Fabricating MOF/Polymer Composites via Freeze Casting for Water Remediation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents and Materials
2.2. Preparation of UiO-66, UiO-66-NH2 and UiO-NO2 Nanoparticles
2.3. Fabrication of Chitosan/UiO-66 Composites via Freeze Casting
2.4. Post-Treatment of Freeze-Dried Chitosan/UiO-66 Monoliths
2.4.1. Base Treatment
2.4.2. Chemical Crosslinking of Chitosan with Glutaraldehyde
2.5. Water Treatment with MCPP Adsorption
2.6. Material Characterization
3. Results and Discussion
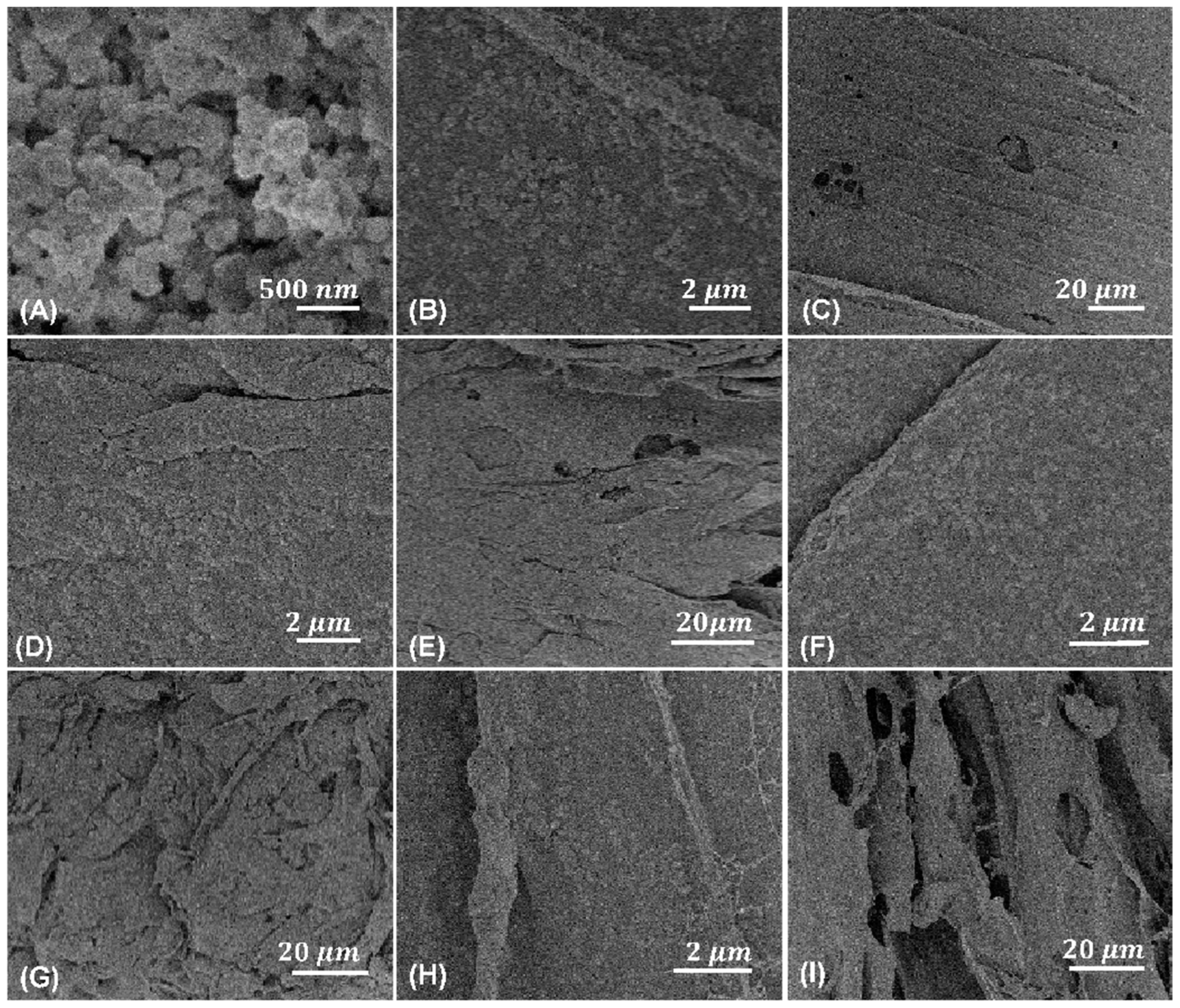
3.1. Freeze-Dried and Post-Treated Chitosan/UiO-66 Composites
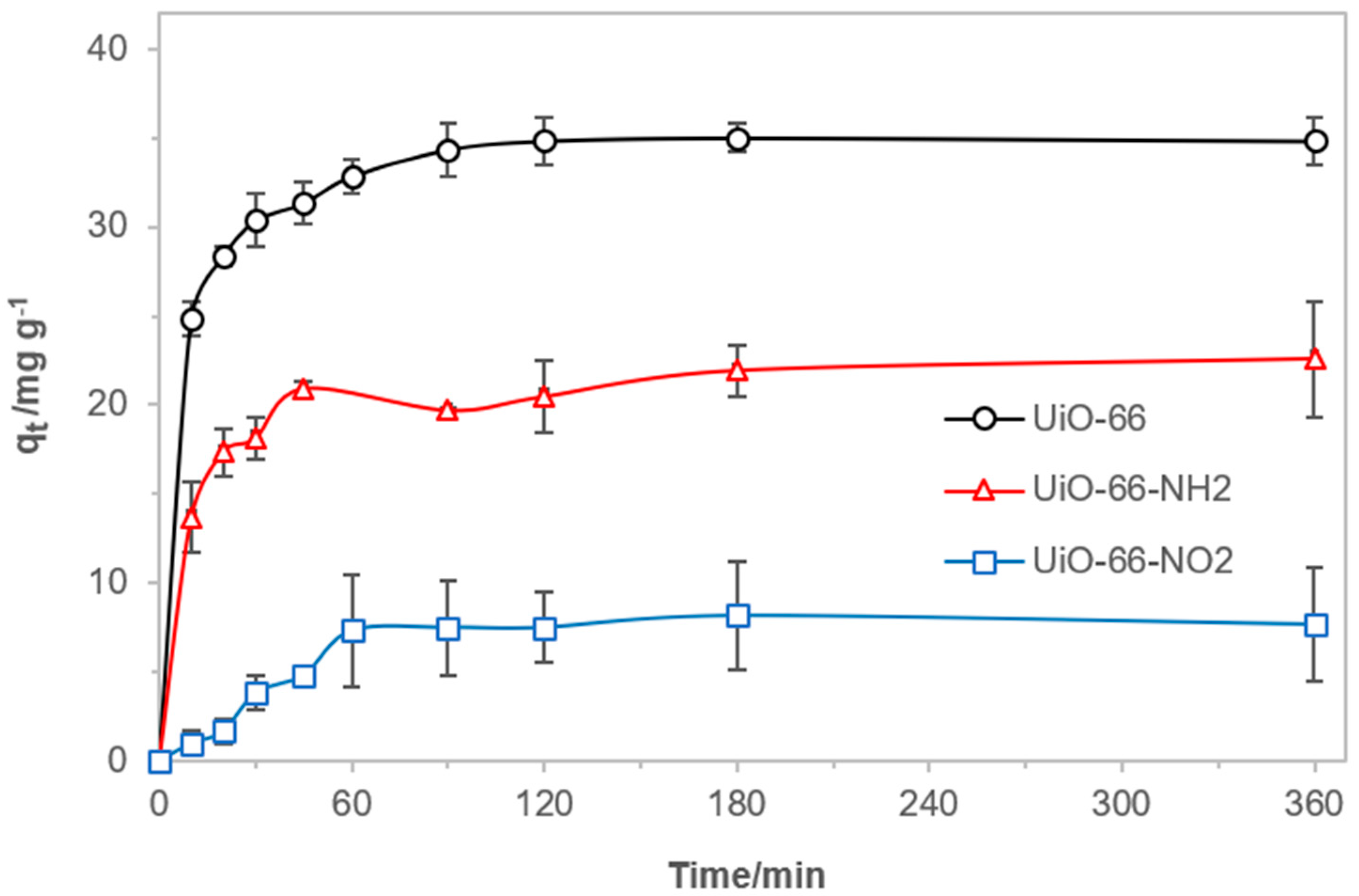
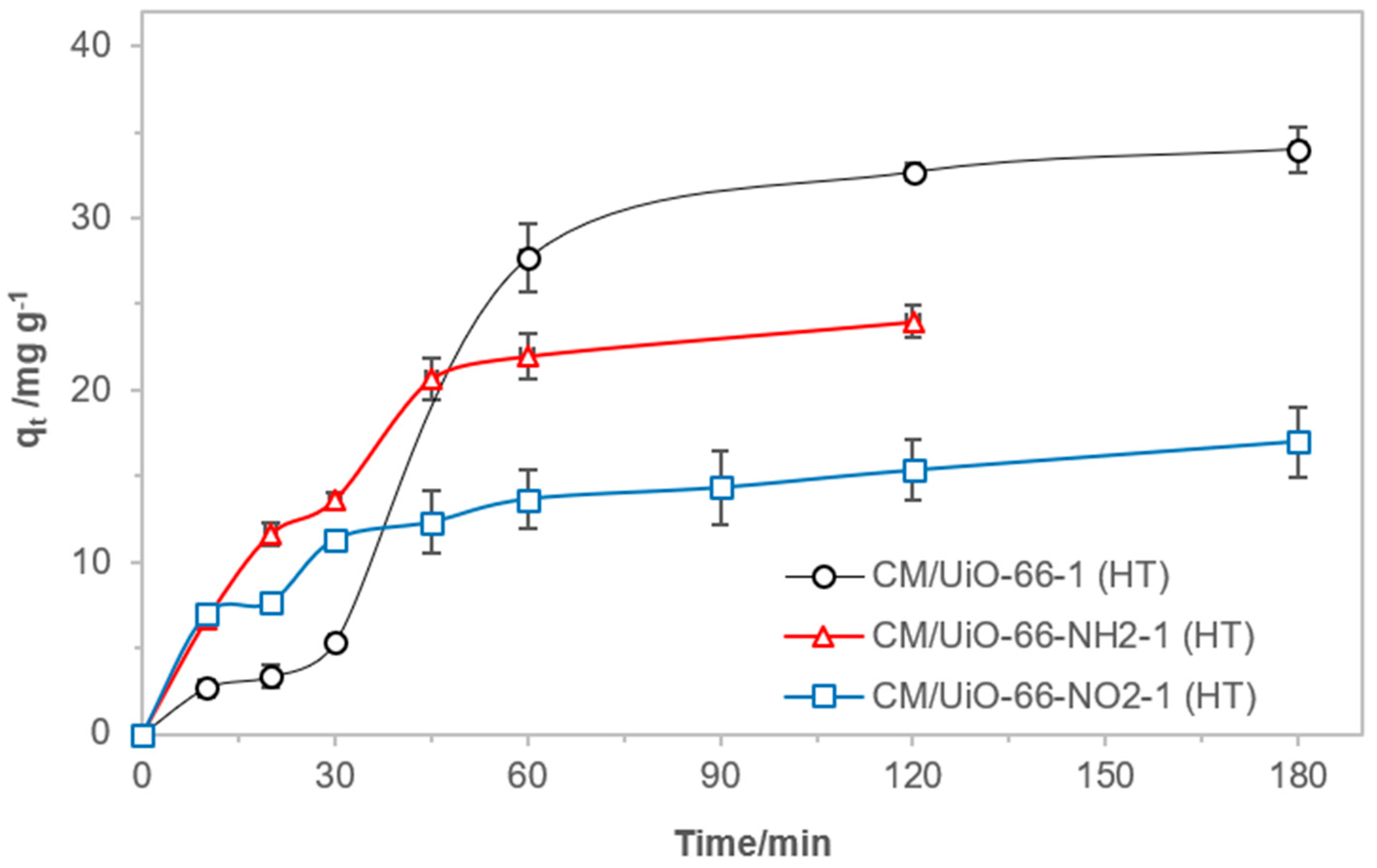
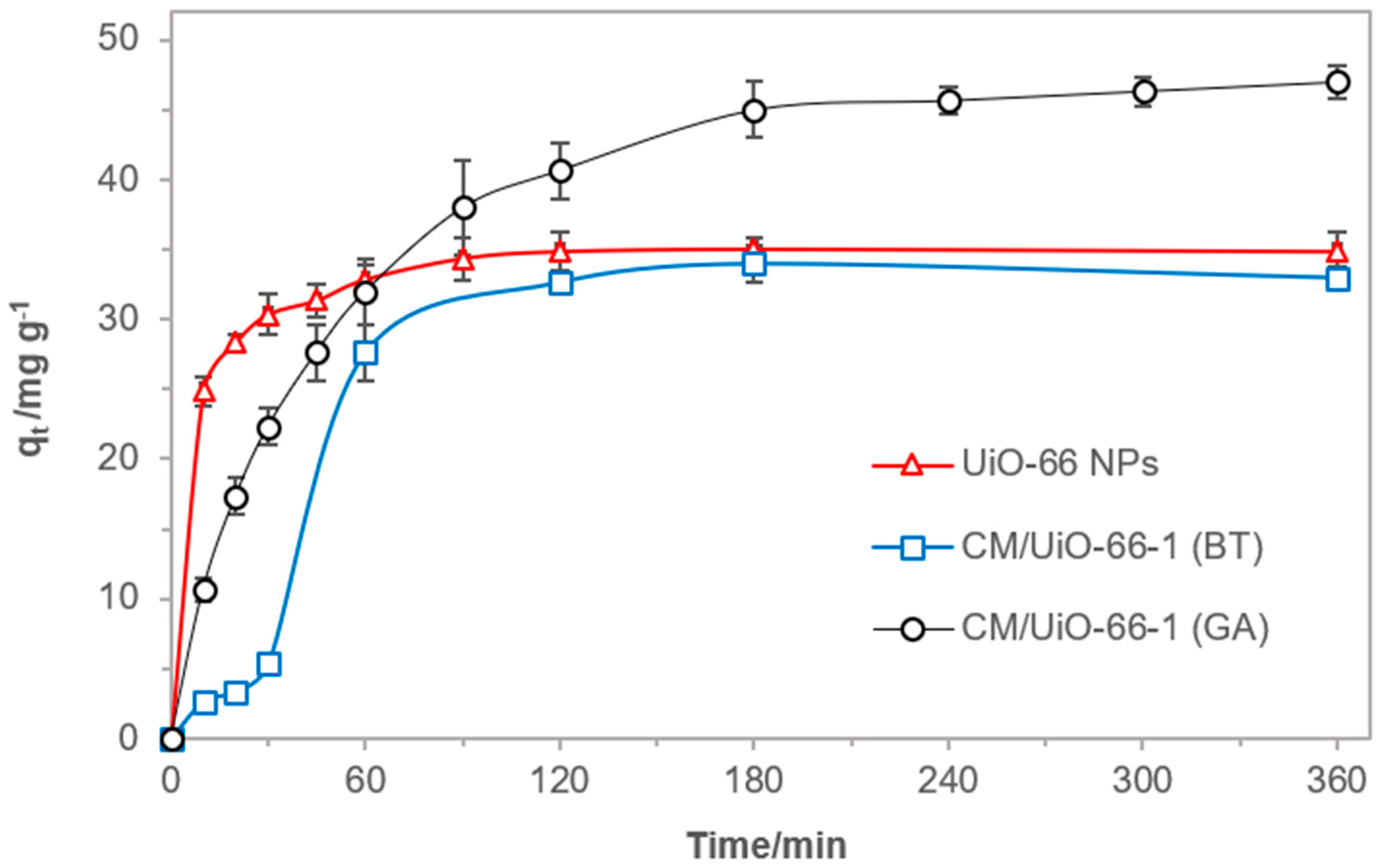
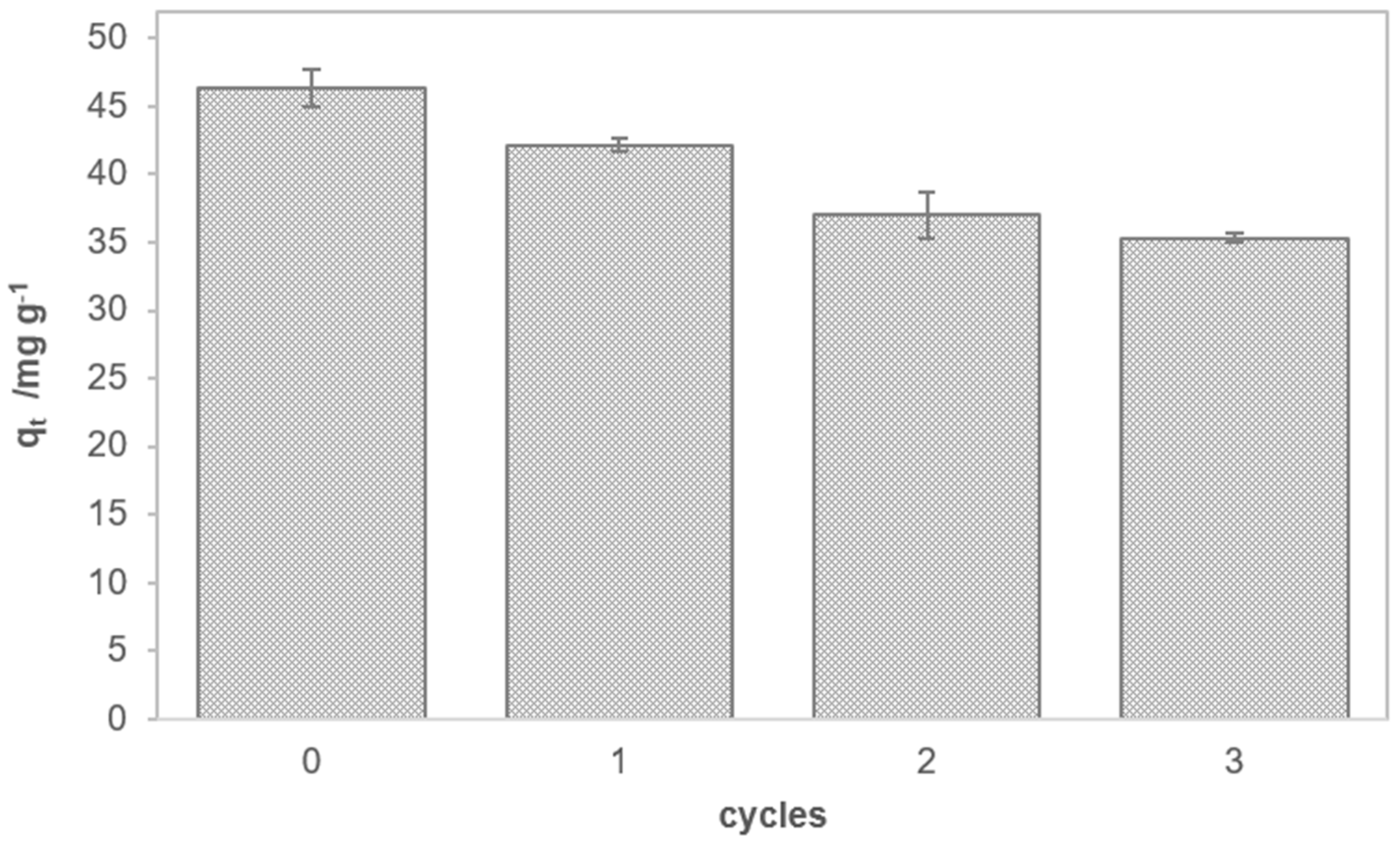
3.2. Adsorption of MCPP from Aqueous Solutions
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Mishra, A.; Clark, J.H. Green Materials for Sustainable Water Remediation and Treatment; The Royal Society of Chemistry: London, UK, 2013. [Google Scholar]
