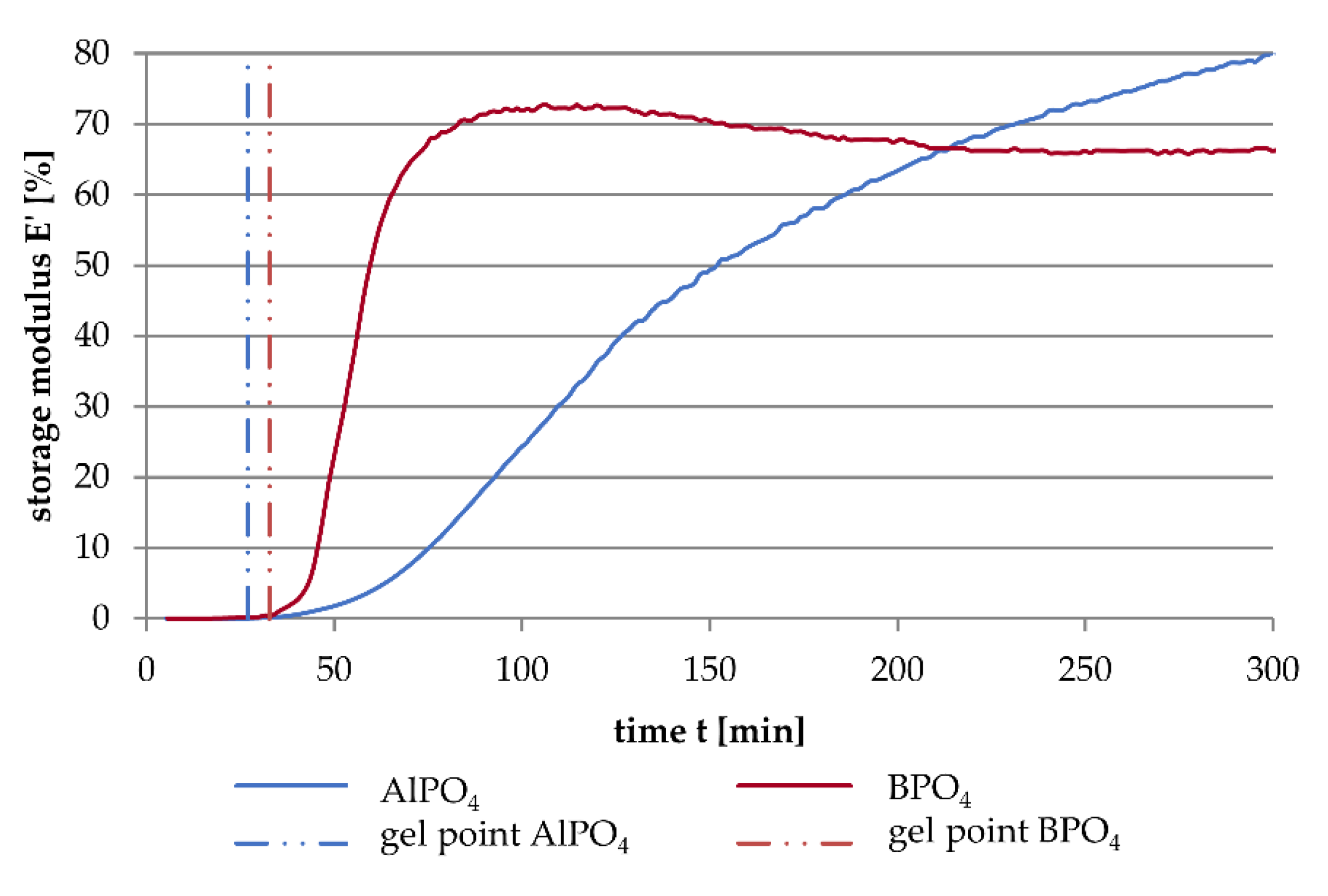
3.1. Dynamic Mechanical Analysis
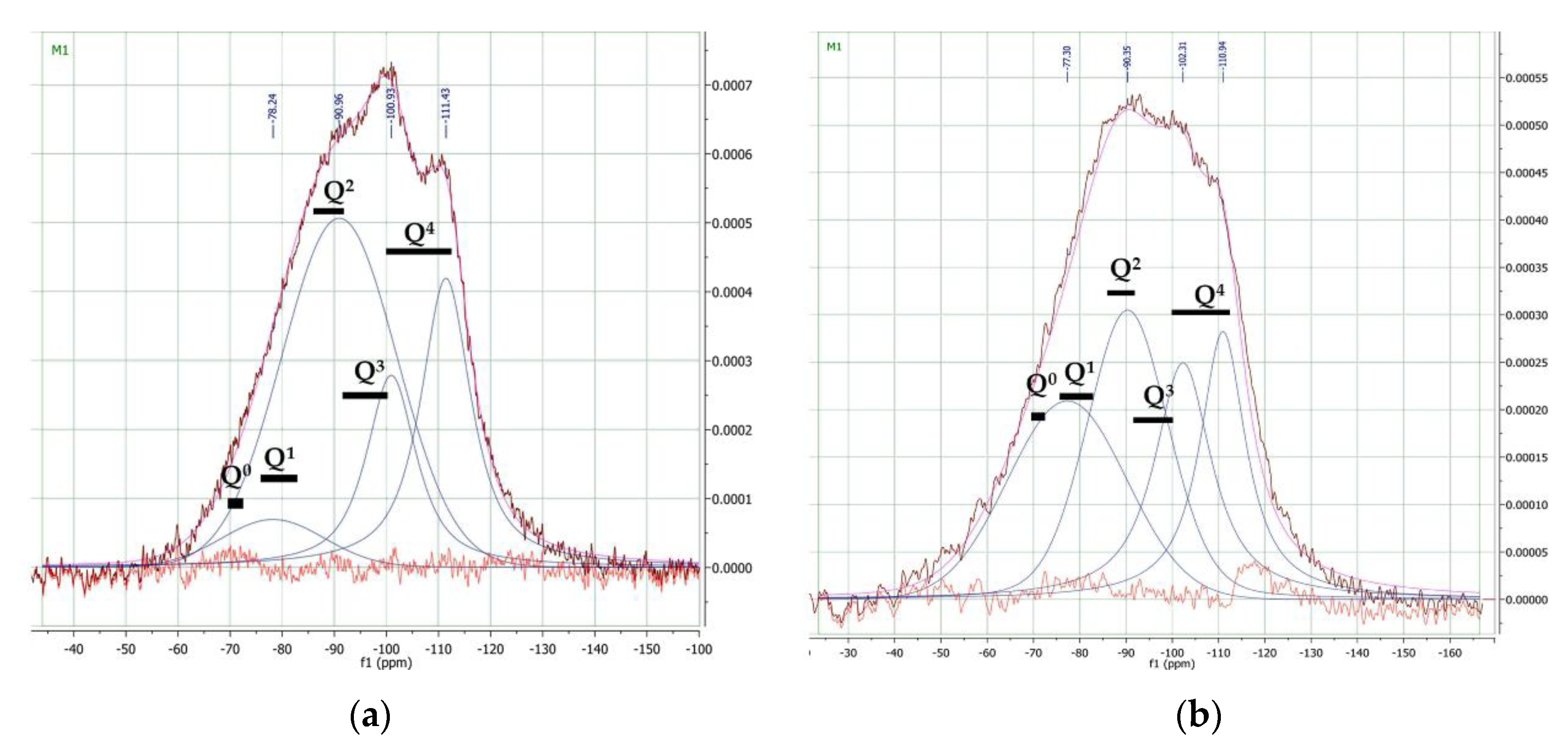
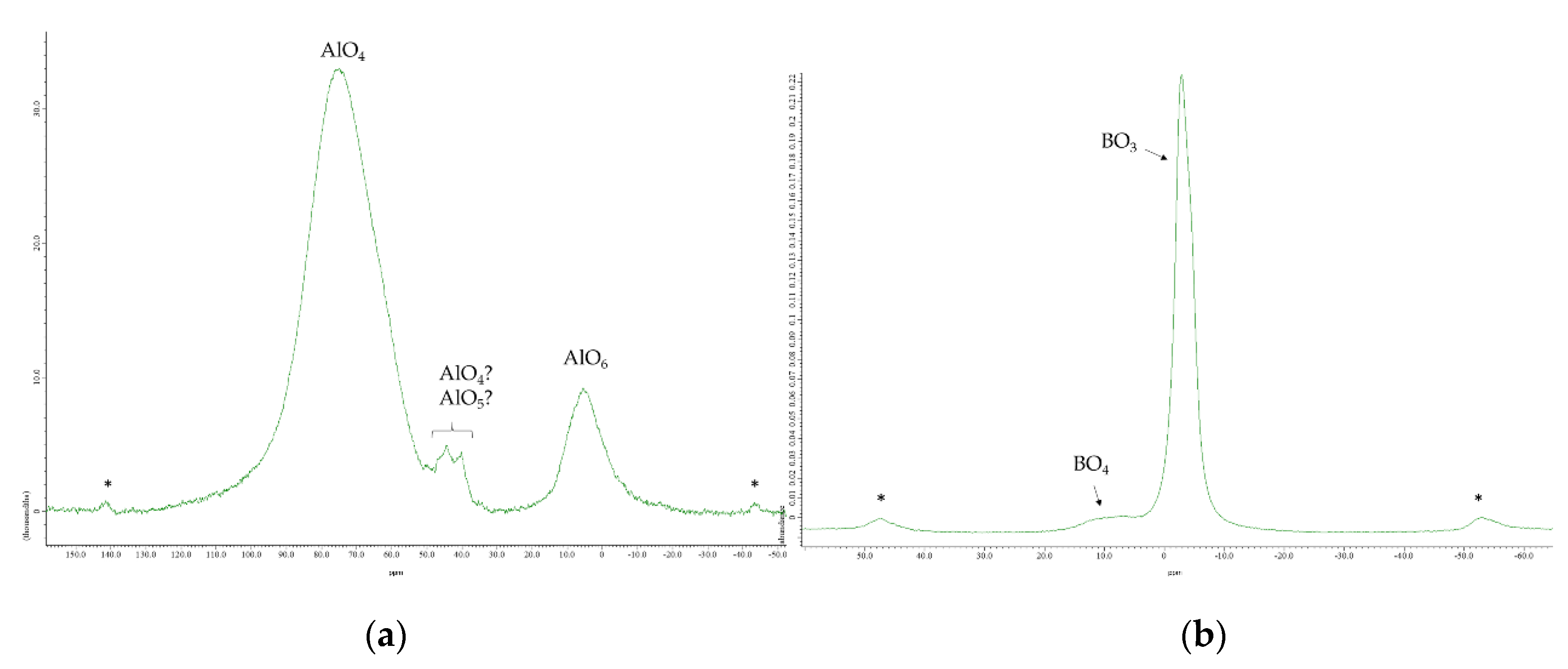
3.2. Nuclear Magnetic Resonance Spectroscopy
4. Discussion
5. Conclusions
- The addition of aluminium orthophosphate (AlPO4) to liquid alkali silicates results in a lower increase of the storage modulus when compared to the addition of boron orthophosphate.
- The different rates in storage modulus development correlate with the linking density and the locations of network-forming condensation reactions.
- Boron orthophosphate (BPO4) addition leads to a fast-growing but rarely linked network, due to network-forming reactions in the periphery of the network.
- Hardening with AlPO4 causes network-forming reactions within the network, leading to the formation of highly linked sub-structures surrounded by liquid waterglass. The consequence is a significantly slower increase in storage modulus.
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Wenda, R.W. Die aktuelle Wochenschau der GDCh. Available online: http://archiv.aktuelle-wochenschau.de/2011/w36/woche36.html (accessed on 9 January 2020).
