Exploring the Processing of Tubular Chromite- and Zirconia-Based Oxygen Transport Membranes
Abstract
1. Introduction
2. Materials and Methods
3. Results
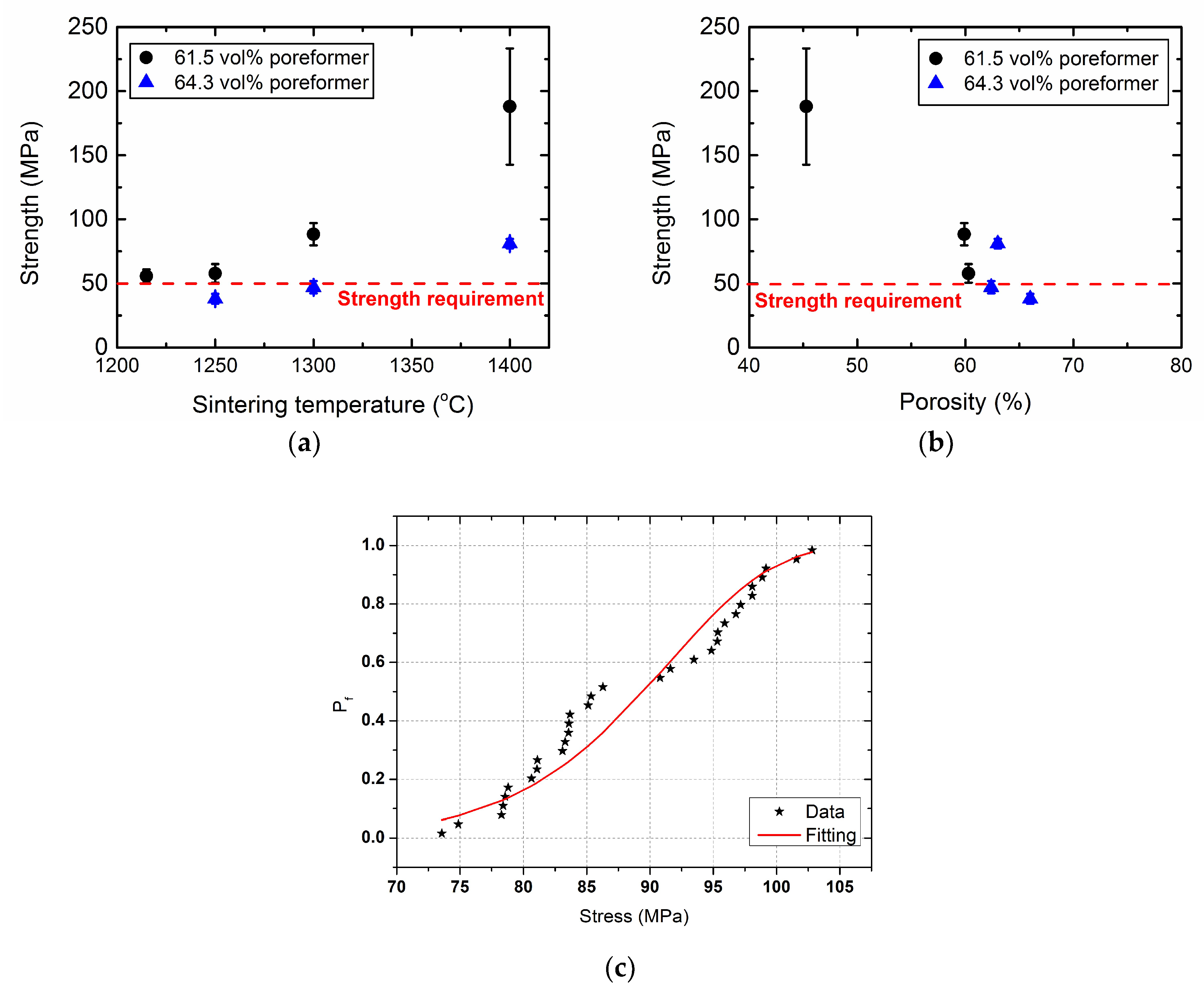
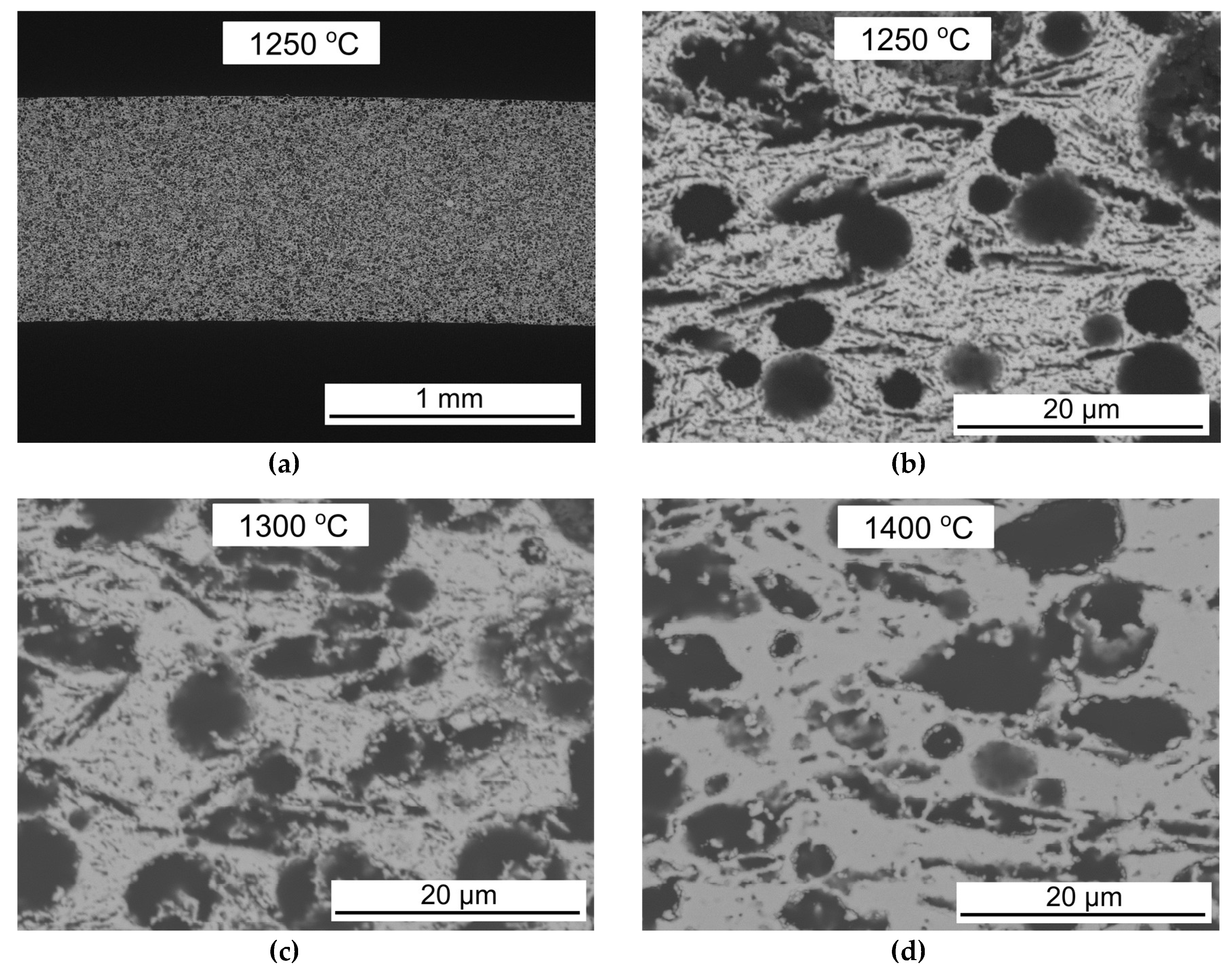
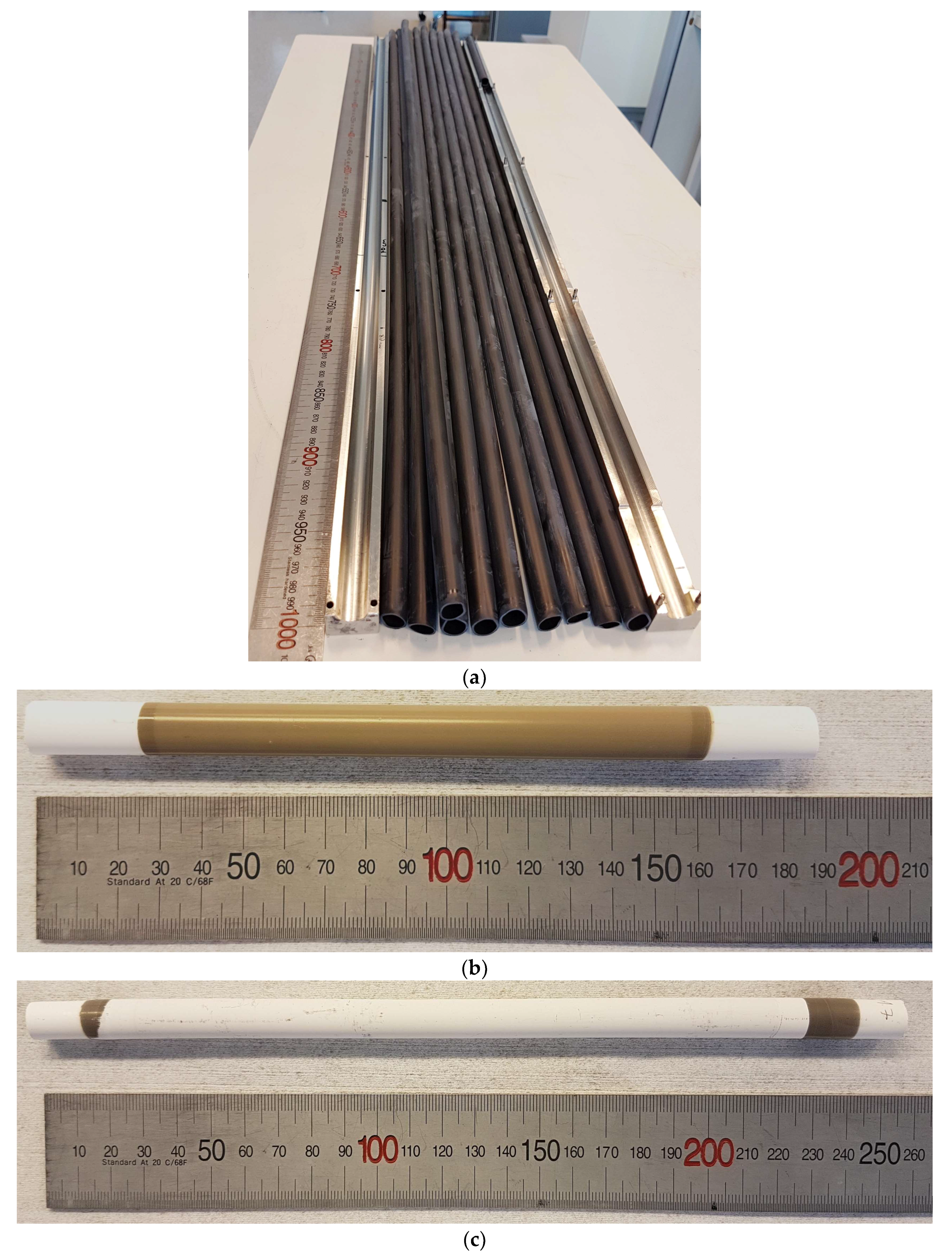
3.1. Porous 3YSZ Support Tubes
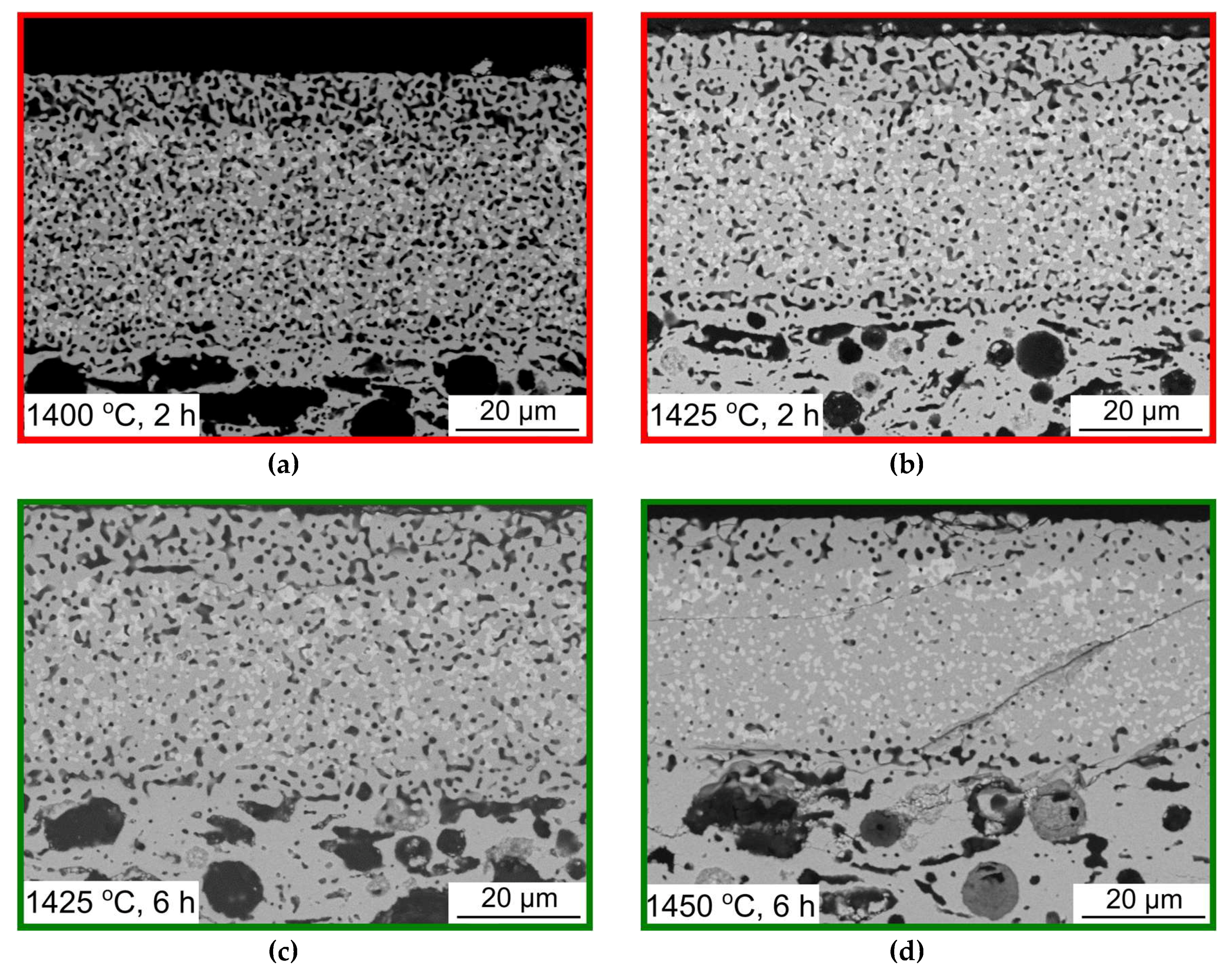
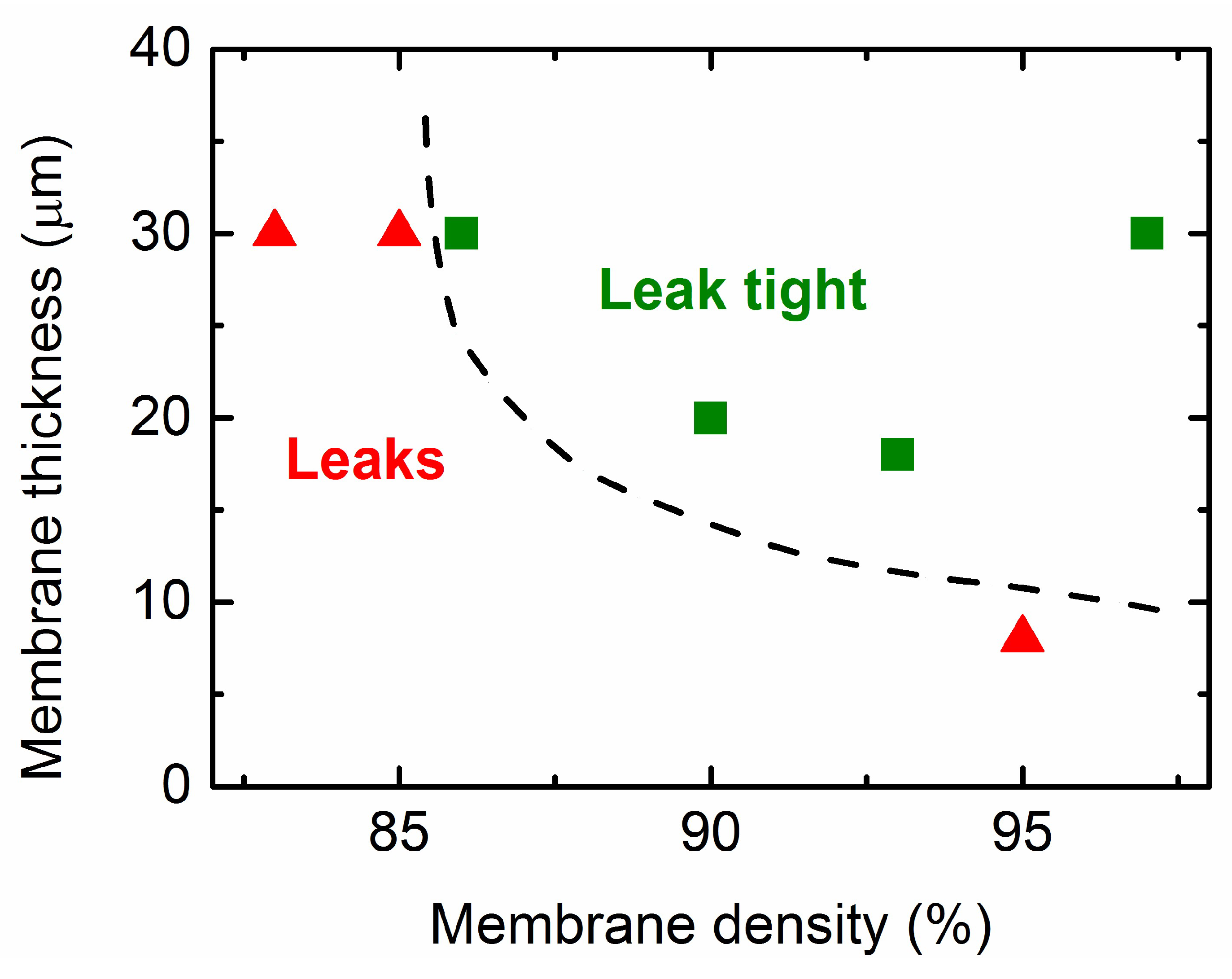
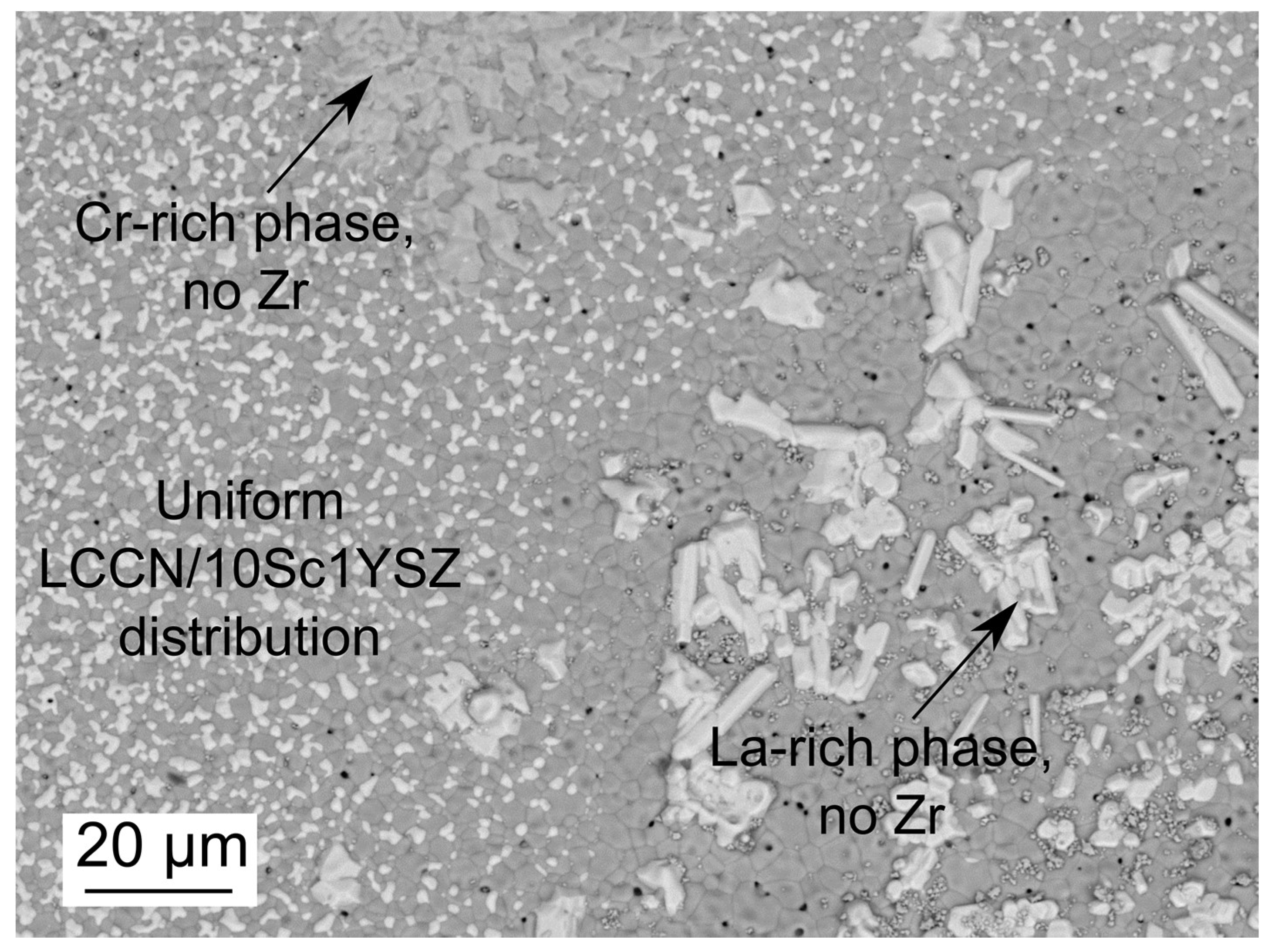
3.2. Co-Sintering of Membrane and Activation Layers on the Porous Support Tubes
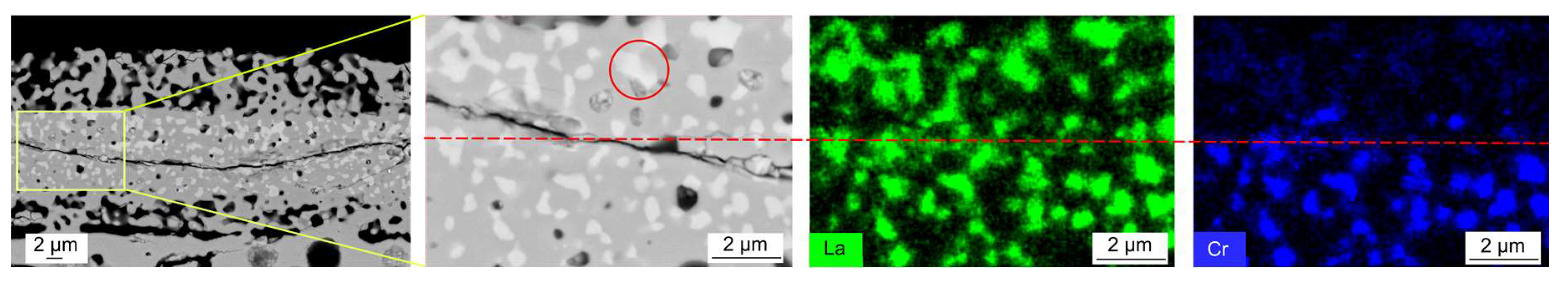
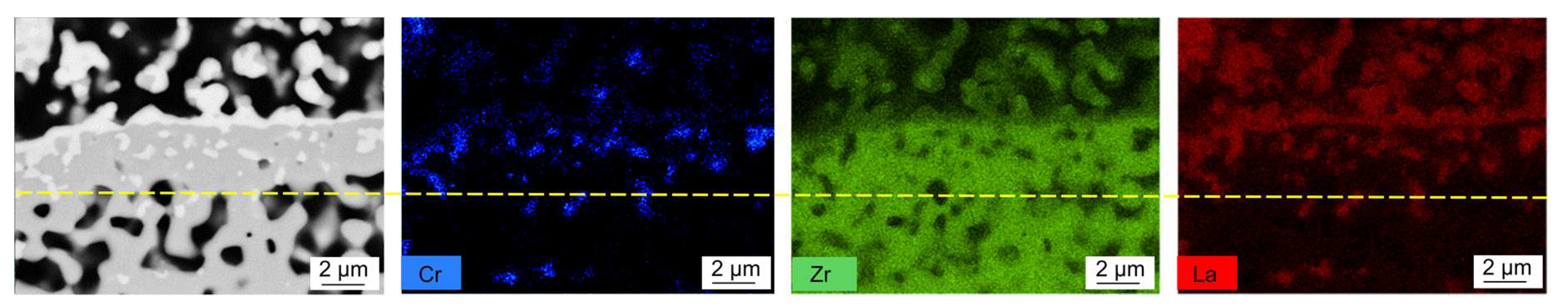
3.3. Cr Evaporation during Co-Sintering
4. Discussion
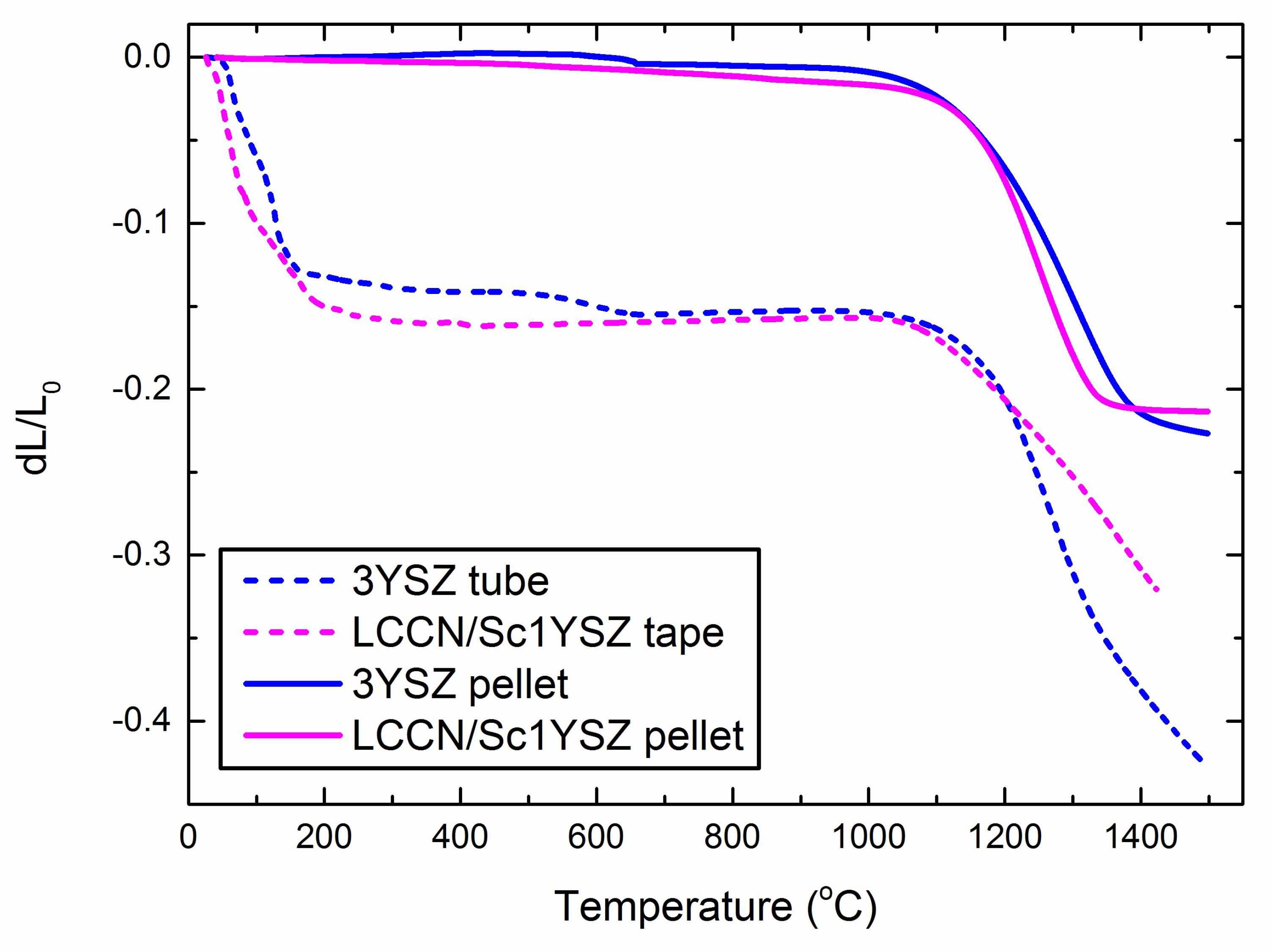
4.1. Ceramic Processing of 3YSZ Porous Support Tubes
4.2. Crack Formation during Co-Sintering of Tubular, Asymmetric Oxygen Transport Membranes
4.3. Evaporation of Cr
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Stadler, H.; Beggel, F.; Habermehl, M.; Persigehl, B.; Kneer, R.; Modigell, M.; Jeschke, P. Oxyfuel coal combustion by efficient integration of oxygen transport membranes. Int. J. Greenh. Gas. Control 2011, 5, 7–15. [Google Scholar] [CrossRef]
- Wei, Y.; Yang, W.; Caro, J.; Wang, H. Dense ceramic oxygen permeable membranes and catalytic membrane reactors. Chem. Eng. J. 2013, 220, 185–203. [Google Scholar] [CrossRef]
- Ahrenfeldt, J.; Egsgaard, H.; Stelte, W.; Thomsen, T.; Henriksen, U.B. The influence of partial oxidation mechanisms on tar destruction in TwoStage biomass gasification. Fuel 2013, 112, 662–680. [Google Scholar] [CrossRef]
- Bouwmeester, H.J.M.; Gellings, P.J. Handbook of Solid State Electrochemistry; CRC Press: Boca Raton, FL, USA, 1997. [Google Scholar]
