Is a Zirconia Dental Implant Safe When It Is Available on the Market?
Abstract
1. Background
1.1. Zirconia, ZrO2
1.1.1. Phase Transformation Toughening, PTT
1.1.2. Low Temperature Degradation (LTD)
1.2. Available ISO Standards for Dental Implants Made from Y-TZP
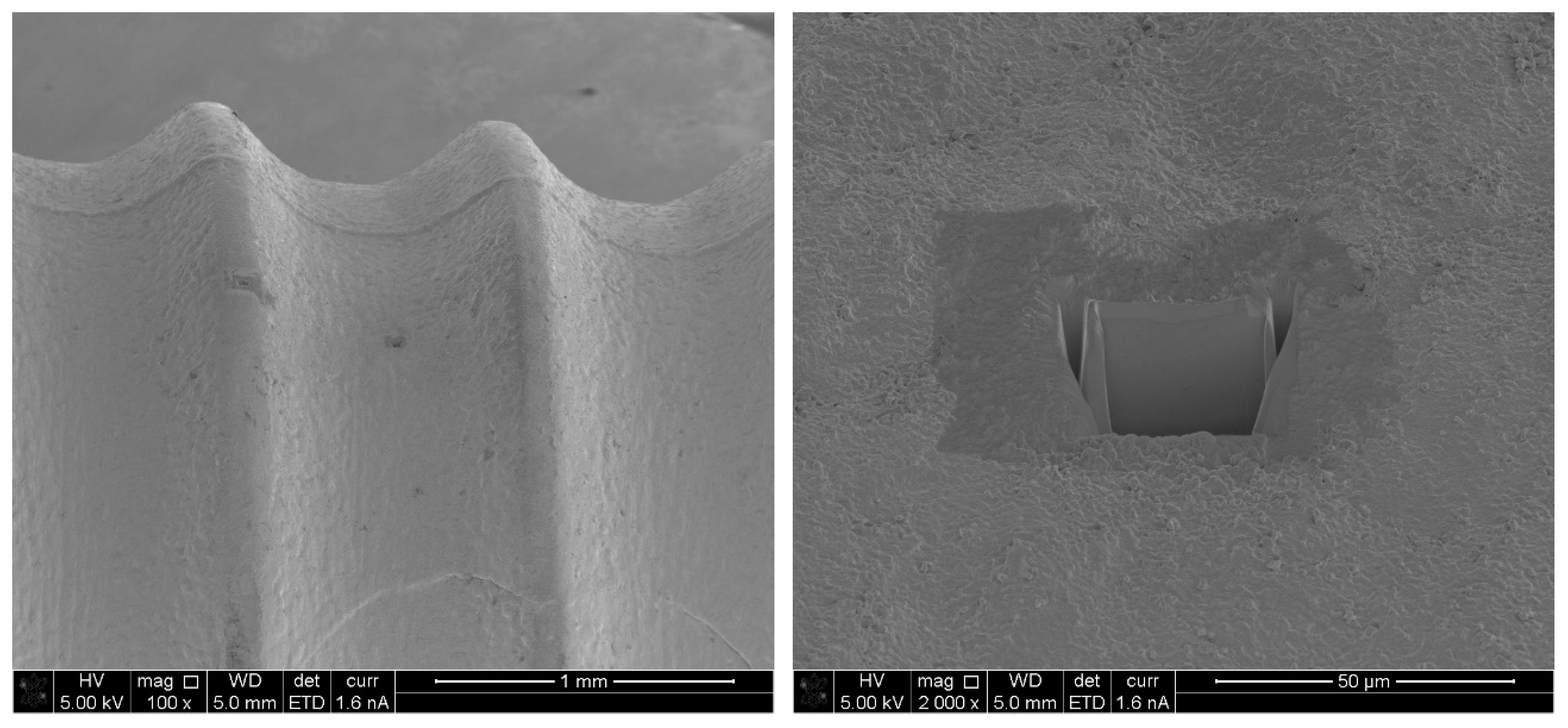
1.2.1. ISO 14801
Dentistry–Implants–Dynamic Fatigue Test for Endosseous Dental Implants
1.2.2. ISO 13356
Implants for Surgery—Ceramic Materials Based on Yttria-Stabilized Tetragonal Zirconia (Y-TZP)
1.3. Methods for Quantification and Visualization of Crystallography
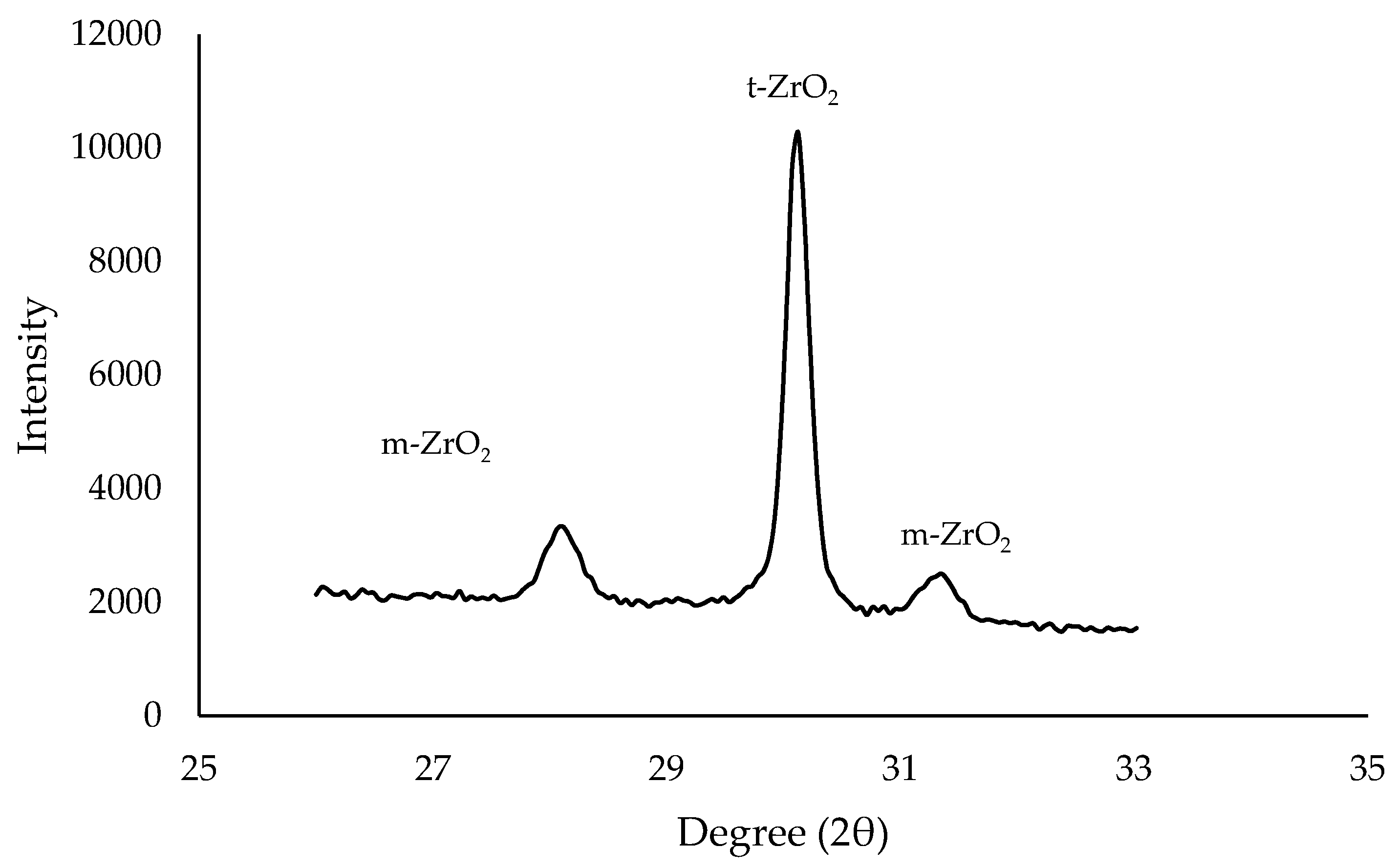
1.3.1. X-ray Diffraction (XRD)
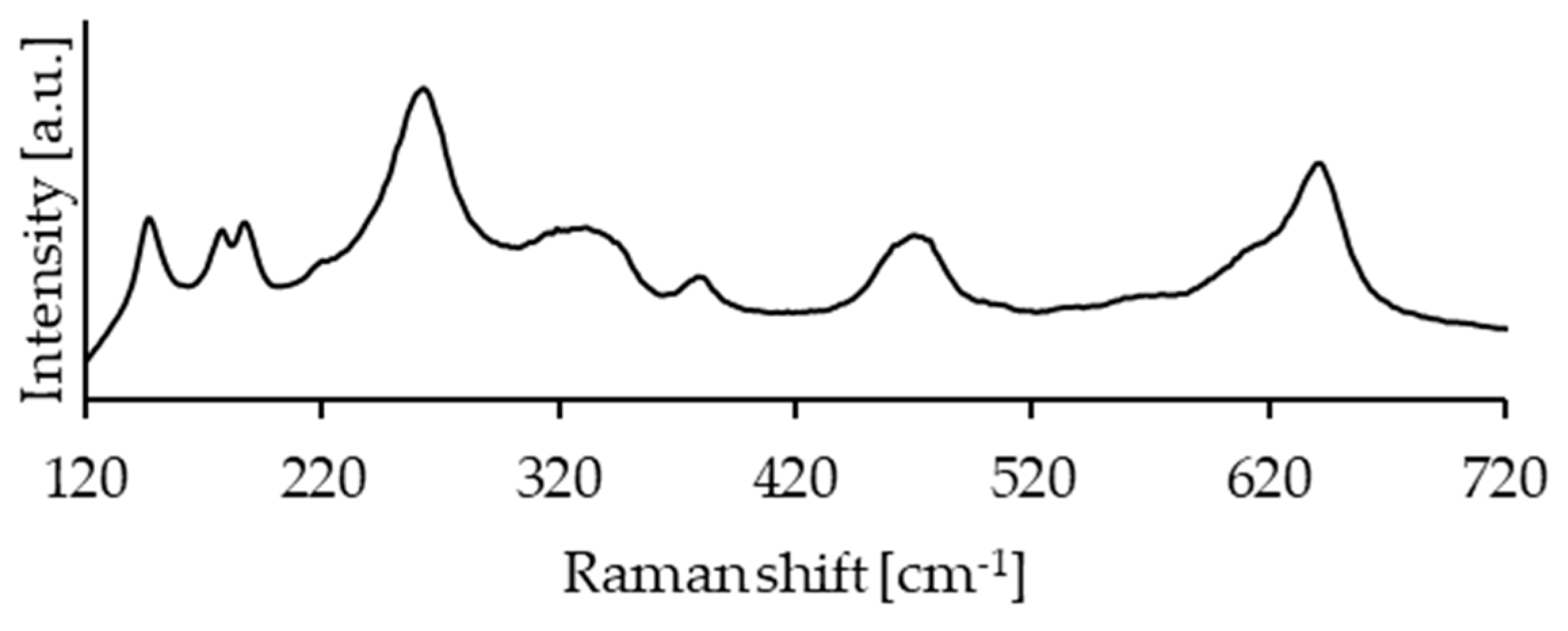
1.3.2. Raman Spectroscopy
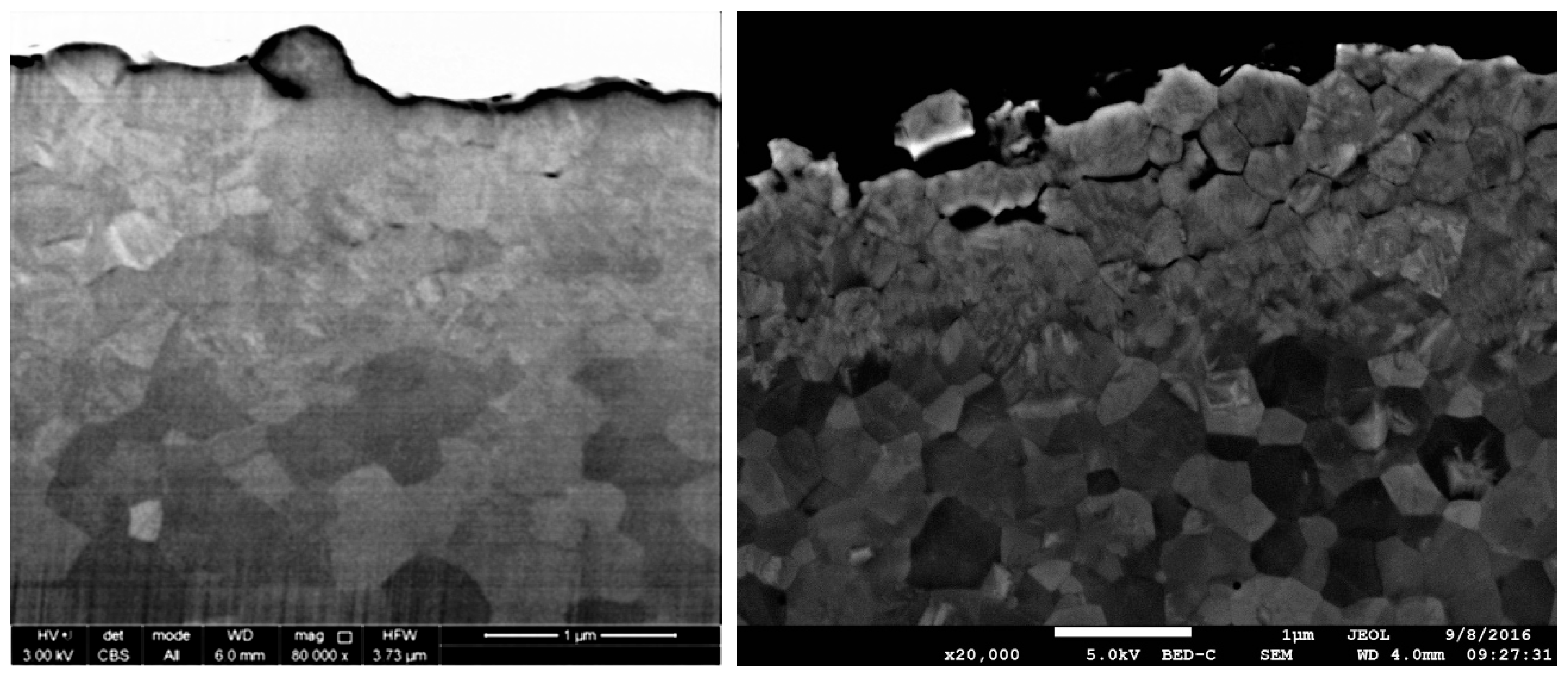
1.3.3. Scanning Electron Microscopy (SEM)
2. Discussion
2.1. Environmental Conditions—Ageing of the Final Product
2.2. Horizontal Shear Forces
2.3. Applied Load and Amount of Loading Cycles
2.4. Final Static Loading to Fracture
3. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Piconi, C.; Maccauro, G. Zirconia as a ceramic biomaterial. Biomaterials 1999, 20, 1–25. [Google Scholar] [CrossRef]
- Kisi, E.H.; Howard, C. Crystal Structures of Zirconia Phases and their Inter-Relation. Key Eng. Mater. 1998, 153, 1–36. [Google Scholar] [CrossRef]
- Zhang, F.; Reveron, H.; Spies, B.C.; Van Meerbeek, B.; Chevalier, J. Trade-off between fracture resistance and translucency of zirconia and lithium-disilicate glass ceramics for monolithic restorations. Acta Biomater. 2019, 91, 24–34. [Google Scholar] [CrossRef] [PubMed]
- Camposilvan, E.; Leone, R.; Gremillard, L.; Sorrentino, R.; Zarone, F.; Ferrari, M.; Chevalier, J. Aging resistance, mechanical properties and translucency of different yttria-stabilized zirconia ceramics for monolithic dental crown applications. Dent. Mater. 2018, 34, 879–890. [Google Scholar] [CrossRef] [PubMed]
- Pieralli, S.; Kohal, R.J.; Jung, R.E.; Vach, K.; Spies, B.C. Clinical Outcomes of Zirconia Dental Implants: A Systematic Review. J. Dent. Res. 2017, 96, 38–46. [Google Scholar] [CrossRef] [PubMed]
