Mechanism of Carbon Contamination in Transparent MgAl2O4 and Y3Al5O12 Ceramics Sintered by Spark Plasma Sintering
Abstract
:1. Introduction
2. Experimental Procedure
3. Results and Discussion
4. Conclusions
- -
- Carbon contamination originates from carbon clusters which are formed by carbon originating from the powder itself and from the SPS environment (papiex® graphite foil, graphite die, graphite felt).
- -
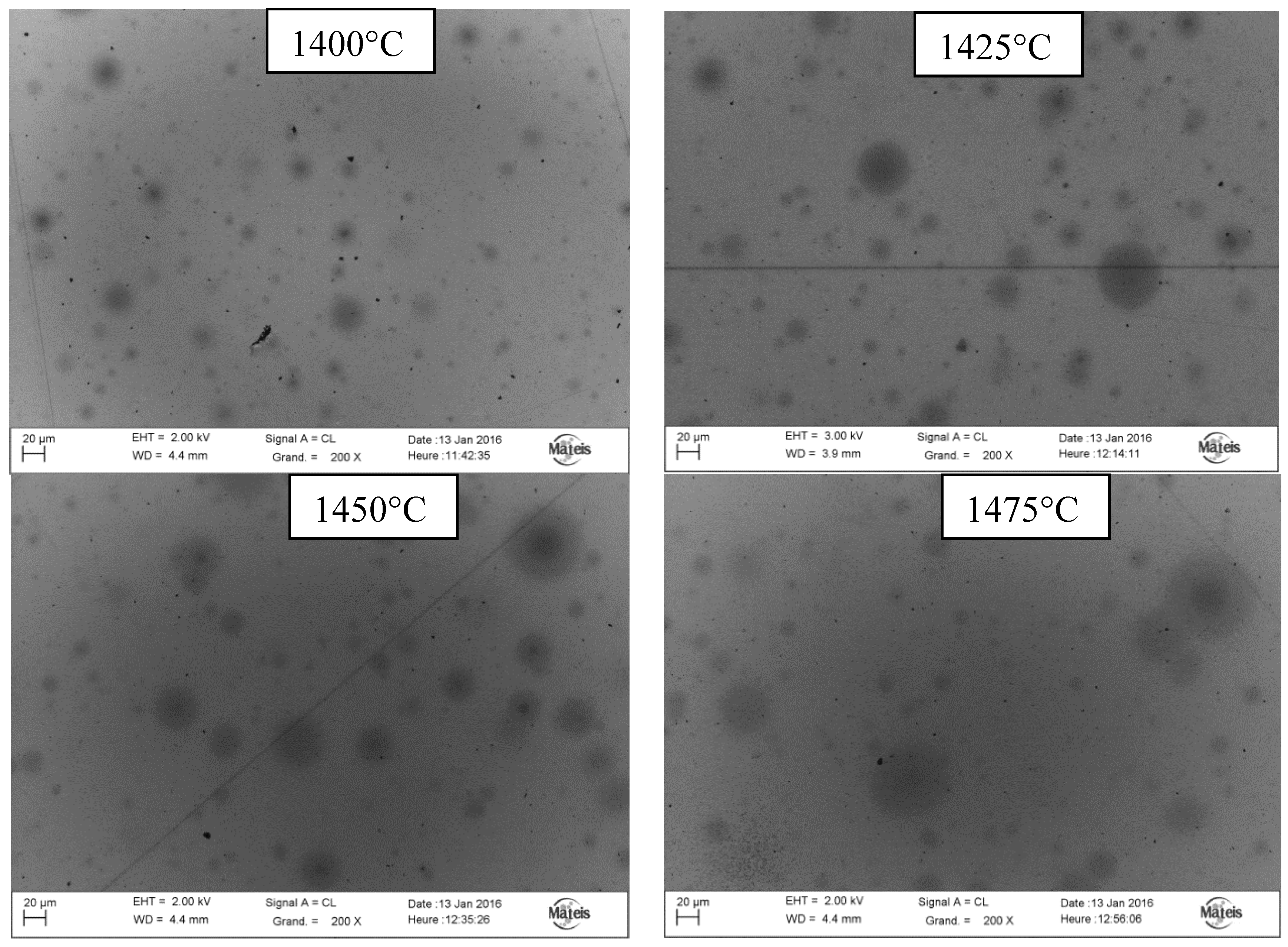
- Longer SPS time and a higher temperature are responsible for carbon diffusion from the carbon clusters. The carbon diffuses in the volume around each cluster, which then increases more and more in size.