- Puig-Arnavat, M.; Soprani, S.; Søgaard, M.; Engelbrecht, K.; Ahrenfeldt, J.; Henriksen, U.B.; Hendriksen, P.V. Integration of mixed conducting membranes in an oxygen–steam biomass gasification process. RSC Adv. 2013, 3, 20843. [Google Scholar] [CrossRef]
- Pippardt, U.; Böer, J.; Kiesel, L.; Kircheisen, R.; Kriegel, R.; Voigt, I. Co-firing technology for the preparation of asymmetric oxygen transporting membranes based on BSCF and Zr-doped BSCF. AIChE J. 2014, 60, 15–21. [Google Scholar] [CrossRef]
- Shao, Z.; Yang, W.; Cong, Y.; Dong, H.; Tong, J.; Xiong, G. Investigation of the permeation behavior and stability of a Ba0.5Sr0.5Co0.8Fe0.2O3−δ. J. Membr. Sci. 2000, 172, 177–188. [Google Scholar] [CrossRef]
- Švarcová, S.; Wiik, K.; Tolchard, J.; Bouwmeester, H.J.M.; Grande, T. Structural instability of cubic perovskite BaxSr1−xCo1−yFeyO3−δ. Solid State Ionics 2008, 178, 1787–1791. [Google Scholar] [CrossRef]
- Brett, D.J.L.; Atkinson, A.; Brandon, N.P.; Skinner, S.J. Intermediate temperature solid oxide fuel cells. Chem. Soc. Rev. 2008, 37, 1568. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.; Yang, W. Critical Factors Affecting Oxygen Permeation Through Dual-phase Membranes, 1st ed.; Elsevier BV: Amsterdam, The Netherlands, 2011; Volume 14. [Google Scholar]
- Artemov, V.G.; Kuritsyna, I.E.; Lebedev, S.P.; Komandin, G.A.; Kapralov, P.O.; Spektor, I.E.; Kharton, V.V.; Bredikhin, S.I.; Volkov, A.A. Analysis of electric properties of ZrO2-Y2O3 single crystals using teraherz IR and impedance spectroscopy techniques. Russ. J. Electrochem. 2014, 50, 690–693. [Google Scholar] [CrossRef]
- Gupta, S.; Mahapatra, M.K.; Singh, P. Lanthanum chromite based perovskites for oxygen transport membrane. Mater. Sci. Eng. R Rep. 2015, 90, 1–36. [Google Scholar] [CrossRef]
