1. Introduction
Geopolymers are commonly obtained at low temperatures (under 100 °C) by the mixing of an aluminosilicate source material with an alkali hydroxide or alkali silicate solution [
1]. The final amorphous network consists of an alternating sequence of tetrahedral [SiO
4] and [AlO
4], and the microstructure is composed of nanoparticulates separated by micro- and mesopores [
2,
3].
The geopolymer matrix, formed by nanoprecipitates that act as a “glue”, can be easily mixed with several fillers in order to produce composite materials suitable for different applications.
The production of composites is indeed of considerable importance to further increase the potentialities of geopolymers. In this respect, geopolymer composites have been already formulated to obtain oxygen carriers suitable for chemical looping combustion (CLC) processes [
4,
5] or catalysts for tar decomposition [
6]. CLC is currently considered one of the best alternatives to reduce the economic cost of CO
2 capture. This technology is based on indirect fuel combustion by use of a solid oxygen carrier, generally a metal oxide having the capability of transporting the oxygen needed for the combustion from an air reactor to a fuel reactor, usually designed as two coupled fluidized beds [
7]. The combustion takes place in the fuel reactor through the reaction between the fuel and the solid oxygen carrier, which is consequently reduced to a lower oxidation state. The reduced oxygen carrier is then transferred to the air reactor, where it is regenerated by oxidation in air at high temperature [
8].
For this purpose, geopolymer matrices were doped with iron, manganese oxides, or a mix of the two, as these metal oxides facilitate the active phases necessary for the process. Indeed, in such composite materials, the active phase was tightly embedded into the geopolymer matrix, endowing the material with the required stability. The production of these materials was aimed at overcoming the major limitations in the production of the oxygen carriers, which normally requires costly or complex processing routes. Oxygen carriers are usually obtained by traditional methods, such as the impregnation of supports with metal salts followed by drying and calcination [
9], or the use of ceramic synthesis, which involves the optimization of a slurry and the subsequent shaping, granulation, drying, and final sintering at high temperature. The unique, one-step production process adopted for the production of the geopolymer carriers results particularly advantageous for time and cost optimization of the entire synthesis, especially in the perspective of large-scale applications, which are heavily affected by cost of materials and by energy demand of the process.
Taking into account that potential oxygen carriers should have a series of requirements, such as high oxygen transport capacity, negligible decline in the performance after repeated cycles, resistance to comminution and agglomeration, large availability and low cost [
8], and an appropriate porosity to support the mentioned requirements, in the present paper new methods to obtain carriers for packed beds were explored. One of the major advantages of geopolymers is the easy, low-cost, and green production process, and the ease of shaping the final materials [
1]. Since geopolymerization is a water-based reaction, it is possible to obtain a custom-tailored porosity. Water content in the starting mixture affects the intrinsic mesoporosity of the geopolymer matrix, since water acts as a pore former during the polycondensation stage [
3]. Furthermore, a lamellar macroporosity can be obtained using an ice-templating technique, as already reported [
10,
11].
The ice-templating technique consists of freezing an aqueous suspension, followed by ice sublimation under reduced pressure. The resulting green body is generally sintered to consolidate the overall architecture, as well as to obtain the desired mechanical and functional properties, while maintaining a high level of porosity.
Unidirectional channel-like (lamellar) porosity is obtained in the case of unidirectional freezing, where pores are the replica of ice crystals [
12,
13,
14].
The process is environmentally friendly, since it uses water as a removable template, is highly versatile, and the resulting structures are highly tunable by changing the process conditions. Indeed, the ice-template can be easily removed through simple thawing and drying, overcoming the problems related to other templating techniques, which require the use of expensive templates, removed through either high temperature or extremely high or low pH conditions [
15].
The authors have already reported on the application of this technique to a water-based sol-gel system able to obtain metakaolin-based geopolymers, avoiding the use of dispersant and binders and a consolidation at high temperature [
10,
11].
The aim of the present work is to extend the use of this technique to the novel geopolymer-Fe/Mn oxides composites, in order to obtain oxygen carriers with specific geometries and porosity for CLC processes conceived with a packed bed configuration.
In fact, the composites were originally conceived and tested as oxygen carriers for CLC processes designed to be used in the form of granules for a fluidized bed configuration.
However, other reactor concepts could be proposed for CLC as twin packed bed reactors. The main advantages of packed bed reactor technology for CLC is that the separation of the gas and particles is intrinsically avoided, the particles are not comminuted small enough, and the pressure drop is limited. Thus, packed bed CLC shows great potential as an alternative for the interconnected fluidized bed system [
16].
As a matter of fact, ice-templating allowed obtainment of composite monoliths with oriented, lamellar macroporosity, as well as millimeter-sized beads with radial porosity, obtained through an injection-solidification method directly in liquid nitrogen. The formation of micrometer sized pores would favor the easy diffusion of bulky molecules inside the shaped beads [
17] in fixed bed reactors.