- -
- Once the kinetics of diffusion is thermally activated (Arrhenius law), the most significant parameter which can explain the light absorption increase is temperature.
- -
- SPS conditions such as the current and insulating felt can also influence the temperature and so impact carbon diffusion.
- -
- Cleaner powders with a low specific surface area can limit the formation of carbon clusters. Lower temperature and shorter heat treatment will also limit their diffusion and preserve the transparency.
Author Contributions
Funding
Conflicts of Interest
References
- Krell, A.; Hutzler, T.; Klimke, J. Transparent Ceramics for Structural Applications, Ceram. Forums Int. 2007, 84, e41–e50. [Google Scholar]
- Bonnefont, G.; Fantozzi, G.; Trombert, S.; Bonneau, L. Fine-grained transparent MgAl2O4 spinel obtained by spark plasma sintering of commercially available nanopowders. Ceram. Int. 2012, 38, 131–140. [Google Scholar] [CrossRef]
- Goldstein, A.; Shames, A.I.; Stevenson, A.J.; Cohen, A.; Vulfson, M. Parasitic Light Absorption Processes in Transparent Polycrystalline MgAl2O4 and YAG. J. Am. Ceram. Soc. 2013, 96, 3523–3529. [Google Scholar] [CrossRef]
- Li, J.G.; Ikegami, T.; Lee, J.H.; Mori, T. Fabrication of Translucent Magnesium Aluminate Spinel Ceramics. J. Am. Ceram. Soc. 2000, 83, 2866–2868. [Google Scholar] [CrossRef]