- Zellmann, H.D. Metaphosphat–modifizierte Silikatbinder als Basis Säurebeständiger Beschichtungsmaterialien. Ph.D. Thesis, Bauhaus University Weimar, Weimar, Germany, 19 September 2008. [Google Scholar]
- Giskow, R. Waterglass and Phosphate—A System for Alchemists? Ceram. forum Int. 2005, 82, 22–26. [Google Scholar]
- Pavlovský, J.; Thomas, B.; Brendler, E.; Polzin, H.; Tilch, W.; Skuta, R.; Jelínek, P. Wasserglasgebundene Formstoffe. Untersuchungen zum Einfluss des Silikatmoduls und der Verdünnung auf die Struktur und die Festigkeitseigenschaften von Wasserglaslösungen und zur Kinetik des Härtungsprozesses. Giess.-Prax. 2005, 3, 82–88. [Google Scholar]
- Roggendorf, H. Strukturmodelle von Wassergläsern? In Referate und Vorträge der 83 Glastech; Tagung der DGG: Amberg, Germany, 2009. [Google Scholar]
- Schuch, K. Steuerung des Aggregationsprozesses in wässrigen Alkalisilikatsolen durch spezielle Gelinitiatoren und moderate Wärmebehandlung zum Aufbau einer stabilen Silikatbeschichtung. Ph.D. Thesis, Bauhaus University Weimar, Weimar, Germany, 21 November 2015. [Google Scholar]
- Roggendorf, H.; Böschel, D. Hydrous sodium silicate glasses obtained by drying sodium silicate solutions. Glass Sci. Technol. 2002, 75, 103–111. [Google Scholar]
- Roggendorf, H.; Wolter, H.; Trempler, J.; Runge, J.; Busse, K.; Adhikari, R.; Kressler, J.; Michler, G.H. Inorganic colloidal glasses–relations between preparation and structure. Eur. J. Glass Sci. Tech. B. Phys. Chem. Glasses 2005, 46, 439–443. [Google Scholar]
- Parr, C.; Auvray, J.M.; Szepizdyn, M.; Wöhrmeyer, C.; Zetterstrom, C. A Review of Bond Systems for Monolithic Castable Refractories. Refract. worldforum 2015, 7, 63–70. [Google Scholar]
- Polzin, H. Die Anwendung kaltselbsthärtender anorganischer Bindersysteme. Giess.-Prax. 2010, 9, 282–287. [Google Scholar]
- Staffel, T.; Wahl, F.; Weber, S.; Glaum, L.; Glaum, R. Kälte und Feuchte—na und? Polym. Alum. als Wasserglashärter 2002, 108, 103–109. [Google Scholar]
- Masoudi, A. Strukturelle Phasenanalyse von chemischen Prozessadditiven in der Silikat-Industrie; Springer Fachmedien Wiesbaden: Wiesbaden, Germany, 2019. [Google Scholar]
- Masoudi, A. Einfluss der Struktur von Aluminium-Metaphosphaten auf die Chemische Härtung von Kalium-Wasserglas-Bindern. Ph.D. Thesis, University of Koblenz-Landau, Koblenz, Germany, 26 April 2019. [Google Scholar]
- Menard, K.P. Dynamic Mechanical Analysis: Practical Introduction, 2nd ed.; Taylor and Francis Group: Boca Raton, FL, USA, 2008; Available online: http://sv.20file.org/up1/608_0.pdf (accessed on 9 January 2020).