Starting from the already tested composite mixture based on a metakaolin-based geopolymer matrix added with a mix of Fe and Mn oxides [
6], the geopolymerization was triggered following the optimized process of freeze-casting reported by the authors [
10]. The slurry has been used to produce both monoliths and beads, then the obtained materials have been characterized in terms of macro- and microstructure, porosity, and specific surface area.
2. Materials and Methods
2.1. Materials and Sample Preparation
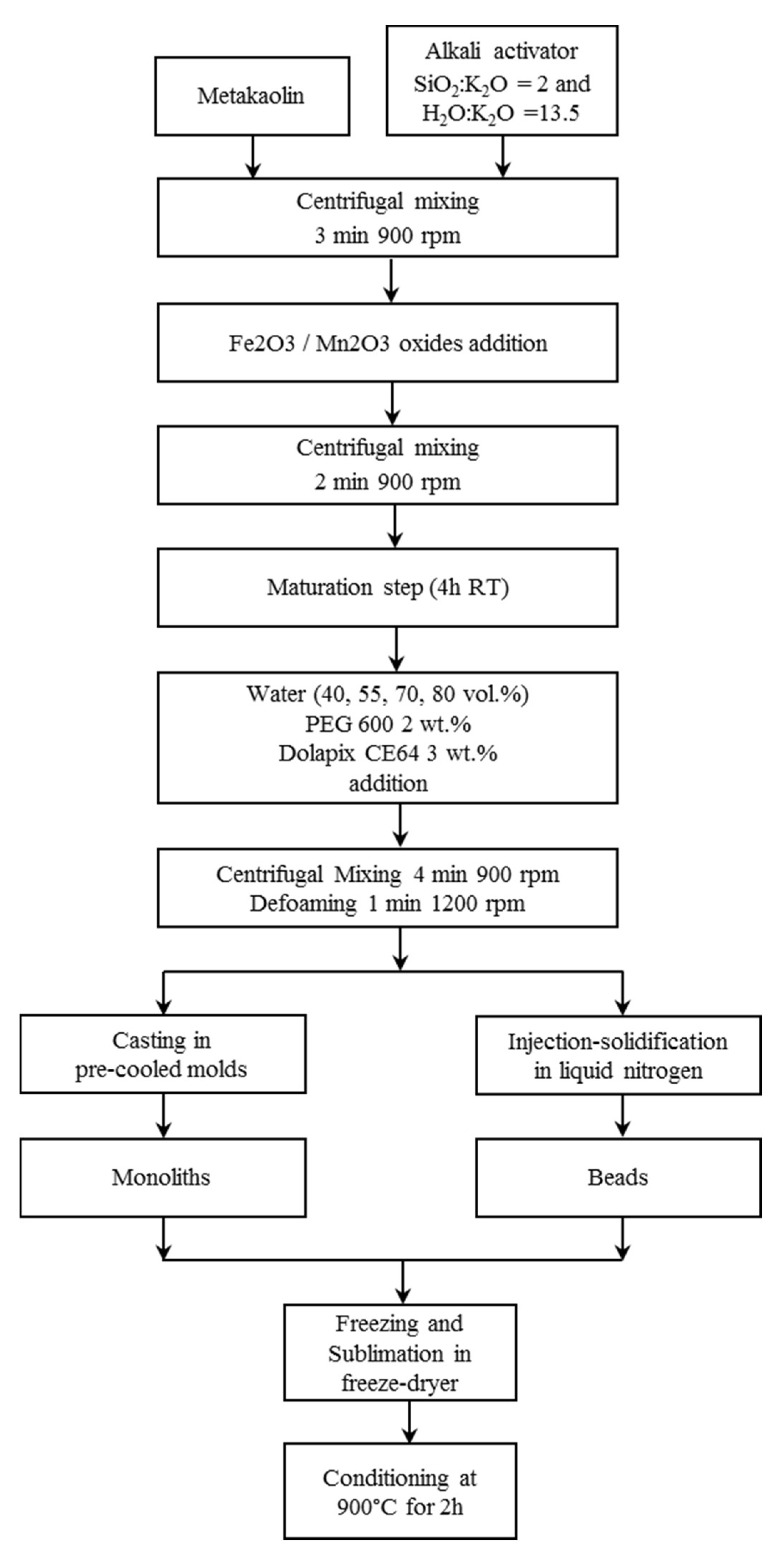
The geopolymer matrix was prepared mixing metakaolin grade M1200S purchased from Imerys (ssa = 25 m2 g−1, d50 = 1.5 µm) with a solution of potassium di-silicate used as alkali activator, having a molar ratio of SiO2:K2O = 2 and H2O:K2O = 13.5. The solution was prepared by dissolving KOH pellets (purity >85% from Sigma-Aldrich) in distilled water and adding fumed silica powder (99.8% from Sigma-Aldrich) under magnetic stirring.