- Gilde, G.; Patel, P.; Patterson, P.; Blodgett, D.; Duncan, D.; Hahn, D. Evaluation of Hot Pressing and Hot Isostastic Pressing Parameters on the Optical Properties of Spinel. J. Am. Ceram. Soc. 2005, 88, 2747–2751. [Google Scholar] [CrossRef]
- Reimanis, I.; Kleebe, H.J. A Review on the Sintering and Microstructure Development of Transparent Spinel (MgAl2O4). J. Am. Ceram. Soc. 2009, 92, 1472–1480. [Google Scholar] [CrossRef]
- Kim, B.N.; Hiraga, K.; Morita, K.; Yoshida, H. Spark plasma sintering of transparent alumina. Scr. Mater. 2007, 57, 607–610. [Google Scholar] [CrossRef]
- Jiang, D.T.; Hulbert, D.M.; Anselmi-Tamburini, U.; Ng, T.; Land, D.; Mukherjee, A.K. Optically transparent polycrystalline Al2O3 produced by spark plasma sintering. J. Am. Ceram. Soc. 2008, 91, 151–154. [Google Scholar] [CrossRef]
- Kim, B.N.; Hiraga, K.; Morita, K.; Yoshida, H. Effects of heating rate on microstructure and transparency of spark-plasma-sintered alumina. J. Eur. Ceram. Soc. 2009, 29, 323–327. [Google Scholar] [CrossRef]
- Kim, B.N.; Hiraga, K.; Morita, K.; Yoshida, H.; Miyazaki, T.; Kagawa, Y. Microstructure and optical properties of transparent alumina. Acta Mater. 2009, 57, 1319–1326. [Google Scholar] [CrossRef]
- Morita, K.; Kim, B.N.; Yoshida, H.; Hiraga, K. Densification behavior of a fine-grained MgAl2O4 spinel during spark plasma sintering (SPS). Scr. Mater. 2010, 63, 565–568. [Google Scholar] [CrossRef]
