Abstract
Most materials expand when heated, which can lead to thermal stress and even failure. Whereas thermomiotic materials exhibit negative thermal expansion, the creation of materials with near-zero thermal expansion presents an ongoing challenge due to the need to optimize thermal and mechanical properties simultaneously. The present work describes the preparation and properties of polymer–ceramic composites with low thermal expansion. Ceramic scaffolds, prepared by freeze-casting of low-thermal-expansion Al2W3O12, were impregnated with poly(methylmethacrylate) (PMMA). The resulting composites can have a coefficient of thermal expansion as low as 2 × 10−6 K−1, and hardness values of 4.0 ± 0.3 HV/5 (39 ± 3 MPa) and 16 ± 3 HV/5 (160 ± 30 MPa) parallel and perpendicular to the ice growth, respectively. The higher hardness perpendicular to the ice growth direction indicates that the PMMA is acting to improve the mechanical properties of the composite.
1. Introduction
Most materials experience a substantial change in volume with change in temperature. Thermal expansion can lead to thermal stress and, consequently, damage and fracture, limiting the usable temperature range of materials [1]. Thermomiotic materials, i.e., materials with negative coefficients of thermal expansion (NTE) [1], offer the potential to create novel near-zero thermal expansion materials by use in combination with positive thermal expansion (PTE) materials in composites [1,2,3]. Such an approach potentially allows precise tailoring of the bulk coefficient of thermal expansion (CTE) in conjunction with other physical properties.
Several oxide ceramics are known to display large negative thermal expansion over broad temperature ranges; the most notable examples are the AM2O8 and A2M3O12 families [4,5,6]. However, there are significant challenges to the incorporation of these materials in composites. Reduction of the thermal expansivity of stiff materials, such as metals and ceramics, by thermomiotic materials necessarily causes significant thermal stresses in both components [7]. The characteristically low stiffness and large CTE of polymers make them attractive targets for combination with thermomiotics. Composites that combine a stiff material with a soft material also can exhibit emergent properties such as high bending strength, as exemplified by nacreous biominerals [8,9,10]. Efforts to incorporate thermomiotic ceramics into composites with polymers have often been complicated by the challenge of effectively dispersing the ceramic into the polymer and of ensuring effective mechanical coupling between the components [11,12].
Herein we describe combining a near-zero thermal expansion material (Al2W3O12) with a polymer (polymethylmethacrylate (PMMA)) to produce composites with 98–34 vol % Al2W3O12 prepared via unidirectional freeze-casting (Figure 1) and subsequent in situ polymerization. Freeze-casting has been used to control or enhance many physical properties of materials [10]; however, studies of its potential applications have largely focused to date on mechanical [9,13,14], transport [15,16,17], and electronic [18,19,20] properties; aside from one preliminary report [21], the thermal expansivity of freeze-cast structures has not received significant attention. Investigations of the thermal expansivity of freeze-cast materials could allow the synthesis of near-zero CTE composites, and understanding the underlying mechanisms of their thermal expansion could lead to increased robustness of freeze-cast composites that are subject to thermal cycling [22].

Figure 1.
The steps of unidirectional freeze-casting: (from left to right): dispersion of ceramic particles (represented by white spheres) in water; anisotropic freezing; removal of ice by sublimation; and sintering [10].
Unlike conventional techniques used to create near-zero CTE composites (such as sintering or sol–gel casting), freeze-casting provides good control of both the loading volume and morphology of the scaffold. In addition, unidirectional freeze-casting can produce anisotropic scaffolds and therefore can lead to anisotropic composites. Anisotropy allows further tailoring of the material, as the properties in a given direction are no longer subject to the bounds imposed on an isotropic bulk material [22,23]. Freeze-casting offers many possibilities for generating controlled microstructure [24,25,26], which, in turn, could allow the synthesis of composites with a wide range of tunable CTEs [27,28].
The present work uses aluminum tungstate (Al2W3O12) as the thermomiotic component. Aluminum tungstate has an orthorhombic structure (space group Pbcn); the CTEs along its b- and c-axes are negative, but expansion along the a-axis is so large and positive that the compound as a whole exhibits a small positive CTE (1.17 × 10−6 K−1 from 50 to 550 °C) [29]. Al2W3O12 belongs to the A2M3O12 family of materials; these materials have CTEs that range from low positive to negative [5,30]. However, efforts to produce near-zero thermal expansion materials by consolidation of A2M3O12 materials into bulk polycrystals often have been complicated by the large thermal stress produced by thermal expansion anisotropy, which leads to microcracking and mechanical weakness [22,31,32]. The creation of polymer–ceramic composites with controllable microstructure—and, therefore, controllable mechanical properties—is highly desirable for this class of materials.
2. Materials and Methods
2.1. Materials
The chemical reagents and their sources were as follows: ammonium bicarbonate (Sigma Aldrich, St. Louis, MO, USA, 99.0%); ammonium tungstate oxide hydrate (Alpha Aesar, Haverhill, MA, USA); aluminum nitrate nonahydrate (Alpha Aesar, Haverhill, MA, USA, 98.0–102.0% pure, as reported on the label); poly(vinyl alcohol) (PVA, Sigma Aldrich, St. Louis, MO, USA, 97%), poly(methylmethacrylate) (PMMA, Sigma Aldrich, St. Louis, MO, USA, 99%), 3-(methacryloyloxy) propyltrimethoxysilane (γ-MPS, Alpha Aesar, Haverhill, MA, USA, 97%), sodium lauryl sulfate (SLS, Fluka, Mexico City, NM, USA, 99%), methyl methacrylate (MMA, Alpha Aesar, Haverhill, MA, USA, 99%), and potassium persulfate (KPS, Sigma Aldrich, St. Louis, MO, USA, 99.95%).