- Tesh, S.J.; Scott, T.B. Nano-Composites for Water Remediation: A Review. Adv. Mater. 2014, 26, 6056–6068. [Google Scholar] [CrossRef] [PubMed]
- Ali, I. New Generation Adsorbents for Water Treatment. Chem. Rev. 2012, 112, 5073–5091. [Google Scholar] [CrossRef] [PubMed]
- Lee, A.; Elam, J.W.; Darling, S.B. Membrane Materials for Water Purification: Design, Development, and Application. Environ. Sci. Water Res. Technol. 2016, 2, 17–42. [Google Scholar] [CrossRef]
- Smith, S.C.; Rodrigues, D.F. Carbon-Based Nanomaterials for Removal of Chemical and Biological Contaminants from Water: A Review of Mechanisms and Applications. Carbon 2015, 91, 122–143. [Google Scholar] [CrossRef]
- Bhatnagar, A.; Sillanpää, M. A Review of Emerging Adsorbents for Nitrate Removal from Water. Chem. Eng. J. 2011, 168, 493–504. [Google Scholar] [CrossRef]
- Herrmann, J.-M. Heterogeneous Photocatalysis: Fundamentals and Applications to the Removal of Various Types of Aqueous Pollutants. Catal. Today 1999, 53, 115–129. [Google Scholar] [CrossRef]
- Zhou, H.-C.; Long, J.R.; Yaghi, O.M. Introduction to Metal-Organic Frameworks. Chem. Rev. 2012, 112, 673–674. [Google Scholar] [CrossRef] [PubMed]
- Li, J.-R.; Sculley, J.; Zhou, H.-C. Metal-Organic Frameworks for Separations. Chem. Rev. 2012, 112, 869–932. [Google Scholar] [CrossRef] [PubMed]
- Howarth, A.J.; Liu, Y.; Li, P.; Li, Z.; Wang, T.C.; Hupp, J.T.; Farha, O.K. Chemical, Thermal and Mechanical Stabilities of Metal−Organic Frameworks. Nat. Rev. Mater. 2016, 1, 15018. [Google Scholar] [CrossRef]
- Cavka, J.H.; Jakobsen, S.; Olsbye, U.; Guillou, N.; Lamberti, C.; Bordiga, S.; Lillerud, K.P. A New Zirconium Inorganic Building Brick Forming Metal Organic Frameworks with Exceptional Stability. J. Am. Chem. Soc. 2008, 130, 13850–13851. [Google Scholar] [CrossRef] [PubMed]