The geopolymer slurry, with a theoretical Si:Al molar ratio equal to 2, was prepared using a planetary centrifugal mixer (Thinky Mixer ARE-500, Thinky, Japan) for 3 minutes at 900 rpm.
Then the metal oxides were added in equal amount to the geopolymer mixture to the extent of 51% by weight of dry solid geopolymer reactants. Mn
2O
3 (Sigma-Aldrich) and Fe
2O
3 (MRC, Toulouse, France) 325 mesh powders were used and mixed with the centrifugal mixer for 2 minutes at 900 rpm. After the preparation, the composite slurries underwent a maturation step of 4 h at room temperature, already reported in [
10], in order to trigger the geopolymerization, but avoiding the complete consolidation of the slurry. Deionized water was then added to the maturated slurry in different amounts (40, 55, 70, 80 vol.%), calculated over the theoretical volume of the geopolymer solid matrix. Dispersant Dolapix CE64 (Zschimmer and Schwarz, Lahnstein, Germany) and binder Polyethylene glycol (PEG 600, Merck, Kenilworth, NJ, USA) were also added in the amount of 3 and 2 wt.% on the dry solid composite, respectively. The mixture was then mixed with the centrifugal mixer for 4 minutes at 900 rpm and a defoaming step (1 minute at 1200 rpm) was lastly added.
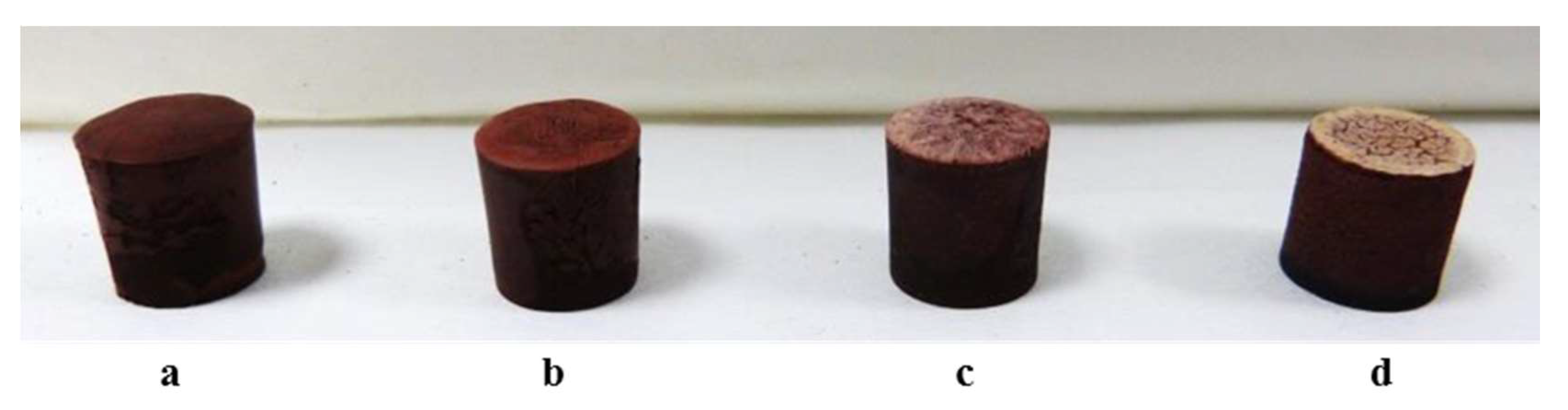