All reagents were used as received except PVA, which was passed through a 100-mesh sieve before use to remove clumps and facilitate dissolution.
2.2. Experimental Methods
2.2.1. Preparation of Aluminum Tungstate Particles
Aluminum tungstate was synthesized by mixing stoichiometric amounts of 0.1 M aluminum nitrate nonahydrate aqueous solution (200 mL) with 0.1 M tungstate oxide hydrate solution (25 mL). The pH of the solution was adjusted to 6–7 with 1.5 M ammonium hydrogen carbonate solution and then stirred for 1 h.
The solution was subsequently centrifuged, and the precipitate was washed twice with ethanol. The precipitate was dried in a desiccator under vacuum for 10 h and then calcined at 750 °C for 1 h in a Lindberg/Blue M TF55035A furnace under dry argon to crystallize the amorphous product. To reduce mean particle size and facilitate dispersion, the powder was ball-milled with WC balls (1:12 powder:ball mass ratio) for 1 h.
2.2.2. Preparation of Aluminum Tungstate Scaffold
A scaffold was made from the resulting powder via freeze-casting. Each slurry had a total volume of 2 mL and was composed of 3 vol % PMMA, 1.5 vol % PVA, and varying known proportions of aluminum tungstate (15 vol %, 41 vol %, or 53 vol %, as noted) in deionized (DI) water. The materials were mixed in a PM200 planetary ball mill with two 1.25 cm WC balls at 100 rpm for 25 min. The slurries were freeze-cast immediately afterwards.
The slurries were poured into a poly(ethylene) mould with a copper base and placed on a cold plate held at −27 ± 1 °C, allowing unidirectional growth of the templating ice. After the samples were completely frozen, they were freeze-dried at −50 °C (trap temperature) and 0.02 bar for 24 h. After sublimation was complete, the green bodies were sintered in air in two stages: the temperature was increased from room temperature to 500 °C at 10 K min−1 and was held at 500 °C for 2 h (to remove the additives), then increased to 1000 °C at 10 K min−1 and held at this sintering temperature for 2 h (to strengthen the scaffold).
2.2.3. Preparation of Aluminum Tungstate/PMMA Composite
After sintering, scaffolds were impregnated with PMMA by emulsion polymerization following our published procedure for the production of alumina–PMMA composites [33]. The scaffolds were rinsed with DI water and dried at room temperature overnight. They were then placed in a refluxing (i.e., evaporating and condensing) solution of 5 mass % γ-MPS in methanol for 2 h and left at room temperature in solution overnight. The scaffolds were rinsed again and dried at 50 °C for 24 h. A polymerization solution (consisting of 27 mL DI water, 13 mL MMA, 0.058 g SLS, and 0.058 g KPS per 1 g of scaffold) was first stirred at 40 °C for 30 min before the temperature was increased to 80 °C. The scaffold was placed in the solution for 7 h. After polymerization the composites were rinsed and dried. The mass of the scaffold was determined before and after impregnation.
2.3. Characterization
2.3.1. Thermal Expansion
Samples were prepared for dilatometric analysis by cutting fragments from the center of dried composites; samples were 2–10 mm in each dimension. Samples were cut by applying a sharp force to a blade in order to produce clean breaks. The sides of each sample were polished with fine-grit sandpaper to obtain a flat surface. Loose particles were removed by blowing compressed air across each sample.
The thermal expansions of the composites were measured using a push-rod dilatometer (NETZSCH, Upper Franconia, Germany, model DIL 402 C). Each sample was cycled three times; each cycle consisted of heating at a rate of 10 K min−1 and cooling at a rate of 5 K min−1. The linear coefficient of thermal expansion was determined from the slopes of the heating and cooling curves from the resulting plots of thermal strain as a function of temperature. Dilatometric cooling curves are, in general, less affected by the formation and healing of microcracks [30], but the results here are similar for heating and cooling (see below) so both were included in the averages.
2.3.2. Thermogravimetric Analysis
The compositions of the samples were determined using a NETZSCH, Germany, TG 209 F3 thermogravimetric analysis (TGA) instrument. Each sample (mass ≈ 15 mg) was heated at 5 K min−1 from 25 °C to 600 °C, then held at 600 ˚C for 30 min (to fully degrade the PMMA) [34] under a dry argon flow (20 mL min−1).
2.3.3. Scanning Electron Microscopy
The internal structures of the scaffolds and composites were examined by SEM. A nanoScience Instruments, USA, Phenom G2 Pro desktop SEM with a back-scattering detector was used to view the morphology and porosity of the samples. SEM images were taken at a working distance of approximately 2 mm with an acceleration voltage of 5 keV.
2.3.4. Density
The densities of the scaffolds and composites were obtained by measurements of the sample volume and mass, giving density to compare with the bulk. The dimensions of the cylinders were measured with calipers and were used to calculate the total volume. The masses of the cylinders were obtained, and porosity was calculated from the literature values for the densities of fully consolidated Al2W3O12 (5.12 g cm−3) and PMMA (1.188 g cm−3) [35,36].
2.3.5. X-ray Diffraction
The degree of crystallinity, phase, and purity of the aluminum tungstate particles were assessed by XRD. A Rigaku, Japan, Ultima IV diffractometer with Cu Kα radiation, a diffracted beam monochromator, and a scintillation detector was used.
2.3.6. Vickers Indentation
The hardness of the composites was measured using Vickers indentation (Model V-100A, Leco Corporation, St. Joseph, MI, USA) with 5 kg loads and 10 s load times. The dimensions of the indentations were measured using an Olympus, Japan, BX51M UM optical microscope.