- Pirou, S.; Bermudez, J.M.; Na, B.T.; Ovtar, S.; Yu, J.H.; Hendriksen, P.V.; Kaiser, A.; Reina, T.R.; Millan, M.; Kiebach, R. Performance and stability of (ZrO2)0.89(Y2O3)0.01(Sc2O3)0.10-LaCr0.85Cu0.10Ni0.05O3−δ oxygen transport membranes under conditions relevant for oxy-fuel combustion. J. Membr. Sci. 2018, 552, 115–123. [Google Scholar] [CrossRef]
- Baumann, S.; Serra, J.M.; Lobera, M.P.; Escolástico, S.; Schulze-Küppers, F.; Meulenberg, W.A. Ultrahigh oxygen permeation flux through supported Ba0.5Sr0.5Co0.8Fe0.2O3−δmembranes. J. Membr. Sci. 2011, 377, 198–205. [Google Scholar] [CrossRef]
- Schulz, M.; Pippardt, U.; Kiesel, L.; Ritter, K.; Kriegel, R. Oxygen permeation of various archetypes of oxygen membranes based on BSCF. AIChE J. 2012, 58, 3195–3202. [Google Scholar] [CrossRef]
- Baumann, S.; Meulenberg, W.A.; Buchkremer, H.P. Manufacturing strategies for asymmetric ceramic membranes for efficient separation of oxygen from air. J. Eur. Ceram. Soc. 2013, 33, 1251–1261. [Google Scholar] [CrossRef]
- Kaiser, A.; Foghmoes, S.P.; Pecanac, G.; Malzbender, J.; Chatzichristodoulou, C.; Glasscock, J.A.; Ramachandran, D.; Ni, D.W.; Esposito, V.; Søgaard, M.; et al. Design and optimization of porous ceramic supports for asymmetric ceria-based oxygen transport membranes. J. Membr. Sci. 2016, 513, 85–94. [Google Scholar] [CrossRef]
- Kwok, K.; Frandsen, H.L.; Søgaard, M.; Hendriksen, P.V. Stress analysis and fail-safe design of bilayered tubular supported ceramic membranes. J. Membr. Sci. 2014, 453, 253–262. [Google Scholar] [CrossRef]
- Klemensø, T.; Boccaccini, D.; Brodersen, K.; Frandsen, H.L.; Hendriksen, P.V. Development of a Novel Ceramic Support Layer for Planar Solid Oxide Cells. Fuel Cells 2014, 14, 153–161. [Google Scholar] [CrossRef]
- Beggel, F.J.; Nauels, N.; Modigell, M. CO2 Separation via the Oxyfuel Process with O2-Transport Membranes in Coal Power Plants; Wiley-VCH-Verl.: Weinheim, Germany, 2011; pp. 405–430. [Google Scholar]