- Pieralli, S.; Kohal, R.J.; Rabel, K.; von Stein-Lausnitz, M.; Vach, K.; Spies, B.C. Clinical outcomes of partial and full-arch all-ceramic implant-supported fixed dental prostheses. A systematic review and meta-analysis. Clin. Oral. Implant. Res. 2018, 29, 224–236. [Google Scholar] [CrossRef]
- Garvie, R.C.; Hannink, R.H.; Pascoe, R.T. Ceramic steel? Nature 1975, 258, 703–704. [Google Scholar] [CrossRef]
- Gupta, T.K.; Lange, F.F.; Bechtold, J.H. Effect of stress-induced phase transformation on the properties of polycrystalline zirconia containing metastable tetragonal phase. J. Mater. Sci. 1978, 13, 1464–1470. [Google Scholar] [CrossRef]
- Deville, S.; Chevalier, J.; Gremillard, L. Influence of surface finish and residual stresses on the ageing sensitivity of biomedical grade zirconia. Biomaterials 2006, 27, 2186–2192. [Google Scholar] [CrossRef]
- Keuper, M.; Eder, K.; Berthold, C.; Nickel, K. Direct evidence for continuous linear kinetics in the low-temperature degradation of Y-TZP. Acta Biomater. 2013, 9, 4826–4835. [Google Scholar] [CrossRef]