3. Results and Discussion
3.1. Scaffold Synthesis
The size of the particles/agglomerates in the slurry has a strong influence on the growth of ice during freeze-casting and, consequently, on the morphology of the final product [33,37]. Larger agglomerates are detrimental to ice growth, as it is more difficult for the growing ice crystals to push such large particles aside [33,37]. Much smaller agglomerates produce inhomogeneous structures, as their higher surface area to volume ratios lead to increased ice nucleation [33]. A mixture of large and small particles (e.g., 400 nm and 40 nm) can lead to good packing during freeze-casting [33,38].
The crude aluminum tungstate particles were composed of agglomerates of smaller particles (as seen from SEM images). The agglomerates ranged in size from 2 to 120 μm, with an average size of 28 μm (see Figure S1). The average size of the individual particles was 0.2 μm. This precursor powder was ball milled to reduce the particle size. After milling, the particles were still found to be aggregates of smaller particles of 0.2 μm. The aggregate size decreased to 0.6 μm, with a range of 0.2 to 2.0 μm (see Figures S2 and S3).
Homogenous dispersions also are essential to obtaining a consistent lamellar structure. Initially, slurries were mixed using a magnetic stir bar. However, inspection of the scaffolds by SEM revealed that there was a higher proportion of large particles at the base of the scaffolds than in the bulk, suggesting that they had either fallen out of suspension before freezing or were never dispersed (see Figure S4). This is undesirable, as a poor dispersion of particles would result in an uneven porosity throughout the sample. In addition, concentration of large particles at the base would influence the initial ice crystal growth and subsequently impact the microstructure. Mixing the slurries using a planetary ball mill was found to decrease sedimentation.
After freeze-casting, the green body was sintered. As there are no reports of aluminum tungstate being freeze-cast, an appropriate sintering temperature needed to be determined to ensure strengthening of the green form without degradation of the Al2W3O12 particles or the scaffold’s microstructure. This temperature must be below the melting point of Al2W3O12, 1260 °C. In addition to strengthening the green body, the sintering should crystallize the material, which had become partially amorphous during ball milling (see Figures S5 and S6).
Samples of powdered aluminum tungstate were sintered at 1100 °C and 1000 °C. XRD of ground samples of the green bodies revealed that both samples became more crystalline after sintering, and neither showed evidence of decomposition, which is consistent with literature reports [39]. Upon sintering, the particles regained the crystallinity lost during ball milling (see Figure 2). While the aluminum tungstate was clearly more crystalline above 1100 °C, the internal structure created was destroyed by sintering at the higher temperature (the sample was slumped and no longer cylindrical). After sintering at 1000 °C, the material was both crystalline and robust.
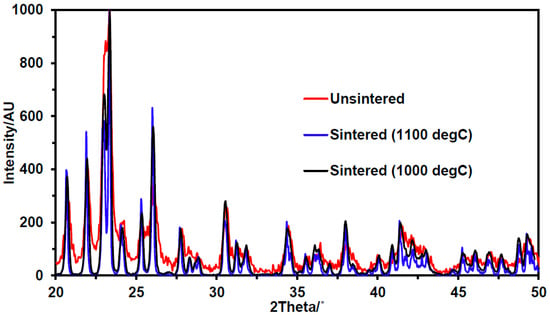
Figure 2.
XRD diffractograms of aluminum tungstate after sintering at various temperatures, showing improvement in crystallinity on sintering.
3.2. Structural Characterization of Scaffolds and Composites
After sintering at 1000 °C, SEM images confirmed that the internal lamellar structure was retained. The average Al2W3O12 grain size increased after sintering. SEM images revealed that the morphology of the aluminum tungstate particles in the scaffolds was distinctly different from that of the particles added to the slurry, being nodular rather than grainy and foliated (see Figure 3). The particles after sintering were somewhat larger, 4 μm vs. 0.6 μm on average. This change is likely due to the relatively high sintering temperature required to restore crystallinity; the rate of aluminum tungstate particle growth has been shown to increase significantly above 830 °C [40], and densification of bulk Al2W3O12 begins to occur rapidly during sintering at ca. 830 °C [41,42].

Figure 3.
SEM images contrasting the structure of the aluminum tungstate particles (a) after and (b, c) before freeze-casting.
Composites were made in which 98, 64, and 34 vol % of the solid was Al2W3O12. Void space remained in the composite after impregnation with PMMA for all scaffolds. The porosities and densities of these composites, as well as their scaffolds of origin, are summarized in Table 1. As the aluminum tungstate content increased, the porosity of the scaffold and composite decreased. The decrease in porosity can also be seen from the SEM images (see Figure 4). The microstructures of the 34 and 64 vol % Al2W3O12 scaffolds can be described as bridged lamellar, with a significant number of aggregates present in the 64 vol % scaffold. The lamellae in the 34 vol % scaffold were ordered on length scales of hundreds of microns, while those in the 64 vol % scaffold showed ordering only over shorter distances. The 98 vol % scaffold also showed a lamellar microstructure, but these lamellae were completely disordered.

Table 1.
Porosity and density of scaffolds and composites.
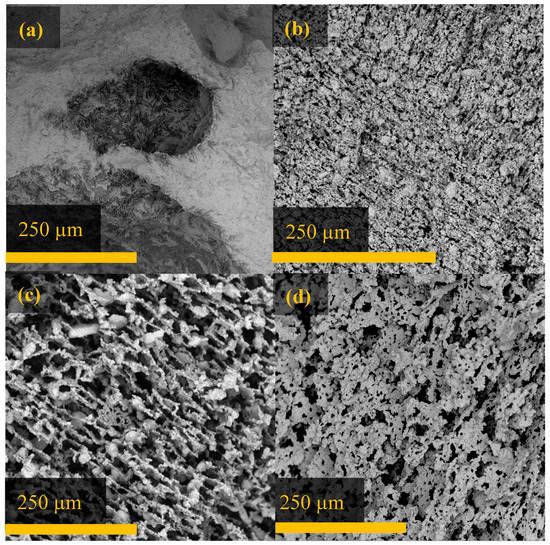
Figure 4.