- Hopp, V.; Sax, A.; Helmus, D.; Quirmbach, P. Adaptation of the dynamic mechanical analysis to determine the gel point during the setting of liquid alkali silicates. Int. J. Appl. Ceram. Technol. 2019, 16, 2331–2341. [Google Scholar] [CrossRef]
- Hofmann, K.; Glasser, W.G. Cure monitoring of an epoxy-amine system by dynamic mechanical analysis (DMTA). Thermochim. Acta 1990, 166, 169–187. [Google Scholar] [CrossRef]
- Kim, S.Y.; Choi, D.G.; Yang, S.M. Rheological analysis of the gelation behavior of tetraethylorthosilane / vinyltriethoxysilane hybrid solutions. Korean J. Chem. Eng. 2002, 19, 190–196. [Google Scholar] [CrossRef][Green Version]
- Gan, H.; Hess, P.C.; Kirkpatrick, J. Phosphorus and boron speciation in K2O-B2O3-SiO2-P2O5 glasses. Geochim. Cosmochim. Acta 1994, 58, 4633–4647. [Google Scholar] [CrossRef]
- Brus, J.; Abbrent, S. Advances in 27Al MAS NMR studies of geopolymers. Annu. Rep. NMR Spectrosc. 2016, 88, 79–147. [Google Scholar]
- Schulz, M. Festkörper-NMR-Untersuchungen zur Strukturaufklärung mesostrukturierter Aluminiumphosphate. Ph.D. Thesis, Friedrich-Schiller-Universität Jena, Jena, Germany, 4 December 2002. [Google Scholar]
- Bleuzen, A.; Barboux-Doeuff, S.; Flaud, P.; Sanchez, C. Rheological study of titanium oxide-based gels. Mater. Res. Bull. 1994, 29, 1223–1232. [Google Scholar] [CrossRef]
- Wijnen, P.W.J.G.; Beelen, T.P.M.; Rummens, C.P.J.; van Santen, R.A. Diffusion- and reaction-limited aggregation of aqueous silicate solutions. J. Non-Cryst. Solids 1991, 136, 119–125. [Google Scholar] [CrossRef][Green Version]

| DMA Parameter | Value |
|---|---|
| Instrument | DMA 242 C, Netzsch |
| Amplitude | 50 µm |
| Maximum dynamic force | 7 N |
| Additional constant portion of the static force | 0 N |
| Proportional factor | 0 |
| Frequencies | 1, 5, 10, 16.666, 20, 25 Hz |
| TA | 10 mass% | 20 mass% | 30 mass% | |||
|---|---|---|---|---|---|---|
| Gel Point | Glass Point | Gel Point | Glass Point | Gel Point | Glass Point | |
| 25 °C | 290 | 42.5 | 258.8 | 14.75 | 173.3 | |
| 35 °C | 77.8 | 27 | 153 | 11 | 146.4 | |
| 45 °C | 39.5 | 23 | 95.5 | 9.2 | 57.3 | |
| TA | 10 mass% | 20 mass% | 30 mass% | |||
|---|---|---|---|---|---|---|
| Gel Point | Glass Point | Gel Point | Glass Point | Gel Point | Glass Point | |
| 25 °C | 295 | 52.4 | 189 | 15.7 | 99.1 | |
| 35 °C | 222.5 | 893.2 | 32.4 | 55.8 | 10.6 | 35.8 |
| 45 °C | 71 | 391 | 17.5 | 31.6 | 9.9 | 22.4 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hopp, V.; Masoudi Alavi, A.; Sax, A.; Quirmbach, P. Influence of Aluminium and Boron Orthophosphate on the Setting and the Resulting Structure of Alkali Silicate Binders for Refractory Application. Ceramics 2020, 3, 1-11. https://doi.org/10.3390/ceramics3010001
Hopp V, Masoudi Alavi A, Sax A, Quirmbach P. Influence of Aluminium and Boron Orthophosphate on the Setting and the Resulting Structure of Alkali Silicate Binders for Refractory Application. Ceramics. 2020; 3(1):1-11. https://doi.org/10.3390/ceramics3010001
Chicago/Turabian StyleHopp, Vanessa, Ali Masoudi Alavi, Almuth Sax, and Peter Quirmbach. 2020. "Influence of Aluminium and Boron Orthophosphate on the Setting and the Resulting Structure of Alkali Silicate Binders for Refractory Application" Ceramics 3, no. 1: 1-11. https://doi.org/10.3390/ceramics3010001
APA StyleHopp, V., Masoudi Alavi, A., Sax, A., & Quirmbach, P. (2020). Influence of Aluminium and Boron Orthophosphate on the Setting and the Resulting Structure of Alkali Silicate Binders for Refractory Application. Ceramics, 3(1), 1-11. https://doi.org/10.3390/ceramics3010001