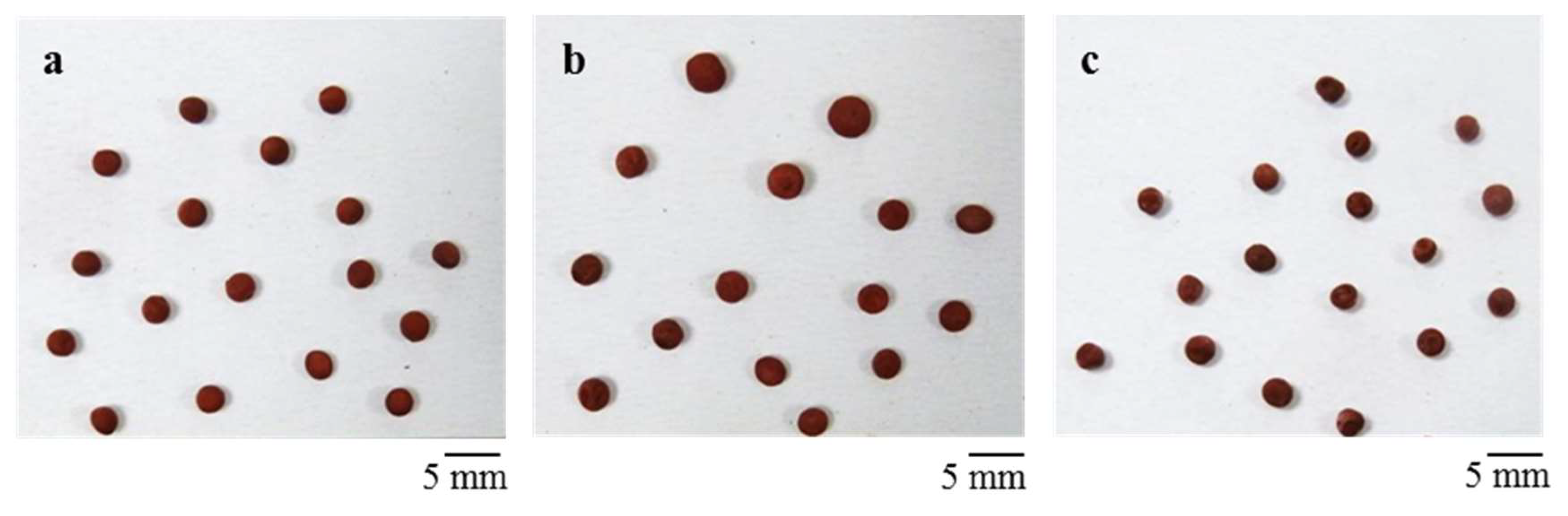
The prepared mixture was then cast in cylindrical plastic molds, which have been pre-cooled on the freeze dryer shelf at −40 °C, to produce monoliths with a diameter of 15 mm and a height of 16 mm. Cast slurries were frozen at −40 °C and the solidified phase was then sublimated at 10 Pa for 24 h, using a freeze-drier apparatus (Edwards Mod. MFD01, Crawley, UK). Conversely, to produce the beads, the mixture was directly dripped in a liquid N2 bath using a peristaltic pump (speed 3 rpm) with a nozzle of 0.6 mm diameter, obtaining ≈2–3 mm sized beads. The beads were then sublimated at 10 Pa for 24 h (Edwards Mod. MFD01, Crawley, UK).
Finally, the monoliths and the beads underwent a thermal treatment in a muffle furnace at 900 °C for 2 h in air, as a conditioning step in accordance to CLC process [
4,
5].
A flow-chart of the freeze-casting process used to produce monoliths and beads is reported in
Figure 1 and the compositions and codes of the produced samples are reported in
Table 1.
2.2. Characterization
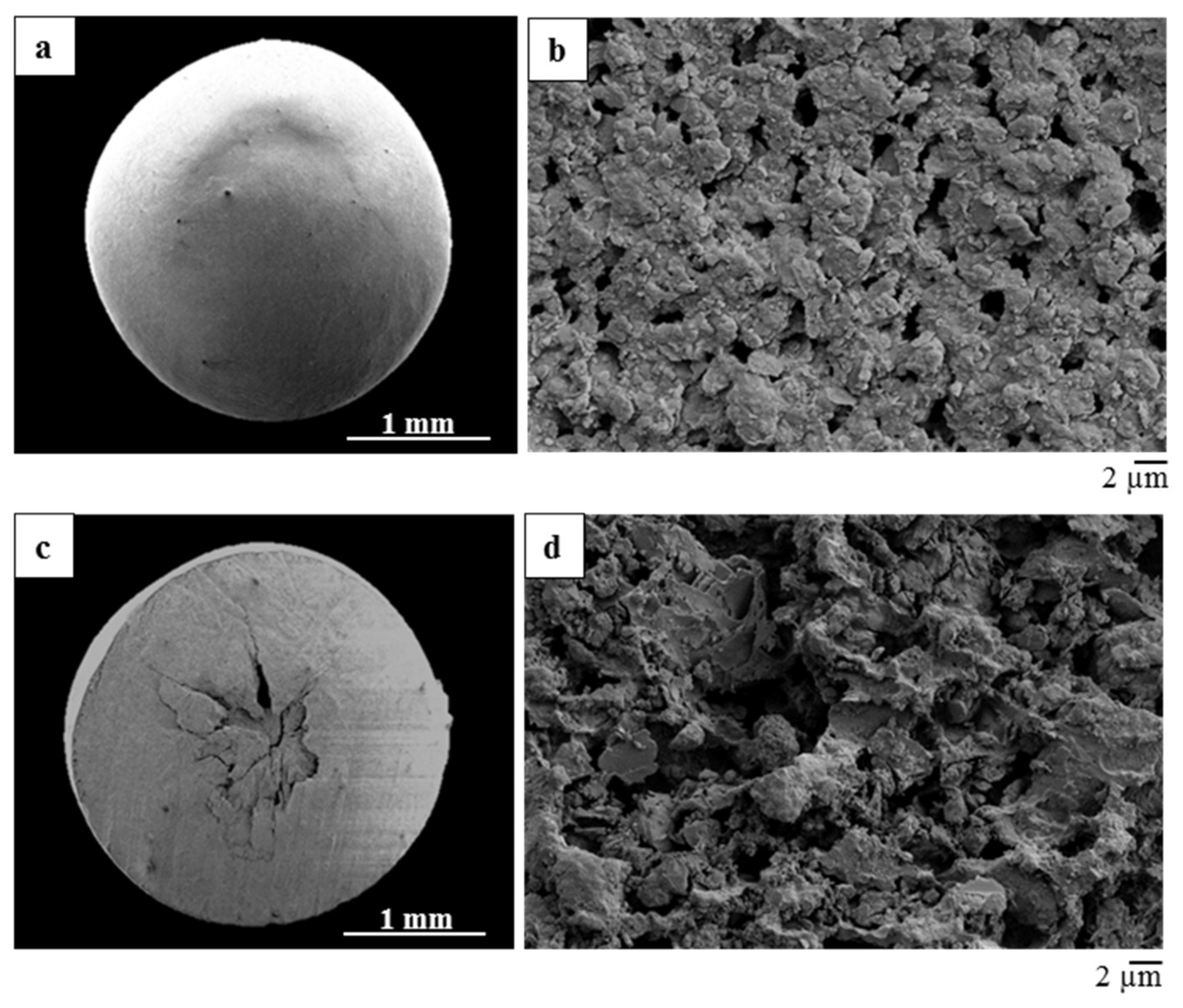
The morphological and macrostructural features of the ice-templated samples were investigated by digital microscopy (3D Digital Microscope RH2000, Hirox, Japan).