- Morita, K.; Kim, B.N.; Yoshida, H.; Hiraga, K.; Sakka, Y. Spectroscopic study of the discoloration of transparent MgAl2O4 spinel fabricated by spark-plasma-sintering (SPS) processing. Acta Mater. 2015, 84, 9–19. [Google Scholar] [CrossRef]
- Frage, N.; Cohen, S.; Meir, S.; Kalabukhov, S.; Dariel, P.M. Spark plasma sintering (SPS) of transparent magnesium-aluminate spinel. J. Mater. Sci. 2007, 42, 3273–3275. [Google Scholar] [CrossRef]
- Morita, K.; Kim, B.N.; Yoshida, H.; Hiraga, K.; Sakka, Y. Influence of pre- and post-annealing on discoloration of MgAl2O4 spinel fabricated by spark-plasma-sintering (SPS). J. Eur. Ceram. Soc. 2016, 36, 2961–2968. [Google Scholar] [CrossRef]
- Morita, K.; Kim, B.N.; Yoshida, H.; Hiraga, K.; Sakka, Y. Distribution of carbon contamination in MgAl2O4 spinel occurring during spark-plasma-sintering (SPS) processing: I—Effect of heating rate and post-annealing. J. Eur. Ceram. Soc. 2018, 38, 2588–2595. [Google Scholar] [CrossRef]
- Morita, K.; Kim, B.N.; Yoshida, H.; Hiraga, K.; Sakka, Y. Distribution of carbon contamination in oxide ceramics occurring during spark-plasma-sintering (SPS) processing: II—Effect of SPS and loading temperatures. J. Eur. Ceram. Soc. 2018, 38, 2596–2604. [Google Scholar] [CrossRef]
- Bernard-Granger, G.; Benameur, N.; Guizard, C.; Nygren, M. Influence of graphite contamination on the optical properties of transparent spinel obtained by spark plasma sintering. Scr. Mater. 2009, 60, 164–167. [Google Scholar] [CrossRef]