SEM images of Al2W3O12 scaffolds before polymerization. Note the increase in porosity going from (a) 98, to (b) 64, then (c) 34 vol % Al2W3O12, shown perpendicular to the direction of ice growth. (The large indentations in the 98 vol % sample, presenting as dark shadows, are due to surface roughness as a result of the cutting of this dense sample.) Image (d) shows the lamella walls of the 34 vol % scaffold.
Note that all composites retained some porosity, even after impregnation with polymer (Figure 5). SEM images revealed that the composites were homogeneously filled with PMMA, indicating that this porosity did not arise from poor monomer transport throughout the scaffold. The residual porosity could be a result of the use of emulsion polymerization to impregnate the scaffold: PMMA produced by emulsion polymerization is always somewhat porous due to the use of a surfactant [43]. Consequently, there would be a significant void space in all composites synthesized by this method. Although void space will not participate directly in thermal expansion, porosity in an ordered microstructure can change the bulk CTE [27]. Porosity can decrease thermal stress in the composite while also decreasing the material’s strength. MMA could be polymerized by free radical polymerization without a surfactant if, for a particular application, void space should be avoided. Alternatively, stronger ceramic–polymer interactions could possibly be achieved by surface modification of the ceramic, as in our recent studies of Al2Mo3O12–polyethylene composites [11].
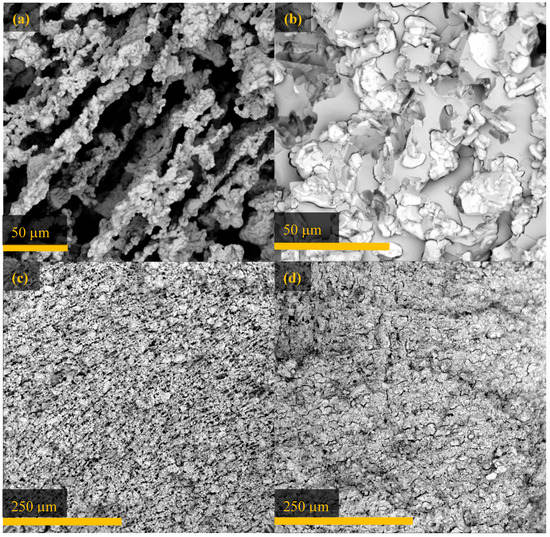
Figure 5.
SEM images of aluminum tungstate: (a) 64 vol % and (c) 98 vol % before and (b) 64 vol % and (d) 98 vol % after infiltration with PMMA, cut perpendicular to the direction of ice growth.
3.3. Thermal Expansion of Composites
The dilatometric thermograms of the composites, measured both parallel and perpendicular to the direction of ice growth, are shown in Figure 6. The first heating curve during each run is excluded as most displayed large negative thermal strains due to desorption of water from the composites and/or consolidation of the PMMA (see Figure 7). (TGA results shown in Figure S7 in Supplementary Material indicated that the composites contained 1 mass % of residual water, likely originating from the polymerization or absorbed from the air.) The composites showed some negative hysteresis when heated above 110 °C, which can be attributed to densification of the surface PMMA layer. SEM images of the composites before and after thermal cycling in the dilatometer show that the PMMA condensed (see Figure 7), resulting in increased porosity. As Figure 7 shows, there exists significant porosity at the interfaces between the PMMA and the Al2W3O12 before cycling. The CTEs of the composites measured parallel and perpendicular to the direction of ice growth are presented in Table 2. These values can be compared to the dilatometric CTE of bulk Al2W3O12 (1.5–2.0 × 10−6 K−1) [44] as the empty scaffolds (which were too fragile to prepare for dilatometric analysis) would be expected to have CTEs within this range. It is important to note that the thermal expansion behavior of the composites remained consistent (and consistently larger than that of bulk Al2W3O12) after the first heating cycle, indicating that the Al2W3O12 remains in effective mechanical contact with the PMMA. That is, during the heating and cooling cycles, the thermal expansions of the two components are significantly counteracted by each other; therefore, the polymer–ceramic interfaces are sufficiently robust to allow transfer of mechanical forces due to thermal expansion. The consistency of the CTEs upon repeated cycling shows that the interfaces are not significantly damaged by the thermal stresses put upon them.
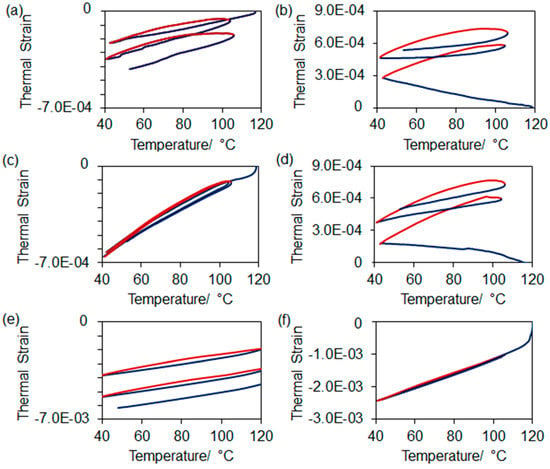
Figure 6.