- Hasan, J.; Jhung, S. Removal of Hazardous Organics from Water Using Metal-Organic Frameworks (MOFs): Plausible Mechanisms for Selective Adsorptions. J. Hazard. Mater. 2015, 283, 329–339. [Google Scholar] [CrossRef] [PubMed]
- Dias, E.M.; Petit, C. Towards the Use of Metal–Organic Frameworks for Water Reuse: A Review of the Recent Advances in the Field of Organic Pollutants Removal and Degradation and the Next Steps in the Field. J. Mater. Chem. A 2015, 3, 22484–22506. [Google Scholar] [CrossRef]
- Fu, Q.; Wen, L.; Zhang, L.; Chen, X.; Pun, D.; Ahmed, A.; Yang, Y.; Zhang, H. Preparation of Ice-Templated MOF–Polymer Composite Monoliths and Their Application for Wastewater Treatment with High Capacity and Easy Recycling. ACS Appl. Mater. Interfaces 2017, 9, 33979–33988. [Google Scholar] [CrossRef] [PubMed]
- Seo, Y.S.; Khan, N.A.; Jhung, S.H. Adsorptive Removal of Methylchlorophenoxypropionic Acid from Water with a Metal-Organic Framework. Chem. Eng. J. 2015, 270, 22–27. [Google Scholar] [CrossRef]