- Pfaff, E.M.; Kaletsch, A.; Broeckmann, C. Design of a Mixed Ionic/Electronic Conducting Oxygen Transport Membrane Pilot Module. Chem. Eng. Technol. 2012, 35, 455–463. [Google Scholar] [CrossRef]
- Ramachandran, D.K.; Søgaard, M.; Clemens, F.; Gurauskis, J.; Kaiser, A. Fabrication and performance of a tubular ceria based oxygen transport membrane on a low cost MgO support. Sep. Purif. Technol. 2015, 147, 422–430. [Google Scholar] [CrossRef]
- Ovtar, S.; Gurauskis, J.; Haugen, A.B.; Chatzichristodoulou, C.; Kaiser, A.; Hendriksen, P.V. Oxygen transport properties of tubular Ce0.9Gd0.1O1.95-La0.6Sr0.4FeO3−δ composite asymmetric oxygen permeation membranes supported on magnesium oxide. J. Membr. Sci. 2017, 523, 576–587. [Google Scholar] [CrossRef]
- Dahl, P.I.; Fontaine, M.-L.; Ahouanto, F.; Denonville, C.; Paulsen, O.; Larring, Y.; Peters, T.; Henriksen, P.P.; Bredesen, R. Fabrication, sealing and high pressure testing of tubular La2NiO4+δ membranes for air separation. Energy Procedia 2012, 23, 187–196. [Google Scholar] [CrossRef]
- Charlas, B.; Frandsen, H.L.; Brodersen, K.; Henriksen, P.V.; Chen, M. Residual stresses and strength of multilayer tape cast solid oxide fuel and electrolysis half-cells. J. Power Sources 2015, 288, 243–252. [Google Scholar] [CrossRef]
- Haugen, A.B.; Gurauskis, J.; Kaiser, A.; Søgaard, M. Graphite and PMMA as pore formers for thermoplastic extrusion of porous 3Y-TZP oxygen transport membrane supports. J. Eur. Ceram. Soc. 2017, 37, 1039–1047. [Google Scholar] [CrossRef]
- Haugen, A.B.; Geffroy, A.; Kaiser, A.; Gil, V. MgO as a non-pyrolyzable pore former in porous membrane supports. J. Eur. Ceram. Soc. 2018, 38, 3279–3285. [Google Scholar] [CrossRef]