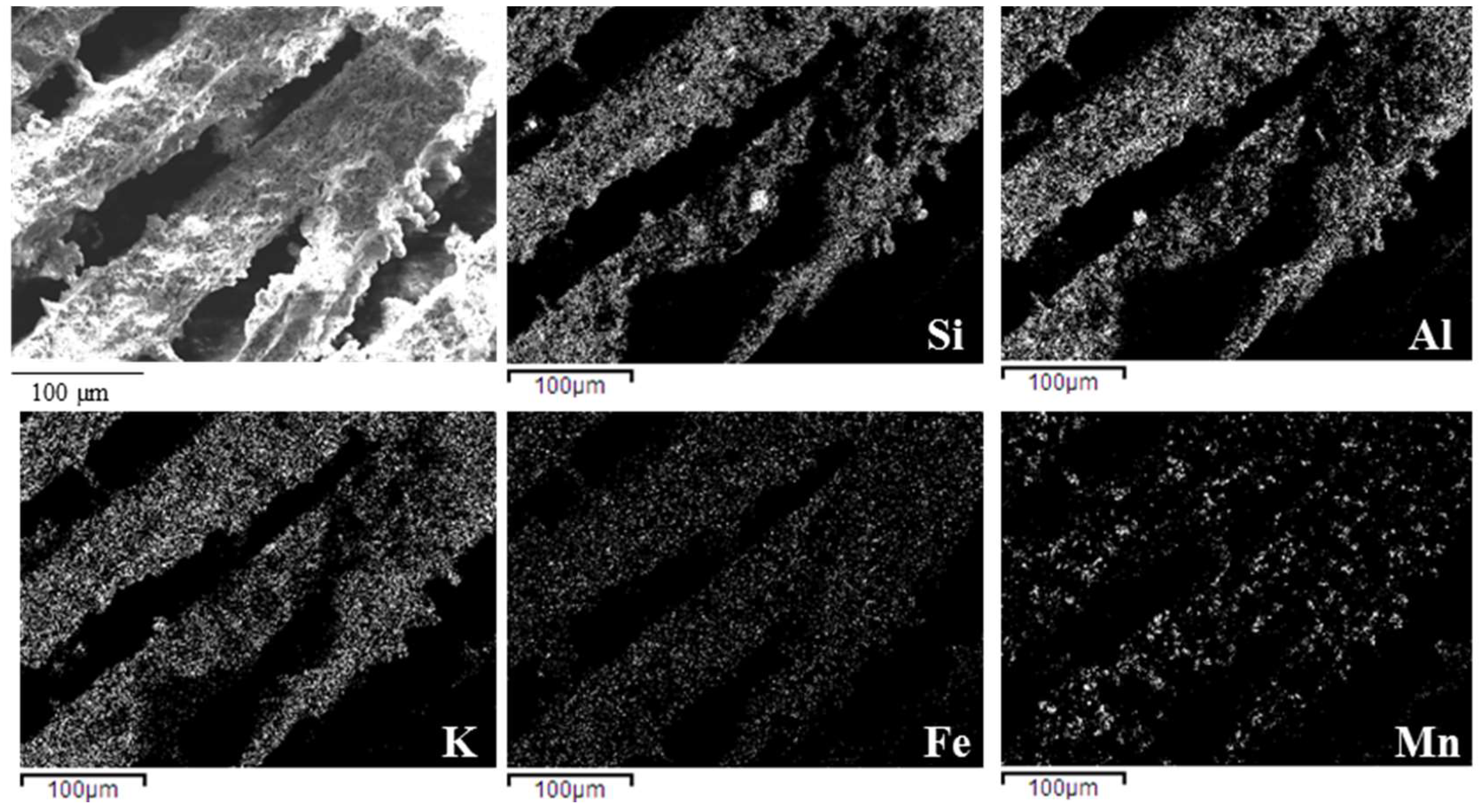
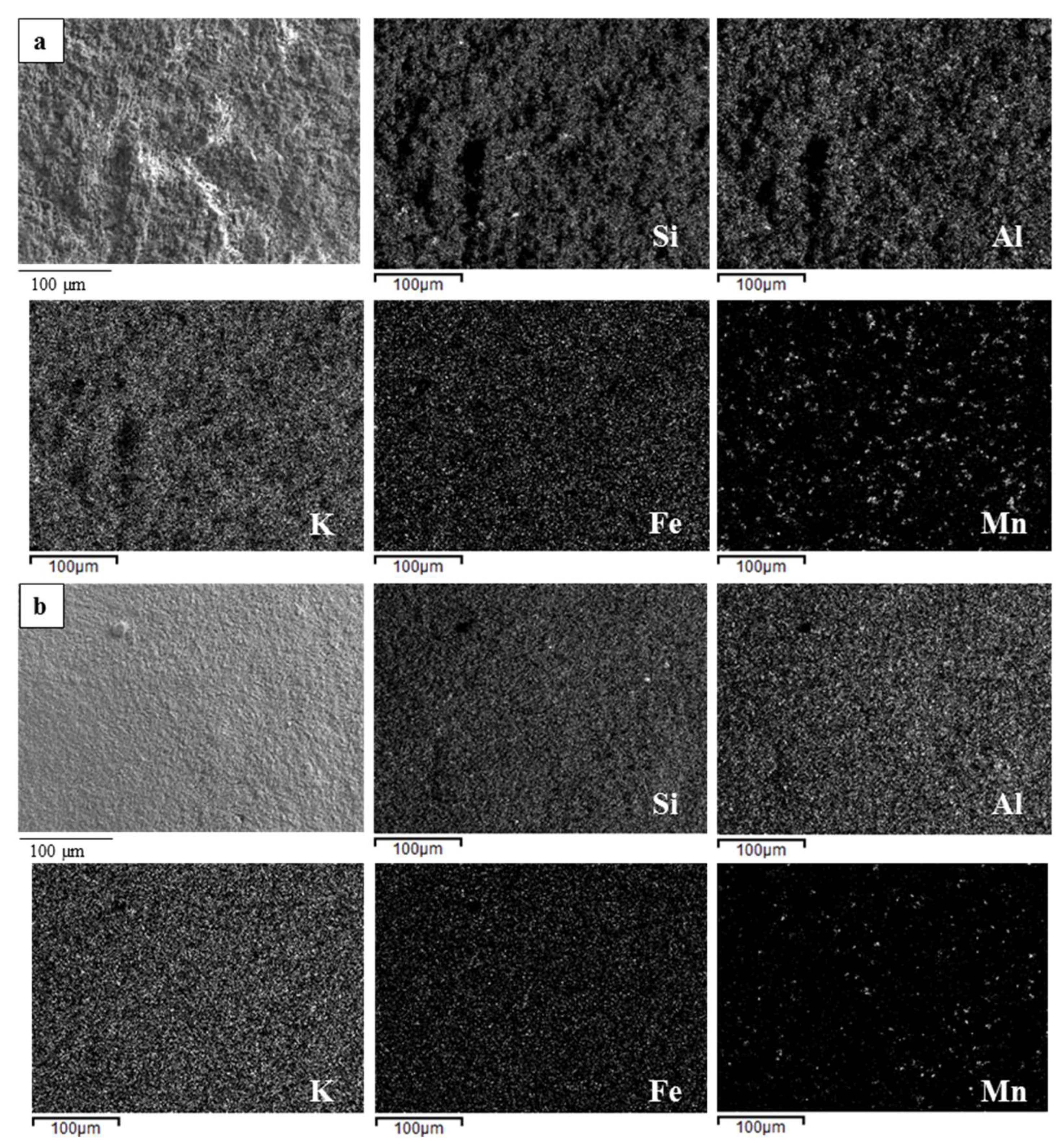
The microstructural features of the composite monoliths and beads were examined by Environmental Scanning Electron Microscopy (E-SEM FEI Quanta 200, FEI Company, Hillsboro, OR, USA) and by Field Emission Gun-Scanning Electron Microscopy (FEG-SEM, ΣIGMA: Zeiss, Germany) equipped with EDS (probe X-Act, INCA Energy 300, Oxford Instruments, Abingdon, UK) used for element mapping. The samples before the observation were coated with a thin layer of gold using a turbo-pumped sputter coater (Quorum Q150T ES, Quorum Technologies Ltd., East Sussex, UK).