- Ahmed, A.; Forster, M.; Clowes, R.; Myers, P.; Zhang, H. Hierarchical Porous Metal–Organic Framework Monoliths. Chem. Commun. 2014, 50, 14314–14316. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, A.; Hasell, T.; Clowes, R.; Myers, P.; Cooper, A.I.; Zhang, H. Aligned Macroporous Monoliths with Intrinsic Microporosity via a Frozen-Solvent-Templating Approach. Chem. Commun. 2015, 51, 1717–1720. [Google Scholar] [CrossRef] [PubMed]
- Zou, H.; Wu, S.; Shen, J. Polymer/Silica Nanocomposites: Preparation, Characterization, Properties, and Applications. Chem. Rev. 2008, 108, 3893–3957. [Google Scholar] [CrossRef] [PubMed]
- Kitao, T.; Zhang, Y.; Kitagawa, S.; Wang, B.; Uemura, T. Hybridiation of MOFs and Polymers. Chem. Soc. Rev. 2017, 46, 3108–3133. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H. Ice Templating and Freeze-Drying for Porous Materials and Their Applications; Wiley-VCH: Weinheim, Germany, 2018. [Google Scholar]
- Deville, S. Freezing Colloids: Observations, Principles, Control, and Use; Springer International Publishing AG: Cham, Switzerland, 2017. [Google Scholar]
- Kandiah, M.; Nilsen, M.H.; Usseglio, S.; Jakobsen, S.; Olsbye, U.; Tilset, M.; Larabi, C.; Quadrelli, E.A.; Bonino, F.; Lillerud, K.P. Synthesis and Stability of Tagged UiO-66 Zr-MOFs. Chem. Mater. 2010, 22, 6632–6640. [Google Scholar] [CrossRef]
- Liu, Y.; Ma, L.; Gao, C. Facile Fabrication of the Glutaraldehyde Cross-Linked Collagen/Chitosan Porous Scaffold for Skin Tissue Engineering. Mater. Sci. Eng. C 2012, 32, 2361–2366. [Google Scholar] [CrossRef]
- Clasen, C.; Wilhelms, T.; Kulicke, W.-M. Formation and Characterization of Chitosan Membranes. Biomacromolecules 2006, 7, 3210–3222. [Google Scholar] [CrossRef] [PubMed]
- Coates, J. Interpretation of Infrared Spectra, a Practical Approach. Encycl. Anal. Chem. 2006. [Google Scholar] [CrossRef]