- Meir, S.; Kalabukhov, S.; Froumin, N.; Dariel, M.P.; Frage, N. Synthesis and Densification of Transparent Magnesium Aluminate Spinel by SPS Processing. J. Am. Ceram. Soc. 2009, 92, 358–364. [Google Scholar] [CrossRef]
- Rubat du Merac, M.; Kleebe, H.-J.; Muller, M.M.; Reimanis, I.E. Fifty Years of Research and Development Coming to Fruition; Unraveling the Complex Interactions during Processing of Transparent Magnesium Aluminate (MgAl2O4) Spinel. J. Am. Ceram. Soc. 2013, 96, 3341–3365. [Google Scholar] [CrossRef]
- Goldstein, A. Correlation between MgAl2O4-Spinel Structure, Processing Factors and Functional Properties of Transparent Parts (Progress Review). J. Eur. Ceram. Soc. 2012, 32, 2869–2886. [Google Scholar] [CrossRef]
- Whittaker, A.G. Diffusion in Microwave-Heated Ceramics. Chem. Mater. 2005, 17, 3426–3432. [Google Scholar] [CrossRef]
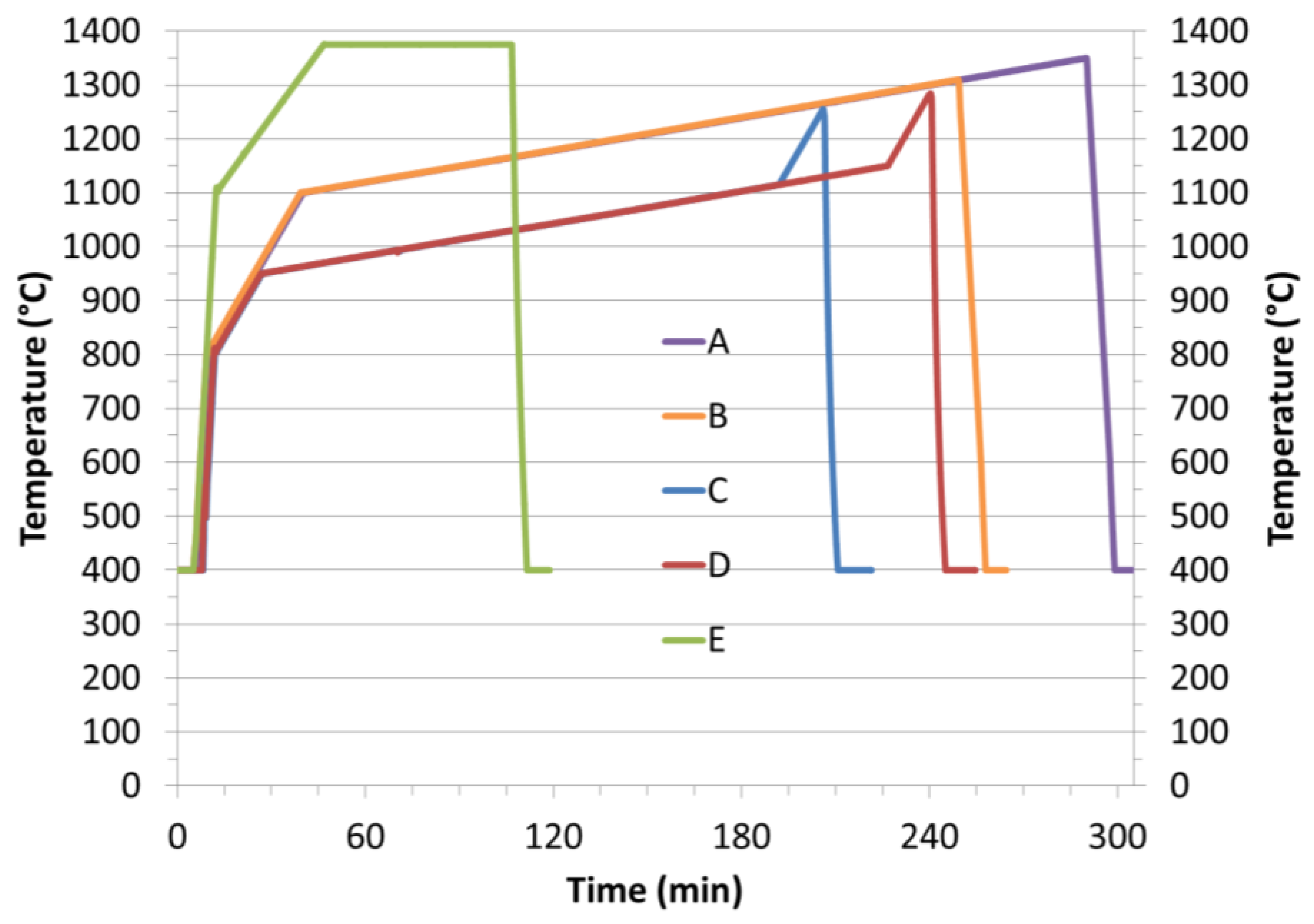

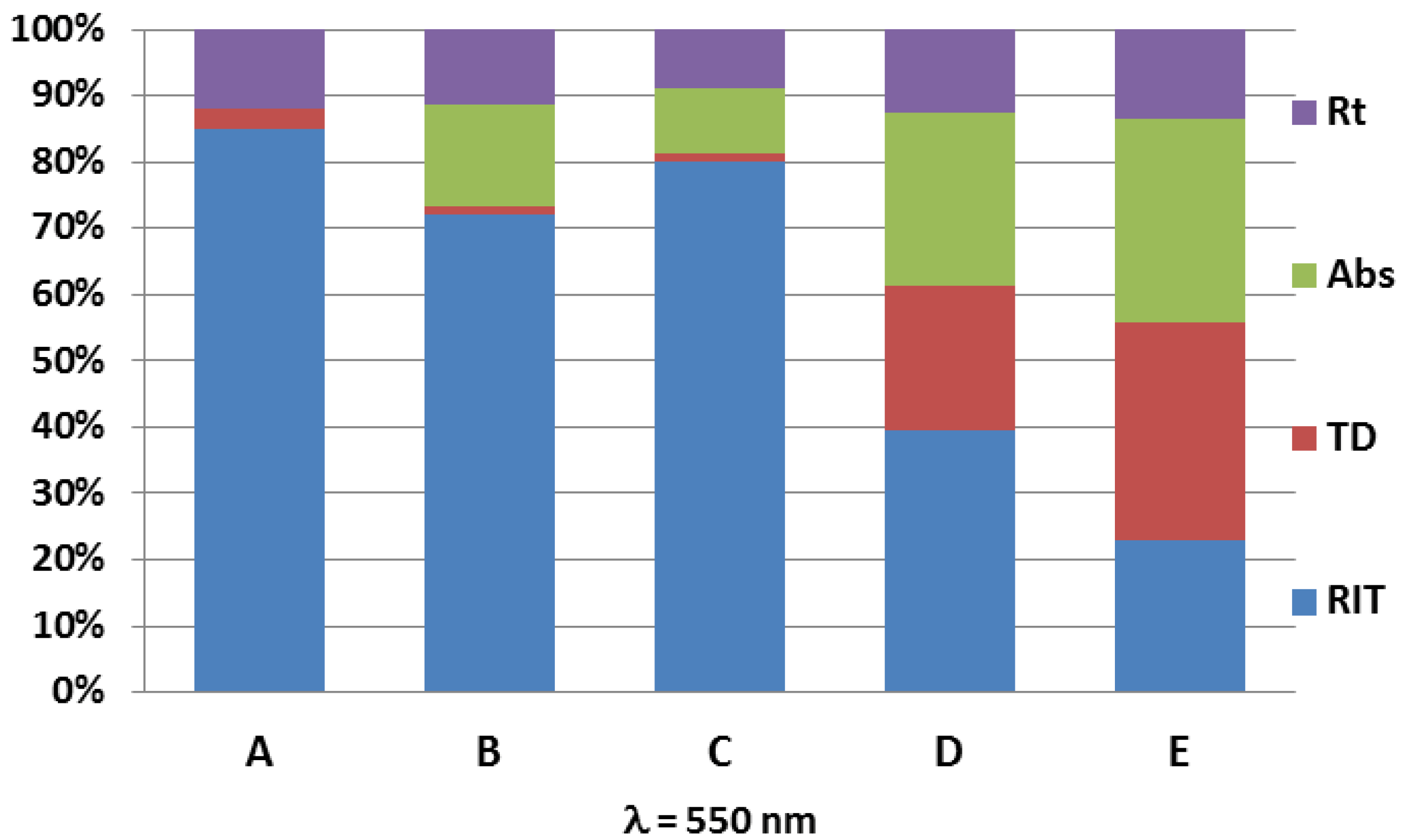

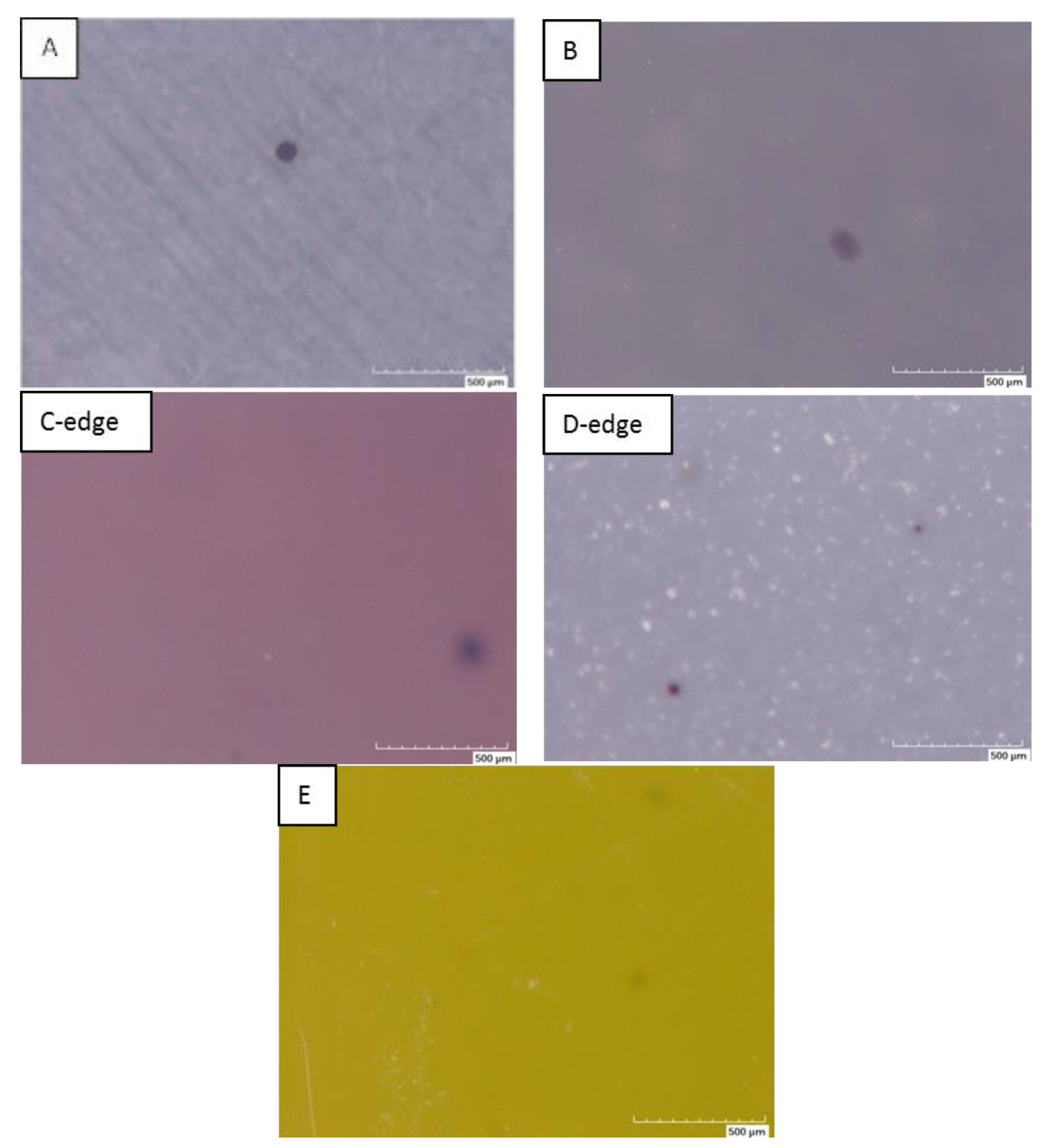
| Samples | Starting Powder | Max Sintering Temperature (°C) | Graphite Felt | Sample Diameter (mm) | Max Irms (A) | Pyrometer |
|---|---|---|---|---|---|---|
| A = MgAl2O4 | S25CRX lot 12 | 1350 | no | 20 | 1390 | Horizontal |
| SSA = 17.1 m2/g | ||||||
| d50 = 29 nm | ||||||
| B = MgAl2O4 | S25CRX lot 14 | 1310 | no | 20 | 1200 | Horizontal |
| SSA = 27.4 m2/g | ||||||
| d50 = 60 nm | ||||||
| C = MgAl2O4 | S30X-W (granulated) | 1250 | yes | 40 | 2850 | Vertical |