The percent values of sample total porosity were calculated according to the Equation (1):
The bulk density of samples was determined by weight-to-volume ratio. The volume was geometrically measured by using a caliper (accuracy ± 0.05 mm). The true density, i.e., mass/volume of the solid, was determined by helium pycnometry (Multivolume pycnometer 1305 by Micrometrics).
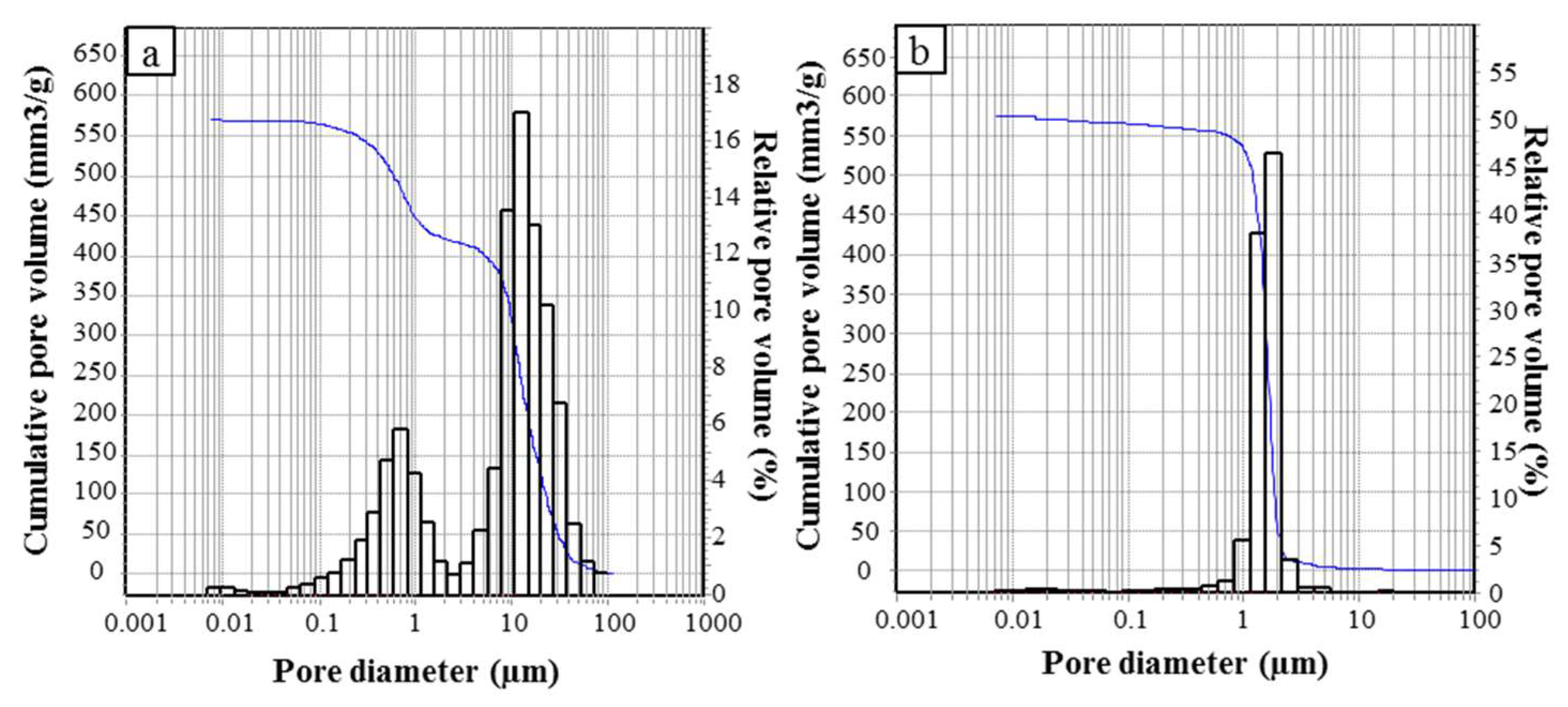
The pore size distribution in the range 0.0058–100 µm was analyzed by mercury intrusion porosimetry (MIP, surface tension = 0.48 N/m and contact angle = 140°, Thermo Finnigan Pascal 140 and Thermo Finnigan Pascal 240), with an experimental error of 4% due to the accuracy of the instrument.
The measurements of specific surface area were conducted using a Thermo ScientificTM Surfer instrument and the specific surface area (SBET) was calculated by the Brunauer–Emmet–Teller method by means of nitrogen adsorption at 77 K. The measurements were performed on monolith pieces and on the entire beads.
4. Conclusions
The freeze-casting technique, applied to geopolymer-Fe/Mn oxide composites, was successful for the production of highly porous monoliths and beads, conceived as potential oxygen carriers for CLC processes with a packed bed configuration.