Dilatometric heating and cooling curves of Al2W3O12/PMMA composites, excluding the first heating curve, measured parallel and perpendicular to the direction of ice growth. The red lines indicate the heating curves while the blue lines indicate the cooling curves. (a) 98 vol % Al2W3O12, parallel to ice growth; (b) 98 vol % Al2W3O12, perpendicular to ice growth; (c) 64 vol % Al2W3O12, parallel to ice growth; (d) 64 vol % Al2W3O12, perpendicular to ice growth; (e) 34 vol % Al2W3O12, parallel to ice growth; (f) 34 vol % Al2W3O12, perpendicular to ice growth.
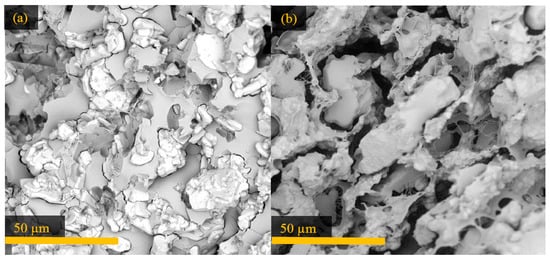
Figure 7.
SEM images of the 34 vol % Al2W3O12 composite (a) before and (b) after cycling in the dilatometer.

Table 2.
Linear coefficient of thermal expansion (CTE) of Al2W3O12–PMMA composites.
The CTEs for each experiment were obtained from the slopes of the linear regions of the thermograms. The CTEs (averaged from 40 to 100 °C) are plotted in Figure 8, in comparison with results predicted from the rule of mixtures [44] and the Turner model [45]. The rule of mixtures is defined as
where αBulk is the CTE of the bulk material and αi and ϕi are the CTE and volume fraction, respectively, of the ith component. The volume of the PMMA component was corrected for water loss as deduced from the TGA data. The Turner model is an extension of the rule of mixtures; it includes the influence of variations in bulk modulus between components. It is given as [45]
where Ki is the bulk modulus of the ith component. The experimental CTEs of the composites match the Turner model significantly better than the rule of mixtures, indicating that the large difference in stiffness between the two components plays a role in the overall thermal expansion (K = 48 GPa vs. 12 GPa for Al2W3O12 and PMMA, respectively [46,47]). Due to their flexible framework structures, thermomiotics typically have lower elastic moduli than would be predicted based on the strengths of their chemical bonds [1,22,48], which, in combination with the large magnitudes of the CTEs of polymers, often complicates efforts to use thermomiotics to counteract positive thermal expansion.
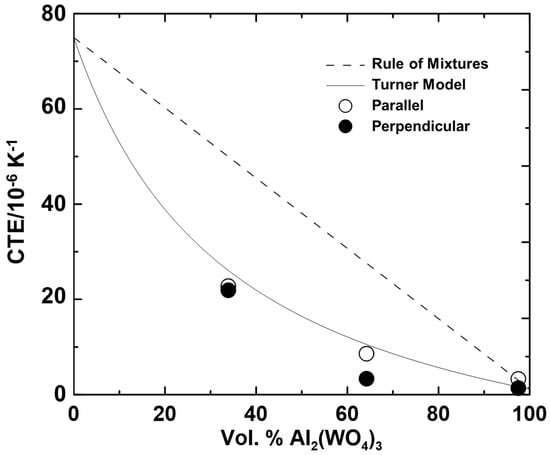
Figure 8.
Experimental linear CTEs of Al2W3O12–PMMA composites from 40 to 100 °C as a function of ceramic volume fraction, compared with CTEs predicted from the rule of mixtures and the Turner model.
The Turner model and rule of mixtures are based on assumptions of homogenous composite microstructure and zero porosity, so they cannot be expected to quantitatively predict the behaviour of the materials like those reported herein. In fact, given two materials of different CTEs and void space, it is possible to design a microstructure with a CTE of arbitrary sign and magnitude [27]. Previous attempts to synthesize composites combining a polymer and a thermomiotic component using, for example, melt compounding, have been limited by their stochastic microstructures to values between that of the rule of mixtures and the Turner model, with the results often lying close to that of the rule of mixtures [11,12,38,49,50,51]. As demonstrated here, the freeze-casting and polymer infiltration allows the stiffer thermomiotic component to effectively contain the polymer component and dominate the thermal expansion of polymer–ceramic composites even when the two components are present in comparable volume fractions.
3.4. Hardness Measurements
The hardness of the 64 vol % Al2W3O12 composite was measured using a Vickers indenter, both perpendicular and parallel to the direction of ice growth. The hardness was 39 ± 3 MPa (4.0 ± 0.3 HV/5) parallel to the ice growth and 160 ± 30 MPa (16 ± 3 HV/5) perpendicular to the ice growth. This composite is quite soft (compared with fully dense polycrystalline Al2W3O12 at 4.9 GPa, as measured by nanoindentation [39]), as expected given its large pore fraction and the relatively low sintering temperature used to preserve the lamellar microstructure. It should be noted that hardness values measured by nanoindentation are expected to be larger than those measured by microindentation, as materials subject to the larger loads of microindentation are more likely to experience microcracking. However, the microhardness of bulk Al2W3O12 has not been reported. Of note, the composite is significantly harder in the direction perpendicular to ice growth than in that parallel. This additional resistance to permanent deformation in the perpendicular direction indicates that the freeze-cast hybrid structure is, to some extent, effective at arresting crack propagation in that direction, as intended [9]. Due to the small dimensions of the composite samples, it was not possible to perform flexural strength measurements. However, the hardness results are consistent with the expected increase in flexural strength for nacre-like composites [9,52,53].