- Lughi, V.; Sergo, V. Low temperature degradation -aging- of zirconia: A critical review of the relevant aspects in dentistry. Dent. Mater. 2010, 26, 807–820. [Google Scholar] [CrossRef] [PubMed]
- Clarke, I.C.; Manaka, M.; Green, D.D.; Williams, P.; Pezzotti, G.; Kim, Y.-H.; Ries, M.; Sugano, N.; Sedel, L.; Delauney, C.; et al. Current status of zirconia used in total hip implants. JBJS 2003, 85, 73–84. [Google Scholar] [CrossRef] [PubMed]
- Sanon, C.; Chevalier, J.; Douillard, T.; Cattani-Lorente, M.; Scherrer, S.S.; Gremillard, L. A new testing protocol for zirconia dental implants. Dent. Mater. 2015, 31, 15–25. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Inokoshi, M.; Batuk, M.; Hadermann, J.; Naert, I.; Van Meerbeek, B.; Vleugels, J. Strength, toughness and aging stability of highly-translucent Y-TZP ceramics for dental restorations. Dent. Mater. 2016, 32, e327–e337. [Google Scholar] [CrossRef] [PubMed]
- Matsui, K.; Yoshida, H.; Ikuhara, Y. Nanocrystalline, Ultra-Degradation-Resistant Zirconia: Its Grain Boundary Nanostructure and Nanochemistry. Sci. Rep. 2014, 4, 4758. [Google Scholar] [CrossRef] [PubMed]
- Nogiwa-Valdez, A.; Rainforth, W.; Zeng, P.; Ross, I.; Rainforth, W. Deceleration of hydrothermal degradation of 3Y-TZP by alumina and lanthana co-doping. Acta Biomater. 2013, 9, 6226–6235. [Google Scholar] [CrossRef] [PubMed]
- Batuk, M.; Hadermann, J.; Vleugels, J.; Chevalier, J.; Olagnon, C.; Van Meerbeek, B.; Zhang, F. Grain-Boundary Engineering for Aging and Slow-Crack-Growth Resistant Zirconia. J. Dent. Res. 2017, 96, 774–779. [Google Scholar]
- ISO 14801: Dentistry-Implants—Dynamic fatigue test for endosseous dental implants. Volume DIN EN ISO 14801:2008-02. Available online: https://www.iso.org/obp/ui/#iso:std:iso:14801:ed-2:v1:en (accessed on 23 October 2019).
- ISO 13356: Implants for surgery—Ceramic materials based on yttria-stabilized tetragonal zirconia (Y-TZP). Volume ISO 13356:2008(E) 2nd Edition. Available online: https://www.iso.org/standard/40166.html (accessed on 23 October 2019).