- Pratt, D.Y.; Wilson, L.D.; Kozinski, J.A. Preparation and Sorption Studies of Glutaraldehyde Cross-Linked Chitosan Copolymers. J. Colloid Interface Sci. 2013, 395, 205–211. [Google Scholar] [CrossRef] [PubMed]
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rogers, C.; Pun, D.; Fu, Q.; Zhang, H. Fabricating MOF/Polymer Composites via Freeze Casting for Water Remediation. Ceramics 2018, 1, 353-363. https://doi.org/10.3390/ceramics1020028
Rogers C, Pun D, Fu Q, Zhang H. Fabricating MOF/Polymer Composites via Freeze Casting for Water Remediation. Ceramics. 2018; 1(2):353-363. https://doi.org/10.3390/ceramics1020028
Chicago/Turabian StyleRogers, Coral, Daniel Pun, Qingshan Fu, and Haifei Zhang. 2018. "Fabricating MOF/Polymer Composites via Freeze Casting for Water Remediation" Ceramics 1, no. 2: 353-363. https://doi.org/10.3390/ceramics1020028
APA StyleRogers, C., Pun, D., Fu, Q., & Zhang, H. (2018). Fabricating MOF/Polymer Composites via Freeze Casting for Water Remediation. Ceramics, 1(2), 353-363. https://doi.org/10.3390/ceramics1020028