- Clemens, F. Thermoplastic Extrusion for Ceramic Bodies. In Extrusion in Ceramics; Händle, F., Ed.; Springer: Berlin, Germany, 2009; pp. 295–311. [Google Scholar]
- Kwok, K.; Kiesel, L.; Frandsen, H.L.; Søgaard, M.; Hendriksen, P.V. Strength characterization of tubular ceramic materials by flexure of semi-cylindrical specimens. J. Eur. Ceram. Soc. 2014, 34, 1423–1432. [Google Scholar] [CrossRef]
- Weibull, W. A statistical distribution function of wide applicability. J. Appl. Mech. 1951, 18, 293–297. [Google Scholar]
- Gurauskis, J.; Ovtar, S.; Kaiser, A.; Sogaard, M.; Hendriksen, P.V. Ceria Based Composite Membranes for Oxygen Separation. ECS Trans. 2014, 64, 251–258. [Google Scholar] [CrossRef]
- Hendriksen, P.V.; Høgsberg, J.R.; Kjeldsen, A.M.; Sørensen, B.F.; Pedersen, H.G. Failure Modes of Thin Supported Membranes; Wiley-Blackwell: Hoboken, NJ, USA, 2008; pp. 347–360. [Google Scholar]
- Ramachandran, D.K.; Kwok, K.; Søgaard, M.; Clemens, F.; Glasscock, J.; Kaiser, A. The role of sacrificial fugitives in thermoplastic extrusion feedstocks on properties of MgO supports for oxygen transport membranes. J. Eur. Ceram. Soc. 2015, 35, 1527–1537. [Google Scholar] [CrossRef]
- Molla, T.T.; Ramachandran, D.K.; Ni, D.W.; Esposito, V.; Teocoli, F.; Olevsky, E.; Bjørk, R.; Pryds, N.; Kaiser, A.; Frandsen, H.L. Modeling constrained sintering of bi-layered tubular structures. J. Eur. Ceram. Soc. 2015, 35, 941–950. [Google Scholar] [CrossRef]
- Mahapatra, M.K.; Lu, K. Seal glass for solid oxide fuel cells. J. Power Sources 2010, 195, 7129–7139. [Google Scholar] [CrossRef]
- Sarantaridis, D.; Atkinson, A. Redox cycling of Ni-based solid oxide fuel cell anodes: A review. Fuel Cells 2007, 7, 246–258. [Google Scholar] [CrossRef]
- Höfer, H.E.; Kock, W.F. Crystal Chemistry and Thermal Behavior in the La(Cr,Ni)O3 Perovskite System. J. Electrochem. Soc. 1993, 140, 2889–2894. [Google Scholar] [CrossRef]