In general, the simultaneous formation of geopolymer intrinsic mesoporosity and macro-porosity by ice growth, together with a final chemical consolidation, was obtained by properly combining the maturation step of the geopolymer reactive system with the water targeted for the ice-templating.
A water amount of 55 vol.% was optimal for the process and suitable for the formation of both monoliths and beads.
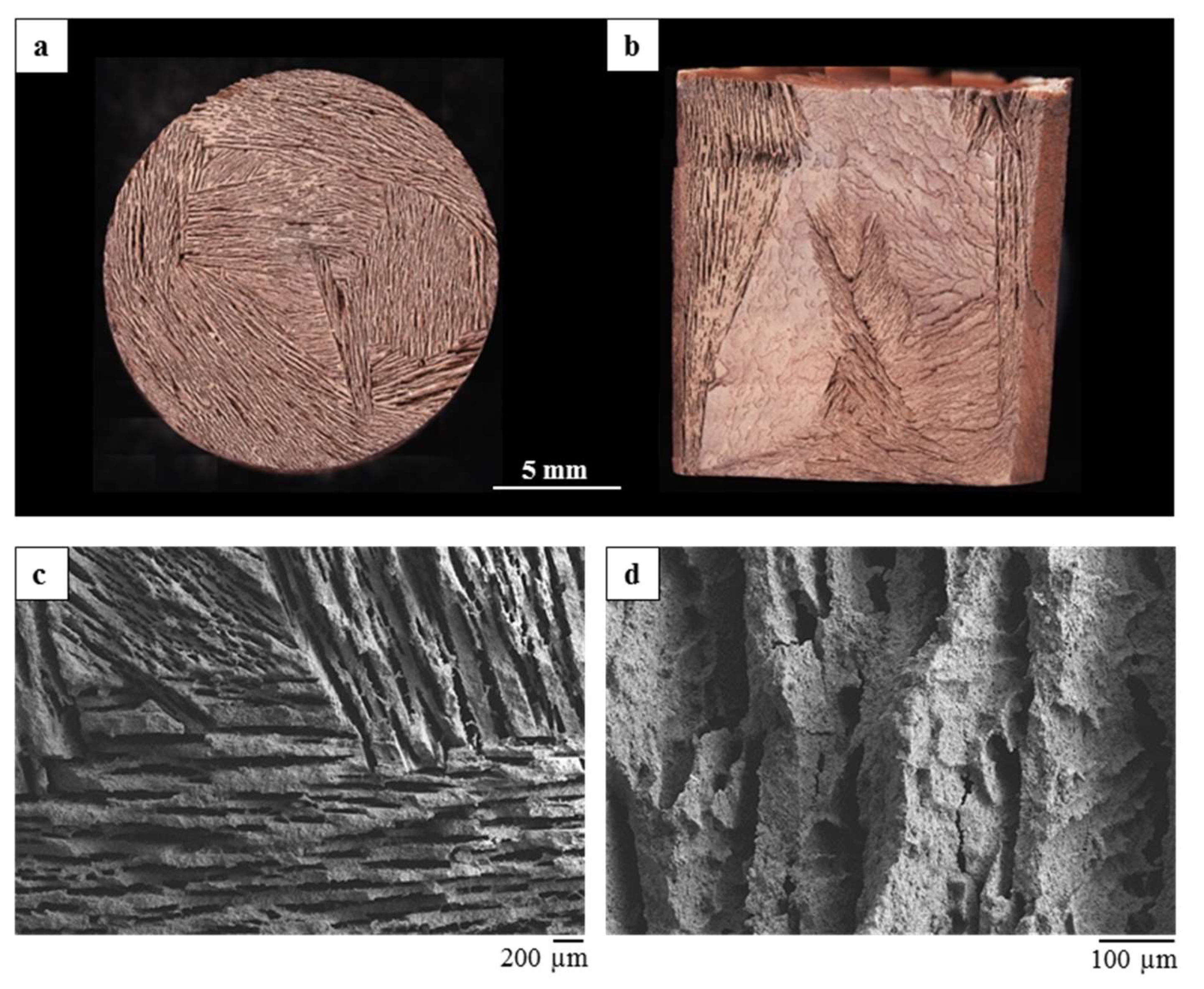
The monoliths showed the formation of a unidirectional lamellar porosity arranged in domains, running form the bottom to the top of the sample. The direct dripping of the mixture in liquid nitrogen lead to the formation of rounded beads with ~2.5 mm diameter.
The differently shaped materials possess a comparable porosity, albeit with a different pore size distribution, due to the development of a different macroporosity during the ice-templating.
In both cases, the process led to obtain porous and homogeneous microstructures, with well-dispersed metal oxides inside the geopolymer matrix.
Therefore, both the carriers could be suitable for a packed bed reactor configuration; studies are ongoing to further improve the process conditions and the differently shaped oxygen carriers will be tested in a lab-scale CLC plant to assess the performances in terms of CO conversion and oxygen transport ability.