4. Conclusions
We produced and characterized composites composed of a freeze-cast thermomiotic ceramic, Al2W3O12, infilled with PMMA. With appropriate ceramic particle size and dispersion in the slurry, and an appropriate sintering temperature of the freeze-cast product, lamellar scaffolds were made successfully. These were infilled with PMMA polymer, and their properties indicate that this method can be used to create composites with controllable thermal expansion. Increased microhardness was measured in the direction perpendicular to ice growth, suggesting that the lamellar microstructure and addition of PMMA were able to improve the material’s resistance to microcrack propagation in that direction. Further development of the freeze-casting method as applied to thermomiotic ceramics could provide a way to compensate for the intrinsically poor mechanical properties of materials with low or negative thermal expansion [1,22,42].
Supplementary Materials
The following are available online at http://www.mdpi.com/2571-6131/2/1/11/s1, Figure S1: Scanning electron micrographs of aluminum tungstate particles prior to ball milling, Figure S2: Scanning electron micrographs of aluminum tungstate after ball milling, Figure S3: Histogram of aluminum tungstate particle sizes after ball milling, Figure S4: SEM images of Al2(WO4)3 scaffolds, illustrating the influence of improved dispersion techniques, Figure S5: XRD pattern of aluminum tungstate particles after calcination at 750 °C for various times, Figure S6: XRD pattern of aluminum tungstate after ball milling for various times, Figure S7: TGA thermogram of an aluminum tungstate-PMMA composite.
Author Contributions
Conceptualization, M.A.W.; methodology, C.P.R. and S.N.E.; formal analysis, S.N.E.; investigation, S.N.E.; data curation, C.P.R.; writing—original draft preparation, S.N.A.; writing—review and editing, M.A.W., C.P.R, and S.N.E.; supervision, C.P.R. and M.A.W.; funding acquisition, M.A.W.
Funding
This research was funded by NSERC Canada, grant number RGPIN-2015-04593.
Acknowledgments
The authors thank Professors M. Obrovac, J. Dahn, R. White, and K. Plucknett for use of their XRD, ball mills, freeze-drying, and Vickers indenter and dilatometry equipment, respectively; A. George for assistance with furnaces; P. Scallion for assistance with SEM; and P. Li for assistance with the sputter coater. The authors gratefully acknowledge the assistance of C. Jin and C. Wang for assistance with the Vickers indenter and T. Hatchard for advice on optimizing the ball milling procedure.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
References
- Romao, C.P.; Miller, K.J.; Whitman, C.A.; White, M.A.; Marinkovic, B.A. Comprehensive Inorganic Chemistry II; Reedijk, J., Poeppelmeier, K., Eds.; Elsevier: Oxford, UK, 2013; Volume 4, pp. 128–151. [Google Scholar]
- Miller, W.; Smith, C.W.; Mackenzie, D.S.; Mackenzie, D.S.; Evans, K.E. Negative thermal expansion: A review. J. Mater. Sci. 2009, 44, 5441–5451. [Google Scholar] [CrossRef]
- Lind, C. Two decades of negative thermal expansion research: Where do we stand? Materials 2012, 5, 1125–1154. [Google Scholar] [CrossRef] [PubMed]
- Mary, T.A.; Evans, J.S.O.; Vogt, T.; Sleight, A.W. Negative thermal expansion from 0.3 to 1050 kelvin in ZrW2O8. Science 1996, 272, 90–92. [Google Scholar] [CrossRef]
- Evans, J.S.O.; Mary, T.A.; Sleight, A.W. Negative thermal expansion in Sc2(WO4)3. J. Solid State Chem. 1998, 137, 148–160. [Google Scholar] [CrossRef]
- Marinkovic, B.M.; Jardim, P.M.; de Avillez, R.R.; Rizzo, F. Negative thermal expansion in Y2Mo3O12. Solid State Sci. 2005, 7, 1377–1383. [Google Scholar] [CrossRef]
- Romao, C.P.; White, M.A. Negative stiffness in ZrW2O8 inclusions as a result of thermal stress. J. Appl. Phys. 2016, 109, 031902. [Google Scholar] [CrossRef]
- Meyer, G. Rigid biological systems as models for synthetic composites. Science 2005, 310, 1144–1147. [Google Scholar] [CrossRef]
- Deville, S.; Saiz, E.; Nalla, R.K.; Tomsia, A.P. Freezing as a path to build complex composites. Science 2006, 311, 515–518. [Google Scholar] [CrossRef]
- Deville, S. The lure of ice-templating: Recent trends and opportunities for porous materials. Scripta Materialia 2018, 147, 119–124. [Google Scholar] [CrossRef]
- Soares, A.R.; Ponton, P.I.; Mancic, L.; d’Almeida, J.R.M.; Romao, C.P.; White, M.A.; Marinkovic, B.A. Al2Mo3O12/polyethylene composites with reduced coefficient of thermal expansion. J. Mater. Sci. 2014, 49, 7870–7882. [Google Scholar] [CrossRef]
- Ponton, P.I.; Prisco, L.P.; Marinkovic, B.A. Effects of low contents of A2M3O12 submicronic thermomiotic-like fillers on thermal expansion and mechanical properties of HDPE-based composites. Polym. Compos. 2018, 39, E1821–E1833. [Google Scholar] [CrossRef]
- Bouville, F.; Maire, E.; Meille, S.; Van de Moortèle, B.; Stevenson, A.J.; Deville, S. Strong, tough and stiff bioinspired ceramics from brittle constituents. Nat. Mater. 2014, 13, 508–514. [Google Scholar] [CrossRef] [PubMed]