- Gupta, S.; Mahapatra, M.K.; Singh, P. Phase transformation, thermal expansion and electrical conductivity of lanthanum chromite. Mater. Res. Bull. 2013, 48, 3262–3267. [Google Scholar] [CrossRef]
- Christie, G.M.; Middleton, P.H.; Steele, B.C.H. Liquid phase sintering, electrical conductivity, and chemical stability of lanthanum chromite doped with calcium and nickel. J. Eur. Ceram. Soc. 1994, 14, 163–175. [Google Scholar] [CrossRef]
- Son, H.J.; Lim, T.H.; Shin, D.R.; Song, R.H.; Kim, S.H. Investigation of Scandia Stabilized Zirconia (ScSZ)—Yttria Stabilized Zirconia (YSZ) Composite Electrolyte for Intermediate Temperature Fuel Cells. Solid State Phenom. 2007, 124–126, 795–798. [Google Scholar] [CrossRef]
- Hirano, M.; Watanabe, S.; Kato, E.; Mizutani, Y.; Kawai, M.; Nakamura, Y. Fabrication, electrical conductivity and mechanical properties of Sc2O3-doped tetragonal zirconia ceramics. Solid State Ionics 1998, 111, 161–169. [Google Scholar] [CrossRef]
- Kaiser, A.; Foghmoes, S.; Chatzichristodoulou, C.; Søgaard, M.; Glasscock, J.A.; Frandsen, H.L.; Hendriksen, P.V. Evaluation of thin film ceria membranes for syngas membrane reactors—Preparation, characterization and testing. J. Membr. Sci. 2011, 378, 51–60. [Google Scholar] [CrossRef]
- Le, S.; Sun, K.N.; Zhang, N.; Zhu, X.; Sun, H.; Yuan, Y.X.; Zhou, X. Fabrication and evaluation of anode and thin Y2O3-stabilized ZrO2 film by co-tape casting and co-firing technique. J. Power Sources 2010, 195, 2644–2648. [Google Scholar] [CrossRef]
- Cheng, T.; Raj, R. Flaw Generation During Constrained Sintering of Metal-Ceramic and Metal-Glass Multilayer Films. J. Am. Ceram. Soc. 1989, 72, 1649–1655. [Google Scholar] [CrossRef]