- Cattani-Lorente, M.; Scherrer, S.; Durual, S.; Sanon, C.; Douillard, T.; Gremillard, L.; Chevalier, J.; Wiskott, A. Effect of different surface treatments on the hydrothermal degradation of a 3Y-TZP ceramic for dental implants. Dent. Mater. 2014, 30, 1136–1146. [Google Scholar] [CrossRef]
- Chevalier, J.; Loh, J.; Gremillard, L.; Meille, S.; Adolfson, E. Low-temperature degradation in zirconia with a porous surface. Acta Biomater. 2011, 7, 2986–2993. [Google Scholar] [CrossRef]
- Garvie, R.C.; Nicholson, P.S. Phase analysis in zirconia systems. J. Am. Ceram. Soc. 1972, 55, 303–305. [Google Scholar] [CrossRef]
- Toraya, H.; Yoshimura, M.; Somiya, S. Calibration Curve for Quantitative Analysis of the Monoclinic-Tetragonal ZrO2System by X-Ray Diffraction. J. Am. Ceram. Soc. 1984, 67, C-119–C-121. [Google Scholar] [CrossRef]
- Keuper, M.; Berthold, C.; Nickel, K.G. Long-time aging in 3mol.% yttria-stabilized tetragonal zirconia polycrystals at human body temperature. Acta Biomater. 2014, 10, 951–959. [Google Scholar] [CrossRef] [PubMed]
- Chevalier, J.; Gremillard, L.; Deville, S. Low-Temperature Degradation of Zirconia and Implications for Biomedical Implants. Annu. Rev. Mater. Res. 2007, 37, 1–32. [Google Scholar] [CrossRef]
- Presser, V.; Keuper, M.; Berthold, C.; Nickel, K.G. Experimental Determination of the Raman Sampling Depth in Zirconia Ceramics. Appl. Spectrosc. 2009, 63, 1288–1292. [Google Scholar] [CrossRef] [PubMed]
- Tabares, J.A.M.; Anglada, M.J. Quantitative Analysis of Monoclinic Phase in 3Y-TZP by Raman Spectroscopy. J. Am. Ceram. Soc. 2010, 93, 1790–1795. [Google Scholar]
- Spies, B.C.; Maass, M.E.; Adolfsson, E.; Sergo, V.; Kiemle, T.; Berthold, C.; Gurian, E.; Fornasaro, S.; Vach, K.; Kohal, R.-J. Long-term stability of an injection-molded zirconia bone-level implant: A testing protocol considering aging kinetics and dynamic fatigue. Dent. Mater. 2017, 33, 954–965. [Google Scholar] [CrossRef] [PubMed]
- Sanon, C.; Chevalier, J.; Douillard, T.; Kohal, R.J.; Coelho, P.G.; Hjerppe, J.; Silva, N.R. Low temperature degradation and reliability of one-piece ceramic oral implants with a porous surface. Dent. Mater. 2013, 29, 389–397. [Google Scholar] [CrossRef] [PubMed]
- Morneburg, T.R.; A Pröschel, P. In vivo forces on implants influenced by occlusal scheme and food consistency. Int. J. Prosthodont. 2003, 16, 481–486. [Google Scholar]
- Delong, R.; Sakaguchi, R.; Douglas, W.; Pintado, M. The wear of dental amalgam in an artificial mouth: A clinical correlation. Dent. Mater. 1985, 1, 238–242. [Google Scholar] [CrossRef]
- Sakaguchi, R.L.; Douglas, W.H.; DeLong, R.; Pintado, M.R. The wear of a posterior composite in an artificial mouth: A clinical correlation. Dent. Mater. 1986, 2, 235–340. [Google Scholar] [CrossRef]
- Po, J.; Kieser, J.; Gallo, L.; Tésenyi, A.; Herbison, P.; Farella, M. Time-Frequency Analysis of Chewing Activity in the Natural Environment. J. Dent. Res. 2011, 90, 1206–1210. [Google Scholar] [CrossRef] [PubMed]
- Rosentritt, M.; Behr, M.; Gebhard, R.; Handel, G. Influence of stress simulation parameters on the fracture strength of all-ceramic fixed-partial dentures. Dent. Mater. 2006, 22, 176–182. [Google Scholar] [CrossRef] [PubMed]
- ISO 6872:2015(E). Dentistry—Ceramic materials. Available online: https://www.iso.org/obp/ui/#iso:std:iso:6872:ed-4:v1:en (accessed on 23 October 2019).

© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Frigan, K.; Chevalier, J.; Zhang, F.; Spies, B.C. Is a Zirconia Dental Implant Safe When It Is Available on the Market? Ceramics 2019, 2, 568-577. https://doi.org/10.3390/ceramics2040044
Frigan K, Chevalier J, Zhang F, Spies BC. Is a Zirconia Dental Implant Safe When It Is Available on the Market? Ceramics. 2019; 2(4):568-577. https://doi.org/10.3390/ceramics2040044
Chicago/Turabian StyleFrigan, Katarina, Jérôme Chevalier, Fei Zhang, and Benedikt Christopher Spies. 2019. "Is a Zirconia Dental Implant Safe When It Is Available on the Market?" Ceramics 2, no. 4: 568-577. https://doi.org/10.3390/ceramics2040044
APA StyleFrigan, K., Chevalier, J., Zhang, F., & Spies, B. C. (2019). Is a Zirconia Dental Implant Safe When It Is Available on the Market? Ceramics, 2(4), 568-577. https://doi.org/10.3390/ceramics2040044