- Munch, E.; Launey, M.E.; Alsem, D.H.; Saiz, E.; Tomsia, A.P.; Ritchie, R.O. Tough, bio-inspired hybrid materials. Science 2008, 322, 1516–1520. [Google Scholar] [CrossRef] [PubMed]
- Schiffres, S.N.; Harish, S.; Maruyama, S.; Shiomi, J.; Malen, J.A. Tunable electrical and thermal transport in ice-templated multilayer graphene nanocomposites through freezing rate control. ACS Nano 2013, 7, 11183–11189. [Google Scholar] [CrossRef] [PubMed]
- Zeng, X.; Yao, Y.; Gong, Z.; Wang, F.; Sun, R.; Xu, J.; Wong, C.P. Ice-templated assembly strategy to construct 3D boron nitride nanosheet networks in polymer composites for thermal conductivity improvement. Small 2015, 16, 6205–6213. [Google Scholar] [CrossRef] [PubMed]
- Wicklein, B.; Kocjan, A.; Salazar-Alvarez, G.; Carosio, F.; Camino, G.; Antonietti, M.; Bergström, L. Thermally insulating and fire-retardant lightweight anisotropic foams based on nanocellulose and graphene oxide. Nat. Nanotech. 2015, 10, 277. [Google Scholar] [CrossRef] [PubMed]
- Zeng, X.; Ye, L.; Yu, S.; Sun, R.; Xu, J.; Wong, C.P. Facile preparation of superelastic and ultralow dielectric boron nitride nanosheet aerogels via freeze-casting process. Chem. Mater. 2015, 27, 5849–5855. [Google Scholar] [CrossRef]
- Zhang, Y.; Bao, Y.; Zhang, D.; Bowen, C.R. Porous PZT ceramics with aligned pore channels for energy harvesting applications. J. Am. Ceram. Soc. 2014, 98, 2980–2983. [Google Scholar] [CrossRef]
- Shao, Y.; El-Kady, M.F.; Lin, C.W.; Zhu, G.; Marsh, K.L.; Hwang, J.Y.; Zhang, Q.; Li, Y.; Wang, H.; Kaner, R.B. 3D Freeze-Casting of Cellular Graphene Films for Ultrahigh-Power-Density Supercapacitors. Adv. Mater. 2016, 28, 6719–6726. [Google Scholar] [CrossRef]
- White, M.A.; Conrad, J.; Chen, R.; Romao, C.; Pereira, A.; Hill, I. Applications of ice-templated ceramics. Int. J. Appl. Ceram. Technol. 2018, 15, 1075–1084. [Google Scholar] [CrossRef]
- Romao, C.P.; Donegan, S.P.; Zwanziger, J.W.; White, M.A. Relationships between elastic anisotropy and thermal expansion in A2Mo3O12 materials. Phys. Chem. Chem. Phys. 2016, 18, 30652–30661. [Google Scholar] [CrossRef] [PubMed]
- Kreher, W.S. Modeling of random microstructural stresses and grain boundary damage in polycrystals. Comp. Mater. Sci. 1996, 7, 147–153. [Google Scholar] [CrossRef]
- Dhainaut, J.; Piana, G.; Deville, S.; Guizard, C.; Klotz, M. Freezing-induced ordering of block copolymer micelles. Chem. Comm. 2014, 50, 12572–12574. [Google Scholar] [CrossRef]
- Ghosh, D.; Banda, M.; Kang, H.; Dhavale, N. Platelets-induced stiffening and strengthening of ice-templated highly porous alumina scaffolds. Scripta Materialia 2016, 125, 29–33. [Google Scholar] [CrossRef]
- Martoïa, F.; Cochereau, T.; Dumont, P.J.J.; Orgéas, L.; Terrien, M.; Belgacem, M.N. Cellulose nanofibril foams: Links between ice-templating conditions, microstructures and mechanical properties. Mater. Design 2016, 104, 376–391. [Google Scholar]
- Sigmund, O.; Torquato, S.J. Design of materials with extreme thermal expansion using a three-phase topology optimization method. Mech. Phys. Solids. 1997, 45, 1037–1067. [Google Scholar] [CrossRef]
- Qu, J.; Kadic, M.; Naber, A.; Wegener, M. Micro-structured two-component 3D metamaterials with negative thermal-expansion coefficient from positive constituents. Sci. Rep. 2017, 7, 40643. [Google Scholar] [CrossRef]
- Imanaka, N.; Hiraiwa, M.; Adachi, G.; Dabkowska, H.; Dabkowski, A. Thermal contraction behavior in Al2(WO4)3 single crystal. J. Cryst. Growth 2000, 200, 176–179. [Google Scholar] [CrossRef]
- Romao, C.P.; Perras, F.A.; Werner-Zwanziger, U.; Lussier, J.A.; Miller, K.J.; Calahoo, C.M.; Zwanziger, J.W.; Bieringer, M.; Marinkovic, B.A.; Bryce, D.L.; et al. Zero thermal expansion in ZrMgMo3O12: NMR crystallography reveals origins of thermoelastic properties. Chem. Mater. 2015, 27, 2633–26476. [Google Scholar] [CrossRef]
- Prisco, L.P.; Romao, C.P.; Fernando, R.; White, M.A.; Marinkovic, B.A. The effect of microstructure on thermal expansion coefficients in powder-processed Al2Mo3O12. J. Mater. Sci. 2013, 48, 2986–2996. [Google Scholar] [CrossRef]
- Romao, C.P.; Miller, K.J.; Johnson, M.B.; Zwanziger, J.W.; Marinkovic, B.A.; White, M.A. Thermal, vibrational, and thermoelastic properties of Y2Mo3O12 and their relations to negative thermal expansion. Phys. Rev. B 2014, 90, 024305. [Google Scholar] [CrossRef]
- Chen, R.; Johnson, M.B.; Plucknett, K.P.; White, M.A. Thermal conductivity of tunable lamellar aluminum oxide/polymethyl methacrylate hybrid composites. J. Mater. Res. 2012, 27, 1869–1876. [Google Scholar] [CrossRef]
- Kashiwagi, T.; Inaba, A.; Brown, J.E.; Hatada, K.; Kitayama, T.; Masuda, E. Effects of weak linkages on the thermal and oxidative degradation of poly(methyl methacrylates). Macromol. 1986, 19, 2160–2168. [Google Scholar] [CrossRef]
- Ivanova, D.; Nikolov, V.; Peshev, P. Solvents for growing Al2(WO4)3 single crystals from high-temperature solutions. J. Alloys Compd. 2007, 430, 356–360. [Google Scholar] [CrossRef]
- Poly(methyl methacrylate); SDS 200336; Millipore Sigma: Saint Louis, MO, USA, 22 January 2019.