- Fleming, P.; Farrell, R.A.; Holmes, J.D.; Morris, M.A. The Rapid Formation of La(OH)3 from La2O3 Powders on Exposure to Water Vapor. J. Am. Ceram. Soc. 2010, 93, 1187–1194. [Google Scholar] [CrossRef]
- Acharya, K.; Mazumder, S.K.; Burra, R.K.; Williams, R.; Haynes, C. System-interaction analyses of solid-oxide fuel cell (SOFC) power-conditioning system. In Proceedings of the 38th IAS Annual Meeting on Conference Record of the Industry Applications Conference, Salt Lake City, UT, USA, 12–16 October 2003; IEEE: Piscataway, NJ, USA, 2003; Volume 3, pp. 2026–2032. [Google Scholar]
- Van Heuveln, F.H.; Bouwmeester, H.J.M. Electrode Properties of Sr-Doped LaMnO3 on Yttria-Stabilized Zirconia. J. Electrochem. Soc. 1997, 144, 134–140. [Google Scholar] [CrossRef]

| Activation Layers | Membrane Layer | ||||
|---|---|---|---|---|---|
| Function | Component | wt % | Function | Component | wt % |
| Solvent | Ethanol (reactant grade, Sigma Aldrich) | 61.8 | Solvent | Ethanol (reactant grade, Sigma Aldrich) | 67.5 |
| Dispersant | Polyvinyl pyrolidone (PVP K30, Sigma Aldrich) | 1.3 | Dispersant | Polyvinyl pyrolidone (PVP K15, Sigma Aldrich) | 1.5 |
| Binder | Polyvinyl butyral (B30 HH Mowital, Kuraray, USA) | 3.1 | Binder | Polyvinyl pyrolidone (PVP K30, Sigma Aldrich) | 1.5 |
| Backbone | 10Sc1YSZ (Daiichi, Japan) calcined at 900 °C | 25.8 | Ionic conductor | 10Sc1YSZ (Daiichi, Japan) calcined at 900 °C | 19.8 |
| Plasticizer | Dibutyl sebacate (Sigma Aldrich) | 2.2 | Electronic conductor | LCCN (Cerpotech, Norway) | 8.8 |
| Pore former | Graphite (UF-1, Graphit Kropfmühl, Germany) | 5.8 | Sintering aid | NiO (product 12359, Alfa Aesar) planetary ball milled for 90 min | 0.9 |
| Sintering temp. (°C) | Gas Permeability Coefficient (m2) | Porosity (vol %) | Pore Size, d10 (µm) | Pore Size, d50 (µm) | Pore Size, d90 (µm) | Average Strength (MPa) |