| SSA = 27.9 m2/g | ||||||
| d50 = 73 nm | ||||||
| D = MgAl2O4 | S30X-W (granulated) | 1280 | yes | 40 | 2950 | Vertical |
| SSA = 27.9 m2/g | ||||||
| d50 = 73 nm | ||||||
| E = Y3Al5O12 | YAG:Ce | 1375 | no | 20 | 1070 | Horizontal |
| SSA = 7 m2/g | ||||||
| d50 = 150 nm |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hammoud, H.; Garnier, V.; Fantozzi, G.; Lachaud, E.; Tadier, S. Mechanism of Carbon Contamination in Transparent MgAl2O4 and Y3Al5O12 Ceramics Sintered by Spark Plasma Sintering. Ceramics 2019, 2, 612-619. https://doi.org/10.3390/ceramics2040048
Hammoud H, Garnier V, Fantozzi G, Lachaud E, Tadier S. Mechanism of Carbon Contamination in Transparent MgAl2O4 and Y3Al5O12 Ceramics Sintered by Spark Plasma Sintering. Ceramics. 2019; 2(4):612-619. https://doi.org/10.3390/ceramics2040048
Chicago/Turabian StyleHammoud, Hussein, Vincent Garnier, Gilbert Fantozzi, Etienne Lachaud, and Solène Tadier. 2019. "Mechanism of Carbon Contamination in Transparent MgAl2O4 and Y3Al5O12 Ceramics Sintered by Spark Plasma Sintering" Ceramics 2, no. 4: 612-619. https://doi.org/10.3390/ceramics2040048
APA StyleHammoud, H., Garnier, V., Fantozzi, G., Lachaud, E., & Tadier, S. (2019). Mechanism of Carbon Contamination in Transparent MgAl2O4 and Y3Al5O12 Ceramics Sintered by Spark Plasma Sintering. Ceramics, 2(4), 612-619. https://doi.org/10.3390/ceramics2040048