- Deville, S.; Saiz, E.; Tomsia, A.P. Ice-templated porous alumina structures. Acta Mater. 2007, 55, 1965–1974. [Google Scholar] [CrossRef]
- Plucknett, K.P.; Munro, C.D. Aqueous Colloidal Characterization and Forming of Multimodal Barium Titanate Powders. J. Am. Cer. Soc. 2009, 92, 2537–2543. [Google Scholar]
- Batista, F.M.C.; La Porta, F.A.; Gracia, L.; Cerdeiras, E.; Mestres, L.; Li, M.S.; Bastista, N.C.; Andres, J.; Longo, E.; Cavalcante, L.S. A joint experimental and theoretical study on the electronic structure and photoluminescence properties of Al2(WO4)3 powders. J. Mol. Struct. 2015, 1081, 381–388. [Google Scholar] [CrossRef]
- Nihtianova, D.; Velichkova, N.; Nikolova, R.; Koseva, I.; Yordanova, A.; Nikolov, V. Characterization of nanosized Al2(WO4)3. Mater. Res. Bull. 2011, 46, 2125–2130. [Google Scholar] [CrossRef]
- Prisco, L.P.; Pontón, P.I.; Guamán, M.V.; Avillez, R.R.; Romao, C.P.; Johnson, M.B.; White, M.A.; Marinkovic, B.A. Assessment of the Thermal Shock Resistance Figures of Merit of Al2W3O12, a Low Thermal Expansion Ceramic. J. Am. Cer. Soc. 2016, 99, 1742–1748. [Google Scholar] [CrossRef]
- Prisco, L.P.; Marzano, M.; Pontón, P.I.; Costa, A.M.L.M.; Neto, C.C.; Sweet, G.; Romao, C.P.; White, M.A.; Marinkov, B.A. Relationship between sintering methods and physical properties of the low positive thermal expansion material Al2W3O12. Int. J. App. Ceram. Tech. 2019, 16, 346–356. [Google Scholar] [CrossRef]
- Gökmen, M.T.; du Prez, F.E. Porous polymer particles: A comprehensive guide to synthesis, characterization, functionalization and applications. Prog. Polym. Sci. 2012, 37, 365–405. [Google Scholar] [CrossRef]
- White, M.A. Thermal Expansion. In Physical Properties of Materials, 3rd ed.; CRC Press: Boca Raton, FL, USA, 2019; pp. 173–186. [Google Scholar]
- Kingery, W.D.; Bowen, H.K.; Uhlmann, D.R. Introduction to Ceramics; John Wiley & Sons: New York, NY, USA, 1960. [Google Scholar]
- Varga, T.; Wilkinson, A.P.; Lind, C.; Bassett, W.A.; Zha, C.-S. High pressure synchrotron x-ray powder diffraction study of Sc2Mo3O12 and Al2W3O12. J. Phys. Condens. Matter 2005, 27, 4271–4283. [Google Scholar] [CrossRef]
- Sinha, M.; Buckley, D.J. Acoustic Properties of Polymers. In Physical Properties of Polymers; Mark, J.E., Ed.; Springer: New York, NY, USA, 2007; pp. 1021–1031. [Google Scholar]
- Romao, C.P. Anisotropic thermal expansion in flexible materials. Phys. Rev. B 2017, 96, 134113. [Google Scholar] [CrossRef]
- Tani, J.; Kimura, H.; Hirota, K.; Kido, H. Thermal expansion and mechanical properties of phenolic resin/ZrW2O8 composites. J. App. Pol. Sci. 2007, 106, 3343–3347. [Google Scholar] [CrossRef]
- Sullivan, L.M.; Lukehart, C.M. Zirconium Tungstate (ZrW2O8)/Polyimide Nanocomposites Exhibiting Reduced Coefficient of Thermal Expansion. Chem. Matter. 2005, 17, 2136–2141. [Google Scholar] [CrossRef]
- Lind, C.; Coleman, M.R.; Kozy, L.C.; Sharma, G.R. Zirconium tungstate/polymer nanocomposites: Challenges and opportunities. Phys. Status Solidi B 2010, 248, 123–129. [Google Scholar] [CrossRef]
- Liu, Q.; Ye, F.; Gao, Y.; Liu, S.; Yang, H.; Zhou, Z. Fabrication of a new SiC/2024Al co-continuous composite with lamellar microstructure and high mechanical properties. J. Alloy Compd. 2014, 585, 146–153. [Google Scholar] [CrossRef]
- Gurbuz, S.N.; Dericioglu, A.F. Effect of reinforcement surface functionalization on the mechanical properties of nacre-like bulk lamellar composites processed by a hybrid conventional method. Mater. Sci. Eng. C 2013, 33, 2011–2019. [Google Scholar] [CrossRef]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