|---|---|---|---|---|---|---|
| 1250 | 1.80 × 10−14 | 60.3 | 0.27 | 1.19 | 1.66 | 57.8 |
| 1300 | 1.00 × 10−14 | 59.9 | 0.34 | 1.31 | 1.69 | 88.3 |
| 1400 | 1.10 × 10−14 | 45.3 | 0.60 | 1.33 | 1.85 | 188 |
| Layer | Material | Thermal Expansion coef. (10−6 K−1) | Reference |
|---|---|---|---|
| Support | 3YSZ | 10.5 | [36] |
| Activation layers | 10Sc1YSZ | ≤10.0 | Based on reports on Sc2O3-ZrO2-Y2O3 [40,41] |
| Composite membrane | 30 vol % LCCN, 70 vol % 10Sc1YSZ | ~9.9 | Volumetric average of 10Sc1YSZ and LCCN (9.5 × 10−6 K−1) |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Haugen, A.B.; Aguilera, L.M.; Kwok, K.; Molla, T.; Andersen, K.B.; Pirou, S.; Kaiser, A.; Hendriksen, P.V.; Kiebach, R. Exploring the Processing of Tubular Chromite- and Zirconia-Based Oxygen Transport Membranes. Ceramics 2018, 1, 229-245. https://doi.org/10.3390/ceramics1020019
Haugen AB, Aguilera LM, Kwok K, Molla T, Andersen KB, Pirou S, Kaiser A, Hendriksen PV, Kiebach R. Exploring the Processing of Tubular Chromite- and Zirconia-Based Oxygen Transport Membranes. Ceramics. 2018; 1(2):229-245. https://doi.org/10.3390/ceramics1020019
Chicago/Turabian StyleHaugen, Astri Bjørnetun, Lev Martinez Aguilera, Kawai Kwok, Tesfaye Molla, Kjeld Bøhm Andersen, Stéven Pirou, Andreas Kaiser, Peter Vang Hendriksen, and Ragnar Kiebach. 2018. "Exploring the Processing of Tubular Chromite- and Zirconia-Based Oxygen Transport Membranes" Ceramics 1, no. 2: 229-245. https://doi.org/10.3390/ceramics1020019
APA StyleHaugen, A. B., Aguilera, L. M., Kwok, K., Molla, T., Andersen, K. B., Pirou, S., Kaiser, A., Hendriksen, P. V., & Kiebach, R. (2018). Exploring the Processing of Tubular Chromite- and Zirconia-Based Oxygen Transport Membranes. Ceramics, 1(2), 229-245. https://doi.org/10.3390/ceramics1020019





